Abstract
Epidemiologic data demonstrate sex differences in autoimmune diseases, immune responses against infection, and anti-tumor immunity, and accumulating evidence suggests a major role for sex hormones in mediating these differences. Here, we review recent advances in understanding how sex hormones regulate T cell responses to alter susceptibility to autoimmunity. While sex hormones can directly alter gene transcriptional programs of T cells, we focus here on how sex hormones alter T cell development and function through their effects on thymic stromal cells and innate cell types. In addition to contributing to our understanding of sex differences, these findings also have implications for the therapeutic use of sex hormones and sex hormone modulators, which are now being prescribed to increasing numbers of patients for a wide variety of indications.
Introduction
Sex differences, defined by distinct chromosome content, unique reproductive organs and sex-determined steroid hormone levels, dictate many aspects of our being. It is now well-documented that our immune responses are critically determined by sex, as illustrated by the predominance of females with multiple autoimmune diseases, where female to male ratios can approach 11:1 (1). Sex dimorphism in anti-tumor immunity and responses to infection/vaccination are also apparent (reviewed in (2)). For instance, in a recent study of men and women receiving trivalent inactivated seasonal influenza vaccine, increased pro-inflammatory cytokines and antibody responses were seen in females (3). Yet despite the preponderance of evidence, disease-related studies have historically ignored the contribution of sex (4). Males or male-derived cells have routinely been used to study many aspects of human health and until the 1990s, females of childbearing age were excluded from drug trials (5). It was not until 2015, after much lobbying primarily by female congressional representatives and scientists, that NIH announced a policy to ensure that sex is considered as a biological variable and that all NIH funded preclinical studies include both males and females. This policy has resulted in a wealth of new data and we are beginning to uncover the underlying immune mechanisms that dictate these differences. Here we provide a brief overview of recent advances in our understanding of sex-dependent immune responses, with a focus on how sex hormones differentially regulate T lymphocytes to alter susceptibility to disease.
Sex hormones and their receptors
Estrogens, progesterone and androgens are the major gonadal sex hormones (reviewed in (6)). Estrogens include estrone, 17β-estradiol (E2) and estriol (E3) and are derived from aromatization of androgens by a single aromatase (P450aro) enzyme. P450aro is expressed in steroidogenic tissue (ovarian granulosa cells in premenopausal women as well as the placenta during pregnancy) and in nonglandular tissue (fat and bone). Progesterone is also produced by ovarian granulosa cells, the adrenal glands, the corpus luteum during the menstrual cycle, and the placenta. The major sources of androgens are the testes and adrenal glands - Leydig cells of the testes are the major source of testosterone in males, and zona reticularis of the adrenal gland produces dehydroepiandrosterone sulfate (DHEAS) in males and female. Testosterone is converted to dihydrotestosterone (DHT), a more biologically active form of testosterone, by 5α-reductase in testosterone’s target tissues (scalp and other peripheral tissues, male reproductive tissues).
The classical sex hormone receptors - the estrogen receptors (ER) ERα and ERβ, the progesterone receptor (PR) isoforms PRA and PRB, and the androgen receptor (AR) - function as hormone activated transcription factors that bind to hormone-response elements in target genes to elicit gene expression (reviewed in (7)). As such, sex hormone/receptor complexes can regulate transcription through direct interactions with specific DNA sequences. Known as hormone response elements, these sequences have been identified at promoters of several genes with critical roles in immune responses. For instance, the interferon-gamma (IFNγ) promoter possesses four putative estrogen response elements, and E2 drives the expression of promoter-reporter constructs in transiently transfected lymphoid cells (8). This finding suggests the possibility that higher estrogen levels in females drive increased T cell IFNγ production and, in this way, predispose females to IFNγ–mediated autoimmune conditions. At the same time, androgen/androgen receptor action in CD4+ T cells may also prevent autoimmunity in males by directly increasing expression of Ptpn1, a phosphatase that inhibits T helper 1 (Th1) differentiation (9). Androgen/androgen receptor complexes can also directly induce anti-inflammatory IL-10 expression by CD4+ T cells, which has been proposed to underlie male protection from central nervous system (CNS) autoimmunity (10). These findings suggest that sex differences in autoimmunity may be attributed to direct alteration of T cell transcriptional profiles by sex hormones.
It is now clear, however, that this paradigm is overly simplistic. First, sex hormone-receptor interactions can exert their effects through DNA-independent mechanisms, such as the activation of cytoplasmic signal transduction pathways (11). GPER1, for example, is a G protein coupled ER localized to the cell membrane that elicits the activation of a variety of cytoplasmic signaling molecules including ERK/MAPK, PKC, PI3K and cAMP (12). Moreover, ERα can also exert its effects through cytoplasmic signaling (13), and activation of non-transcriptional signaling mechanisms have also been described for PR and AR (14, 15). In addition to activating signal transduction cascades, sex hormones can also alter gene expression through their effects on epigenetic modifications (16). In T regulatory cells, for example, androgen exposure alters acetylation of histone H4 at the FOXP3 locus (17).
Second, sex hormones shape T cell responses not only through direct effects on T cells, but also indirectly through modulation of other immune cell types. While there are some notable differences between human and mouse cells in sex hormone receptor expression patterns, ERs, PRs and ARs have been identified at the RNA and/or protein level not only in T cells, but also in thymic epithelial cells (18-20), B cells (21), dendritic cells, macrophages and monocytes, natural killer (NK) cells, Type 2 innate lymphoid cells (ILC2s) and granulocytes, including neutrophils, eosinophils and mast cells (reviewed in (22)). In the following sections, we highlight recent studies showing how sex hormones control T cell phenotype/function during T cell development in the thymus by modulating thymic epithelial cells. We also discuss the hormone-driven interactions of mast cells and ILC2s that can alter the differentiation of CD4+ T cells in the periphery.
Sex hormones, thymic epithelial cells, and T cell development
The thymus is a critical site for generating a diverse T cell repertoire while maintaining self-tolerance, and sex hormones play a critical role in shaping thymic function (Figure 1). It has been recognized for decades that sex hormones influence the thymus: thymic enlargement occurs with castration of male mice, while shrinkage occurs with androgen administration (23). In parallel, increased thymic output is seen in hypogonadal men, and this is reversed with androgen replacement therapy (24). Indeed, multiple studies have linked sex steroids with thymic involution that occurs with puberty and normal aging (reviewed in (25)).
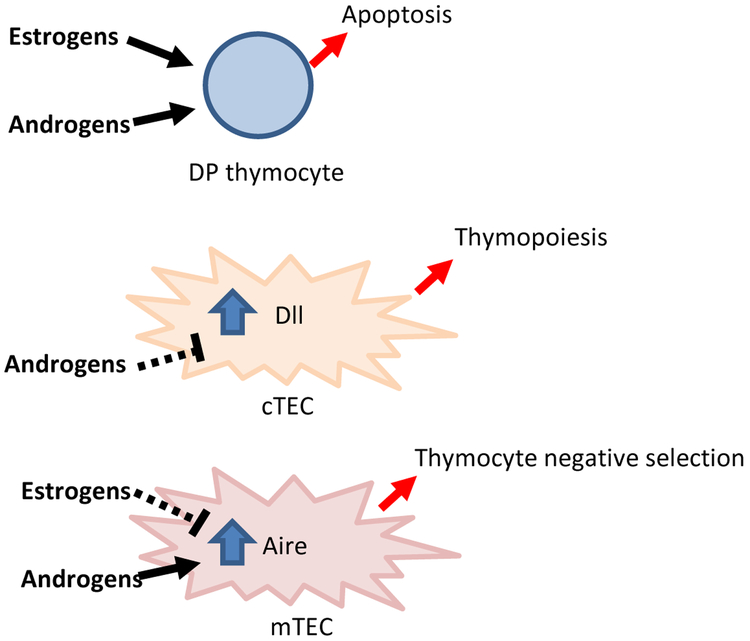
Figure 1.
Sex hormone effects on thymus cell types. Sex hormones induce apoptosis of double positive (DP) progenitors. Additionally, sex hormones directly modulate gene expression in thymic epithelial cells. Androgens downregulate Dll expression in cortical thymic epithelial cells (cTECs) to inhibit thymopoiesis while upregulating Aire in mTECs to promote negative selection of self-reactive T cells. Estrogen downregulates Aire in mTEC to inhibit thymocyte negative selection.
In the thymus, androgens can directly mediate apoptosis of developing T cells (especially double positive thymocytes) through the release of TNF-alpha (26). However, sex steroid receptor expression is significantly higher on thymic epithelial cells rather than thymocytes, and thymic atrophy has consequently been attributed to sex steroid interactions with thymic epithelial cells (18). These cells can be further categorized into cortical and medullary thymic epithelial cells (cTECs and mTECs), based on their anatomic localization within the thymus and their distinct functions in shaping the T cell repertoire. Notably, recent studies have demonstrated that sex hormones control both cTEC and mTEC function.
An important function of cTECs is to support thymopoiesis through the expression of delta-like 4 (Dll4) (27). Dll4 plays a non-redundant role in thymic T cell development since thymic epithelial cell-specific deletion of Dll4 resulted in complete block of T cell development at the CD44+ CD25- double negative (DN) 1 stage. Recently, it was reported that testosterone administration decreased Dll4 expression by cTECs in 3.5 week old mice (28). The mechanism for this downregulation appears to be direct, since androgen response elements are found in the promoter of the Dll4 gene and androgen/androgen receptor complexes were localized to the Dll4 promoter by chromatin immunoprecipitation (28). Consistent with a role for androgens in downregulating Dll4 expression, lower Dll4 gene expression has been noted in male cTECs compared with female (29). Mice heterozygous for thymic epithelial cell-specific Dll4 deletion showed decreased total thymic cellularity and lack of progression of thymocytes, suggesting that quantitative decreases in Dll4 expression negatively affect thymopoiesis. Conversely, sex steroid blockade was associated with increased Dll4 expression, increased thymic size and increased numbers of all thymocyte subsets.
These findings suggest that sex steroid ablation may be beneficial in clinical conditions characterized by loss of thymic function. Loss of thymic function not only occurs with aging, but is also consequence of radiation, chemotherapy, glucocorticoid administration, infection, and other states of “stress.” Decreased thymic function, particularly in the setting of bone marrow transplantation, can be associated with increased susceptibility to opportunistic infection and malignancy. Thus, there is a need for therapeutic strategies that enhance thymic function in these states. Indeed, sex steroid ablation accelerated recovery of thymic cellularity in mice subjected to sublethal total body irradiation and resulted in improved T cell-mediated viral clearance in these mice (28). These findings add to a previous report that sex steroid blockade enhances thymopoiesis and thymic output in mice after conditioning regimen-induced thymic epithelial cell damage (30, 31). In this study, sex steroid blockade and keratinocyte growth factor (KGF) administration in combination had additive effects, suggesting that this regimen may be an effective means to improve thymic function.
In addition to regulating thymocyte development through their effects on cTECs, sex steroids also regulate mTEC function. mTECs play a major role in the maintenance of self-tolerance toward tissue-specific antigens through their expression of the autoimmune regulator (AIRE) gene. AIRE promotes the promiscuous expression of tissue self-antigens (TSAs) by mTECs (32), and presentation of these TSAs to developing T cells results in negative selection of developing T cells that recognize these TSAs with high affinity. In addition to eliminating autoreactive T cell clones, AIRE also maintains self-tolerance by diverting autoreactive T cells into the regulatory T cell (Treg) lineage. The importance of AIRE in self-tolerance is clearly illustrated by the development of multi-organ autoimmunity in Aire-deficient patients and mice.
Notably, recent work has demonstrated a prime role for sex hormones in modulating Aire expression. Androgens enforce thymic self-tolerance mechanisms by upregulating Aire expression in mTECs (33, 34). mTECs express androgen receptor, and androgen/androgen receptor complexes bind directly to the Aire promoter to upregulate its expression (33). Increased Aire expression drives increased TSA expression, which results in more efficient negative selection of self-reactive T cells. As a consequence, mice administered dihydrotestosterone express higher levels of Aire and are protected from central nervous system (CNS) autoimmunity in an Aire-dependent manner.
At the same time, estrogens inhibit thymic self-tolerance mechanisms by decreasing Aire expression (34). Interestingly, this inhibition does not occur through a direct effect of estrogen/estrogen receptor on the AIRE promoter. Instead estrogen appears to downregulate Aire expression through epigenetic effects on DNA methylation, although the mechanism by which estrogen alters DNA methylation remains to be determined. Estrogen administration increased the production of anti-thyroid antibodies in experimental autoimmune thyroiditis (EAT), and thymectomy abolished this effect. Importantly, Aire expression in mice and human thymus is higher in males compared to females (33, 34). Thus, sex hormone modulation of Aire in the thymus is an important mechanism by which sex bias occurs in autoimmunity: increased levels of androgen and decreased levels of estrogen promote Aire expression in males to protect against autoimmunity.
While negative selection of self-reactive T cells is classically considered an event that occurs in the thymic medulla under the control of Aire+ mTECs, it is worth noting that negative selection of ubiquitous self-antigen-reactive T cells also occurs in the thymic cortex (35). Interestingly, cTECs are not the main antigen presenting cell mediating clonal deletion of T cells in the cortex. Instead, dendritic cells appear to play a major role, since ablation of dendritic cells reduced the number of thymocytes expressing activated caspase 3. Whether sex steroids also play a role in T cell negative selection within the cortex, and whether this mechanism contributes to sex differences in autoimmune predisposition, is currently not known and will require further study.
Sex hormones, mast cells, Type 2 innate lymphoid cells (ILC2s), and regulation of T cells in the periphery
Sex hormones exert profound and direct effects on most innate immune cell populations. Cell development, cytokine and chemokine production, expression of TLRs and prostaglandins, migratory abilities and signaling intermediates have all been identified as targets of sex hormone modulation in these cells (reviewed in (36)). Here we focus on a hormone-mediated interaction between mast cells and ILC2s that regulates T helper cell differentiation and contributes to protection in a sex-dimorphic autoimmune disease.
Mast cells are tissue resident cells that are best studied in the context of allergic inflammation (reviewed in (37)). Upon FcεRI cross-linking by IgE/antigen complexes, mast cells release preformed mediators such as histamine, lysozymes and proteases. Expression of newly synthesized immune modulators can also be elicited by cytokine receptor and TLR activation. Importantly, mast cell activation is also under the control of sex hormones. Estrogen in particular has been implicated in the increased severity of allergic disease in females (38). Estrogen can directly elicit mast cell degranulation corresponding to worsening of allergic asthma during high estrogen phases of the menstrual cycle in females.
ILC2s also play a seminal role in allergic inflammation and are members of a heterogeneous group of tissue resident innate immune cells that include the non-cytotoxic subsets (ILC1 and ILC3), as well as the cytotoxic Natural Killer (NK) cell subset (39). ILC2s are distinguished from other ILC subsets (CD45+, Lineage-, IL7R+) by the expression of GATA3, ST2, the IL-33 receptor, and production of Th2-associated cytokines including IL-5, IL-13 and IL-9. They are required for the development of strong Th2 responses that drive pathologic inflammation in allergic disease. Somewhat surprisingly there is still relatively limited information regarding the direct effects of hormones on ILC subsets. Uterine ILC1 subpopulations (CD45+CD3−CD19−CD11blow/− NK1.1+ NKp46+), distinguished by their expression of Eomes, CD49a and CXCR6, exhibit differing abundance and transcriptomes during pre-puberty, sexual maturity, pregnancy and post-partum, suggesting hormonal exposure affects numbers and functional potential (40). ERs and ARs are expressed by ILC2s and regulate population size in the uterus and lungs (41, 42). Consistent with increased allergic asthma incidence in females, numbers of circulating ILC2s are higher in females compared to male asthmatics (43). In mice, post-pubescent female mice have significantly more ILC2 precursors and lung ILC2s than age-matched males (42, 44, 45).
A sex-specific interaction between mast cells and ILC2s was recently observed in studies of experimental autoimmune encephalomyelitis (EAE), the mouse model of the CNS autoimmune demyelinating disease, multiple sclerosis (MS) (46) (Figure 2). EAE is induced by immunization with myelin peptides in complete Freund’s adjuvant or adoptive transfer of encephalitogenic T cells. As in MS, myelin-specific Th1 and Th17 cells enter the brain and/or spinal cord and orchestrate an immune attack on the myelin sheath, a structure that assists in nerve impulse conduction, as well as on the myelin-producing oligodendrocytes. Although most EAE studies have used C57BL/6 mice, SJL mice uniquely recapitulate two important features of MS (47). First, they develop a relapsing-remitting course of disease, the most common form of MS. In addition, they exhibit sex dimorphism: females are more susceptible to severe disease than males. Immunized SJL females generate a strong Th17 anti-myelin response but surprisingly, resistant males do not fail to respond but rather produce a Th2 response that is non-pathogenic in this setting (46). This difference is clearly influenced by testosterone: males are more susceptible to disease after castration or treatment with AR antagonists (48, 49). Male mice also show increased EAE incidence with advancing age corresponding to decreasing testosterone levels (50). Administration of testosterone to SJL females prior to EAE induction attenuates the pathologic T cell response to one that is IL-4- and IL-10-dominated (48, 51-54).
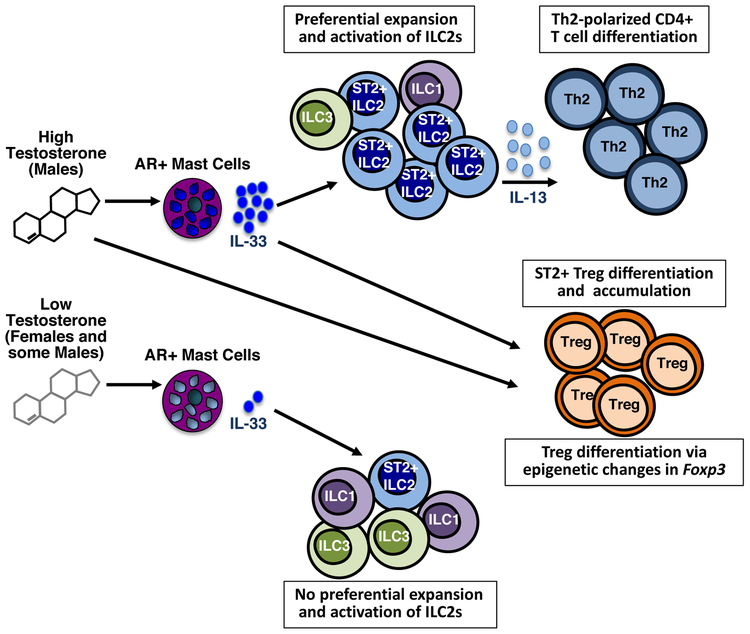
Figure 2.
Proposed regulation of peripheral T cell responses by androgens. Under conditions of relatively high testosterone in males, this hormone acts directly to induce IL-33 expression by mast cells. IL-33 activates the preferential expansion of IL-13-producing ILC2s. IL-13 in turn drives Th2 cell differentiation. Normal adult females express testosterone, but at levels 7-8 times lower than adult males. This reduced level is below the threshold to trigger a strong IL-33 response. Exposure to high testosterone during cell development may alter the Il33 locus increasing chromatin accessibility and expression potential in males. Testosterone promotes Treg cell expansion and function: Indirectly, through induction of IL-33, an ST2+ subset of Treg cells undergo differentiation and local accumulation in tissues. Testosterone may also nfluence acquisition of chromatin modifications in the Foxp3 gene that promote increased Foxp3 expression.
The Th2 anti-myelin response in SJL males is influenced by testosterone-dependent actions on mast cells. Within the CNS, mast cells are normal residents of the meninges, brain and spinal cord and are activated early post-disease induction. In females, mast cells produce pro-inflammatory cytokines such as TNF and IL-1β, which collectively alter the normally restrictive blood brain barrier (55). Mast cell-derived IL-1β also promotes T cell encephalitogenicity (56). In contrast, under the influence of relatively high testosterone levels, mast cell activation in males results in the production of IL-33, an IL-1 family cytokine, but not TNF or IL-1β (46). IL-33 activates ILC2s to proliferate and to produce IL-13, a cytokine essential for robust Th2 cell differentiation (57, 58). It is hypothesized that the lower testosterone levels in females are not sufficient to induce a threshold amount of IL-33 production by mast cells to trigger this IL-33/ILC2 Th2 differentiation pathway. Indeed an immunization-induced increase in ILC2s in the CNS is observed in males but is much reduced in females. The protective consequences of this mast cell-IL-33/ILC2 pathway was validated in experiments in which susceptible mice were treated with IL-33. Administration of IL-33 to female mice induces significant increases in IL-13-producing ILC2s in the lymph nodes and CNS. If given prior to disease a primary Th2 anti-myelin response is generated and EAE is diminished. Strikingly, IL-33 treatment of mice with established disease converts the ongoing Th17 response one dominated by Th2 cells, demonstrating the plasticity of these encephalitogenic T cells, and prevents relapses (46).
Testosterone’s effects are direct as shown by the ability of this hormone to elicit IL-33 in AR+ male-derived mast cell lines. However, it is likely that testosterone has other effects during mast cell development that poise mast cells for IL-33 expression potential, perhaps at the epigenetic level. This idea is based on the observation that although both male- and female-derived mast cells express the androgen receptor, testosterone elicits inducible IL-33 only in male-derived cells (46). This male- and female-specific cytokine pattern-IL-33 induction in males and TNF/IL-1β production in females-also occurs with other modes of mast cell activation including FceR1 cross-linking. These data indicate that the ability to express a particular array of cytokines may be programmed by prolonged sex hormone exposure starting early in life and is not due solely to acute sex hormone-receptor interactions.
Other T cells may be targets of testosterone-induced IL-33 production. A subset of IL-33 receptor+ (ST2+) T regulatory cells was recently described that limit inflammation in a model of inflammatory bowel disease, an activity dependent on IL-33 (59). Decreases in Foxp3+ Tregs also correspond with declining testosterone levels and increased EAE severity in aging C57BL/6 mice (50).
Finally, there is evidence that the effects of sex hormones on mast cells and ILC2s are tissue specific. In contrast to the increased numbers and activity of ILC2s in the CNS of males after EAE induction, testosterone limits ILC2 expansion and activation in the lung in allergic asthma models (44). This inhibitory effect is thought to contribute to the reduced incidence of allergic asthma in post-pubescent males relative to females.
Chromosomal and environmental contributions to sex differences
While our discussion in this review has focused on hormonal differences, evidence now exists that other sex differences also contribute to sex dimorphism in the immune response. X chromosome contributions to autoimmunity are suggested by the observation that 47XXY males with Klinefelter syndrome who are equally susceptible to SLE as XX females (60). In contrast, 45X females with Turner syndrome rarely develop SLE (61). Studies using uniquely informative mouse models strongly support the hypothesis that the X chromosome promotes autoimmune disease susceptibility independent of hormones (62). “Four core genotype” (FCG) mice allow the effects of the sex chromosome complement to be determined without the confounding effects of gonadal type and are derived by crossing XX females and XY-Sry males. In XY-Sry mice, Sry, the testes-determining factor gene, is deleted from the Y chromosome and inserted as a transgene onto an autosomal chromosome. Because Sry is autosomal, the chromosomal sex of these mice is independent of gonadal (hormone-related) sex: both XX-Sry and XY-Sry progeny are gonadal males and mice lacking Sry, XX and XY- progeny, are gonadal females. Voskuhl and colleagues showed that XX females are more susceptible to EAE and systemic lupus erythematosus (SLE) than XY- females mice (63). Furthermore, gonadectomized XX females and XX-Sry males exhibit more severe disease than gonadectomized XY females or XY males, suggesting X chromosome encoded genes do not depend on a “female hormonal environment” to exert their influence. The mechanism by which the XX chromosome complement predisposes to autoimmunity is not completely clear, but more severe disease in XX and XX-Sry mice was associated with increased myeloid cell expression of IL-13Ralpha2, an X chromosome-linked gene that limits T helper 2 responses (63).
In addition to distinct sex chromosome complements, sex differences in microbiota may also underlie differences in autoimmune susceptibility. This is nicely illustrated by a seminal study in the NOD mouse model of type 1 diabetes, which exhibits a female bias in susceptibility (64). Danska et al. showed that males and females have distinct populations of commensal bacteria in their gut. Treatment with broad-spectrum antibiotics or housing in a germ free facility eliminates this sex difference and transfer of male gut extracts to females confers protection. It is worth noting that the male-associated microbes that confer protection in NOD mice appear to be androgen-dependent, suggesting that hormones play a pervasive role in mediating sex differences.
Clinical implications of sex hormones and the immune response
Understanding the role of sex hormones has recently taken on new urgency given the rise in the number of patients undergoing treatment with sex hormones and sex hormone modulators for a variety of conditions. Androgen preparations, for example, are currently FDA approved for a number of androgen deficiency syndromes (e.g., Klinefelter’s syndrome, bilateral torsion, testicular failure due to chemotherapy) in males. Additionally, testosterone is also being used extensively by men with low testosterone levels due to aging, despite lack of evidence for its safety and efficacy for this condition. From 2009 to 2013, the number of patients receiving a prescription for testosterone increased from 1.3 million to 2.3 million (65). Another example is the increasing use of aromatase inhibitors for disorders of growth and development in children, estrogen-responsive cancers, and other indications (66). Given their widespread use, it is important from a public health perspective to understand the effects of sex hormone modulation on multiple physiologic systems.
With the accumulating evidence for their importance in regulating immune function, it is not surprising that administration of sex hormone-modulating therapies have had unintended immune consequences. An excellent example of this is with prostate cancer, in which androgen ablative therapy is a standard palliative treatment for advanced prostate cancer for the purpose of depriving hormone-dependent prostate cancer cells of testosterone. Concomitantly, androgen ablation has been reported to increase T cell infiltration of prostate tumor tissue 7-28 days after administration (67), suggesting that its efficacy as a treatment may also be immune dependent. In support of this, androgen ablation in a mouse model of prostate cancer promoted effector function of prostate-specific T cells (68). Interestingly, androgen ablation has also been associated with upregulation of PD-L1 on dendritic cells and PD-1 on T cells immune cells (69). Whether combination of anti-PD-1 IgG4 (pembrolizumab) and an anti-androgen (enzalutamide) will have additive effects in prostate cancer is currently under investigation (NCT02861573). Thus, understanding how sex hormones affect immune responses is important for making informed decisions about the use of sex hormone modulators and for optimizing treatment strategies for immune-mediated diseases.
Conclusions
Sex differences in immune responses are well-documented, and what underlies these mechanisms is the subject of active investigation. It is now clear that sex hormones play a major role in shaping T cell responses. In addition to their direct effects on T cell transcriptional profiles, sex hormones also influence T cell responses through controlling gene expression in thymic epithelial cells and regulating innate immune cells. Despite recent progress, however, multiple outstanding questions remain regarding sex hormones and their influence on immune cells and their mediators. Estrogen’s effects in autoimmunity are highly complex, for instance, and can be either pro- or anti-inflammatory in different contexts. In EAE mouse models, estrogens have paradoxical anti-inflammatory roles by inhibiting Th1/Th17 differentiation and decreasing chemokine expression (70, 71). In SLE models, on the other hand, estrogens have apparent pro-inflammatory roles and are sufficient to induce disease phenotype (72). These distinct effects may reflect differential activation of ERα vs. ERβ vs. GPER1, although how this leads to reciprocal outcomes require further clarification (73). Another major area of interest is the mechanisms by which sex hormones act to influence immune cell development and function. For instance, one way in which sex hormones function in immune cells is by altering epigenetic modifications, but how sex hormones bring about this change remains unclear. Finally, given the effects of sex hormones on multiple tissue types, devising approaches to restrict sex hormone effects to immune cell types of interest will be an important step in using sex hormone modulation effectively as an immunotherapy.
Acknowledgments
This work was supported by NIH National Multiple Sclerosis Society Grants RG 4684A5/1 and RG 5281-A-3 and NIH R21 NS081598, RO1AI12829 (to MAB) and NIH R01 NS079683 and NIH R01 NS107851, APS1 Foundation, and the Parker Institute for Immunotherapy (to MAS).
References
- 1.Whitacre CC. 2001. Sex differences in autoimmune disease. Nat Immunol 2:777–80. [DOI] [PubMed] [Google Scholar]
- 2.Klein SL, Flanagan KL. 2016. Sex differences in immune responses. Nat Rev Immunol 16:626–38. [DOI] [PubMed] [Google Scholar]
- 3.Furman D, Hejblum BP, Simon N, Jojic V, Dekker CL, Thiebaut R, Tibshirani RJ, Davis MM. 2014. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci U S A 111:869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beery AK, Zucker I. 2011. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev 35:565–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu KA, Mager NA. 2016. Women's involvement in clinical trials: historical perspective and future implications. Pharm Pract (Granada) 14:708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller WL, Auchus RJ. 2011. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev 32:81–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cleve A, Fritzemeier KH, Haendler B, Heinrich N, Moller C, Schwede W, Wintermantel T. 2012. Pharmacology and clinical use of sex steroid hormone receptor modulators. Handb Exp Pharmacol doi: 10.1007/978-3-642-30726-3_24:543–87. [DOI] [PubMed] [Google Scholar]
- 8.Fox HS, Bond BL, Parslow TG. 1991. Estrogen regulates the IFN-gamma promoter. J Immunol 146:4362–7. [PubMed] [Google Scholar]
- 9.Kissick HT, Sanda MG, Dunn LK, Pellegrini KL, On ST, Noel JK, Arredouani MS. 2014. Androgens alter T-cell immunity by inhibiting T-helper 1 differentiation. Proc Natl Acad Sci U S A 111:9887–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liva SM, Voskuhl RR. 2001. Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J Immunol 167:2060–7. [DOI] [PubMed] [Google Scholar]
- 11.Hammes SR, Levin ER. 2007. Extranuclear steroid receptors: Nature and actions. Endocrine Reviews 28:726–741. [DOI] [PubMed] [Google Scholar]
- 12.Tamaki M, Konno Y, Kobayashi Y, Takeda M, Itoga M, Moritoki Y, Oyamada H, Kayaba H, Chihara J, Ueki S. 2014. Expression and functional roles of G-protein-coupled estrogen receptor (GPER) in human eosinophils. Immunol Lett 160:72–78. [DOI] [PubMed] [Google Scholar]
- 13.Ueda K, Karas RH. 2013. Emerging evidence of the importance of rapid, non-nuclear estrogen receptor signaling in the cardiovascular system. Steroids 78:589–96. [DOI] [PubMed] [Google Scholar]
- 14.Leonhardt SA, Boonyaratanakornkit V, Edwards DP. 2003. Progesterone receptor transcription and non-transcription signaling mechanisms. Steroids 68:761–70. [DOI] [PubMed] [Google Scholar]
- 15.Estrada M, Espinosa A, Muller M, Jaimovich E. 2003. Testosterone stimulates intracellular calcium release and mitogen-activated protein kinases via a G protein-coupled receptor in skeletal muscle cells. Endocrinology 144:3586–97. [DOI] [PubMed] [Google Scholar]
- 16.Mann M, Cortez V, Vadlamudi RK. 2011. Epigenetics of estrogen receptor signaling: role in hormonal cancer progression and therapy. Cancers (Basel) 3:1691–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walecki M, Eisel F, Klug J, Baal N, Paradowska-Dogan A, Wahle E, Hackstein H, Meinhardt A, Fijak M. 2015. Androgen receptor modulates Foxp3 expression in CD4+CD25+Foxp3+ regulatory T-cells. Mol Biol Cell 26:2845–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumar N, Shan LX, Hardy MP, Bardin CW, Sundaram K. 1995. Mechanism of androgen-induced thymolysis in rats. Endocrinology 136:4887–93. [DOI] [PubMed] [Google Scholar]
- 19.Olsen NJ, Olson G, Viselli SM, Gu X, Kovacs WJ. 2001. Androgen receptors in thymic epithelium modulate thymus size and thymocyte development. Endocrinology 142:1278–83. [DOI] [PubMed] [Google Scholar]
- 20.Staples JE, Gasiewicz TA, Fiore NC, Lubahn DB, Korach KS, Silverstone AE. 1999. Estrogen receptor alpha is necessary in thymic development and estradiol-induced thymic alterations. J Immunol 163:4168–74. [PubMed] [Google Scholar]
- 21.Altuwaijri S, Chuang KH, Lai KP, Lai JJ, Lin HY, Young FM, Bottaro A, Tsai MY, Zeng WP, Chang HC, Yeh S, Chang C. 2009. Susceptibility to autoimmunity and B cell resistance to apoptosis in mice lacking androgen receptor in B cells. Mol Endocrinol 23:444–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kadel S, Kovats S. 2018. Sex Hormones Regulate Innate Immune Cells and Promote Sex Differences in Respiratory Virus Infection. Front Immunol 9:1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olsen NJ, Watson MB, Henderson GS, Kovacs WJ. 1991. Androgen deprivation induces phenotypic and functional changes in the thymus of adult male mice. Endocrinology 129:2471–6. [DOI] [PubMed] [Google Scholar]
- 24.Olsen NJ, Kovacs WJ. 2011. Evidence that androgens modulate human thymic T cell output. J Investig Med 59:32–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hince M, Sakkal S, Vlahos K, Dudakov J, Boyd R, Chidgey A. 2008. The role of sex steroids and gonadectomy in the control of thymic involution. Cell Immunol 252:122–38. [DOI] [PubMed] [Google Scholar]
- 26.Guevara Patino JA, Marino MW, Ivanov VN, Nikolich-Zugich J. 2000. Sex steroids induce apoptosis of CD8+CD4+ double-positive thymocytes via TNF-alpha. Eur J Immunol 30:2586–92. [DOI] [PubMed] [Google Scholar]
- 27.Koch U, Fiorini E, Benedito R, Besseyrias V, Schuster-Gossler K, Pierres M, Manley NR, Duarte A, Macdonald HR, Radtke F. 2008. Delta-like 4 is the essential, nonredundant ligand for Notch1 during thymic T cell lineage commitment. J Exp Med 205:2515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Velardi E, Tsai JJ, Holland AM, Wertheimer T, Yu VW, Zakrzewski JL, Tuckett AZ, Singer NV, West ML, Smith OM, Young LF, Kreines FM, Levy ER, Boyd RL, Scadden DT, Dudakov JA, van den Brink MR. 2014. Sex steroid blockade enhances thymopoiesis by modulating Notch signaling. J Exp Med 211:2341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dumont-Lagace M, St-Pierre C, Perreault C. 2015. Sex hormones have pervasive effects on thymic epithelial cells. Sci Rep 5:12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly RM, Highfill SL, Panoskaltsis-Mortari A, Taylor PA, Boyd RL, Hollander GA, Blazar BR. 2008. Keratinocyte growth factor and androgen blockade work in concert to protect against conditioning regimen-induced thymic epithelial damage and enhance T-cell reconstitution after murine bone marrow transplantation. Blood 111:5734–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnard AL, Chidgey AP, Bernard CC, Boyd RL. 2009. Androgen depletion increases the efficacy of bone marrow transplantation in ameliorating experimental autoimmune encephalomyelitis. Blood 113:204–13. [DOI] [PubMed] [Google Scholar]
- 32.Anderson MS, Su MA. 2016. AIRE expands: new roles in immune tolerance and beyond. Nat Rev Immunol 16:247–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu ML, Bakhru P, Conley B, Nelson JS, Free M, Martin A, Starmer J, Wilson EM, Su MA. 2016. Sex bias in CNS autoimmune disease mediated by androgen control of autoimmune regulator. Nat Commun 7:11350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dragin N, Bismuth J, Cizeron-Clairac G, Biferi MG, Berthault C, Serraf A, Nottin R, Klatzmann D, Cumano A, Barkats M, Le Panse R, Berrih-Aknin S. 2016. Estrogen-mediated downregulation of AIRE influences sexual dimorphism in autoimmune diseases. J Clin Invest 126:1525–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCaughtry TM, Baldwin TA, Wilken MS, Hogquist KA. 2008. Clonal deletion of thymocytes can occur in the cortex with no involvement of the medulla. J Exp Med 205:2575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaillon S, Berthenet K, Garlanda C. 2017. Sexual Dimorphism in Innate Immunity. Clin Rev Allergy Immunol doi: 10.1007/s12016-017-8648-x. [DOI] [PubMed] [Google Scholar]
- 37.Galli SJ, Tsai M, Marichal T, Tchougounova E, Reber LL, Pejler G. 2015. Approaches for analyzing the roles of mast cells and their proteases in vivo. Adv Immunol 126:45–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonds RS, Midoro-Horiuti T. 2013. Estrogen effects in allergy and asthma. Curr Opin Allergy Clin Immunol 13:92–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klose CS, Artis D. 2016. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol 17:765–74. [DOI] [PubMed] [Google Scholar]
- 40.Filipovic I, Chiossone L, Vacca P, Hamilton RS, Ingegnere T, Doisne JM, Hawkes DA, Mingari MC, Sharkey AM, Moretta L, Colucci F. 2018. Molecular definition of group 1 innate lymphoid cells in the mouse uterus. Nat Commun 9:4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bartemes K, Chen CC, Iijima K, Drake L, Kita H. 2018. IL-33-Responsive Group 2 Innate Lymphoid Cells Are Regulated by Female Sex Hormones in the Uterus. J Immunol 200:229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laffont S, Blanquart E, Guery JC. 2017. Sex Differences in Asthma: A Key Role of Androgen-Signaling in Group 2 Innate Lymphoid Cells. Front Immunol 8:1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cephus JY, Stier MT, Fuseini H, Yung JA, Toki S, Bloodworth MH, Zhou W, Goleniewska K, Zhang J, Garon SL, Hamilton RG, Poloshukin VV, Boyd KL, Peebles RS Jr., Newcomb DC. 2017. Testosterone Attenuates Group 2 Innate Lymphoid Cell-Mediated Airway Inflammation. Cell Rep 21:2487–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laffont S, Blanquart E, Savignac M, Cenac C, Laverny G, Metzger D, Girard JP, Belz GT, Pelletier L, Seillet C, Guery JC. 2017. Androgen signaling negatively controls group 2 innate lymphoid cells. J Exp Med 214:1581–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kadel S, Ainsua-Enrich E, Hatipoglu I, Turner S, Singh S, Khan S, Kovats S. 2018. A Major Population of Functional KLRG1(-) ILC2s in Female Lungs Contributes to a Sex Bias in ILC2 Numbers. Immunohorizons 2:74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Russi AE, Ebel ME, Yang Y, Brown MA. 2018. Male-specific IL-33 expression regulates sex-dimorphic EAE susceptibility. Proc Natl Acad Sci U S A 115:E1520–E1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Papenfuss TL, Rogers CJ, Gienapp I, Yurrita M, McClain M, Damico N, Valo J, Song F, Whitacre CC. 2004. Sex differences in experimental autoimmune encephalomyelitis in multiple murine strains. J Neuroimmunol 150:59–69. [DOI] [PubMed] [Google Scholar]
- 48.Bebo BF Jr., Schuster JC, Vandenbark AA, Offner H. 1999. Androgens alter the cytokine profile and reduce encephalitogenicity of myelin-reactive T cells. J Immunol 162:35–40. [PubMed] [Google Scholar]
- 49.Voskuhl RR, Palaszynski K. 2001. Sex hormones in experimental autoimmune encephalomyelitis: implications for multiple sclerosis. Neuroscientist 7:258–70. [DOI] [PubMed] [Google Scholar]
- 50.Matejuk A, Hopke C, Vandenbark AA, Hurn PD, Offner H. 2005. Middle-age male mice have increased severity of experimental autoimmune encephalomyelitis and are unresponsive to testosterone therapy. J Immunol 174:2387–95. [DOI] [PubMed] [Google Scholar]
- 51.Bebo BF Jr., Schuster JC, Vandenbark AA, Offner H. 1998. Gender differences in experimental autoimmune encephalomyelitis develop during the induction of the immune response to encephalitogenic peptides. J Neurosci Res 52:420–6. [DOI] [PubMed] [Google Scholar]
- 52.Bebo BF Jr., Zelinka-Vincent E, Adamus G, Amundson D, Vandenbark AA, Offner H. 1998. Gonadal hormones influence the immune response to PLP 139-151 and the clinical course of relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol 84:122–30. [DOI] [PubMed] [Google Scholar]
- 53.Dalal M, Kim S, Voskuhl RR. 1997. Testosterone therapy ameliorates experimental autoimmune encephalomyelitis and induces a T helper 2 bias in the autoantigen-specific T lymphocyte response. J Immunol 159:3–6. [PubMed] [Google Scholar]
- 54.Palaszynski KM, Loo KK, Ashouri JF, Liu HB, Voskuhl RR. 2004. Androgens are protective in experimental autoimmune encephalomyelitis: implications for multiple sclerosis. J Neuroimmunol 146:144–52. [DOI] [PubMed] [Google Scholar]
- 55.Christy AL, Walker ME, Hessner MJ, Brown MA. 2013. Mast cell activation and neutrophil recruitment promotes early and robust inflammation in the meninges in EAE. J Autoimmun 42:50–61. [DOI] [PubMed] [Google Scholar]
- 56.Russi AE, Walker-Caulfield ME, Guo Y, Lucchinetti CF, Brown MA. 2016. Meningeal mast cell-T cell crosstalk regulates T cell encephalitogenicity. J Autoimmun 73:100–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lefrancais E, Duval A, Mirey E, Roga S, Espinosa E, Cayrol C, Girard JP. 2014. Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proc Natl Acad Sci U S A 111:15502–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Halim TY, Steer CA, Matha L, Gold MJ, Martinez-Gonzalez I, McNagny KM, McKenzie AN, Takei F. 2014. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity 40:425–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schiering C, Krausgruber T, Chomka A, Frohlich A, Adelmann K, Wohlfert EA, Pott J, Griseri T, Bollrath J, Hegazy AN, Harrison OJ, Owens BMJ, Lohning M, Belkaid Y, Fallon PG, Powrie F. 2014. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature 513:564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scofield RH, Bruner GR, Namjou B, Kimberly RP, Ramsey-Goldman R, Petri M, Reveille JD, Alarcon GS, Vila LM, Reid J, Harris B, Li S, Kelly JA, Harley JB. 2008. Klinefelter's syndrome (47,XXY) in male systemic lupus erythematosus patients: support for the notion of a gene-dose effect from the X chromosome. Arthritis Rheum 58:2511–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cooney CM, Bruner GR, Aberle T, Namjou-Khales B, Myers LK, Feo L, Li S, D'Souza A, Ramirez A, Harley JB, Scofield RH. 2009. 46,X,del(X)(q13) Turner's syndrome women with systemic lupus erythematosus in a pedigree multiplex for SLE. Genes Immun 10:478–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arnold AP, Chen X. 2009. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol 30:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith-Bouvier DL, Divekar AA, Sasidhar M, Du S, Tiwari-Woodruff SK, King JK, Arnold AP, Singh RR, Voskuhl RR. 2008. A role for sex chromosome complement in the female bias in autoimmune disease. J Exp Med 205:1099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS. 2013. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339:1084–8. [DOI] [PubMed] [Google Scholar]
- 65.Anonymous. FDA Drug Safety Communication: FDA cautions about using testosterone products for low testosterone due to aging; requires labeling change to inform of possible increased risk of heart attack and stroke with use. http://www.fda.gov/downloads/Drugs/DrugSafety/UCM436270.pdf. [DOI] [PubMed]
- 66.Shulman DI, Francis GL, Palmert MR, Eugster EA, Lawson Wilkins Pediatric Endocrine Society D, Therapeutics C. 2008. Use of aromatase inhibitors in children and adolescents with disorders of growth and adolescent development. Pediatrics 121:e975–83. [DOI] [PubMed] [Google Scholar]
- 67.Mercader M, Bodner BK, Moser MT, Kwon PS, Park ES, Manecke RG, Ellis TM, Wojcik EM, Yang D, Flanigan RC, Waters WB, Kast WM, Kwon ED. 2001. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A 98:14565–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Drake CG, Doody AD, Mihalyo MA, Huang CT, Kelleher E, Ravi S, Hipkiss EL, Flies DB, Kennedy EP, Long M, McGary PW, Coryell L, Nelson WG, Pardoll DM, Adler AJ. 2005. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell 7:239–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bishop JL, Sio A, Angeles A, Roberts ME, Azad AA, Chi KN, Zoubeidi A. 2015. PD-L1 is highly expressed in Enzalutamide resistant prostate cancer. Oncotarget 6:234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lelu K, Laffont S, Delpy L, Paulet PE, Perinat T, Tschanz SA, Pelletier L, Engelhardt B, Guery JC. 2011. Estrogen receptor alpha signaling in T lymphocytes is required for estradiol-mediated inhibition of Th1 and Th17 cell differentiation and protection against experimental autoimmune encephalomyelitis. J Immunol 187:2386–93. [DOI] [PubMed] [Google Scholar]
- 71.Spence RD, Wisdom AJ, Cao Y, Hill HM, Mongerson CR, Stapornkul B, Itoh N, Sofroniew MV, Voskuhl RR. 2013. Estrogen mediates neuroprotection and anti-inflammatory effects during EAE through ERalpha signaling on astrocytes but not through ERbeta signaling on astrocytes or neurons. J Neurosci 33:10924–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feng F, Nyland J, Banyai M, Tatum A, Silverstone AE, Gavalchin J. 2010. The induction of the lupus phenotype by estrogen is via an estrogen receptor-alpha-dependent pathway. Clin Immunol 134:226–36. [DOI] [PubMed] [Google Scholar]
- 73.Voskuhl RR, Gold SM. 2012. Sex-related factors in multiple sclerosis susceptibility and progression. Nat Rev Neurol 8:255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]