Abstract
A hallmark of long-term memory formation is the requirement for protein synthesis. Administration of protein synthesis inhibitors impairs long-term memory formation without influencing short-term memory. Rapamycin is a highly specific inhibitor of target of rapamycin complex 1 (TORC1) that has been shown to block protein synthesis and impairs long-term memory. In addition to regulating protein synthesis, TORC1 also phosphorylates Unc-51-like autophagy activating kinase-1 (Ulk-1) to suppress autophagy. As autophagy can be activated by rapamycin (and rapamycin inhibits long-term memory), we aimed to test the hypothesis that autophagy inhibitors would enhance long-term memory. To examine if learning alters autophagasome number, male reporter mice carrying the GFP-LC3 transgene were used. Using these mice, we observed that training in the Morris water maze task increases the number of autophagosomes, a finding contrary to our expectations. For learning and memory studies, male Long Evans rats were used due to their relatively larger size (compared to mice), making it easier to perform intrahippocampal infusions in awake, moving animals. When the autophagy inhibitors 3-methyladenine (3-MA) or Spautin-1 were administered bilaterally into the hippocampii prior to training in the Morris water maze task, the drugs did not alter learning. In contrast, when memory was tested 24 hr later by a probe trial, significant impairments were observed. In addition, intrahippocampal infusion of an autophagy activator peptide (TAT-Beclin-1) improved long-term memory. These results indicate that autophagy is not necessary for learning, but it is required for long-term memory formation.
Keywords: autophagy, hippocampus, long-term memory, water maze
Graphical Abstract
Using GFP-LC3 transgenic mice, we provide evidence that spatial learning increases autophagosome formation in hippocampal neurons. Further, we show that intrahippocampal infusion of inhibitors of autophagy do not affect spatial learning, but impair long-term spatial memory. In contrast, post-training infusion of the activator of autophagy Tat-Beclin1 peptide improves long-term memory.
Introduction
The formation of memory is initiated by learning-related increases in neural activity. Enhanced activity causes the release of neurotransmitters, which engage post-synaptic receptors to alter intracellular second messenger signaling (Atkins et al., 1998; Bibb et al., 2010; Chen et al., 2011; Kandel et al., 2014; Park et al., 2014; Zhang et al., 2013). A large body of evidence indicates that these second messenger signaling molecules elicit covalent modifications of existing proteins that underlie short-term memory (memories lasting for minutes to hours) (Giese and Mizuno, 2013; Roberson and Sweatt, 2001; Sweatt, 2016). Long-term memory (memories lasting for days-to-weeks and longer), by comparison, is dependent on new gene expression and protein synthesis that enhance the efficacy of specific neuronal circuit(s) by causing enhanced neurotransmitter release and morphological changes (e.g. formation of new synaptic connections) (Abel and Klann, 2013; Alberini, 1999; Alberini and Kandel, 2015; Bailey et al., 2015; Knierim, 2015; Runyan and Dash, 2004; Sacktor, 2012; Sekeres et al., 2010). Available amino acids, lipids and other molecules are used as building blocks for these changes. These materials exist in readily accessible pools or can be derived from existing proteins (and/or organelles). Existing proteins are primarily degraded by two intracellular catabolic systems: proteasomal degradation and autophagy. The proteasome system degrades cellular proteins that have been tagged by ubiquitin. Previous studies have demonstrated the role of proteasome activity in memory formation in Aplysia and in rodents (Chain et al., 1999; Hegde et al., 1997; Lopez-Salon et al., 2001). Autophagy is a lysosome-dependent degradation process that helps maintain cellular homeostasis by degrading damaged or improperly folded proteins and protein aggregates. In addition, dysfunctional organelles such as mitochondria are also degraded and the proteins, lipids, and nucleotides recycled. However, the role of autophagy in memory formation has not been examined.
It has been demonstrated that impaired autophagic machinery can lead to the accumulation/aggregation of intracellular proteins and damaged organelles causing neurodegeneration (Lionaki et al., 2015; Menzies et al., 2015; Rubinsztein, 2006). Three types of autophagy have been identified: chaperone-mediated autophagy, microautophagy and macroautophagy (Todde et al., 2009). Chaperone-mediated autophagy targets specific proteins (e.g. proteins containing KFERQ motifs) directly into the lysosome for degradation. This process is thought to be activated during starvation and degrades those proteins not critical for survival. Microautophagy involves the direct engulfment of cellular components by the lysosomes for degradation and is thought to play a role in recycling amino acids and other molecules. Macroautophagy (referred to hereafter as simply autophagy) is a highly regulated process that involves the formation of autophagasomes, a double membrane vesicle that grows around and encapsulates cytoplasmic contents including protein aggregates and damaged organelles. Autophagasomes then fuse with endosomes and lysosomes, leading to degradation of the captured material.
Autophagy is initiated by dephosphorylation of Unc-51-like autophagy activating kinase 1 (Ulk-1), and is suppressed by the phosphorylation of Ulk-1 by the target of rapamycin complex 1 (TORC1). Dephosphorylated Ulk-1 leads to the nucleation and assembly of the initial phagophore membrane by the Beclin 1- class III phosphatidylinositol 3-kinase (class III PI3K) complex (Hara et al., 2008; Nazarko and Zhong, 2013). The expansion and closure of the autophagosome is dependent on multiple proteins, including phosphatidylethanolamine-conjugated LC3 (LC3-II) which is generated from the cytosolic precursor LC3-I. The number of autophagosomes has been shown to correlate with the generation of LC3-II, and its levels have been widely used as a surrogate marker of autophagosome formation (Kabeya et al., 2000). Previous studies by us and others have shown that inhibition of TORC1 activity impairs protein translation and perturbs long-term memory formation (Dash et al., 2006; Stoica et al., 2011). As impaired TORC1 activity has been shown to also increase autophagy, we hypothesized that behavioral training would suppress autophagy and that inhibition of autophagy would facilitate memory formation. Contrary to our expectations, we report that infusions of the autophagy inhibitors 3-MA and Spautin-1 into the hippocampus impair, whereas the autophagy activator peptide TAT-Beclin1 improves, spatial memory.
Materials and Methods
Materials
Male Long-Evans rats (320–380 g; RRID:RGD_60991) were purchased from Charles River Laboratories. GFP-LC3 mice (Mizushima et al., 2004) (in a C57BL/6N background) were provided by the RIKEN BRC through the National Bio-Resource Project of the MEXT, Japan (RRID:IMSR_RBRC00806). 3-methyladenine (3-MA) was obtained from Tocris (#3977). Spautin-1 was purchased from Cellagen Technology (#C3430).
| Antibody | Immunogen | Company, catalogue # | Dilution |
|---|---|---|---|
| LC3B (D11) XP | N-terminus of human LC3B | Cell Signaling #3868, Rabbit mAb RRID:AB_2137707 | 1:1000 |
| Beclin-1 | Peptide surrounding Thr72 of human beclin-1 | Cell Signaling #3738, Rabbit polyclonal RRID:AB_490837 | 1:1000 |
| PI3 Kinase Class III (D4E2) | N-terminus of human PI3K class III | Cell Signaling #3358, Rabbit mAb RRID:AB_2299768 | 1:1000 |
| β-Actin | Ac-Asp-Asp-Asp-Ile-Ala-Ala-Leu-Val-Ile-Asp-Asn-Gly-Ser-Gly-Lys, conjugated to KLH. | Sigma #A2228, mouse mAb RRID:AB_476697 | 1:5000 |
| Atg16L1 (D6D5) | N-terminus of human Atg16L1 protein | Cell Signaling #8089, Rabbit mAb RRID:AB_10950320 | 1:100 |
| GFP | aa1-246 of Aequorea victoria | Abcam Ab1218, Mouse mAb RRID:AB_298911 | 1:250 |
Antibody Characterization
The antibodies used in these experiments were verified for their immunoreactivity by western blotting against rat brain extracts. The apparent molecular weight and pattern of the immunoreactive band(s) was compared to previous publications. For LC3B (RRID:AB_2137707), bands corresponding to 16 kDa (LC3-II) and 18 kDa (LC3-1) were detected (Russo et al., 2011). The Beclin-1 antibody (RRID:AB_490837) gave rise to a band of 60 kDa (Russo et al., 2011), whereas PI3 kinase class III (RRID:AB_2299768) was detected at 100 kDa (Devereaux et al., 2013). β-Actin (RRID:AB_476697) was detected as a 42 kDa immunoreactive band (Simiczyjew et al., 2014). The Atg16L1 antibody (RRID:AB_10950320) recognized two bands corresponding to approximately 65 and 70 kDa (Zhai et al., 2014). Immunoreactivity for GFP (RRID:AB_298911) in the GFP-LC3 mice was compared to that seen in previous publications to ensure consistency in staining (Au et al., 2010). No signal was detected in WT C57BL/6 mice lacking the GFP transgene.
Animals
All protocols involving the use of animals were in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee. The male Long Evans rats (10–12 weeks of age) and GFP-LC3 mice (12 weeks of age) used in this study were housed two to a cage in a controlled environment, restricted access animal room designed to contain a single species. The animal care facility is AAALAC-accredited and under the supervision of a full-time staff of veterinarians. Mice and rats were given ad libitum access to food (irradiated PicoLab Rodent Diet 20) and water, and were maintained on a standard 12hr dark-light cycle. The bedding (Bed-o’Cobs 1/8″ irradiated corncob contact bedding), is changed twice a week by the vivarium staff. Sentinel animals are maintained by the animal care facility and used to determine the health of the animals in terms of pathogens and infestations.
Homozygous GFP-LC3 mice were bred to generate the offspring used in this study. Two females (approximately 8 weeks of age) are placed in the same housing cage as one male. The male is removed once pups are born. At 10 days of age, a small ear punch is made for the preparation of DNA for genotyping to confirm the presence of the transgene. The primers utilized were:
5′-ATAACTTGCTGGCCTTTCCACT-3′
5′-CGGGCCATTTACCGTAAGTTAT-3′
5′-GCAGCTCATTGCTGTTCCTCAA-3′
Primers 1 and 3 give rise to a 350bp product that is indicative of wild-type, primers 1 and 2 amplify the transgene and give rise to a 250 bp product. Hemizygous animals give rise to both the 250bp and 350 bp products. Mice were weaned at 21 days. Siblings were housed together in a single cage.
Intrahippocampal infusion and drug administration
For infusion studies, rats were used as their relatively larger brain (compared to mice) makes it easier to accurately implant the guide cannulae, and to infuse the animals while awake. Rats were anesthetized with 5% isoflurane in a 1:1 N2O/O2 mixture and then maintained with 2.5% isoflurane/1:1 air/O2 mixture via face mask. Bilateral guide cannulas aimed at the dorsal hippocampus (anteroposterior, −3.8 mm from bregma; lateral, ±2.0 mm from midline; ventral, −2.0 mm from the dura), were implanted. Rats were closely monitored during the recovery from surgery until full righting was restored. In addition, rats were monitored for any overt signs of pain such as vocalization and irritability prior to being returned to the vivarium. Rats were then allowed to recover in their home cages for 10 days. Rats were monitored and weighed daily and observed for fluid and food intake for the first five days after surgery. Signs of distress include labored respiration, lying on their side, lack of spontaneous movement, weight loss and/or vocalization to touch. Irritation at the incision site was treated topically with Tritop ointment. After the ten day recovery period, animals were randomly assigned to receive either the study drug or vehicle. Injection cannulas extended 1.20 mm beyond the tips of the guides, yielding a total depth of 3.20 mm below the dura. All injections (1.1 μl/hippocampus of either drug or vehicle) were performed in freely moving animals at a rate of 0.2 μl/min via dual syringe infusion pump. 3-MA was prepared in at stock concentrations of 180mM in sterile saline. Spautin1 was prepared in DMSO then diluted in saline to a final concentration of 450μM in 10% DMSO. After the completion of the behavioral experiments, cannulae placement was verified. No animals were removed from the study due to poor cannulae placement.
Morris Water Maze
All behavioral experiments were performed by an experimenter who was kept blind to the treatment schedule. All of our behavioral experiments are carried out at the same time each day. Groups of animals were trained between 8 –11 am or 2–5 pm. Animals trained in the morning were tested the following morning (24 hr post-training). Similarly, animals trained in the afternoon were tested the following afternoon. For experiments to examine the behavioral consequences of intrahippocampal 3-MA infusion, a total of 20 rats were used (3MA: n=9; vehicle: n=11). For examining the effect of intrahippocampal Spautin-1 infusion, twenty-one rats were used (Spautin-1: n=10; vehicle: n=11). One hour after drug (or vehicle) infusion, animals were trained in the hidden platform version of the Morris water maze task (Guzowski and McGaugh, 1997; Schenk and Morris, 1985). Water maze training was carried out as a single block of 12 trials, with each trial separated by a period of 4 minutes. The platform remained in a constant position across all trials. Each trial started by placing the animal in one of four randomly chosen locations, facing the wall of the tank. Animals were allowed to search for the platform for 60 s. If an animal failed to find the platform, it was placed there by the experimenter. Animals were allowed to remain on the platform for a period of 30 s before being returned to a warming cage between trials. At 24 h after training, the animals were tested for memory retention by a probe trial in which the hidden platform was removed from the maze and the animals were allowed to search for a period of 60 s. Animals were monitored by a video camera linked to tracking software (Ethovision, Noldus; RRID:SCR_000441).
For testing the consequences of intrahippocampal infusion of the TAT-Beclin1 peptide, rats received a block of ten trials, and two days later, another block of 4 trials (to ensure that all animals had learned and remember the location of the hidden platform). Immediately following the 4 reminder trials, rats were randomly assigned to receive bilateral infusions (1.0 μl/side @ 0.25 μl/min) of either 6.0ng TAT-Beclin-1 peptide (n=10) or an equimolar amount of TAT peptide alone (n=9). Twenty-four hours later, the time to find the hidden platform was recorded and used as an index of memory.
Western Blotting
To determine the effects of 3-MA and Spautin-1 in vivo, anesthetized rats were injected with study drug into one dorsal hippocampus (anteroposterior, −3.8 mm from bregma; lateral, ±2.0 mm from midline; ventral, −3.2 mm from the dura) while vehicle was simultaneously infused into the contralateral hippocampus (n=4/group). Hippocampal punches (surrounding the site of infusion) were excised 3hr after the infusion and protein extracts prepared. For all westerns except LC3, proteins were resolved in NuPAGE 4–12% Bis-Tris gels (Thermo Scientific, # NP0323PK2) and transferred to Immobilon-P PVDF membranes (Millipore, # IPVH00010). For LC3 western blots, proteins were resolved in 16% Novex Tris-Glycine gels. Membranes were blocked with 5% BSA in PBS-T (PBS + 0.05% Tween 20) for 60 min at room temperature (RT). Membranes were incubated in primary antibodies diluted in PBST overnight at 4° C. After extensive washing in PBST, membranes were incubated in species-specific secondary antibodies conjugated to horseradish peroxidase for 60 min at RT. Immunoreactivity was detected using an enhanced chemiluminescence (ECL) substrate and images captured using X-ray film. Optical densities of the resultant bands were quantified using ImageJ software (freely available from NIH; RRID:SCR_003070) and normalized against the levels of β-Actin. Mean values for the vehicle controls were calculated and results presented as percent vehicle. Western blot results were replicated using at least two separate gels.
Immunohistochemistry
Coronal brain sections (40 μm) were collected using a cryostat. Brain sections were incubated with primary antibodies overnight at 4°C in PBS containing 0.25% Triton X-100 with 2% BSA and 2.5% normal goat serum. Brain sections were extensively washed in PBST and then incubated for 1 hr in species-specific secondary antibodies conjugated to Alexa-fluors. Images were obtained using either a Zeiss Axiovert fluorescent microscope or a Zeiss LSM 510 confocal microscope. Settings for image acquisition were maintained across images. Double-label immunohistochemistry was carried out as described above, with both primary antibodies incubated simultaneously.
Secondary antibodies (e.g. goat anti-rabbit (RRID:AB_10373124), goat anti-mouse (RRID:AB_2534072)) were raised in a single species, coupled to different fluorochromes (e.g. Alexa488; Alexa568), and incubated with the tissue simultaneously. Double-label images on the confocal microscope were acquired sequentially, then split into red and green channels for presentation. Immunohistochemical analysis of NeuN (RRID:AB_2314891), GFAP (RRID:AB_2109645) and MAP2 (RRID:AB_309685) was performed 2 weeks after training and testing) and was performed using three sections/animal from 5 randomly chosen animals/group.
Quantification of autophagosomes
To examine the effect of water maze training on autophagosome numbers, transgenic mice expressing GFP-LC3 were utilized (Mizushima et al., 2004). GFP-LC3 mice (n=4/group) were trained to criterion (3 consecutive trials finding the platform in under 15 seconds) in the Morris water maze task to ensure learning. These mice were euthanized 15 min after training by sodium pentobarbital overdose followed by transcardial perfusion with 4% paraformaldehyde. A group of untrained GFP-LC3 littermates (n=4) were euthanized as described above and used as controls. Brains were removed and coronal tissue sections (corresponding to approximately −2.0 from bregma) selected for GFP immunohistochemistry. Two non-overlapping images of GFP immunoreactivity in the CA1/CA2 subfield (250μm2) were acquired from the dorsal hippocampus (3 sections each animal) from trained and untrained GFP-LC3 mice (n=4/group). Images were threshold adjusted using Image J. Studies have shown that autophagic-triggering stimuli cause aggregation of LC3-II on autophagosome membranes (Kabeya et al., 2000; Kouroku et al., 2007). In GFP-LC3 mice, this results in GFP-LC3-decorated autophagosomes that can be detected using fluorescent microscopy. As previously described, we focused our analysis on structures within the cell soma or primary apical dendrite of CA1/CA2 pyramidal neurons that had a fluorescent intensity >2 times the intensity of the dendrite, circular, and 0.5 – 2μm in diameter. These parameters were used to identify GFP-LC3 puncta and are consistent with that recommended by Klionski et al., (Klionsky et al., 2016). The total number of GFP-LC3 punta/250μm2 was counted and averaged across the two images/section by an experimenter blind to the group designations. The three sections per animal were averaged to give the number of GFP-LC3 punta/250μm2 for each animal.
Statistical analysis
The number of animals used for each of the experiments is based on our prior experience with the techniques, and is consistent with that utilized by other laboratories (Blum et al., 1999; Dash et al., 2006; Ge et al., 2010; Guzowski and McGaugh, 1997). The behavioral studies presented in this manuscript were carried out using 9–11 animals/group. Using previously collected data (i.e. latency to platform during a probe trial, number of platform crossings), it was found that the standard deviation of the residuals could be anticipated to be between 16% and 21% of the mean. Assuming a minimally detectable effect size of 30% between 2 groups (drug and vehicle), the number of animals required to obtain statistical significance (with a power of 0.9 and an alpha value of 0.05) was found to range between 8 to 12 animals/group. For this reason, we utilized group sizes ranging from 9–11 animals to assess the behavioral consequences of manipulating autophagy. For our biochemistry measures (including counting autophagosomes after behavioral training), we utilized 4 animals/group. This is consistent with the results from a sample size calculation using preliminary western blot data which indicated that an n=4 would be sufficient to detect a minimum difference in means that is three times the estimated standard deviation in a 2 group comparison (with a power of 0.9 and an alpha of 0.05). All data was subjected a Kolmogorov-Smirnov test for normality and an equal variance test. Statistical significance was determined by either repeated-measures two-way ANOVAs followed by appropriate post-hoc analysis (with adjustment for multiple comparisons by the Holm-Sidak method), or by a two-tailed Student’s t-test (for either paired (western blots) or unpaired (memory tests) variables). Comparisons that did not have a normal distribution were compared using a non-parametric analysis (e.g. Mann-Whitney Rank Sum test). Grouped data was examined for outliers (defined as >2 standard deviations of the mean). No values were found to meet this criterion. Data were considered significant at P ≤ 0.05.
Results
Spatial learning increases GFP-LC3 punctae
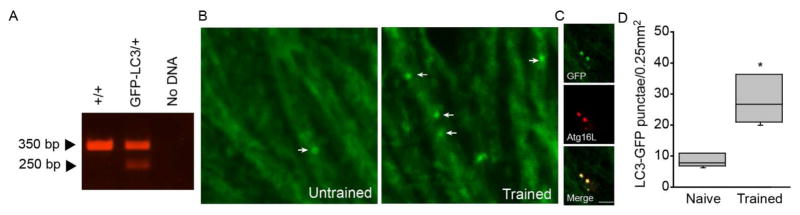
It has been previously demonstrated that spatial learning induces molecular cascades in the CA1/CA2 subfield of the dorsal hippocampus (Blum et al., 1999). To examine the influence of spatial learning on the number of autophagosomes in the CA1/CA2 subfield of the dorsal hippocampus, hemizygous GFP-LC3 mice (n=4/group) were used. Mice were genotyped using DNA prepared from ear punches. The presence of the GFP-LC3 transgene was confirmed by RT-PCR as described in the Methods section. Figure 1A shows that, as expected, primers 1 and 3 gave rise to a 350 bp product, indicative of wild-type. Primers 1 and 2 give rise to a 250 bp product corresponding to the GFP-LC3 transgene. Hemizygous mice display both bands and were used for these studies. Hemizygous GFP-LC3 mice were trained in the Morris water maze to criterion, then euthanized to examine GFP-LC3 puncta by immunohistochemistry. Representative confocal images of GFP staining in the CA1/CA2 apical dendrites from an untrained and a trained GFP-LC3 mouse are shown (Figure 1B). Colocalization of the GFP-LC3 signal with Atg16L (a component of the Atg5/Atg12 autophagosome complex), indicated that the observed GFP-LC3 puncta are likely autophagasomes (Figure 1C). When the number of GFP-LC3 autophagosomes were counted, a significant increase was detected in trained GFP-LC3 mice compared to their untrained counterparts (Figure 1D; two-tailed t-test, t = −3.849, n=4, p=0.012).
Figure 1. Spatial learning increases autophagy.
A) PCR validation of the GFP-LC3 transgene in hemizygous mice. The wild-type gene is indicated by a 350 bp product, whereas the 250 bp band is amplified from the transgene. Hemizygous have both the 350 and 250 bp bands, corresponding to one wild-type allele and the transgene. B) Representative confocal images showing GFP immunoreactivity in the dendrites of CA1/CA2 pyramidal neurons from untrained and Morris water maze trained GFP-LC3 mice. Punctated GFP staining (arrows) appears to be increased as a result of behavioral training, suggesting increased autophagy. C) Confocal images of double-label immunohistochemistry showing colocalization (yellow) of GFP (green) and the autophagy protein Atg16L (red). Scale bars, 10μm. D) Summary results showing that the number of GFP-LC3 puncta was significantly increased as a result of spatial learning. Median is indicated by horizontal line. Error bars represent standard deviation. *, p<0.05.
Intrahippocampal administration of 3-MA or Spautin-1 inhibits autophagy
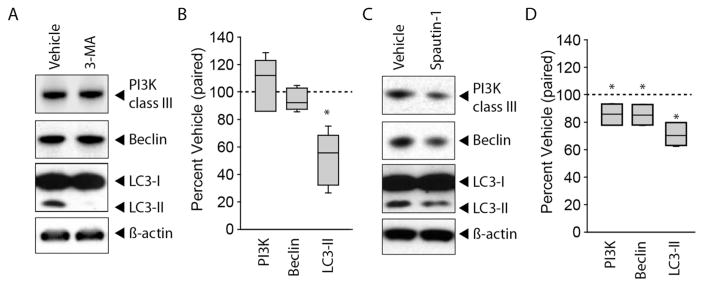
3-MA, which blocks the formation of the autophagosome by inhibiting class III PI3K, has been widely used to study the role of autophagy (Petiot et al., 2000; Seglen and Gordon, 1982). To test the consequences of intrahippocampal 3-MA, individual animals (n=6) were injected with 1.1 μl of 180mM 3-MA to one hippocampus while an equal volume of vehicle (1.1 μl) was simultaneously infused into the contralateral hippocampus (allowing for a within subject comparison). Assuming a volume of 50 μl for the dorsal hippocampus, the equilibrium concentration of 3-MA after infusion is expected to be 3.9 mM. Cell culture studies that have used 3-MA to inhibit autophagy use doses from 4–20 mM (Steiger-Barraissoul and Rami, 2009; Wu et al., 2010). Hippocampal punches (surrounding the site of infusion) were excised 3hr after the infusion and protein extracts prepared. Western blots were carried out using β-Actin as a loading control (Figure 2A). As each animal served as its own control, immunoreactivity was compared between the vehicle- and drug-infused hippocampi using paired t-tests. For presentation purposes, the immunoreactivity of each drug-infused hippocampus was calculated as a percent of its paired vehicle-infused hippocampus. Figure 2B shows that by comparison to the vehicle-infused hippocampus (represented as a dotted line representing 100%), 3-MA significantly decreased the levels of LC3-II (two-tailed t-test for paired variables, t= 4.916, n=6, p=0.005), but had no significant effect on the immunoreactivity of either PI3K (two-tailed t-test for paired variables, t= 0.418, n=6, p=0.697) or Beclin-1 (two-tailed t-test for paired variables, t= −1.164, n=6, p=0.297).
Figure 2. Intrahippocampal administration of 3-MA or Spautin-1 inhibits autophagy.
A) Representative images of western blots and B) summary data showing the immunoreactivity of class III PI3K, Beclin-1, LC3, and β-Actin in hippocampal extracts taken from an animal infused with 3-MA to one hippocampus while an equal volume of vehicle was infused to the contralateral hippocampus of the same animal. 3-MA significantly decreased LC3-II immunoreactivity. C) Representative western blots for class III PI3K, Beclin-1, LC3 and β-Actin in animals receiving intrahippocampal Spautin-1 to one hippocampus while simultaneously receiving vehicle to the contralateral hippocampus of the same animal. D) Summary data showing that Spautin-1 infusion significantly decreased LC3-II immunoreactivity as well as the levels of class III PI3K and Beclin-1. Median for each data set is indicated by horizontal line. Error bars represent standard deviation. Dotted line indicates vehicle value (100%). *, p<0.05.
Spautin-1 is an inhibitor of autophagy that blocks the peptidase activity of Ubiquitin carboxyl-terminal hydrolase 10 (USP10) and Ubiquitin carboxyl-terminal hydrolase 13 (USP13), leading to increased ubiquitination and proteasomal degradation of Beclin-1-containing class III PI3K complexes (Liu et al., 2011). To assess the in vivo consequences of Spautin-1 administration, Spautin-1 (1.1 μl of 450 μM) was injected into one hippocampus and the levels of class III PI3K, Beclin-1, and LC3-II compared to those seen in the vehicle (10% DMSO) -injected contralateral hippocampus of the same animal (n=4; Figure 2C). This dose of Spautin-1 is expected to reach an equilibrium concentration of 9.7 μM. Previous cell culture studies have used 10 μM Spautin-1 to inhibit autophagy (Shao et al., 2014). Figure 2D shows that Spautin-1 modestly, but significantly, reduces the levels of class III PI3K (two-tailed t-test for paired variables, t= −3.366, n=4, p=0.044), Beclin-1 (two-tailed t-test for paired variables, t= −4.056, n=4, p=0.027), and the conversion of LC3-I to LC3-II (two-tailed t-test for paired variables, t= −5.473, n=4, p=0.012), after correction for loading using β-Actin.
Inhibition of autophagy impairs long-term spatial memory
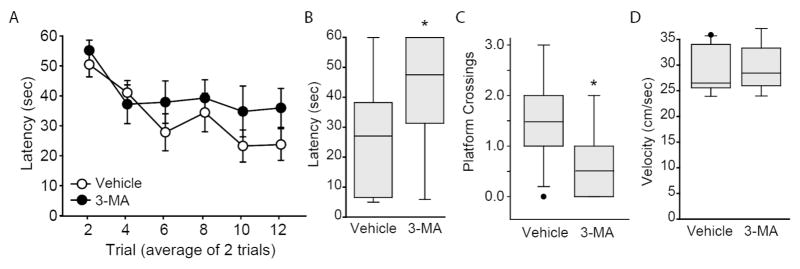
Based on the in vivo biochemical effects of 3-MA and Spautin-1, we next examined if these autophagy inhibitors can influence spatial learning or memory. Male Long Evans rats were implanted with guide cannulae aimed at the dorsal hippocampus and allowed to recover for 10 days. Animals were randomly assigned to receive either 3-MA (1.1 μl of 180 mM 3-MA/side; n=9) or vehicle (n=11). Bilateral infusions (at 0.20 μl/min) were carried out using infusion needles that extended 1.85 mm beyond the end of the guides. One hour after infusion, a single block of 12 training trials (separated by a 4 min ITI) was given in the Morris water maze task as described previously (Blum et al., 1999; Guzowski and McGaugh, 1997) by an experimenter blind to the treatment groups. For presentation purposes, the latency recorded for two trials was averaged. Figure 3A shows that the latency to locate the hidden platform did not significantly differ between the two groups over the course of training (two-way repeated measures ANOVA, F(1,18)=2.52, p=0.13), indicating no influence of the drug on learning. Probe trials in which the hidden platform was removed from the tank and the animals allowed to search for a period of 60 sec were administered 24 hr after training. Time to first platform location (Figure 3B), and number of platform crossings (Figure 3C) are shown. The results show that 3-MA-infused animals required significantly more time to cross the location of the hidden platform (two-tailed t-test for unpaired variables, t=2.967, p=0.009) and crossed the platform significantly less frequently (Mann-Whitney Rank Sum Test, T=64, p=0.016), than did vehicle-infused controls. These differences were not attributable to differences in swimming speed (two-tailed t-test for unpaired variables, t=−0.117, p=0.908; Figure 3D).
Figure 3. Intrahippocampal infusion of 3-MA impairs long-term spatial memory.
A) Rats were given bilateral infusions of either 3-MA (1.1. μl of 180 mM stock/side; n=9) or vehicle (n=11), then given 12 training trials in the Morris water maze task. Training data are presented as mean ± SEM. Memory was tested 24hr after training. Rats infused with 3-MA had impaired spatial memory as indicated by B) increased latency and C) fewer platform crossings. D) No differences between the groups were observed in swimming speed. Median for each data set is indicated by horizontal line. Error bars represent 90th and 10th percentile. *, p<0.05.
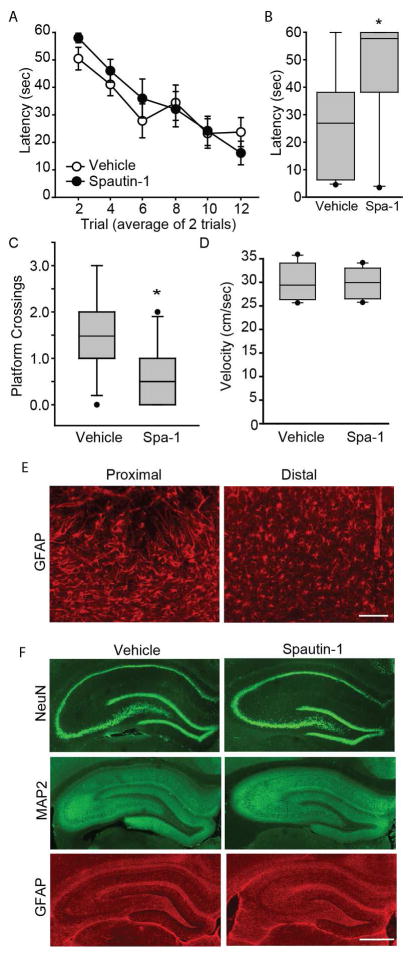
To examine the influence of Spautin-1 on hippocampus-dependent learning and memory, cannulated rats were randomly assigned to be bilaterally infused with either Spautin-1 (1.1 μl of a 450 μM stock; n=10), or an equal volume of vehicle (n=11). After a 1 hr recovery period, animals were trained and tested in the water maze task as described above. Figure 4A shows that there was no significant difference in latency to locate the hidden platform over the course of training between the two groups (two-way repeated measures ANOVA F(1,19)=0.33, p=0.575). Quantification of probe trial performance (given 24hr after training) revealed that Spautin-1 treated animals took significantly longer time to cross the previous location of the platform (two-tailed t-test for unpaired variables, t=−2.97; p=0.008; Figure 4B) and crossed the platform less frequently (Mann-Whitney Rank Sum Test, T=81.50, p=0.034; Figure 4C) than simultaneously tested, vehicle-infused animals. No significant differences in swimming speed were detected between the two groups (two-tailed t-test for unpaired variables, t=−0.45, p=0.655; Figure 4D).
Figure 4. Intrahippocampal infusion of Spautin-1 impairs long-term spatial memory.
A) Rats were given bilateral infusions of either Spautin-1 (1.1 μl of 450 μM stock/side; n=10) or vehicle (n=11). One hr later, rats were given 12 training trials in the abbreviated version of the Morris water maze task. Training data are presented as mean ± SEM. Memory was tested 24hr after training. Rats infused with Spautin-1 had impaired spatial memory as indicated by B) increased latency and C) fewer platform crossings. D) Swimming speed was not different between the Spautin-1- and vehicle-infused animals. Median for each data set is indicated by horizontal line. Error bars represent 90th and 10th percentile. *, p<0.05. E) Representative photomicrographs showing activated astrocytes (immunostained with GFAP) in the cortex immediately adjacent to the cannulation site when examined 2 weeks post-infusion. Distal to the cannulation site, ramified “resting” astrocytes were observed. Scale bar, 100μm. F) Representative photomicrographs of 2-week post-infusion hippocampi immunostained for NeuN (neurons), MAP2 (dendrites), and GFAP (astrocytes). No differences were observed between the vehicle- and Spautin-1-infused hippocampi. Scale bar, 1mm.
As there is accumulating evidence of the interplay between autophagy and apoptosis, (Marino et al., 2014) it is possible that the memory-impairing effects of inhibiting autophagy we observed could be attributed to drug-induced neuronal loss and/or damage. To examine this possibility, sections from Spautin-1 infused animals (2 weeks post-infusion) were immunostained for NeuN (a marker of neurons), microtubule associated protein-2 (MAP2, a marker of dendrites) and glial fibrillary acidic protein (GFAP, a marker of astrocytes). Although immediately proximal to the cannulation site some reactive gliosis could be observed in the cortex (Figure 4E), this appeared to be injection injury-related as it was observed in in both the vehicle- and drug-infused animals. Figure 4F shows that no overt cell loss, dendritic damage, or gliosis was seen in either the vehicle or drug treated animals.
Activation of autophagy improves memory
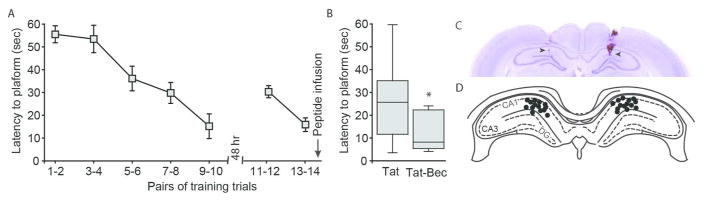
Previously, it has been shown that a peptide derived from the Golgi-associated plant pathogenesis-related protein 1 (GAPR-1) binding domain of Beclin-1 can activate autophagy by releasing Beclin-1 from the Beclin-GAPR-1 complex. In addition, when this peptide is attached to HIV-TAT (a cell penetrating peptide), it activates autophagy both in vitro and when administered to rodents (Shoji-Kawata et al., 2013). In order to examine if this peptide can influence memory, cannulated rats were trained in the water maze. For this study, rats received a block of ten trials, and two days later, another block of 4 reminder trials to ensure that all rats had learned and remember the location of the platform (Figure 5A). Immediately following the 4th reminder trial, rats were randomly assigned to one of two groups: TAT-Beclin1 peptide or TAT alone peptide. TAT-Beclin1 group received bilateral infusions (1.0 μl/side @ 0.25 μl/min) of 6.0 ng TAT-Beclin-1. Control animals received an equimolar amount of TAT peptide (1.0 μl/side of 2.5 ng). We have previously utilized the TAT sequence as a control, and have not observed any demonstrable influences of this peptide on long-term memory (Blum and Dash, 2004). Memory was tested 24 hr later. Figure 5B shows that trained animals treated with TAT-Beclin-1 peptide found the hidden platform significantly faster (two-tailed t-test for unpaired variables, t=−2.284, p=0.036) than animals treated with TAT alone, suggesting enhanced memory.
Figure 5. Post-training, intrahippocampal infusion of TAT-Beclin-1 improves long-term spatial memory.
A) Rats were trained in the Morris water maze, then randomly assigned to receive either 6.0ng/side TAT-Beclin-1 peptide or an equimolar amount of TAT alone peptide. Training data are presented as mean ± SEM. Memory was tested 24hr after training. B) When tested for their memory, animals infused with TAT-Beclin-1 found the hidden platform significantly faster than those treated with TAT. Median for each data set is indicated by horizontal line. Error bars represent 90th and 10th percentile. *, p<0.05. C) Representative image of a cresyl violet stained tissue section indicating the terminus of the infusion (arrow heads). D) All infusion sites were found to be within the dorsal hippocampus (black dots).
After the completion of memory testing, animals were euthanized and brains examined for cannulae location. The representative image of a brain section (stained with cresyl violet) indicates the location of the infusion site (arrowheads; Figure 5C). The summary data presented in Figure 5D shows that all animals used in this study had infusion sites (black circles) that terminated in the dorsal hippocampus.
Discussion
Using pharmacological agents as a well as GFP-LC3 mice, we investigated the role of autophagy in spatial memory formation. The results presented herein revealed three key findings: 1) Spatial learning increases the number of GFP-LC3 autophagosomes in the hippocampus; 2) blockade of autophagy in the hippocampus does not interfere with spatial learning; and 3) autophagy is required for long-term memory. These conclusions are based upon the memory impairing effects of intrahippocampal administration of 3-MA and Spautin-1, and administration of an autophagy inducing peptide which improved memory.
3-MA is a widely used inhibitor of autophagy, whose mechanism of action has been linked to its ability to inhibit class III PI3K (Seglen and Gordon, 1982). However, recent studies have revealed that 3-MA has differential effects on autophagy (i.e. activating or inhibiting) depending on the cellular context and duration of exposure. These effects are thought to be due to its ability to potently, but transiently inhibit class III PI3K (thereby inhibiting autophagy) while persistently inhibiting class I PI3K (thereby activating autophagy) (Wu et al., 2010). In addition, 3-MA has been found to stimulate PKA (via its capacity to increase intracellular cAMP levels) in adipocytes (Heckmann et al., 2013). Although this effect has not been examined in neurons, an increase in cAMP levels would be anticipated to improve long-term memory, (Barad et al., 1998) an effect opposite to that observed here. Due to these potentially confounding actions of 3-MA, we verified our findings using Spautin-1. Spautin-1 (specific and potent autophagy inhibitor-1) has been shown to directly and potently inhibit the peptidase activity of UPS10 and UPS13, resulting in increased Beclin 1 and class III PI3K ubiquitination and degradation (Liu et al., 2011). Consistent with this, we observed that Spautin-1 reduces Beclin-1 and class III PI3K levels. As no other mechanisms of action have been identified for Spautin-1, its ability to also impair long-term memory provides further evidence of a role for autophagy in memory. Of interest, neither 3-MA nor Spautin-1 influenced learning, which indicates that these autophagy inhibitors do not cause aberrant neuronal activity nor cell death. Consistent with this, histopathological analysis did not reveal any visible cell loss/damage in Spautin-1-treated animals as compared to vehicle controls (Figure 4).
A recent report has demonstrated that restoration of autophagic flux is sufficient to improve memory in animals exposed to chronic unpredictable stress (Gu et al., 2014). In aging, reduced autophagic flux is thought to cause accumulation of dysfunctional or damaged molecules/organelles, leading to age-related memory decline, (Gkikas et al., 2014) an effect that can be alleviated by activation of autophagy. For example, in aged Drosophilla, spermidine-induced autophagy reduces the aggregation of ubiquitinated proteins and improves memory (Gupta et al., 2013). Using TgCRND8 mice (a model of Alzheimer’s Disease), Yang et al., found that increasing autophagic-lysosomal function reduced the accumulation of β-amyloid deposits and prevented the development of memory dysfunction (Yang et al., 2011). While these and other studies have demonstrated a critical role for autophagy in pathologies that are associated with memory dysfunction, the present study is, to the best of our knowledge, the first to observe a training-related increase in autophagosome formation and ascribe a role for autophagy in long-term memory formation in normal animals.
A large body of evidence supports the premise that long-term memory requires the synthesis of new proteins for synaptic remodeling and morphological changes (Bourtchuladze et al., 1994; Dash et al., 1990; Kandel et al., 2014; Sacktor, 2012). One function of autophagy is to degrade cellular proteins to generate amino acids that can be used for subsequent protein synthesis (Glick et al., 2010; Shen and Ganetzky, 2009). Thus, it is plausible that the degradation of proteins by autophagy is required for recycling proteins for new protein synthesis. Alternatively, autophagy can selectively degrade molecules that repress memory (e.g. transcriptional repressors) thereby facilitating long-term memory formation (Shehata et al., 2012). This suggests that stimulation of autophagy may be beneficial for long-term memory formation. Consistent with this possibility, we found that intrahippocampal administration of the autophagy activator TAT-Beclin-1 enhances spatial long-term memory (Figure 5). At present, however, it is not known if autophagy plays a general role in protein recycling for protein synthesis and synapse formation, or if specific proteins are targeted for autophagy that facilitate memory formation. Further, as only male animals were used in order to provide proof-in-principle evidence that autophagy plays a role in memory formation, it is not known if this role differs between males and females. These questions will need to be the subject of future studies.
Significance Statement.
Autophagy is an intracellular degradation process by which cells recycle cytoplasmic components in order to respond to energy needs, remove damaged organelles, and eliminate pathogens. Recent studies have shown that impaired autophagy is linked to a number of diseases ranging from asthma to Parkinson’s Disease. However, whether autophagy plays a role in learning and memory has not been fully explored. In this paper, we demonstrate that compounds that inhibit autophagy impair, whereas activation of autophagy facilitates, long-term memory. These findings may have implications in the treatment of diseases associated with memory dysfunction.
Acknowledgments
Support: This work was supported by the National Institutes of Health under grant numbers NS090935 and NS087149.
The authors thank Dr. Noboru Mizushima of the Department of Biochemistry and Molecular Biology, The University of Tokyo, Japan for allowing us to use the GFP-LC3 mice for these studies.
Footnotes
Conflicts of Interest Statement. The authors report no conflicts of interest.
Author’s Roles. All authors had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Conceptualization, P.K.D.; Methodology, P.K.D.; Investigation, M.J.H, J.Z., K.T.T., N.S.R., K.N.H., J.S.M., and A.N.M.; Formal Analysis, P.K.D., K.N.H., and A.N.M.; Writing – Original Draft, P.K.D. and A.N.M.; Writing – Review & Editing, M.J.H., J.Z., K.T.T., N.S.R., K.N.H., A.N.M., and P.K.D.; Visualization, P.K.D. and A.N.M.; Supervision, P.K.D.; Funding Acquisition, P.K.D.
References
- Abel T, Klann E. Molecular and cellular cognition: Neurobiology of Learning and Memory Special Issue 2013. Neurobiol Learn Mem. 2013;105:1–2. doi: 10.1016/j.nlm.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Alberini CM. Genes to remember. J Exp Biol. 1999;202(Pt 21):2887–2891. doi: 10.1242/jeb.202.21.2887. [DOI] [PubMed] [Google Scholar]
- Alberini CM, Kandel ER. The regulation of transcription in memory consolidation. Cold Spring Harb Perspect Biol. 2015;7:a021741. doi: 10.1101/cshperspect.a021741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- Au AK, Bayir H, Kochanek PM, Clark RS. Evaluation of autophagy using mouse models of brain injury. Biochim Biophys Acta. 2010;1802:918–923. doi: 10.1016/j.bbadis.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CH, Kandel ER, Harris KM. Structural Components of Synaptic Plasticity and Memory Consolidation. Cold Spring Harb Perspect Biol. 2015;7:a021758. doi: 10.1101/cshperspect.a021758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barad M, Bourtchouladze R, Winder DG, Golan H, Kandel E. Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc Natl Acad Sci U S A. 1998;95:15020–15025. doi: 10.1073/pnas.95.25.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb JA, Mayford MR, Tsien JZ, Alberini CM. Cognition enhancement strategies. J Neurosci. 2010;30:14987–14992. doi: 10.1523/JNEUROSCI.4419-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum S, Dash PK. A cell-permeable phospholipase Cgamma1-binding peptide transduces neurons and impairs long-term spatial memory. Learn Mem. 2004;11:239–243. doi: 10.1101/lm.74104. [DOI] [PubMed] [Google Scholar]
- Blum S, Moore AN, Adams F, Dash PK. A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory. J Neurosci. 1999;19:3535–3544. doi: 10.1523/JNEUROSCI.19-09-03535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP- responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Chain DG, Schwartz JH, Hegde AN. Ubiquitin-mediated proteolysis in learning and memory. Mol Neurobiol. 1999;20:125–142. doi: 10.1007/BF02742438. [DOI] [PubMed] [Google Scholar]
- Chen DY, Stern SA, Garcia-Osta A, Saunier-Rebori B, Pollonini G, Bambah-Mukku D, Blitzer RD, Alberini CM. A critical role for IGF-II in memory consolidation and enhancement. Nature. 2011;469:491–497. doi: 10.1038/nature09667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash PK, Hochner B, Kandel ER. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature. 1990;345:718–721. doi: 10.1038/345718a0. [DOI] [PubMed] [Google Scholar]
- Dash PK, Orsi SA, Moore AN. Spatial memory formation and memory-enhancing effect of glucose involves activation of the tuberous sclerosis complex-Mammalian target of rapamycin pathway. J Neurosci. 2006;26:8048–8056. doi: 10.1523/JNEUROSCI.0671-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereaux K, Dall’Armi C, Alcazar-Roman A, Ogasawara Y, Zhou X, Wang F, Yamamoto A, De CP, Di PG. Regulation of mammalian autophagy by class II and III PI 3-kinases through PI3P synthesis. PLoS One. 2013;8:e76405. doi: 10.1371/journal.pone.0076405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Dong Z, Bagot RC, Howland JG, Phillips AG, Wong TP, Wang YT. Hippocampal long-term depression is required for the consolidation of spatial memory. Proc Natl Acad Sci U S A. 2010;107:16697–16702. doi: 10.1073/pnas.1008200107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese KP, Mizuno K. The roles of protein kinases in learning and memory. Learn Mem. 2013;20:540–552. doi: 10.1101/lm.028449.112. [DOI] [PubMed] [Google Scholar]
- Gkikas I, Petratou D, Tavernarakis N. Longevity pathways and memory aging. Front Genet. 2014;5:155. doi: 10.3389/fgene.2014.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221:3–12. doi: 10.1002/path.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu HF, Nie YX, Tong QZ, Tang YL, Zeng Y, Jing KQ, Zheng XL, Liao DF. Epigallocatechin-3-gallate attenuates impairment of learning and memory in chronic unpredictable mild stress-treated rats by restoring hippocampal autophagic flux. PLoS One. 2014;9:e112683. doi: 10.1371/journal.pone.0112683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta VK, Scheunemann L, Eisenberg T, Mertel S, Bhukel A, Koemans TS, Kramer JM, Liu KS, Schroeder S, Stunnenberg HG, Sinner F, Magnes C, Pieber TR, Dipt S, Fiala A, Schenck A, Schwaerzel M, Madeo F, Sigrist SJ. Restoring polyamines protects from age-induced memory impairment in an autophagy-dependent manner. Nat Neurosci. 2013;16:1453–1460. doi: 10.1038/nn.3512. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, McGaugh JL. Antisense oligodeoxynucleotide-mediated disruption of hippocampal cAMP response element binding protein levels impairs consolidation of memory for water maze training. Proc Natl Acad Sci U S A. 1997;94:2693–2698. doi: 10.1073/pnas.94.6.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T, Takamura A, Kishi C, Iemura S, Natsume T, Guan JL, Mizushima N. FIP200, a ULK-interacting protein, is required for autophagosome formation in mammalian cells. J Cell Biol. 2008;181:497–510. doi: 10.1083/jcb.200712064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann BL, Yang X, Zhang X, Liu J. The autophagic inhibitor 3-methyladenine potently stimulates PKA-dependent lipolysis in adipocytes. Br J Pharmacol. 2013;168:163–171. doi: 10.1111/j.1476-5381.2012.02110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde AN, Inokuchi K, Pei W, Casadio A, Ghirardi M, Chain DG, Martin KC, Kandel ER, Schwartz JH. Ubiquitin C-terminal hydrolase is an immediate-early gene essential for long-term facilitation in Aplysia. Cell. 1997;89:115–126. doi: 10.1016/s0092-8674(00)80188-9. [DOI] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER, Dudai Y, Mayford MR. The molecular and systems biology of memory. Cell. 2014;157:163–186. doi: 10.1016/j.cell.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12:1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ. From the GPS to HM: Place cells, grid cells, and memory. Hippocampus. 2015;25:719–725. doi: 10.1002/hipo.22453. [DOI] [PubMed] [Google Scholar]
- Kouroku Y, Fujita E, Tanida I, Ueno T, Isoai A, Kumagai H, Ogawa S, Kaufman RJ, Kominami E, Momoi T. ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 2007;14:230–239. doi: 10.1038/sj.cdd.4401984. [DOI] [PubMed] [Google Scholar]
- Lionaki E, Markaki M, Palikaras K, Tavernarakis N. Mitochondria, autophagy and age-associated neurodegenerative diseases: New insights into a complex interplay. Biochim Biophys Acta. 2015;1847:1412–1423. doi: 10.1016/j.bbabio.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Liu J, Xia H, Kim M, Xu L, Li Y, Zhang L, Cai Y, Norberg HV, Zhang T, Furuya T, Jin M, Zhu Z, Wang H, Yu J, Li Y, Hao Y, Choi A, Ke H, Ma D, Yuan J. Beclin1 controls the levels of p53 by regulating the deubiquitination activity of USP10 and USP13. Cell. 2011;147:223–234. doi: 10.1016/j.cell.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Salon M, Alonso M, Vianna MR, Viola H, Mello e Souza, Izquierdo I, Pasquini JM, Medina JH. The ubiquitin-proteasome cascade is required for mammalian long-term memory formation. Eur J Neurosci. 2001;14:1820–1826. doi: 10.1046/j.0953-816x.2001.01806.x. [DOI] [PubMed] [Google Scholar]
- Marino G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol. 2014;15:81–94. doi: 10.1038/nrm3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies FM, Fleming A, Rubinsztein DC. Compromised autophagy and neurodegenerative diseases. Nat Rev Neurosci. 2015;16:345–357. doi: 10.1038/nrn3961. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarko VY, Zhong Q. ULK1 targets Beclin-1 in autophagy. Nat Cell Biol. 2013;15:727–728. doi: 10.1038/ncb2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park AJ, Havekes R, Choi JH, Luczak V, Nie T, Huang T, Abel T. A presynaptic role for PKA in synaptic tagging and memory. Neurobiol Learn Mem. 2014;114:101–112. doi: 10.1016/j.nlm.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petiot A, Ogier-Denis E, Blommaart EF, Meijer AJ, Codogno P. Distinct classes of phosphatidylinositol 3′-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J Biol Chem. 2000;275:992–998. doi: 10.1074/jbc.275.2.992. [DOI] [PubMed] [Google Scholar]
- Roberson ED, Sweatt JD. Memory-forming chemical reactions. Rev Neurosci. 2001;12:41–50. doi: 10.1515/revneuro.2001.12.1.41. [DOI] [PubMed] [Google Scholar]
- Rubinsztein DC. The roles of intracellular protein-degradation pathways in neurodegeneration. Nature. 2006;443:780–786. doi: 10.1038/nature05291. [DOI] [PubMed] [Google Scholar]
- Runyan JD, Dash PK. Inhibition of hippocampal protein synthesis following recall disrupts expression of episodic-like memory in trace conditioning. Hippocampus. 2004;15:333–339. doi: 10.1002/hipo.20055. [DOI] [PubMed] [Google Scholar]
- Russo R, Berliocchi L, Adornetto A, Varano GP, Cavaliere F, Nucci C, Rotiroti D, Morrone LA, Bagetta G, Corasaniti MT. Calpain-mediated cleavage of Beclin-1 and autophagy deregulation following retinal ischemic injury in vivo. Cell Death Dis. 2011;2:e144. doi: 10.1038/cddis.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor TC. Memory maintenance by PKMzeta--an evolutionary perspective. Mol Brain. 2012;5:31. doi: 10.1186/1756-6606-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk F, Morris RG. Dissociation between components of spatial memory in rats after recovery from the effects of retrohippocampal lesions. Exp Brain Res. 1985;58:11–28. doi: 10.1007/BF00238949. [DOI] [PubMed] [Google Scholar]
- Seglen PO, Gordon PB. 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Natl Acad Sci U S A. 1982;79:1889–1892. doi: 10.1073/pnas.79.6.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekeres MJ, Neve RL, Frankland PW, Josselyn SA. Dorsal hippocampal CREB is both necessary and sufficient for spatial memory. Learn Mem. 2010;17:280–283. doi: 10.1101/lm.1785510. [DOI] [PubMed] [Google Scholar]
- Shao S, Li S, Qin Y, Wang X, Yang Y, Bai H, Zhou L, Zhao C, Wang C. Spautin-1, a novel autophagy inhibitor, enhances imatinib-induced apoptosis in chronic myeloid leukemia. Int J Oncol. 2014;44:1661–1668. doi: 10.3892/ijo.2014.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehata M, Matsumura H, Okubo-Suzuki R, Ohkawa N, Inokuchi K. Neuronal stimulation induces autophagy in hippocampal neurons that is involved in AMPA receptor degradation after chemical long-term depression. J Neurosci. 2012;32:10413–10422. doi: 10.1523/JNEUROSCI.4533-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Ganetzky B. Autophagy promotes synapse development in Drosophila. J Cell Biol. 2009;187:71–79. doi: 10.1083/jcb.200907109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji-Kawata S, Sumpter R, Leveno M, Campbell GR, Zou Z, Kinch L, Wilkins AD, Sun Q, Pallauf K, MacDuff D, Huerta C, Virgin HW, Helms JB, Eerland R, Tooze SA, Xavier R, Lenschow DJ, Yamamoto A, King D, Lichtarge O, Grishin NV, Spector SA, Kaloyanova DV, Levine B. Identification of a candidate therapeutic autophagy-inducing peptide. Nature. 2013;494:201–206. doi: 10.1038/nature11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simiczyjew A, Mazur AJ, Popow-Wozniak A, Malicka-Blaszkiewicz M, Nowak D. Effect of overexpression of beta- and gamma-actin isoforms on actin cytoskeleton organization and migration of human colon cancer cells. Histochem Cell Biol. 2014;142:307–322. doi: 10.1007/s00418-014-1199-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger-Barraissoul S, Rami A. Serum deprivation induced autophagy and predominantly an AIF-dependent apoptosis in hippocampal HT22 neurons. Apoptosis. 2009;14:1274–1288. doi: 10.1007/s10495-009-0396-9. [DOI] [PubMed] [Google Scholar]
- Stoica L, Zhu PJ, Huang W, Zhou H, Kozma SC, Costa-Mattioli M. Selective pharmacogenetic inhibition of mammalian target of Rapamycin complex I (mTORC1) blocks long-term synaptic plasticity and memory storage. Proc Natl Acad Sci U S A. 2011;108:3791–3796. doi: 10.1073/pnas.1014715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweatt JD. Neural Plasticity & Behavior - Sixty Years of Conceptual Advances. J Neurochem. 2016;139:179–199. doi: 10.1111/jnc.13580. [DOI] [PubMed] [Google Scholar]
- Todde V, Veenhuis M, van der Klei IJ. Autophagy: principles and significance in health and disease. Biochim Biophys Acta. 2009;1792:3–13. doi: 10.1016/j.bbadis.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Wu YT, Tan HL, Shui G, Bauvy C, Huang Q, Wenk MR, Ong CN, Codogno P, Shen HM. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J Biol Chem. 2010;285:10850–10861. doi: 10.1074/jbc.M109.080796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DS, Stavrides P, Mohan PS, Kaushik S, Kumar A, et al. Reversal of autophagy dysfunction in the TgCRND8 mouse model of Alzheimer’s disease ameliorates amyloid pathologies and memory deficits. Brain. 2011;134:258–277. doi: 10.1093/brain/awq341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Z, Wu F, Dong F, Chuang AY, Messer JS, Boone DL, Kwon JH. Human autophagy gene ATG16L1 is post-transcriptionally regulated by MIR142–3p. Autophagy. 2014;10:468–479. doi: 10.4161/auto.27553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Chen G, Kuang H, Tsien JZ. Mapping and deciphering neural codes of NMDA receptor-dependent fear memory engrams in the hippocampus. PLoS One. 2013;8:e79454. doi: 10.1371/journal.pone.0079454. [DOI] [PMC free article] [PubMed] [Google Scholar]