Abstract
Background: The eighth edition of the American Joint Committee on Cancer (AJCC) staging system has reclassified up to one third of differentiated thyroid cancer patients into one of the younger prognostic stage groups (<55 years of age at diagnosis, stage I or stage II). This reclassification widens the spectrum of disease in these lower stages without significantly impacting overall disease-specific survival (DSS) for the entire stage group. However, the optimistic DSS estimates in the <55-year-old stage groups may not accurately reflect the prognosis of individual patients with American Thyroid Association (ATA) high-risk features. Therefore, the aim of this study was to integrate the ATA risk classification system into the eighth edition AJCC staging system to refine and individualize DSS estimates for differentiated thyroid cancer patients aged <55 years at diagnosis.
Methods: Using the Memorial Sloan Kettering Cancer Center tumor registry, 4881 adult DTC patients aged <55 years at diagnosis receiving initial therapy between 1980 and 2016 were retrospectively analyzed. Using Memorial Sloan Kettering Cancer Center registry coded data, all patients were assigned an eighth edition AJCC stage (I or II), ATA risk of recurrence (low, intermediate, or high), and age group at diagnosis (younger patients defined as ≤45 years old, older patients defined as 45–55 years old). The primary outcome was 10-year DSS.
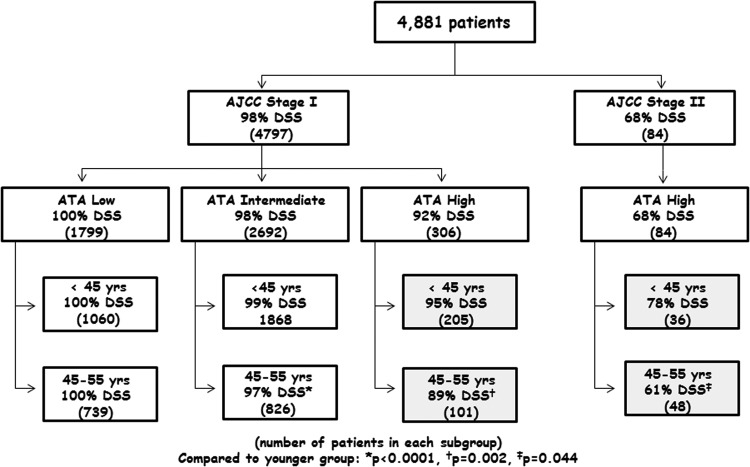
Results: A total of 122 (2.5%) disease-related deaths were observed in the cohort of 4881 patients during a median follow-up of 6.6 years. Integration of the AJCC stage, ATA risk, and age groups identified six subgroups with differing outcomes: (i) stage I/ATA low risk, younger and older, 100% DSS; (ii) stage I/ATA intermediate risk, younger and older, 98% DSS; (iii) stage I/ATA high risk, younger, 95% DSS; (iv) stage I/ATA high risk, older, 89% DSS; (v) stage II/ATA high risk, younger, 78% DSS; and (vi) stage II/ATA high risk, older, 61% DSS.
Conclusions: Integration of AJCC stage, ATA risk, and age group (i) identifies six subgroups of patients with progressively worse DSS as AJCC stage, ATA risk, and age increases, and (ii) provides a more individualized estimate of DSS, especially in ATA high-risk patients.
Keywords: : AJCC, differentiated, thyroid cancer, recurrence, stratification, survival
Introduction
The clinical management of patients with differentiated thyroid cancer (DTC) requires appropriate risk stratification based on estimates of recurrence and disease-specific survival (DSS) (1). The most widely used system for predicting DSS in DTC is the American Joint Committee on Cancer/tumor-node-metastasis staging system (AJCC/TNM; www.cancerstaging.org). To characterize expected clinical outcomes further, the American Thyroid Association (ATA) risk-stratification system is commonly used to estimate the risk of disease recurrence (2). Recently, the AJCC staging system has undergone major modifications to reflect an evolving understanding of the clinicopathologic factors associated with DSS (3). These modifications include raising the age cutoff for staging purposes from 45 to 55 years of age at diagnosis, removing microscopic extrathyroidal extension seen only on histological examination from the definition of T3 disease, and removing central or lateral lymph node metastasis from stage III or IV disease, respectively (3,4).
Recent studies show that the eighth edition AJCC staging system moves up to one third of patients into lower stage groups, resulting in a greater separation of the prognostic stage group survival curves and improved prediction of DSS (5–7). Despite the movement of a large number of patients from higher to lower stages, the 5- and 10-year DSS of the lowest prognostic stage groups appears to be unchanged (5,6). While patients aged >55 years are categorized into one of four prognostic stage groups, patients aged <55 years are simply classified as either stage I (no distant metastases) or stage II (distant metastases identified up to four months after thyroidectomy). As a result, the <55-year-old stage I group now includes patients with a wide spectrum of disease, ranging from unifocal, intrathyroidal papillary microcarcinomas to poorly differentiated tumors with gross extrathyroidal extension into major neck structures. Thus, there is concern that the very optimistic 10-year DSS expected for the entire cohort of stage I patients may not be applicable to the subset of ATA high-risk patients included in the eighth edition AJCC stage I prognostic group (8,9).
While AJCC staging has traditionally been used as a tool to describe population-based outcomes for large groups of patients, the eighth edition promotes the inclusion of additional prognostic factors and development of risk-assessment models that allow the AJCC prognostic stage groups to inform more personalized outcome predictions (10). Although the ATA risk-stratification system was designed and validated to provide individualized estimates of the risk of disease recurrence, it includes several recognized clinicopathologic variables that influence disease survival. Therefore, the goal of this study was to expand the use of the ATA risk-stratification system to refine the DSS predictions of the eighth edition AJCC system in patients aged <55 years age at the time of diagnosis. It was hypothesized that the ATA risk-stratification system could be used to identify subsets of patients within the younger prognostic groups who are likely to have a better or worse DSS than that predicted for the entire cohort.
Methods
After receiving Institutional Review Board approval, data from the Memorial Sloan Kettering Cancer Center (MSKCC) tumor registry were retrospectively reviewed, corresponding to 10,611 consecutive patients with DTC with initial therapy initiated between 1980 and 2016. In contrast to previous publications using the MSKCC surgical database, which included only patients receiving their initial therapy at the center (11,12), the MSKCC tumor registry includes patients treated at MSKCC at any point in their disease course, including those who had their primary surgery elsewhere and were subsequently referred to the center for treatment or follow-up. After exclusion of patients aged ≥55 years (n = 3767), those with less than two years of follow-up from the time of tumor diagnosis (n = 1362), those with anaplastic or medullary thyroid cancer (n = 204), and those with incomplete data for staging or recurrence risk (n = 397), 4881 patients remained for analysis.
Using the MSKCC registry data, patients were assigned as AJCC stage I if they had no evidence of distant metastases (M0/Mx) and stage II in the setting of distant metastases (M1) at diagnosis. The assignment of ATA risk of recurrence category followed the original 2009 ATA risk-stratification system, which differs in a few ways from the most recent “ATA 2009 Risk Stratification System with Proposed Modifications” published in the 2015 ATA guidelines. The 2009 ATA risk-stratification system was used due to inherent limitations in the available clinicopathologic data in the MSKCC registry data, which did not allow for the assignment of ATA risk using the most recently updated definitions. Therefore, ATA risk of recurrence was assigned as follows: high—distant metastases (M1) or gross extrathyroidal extension (T3b, T4a, T4b); intermediate—aggressive histology (including all follicular or Hürthle cell carcinomas), lymph node metastasis (N1a or N1b), or microscopic extrathyroidal extension; and low—absence of any of the above features.
While follicular and Hürthle cell carcinomas without vascular invasion generally carry an excellent prognosis, it was not possible to evaluate vascular invasion consistently in these tumors using MSKCC registry data. Therefore, all follicular and Hürthle cell carcinomas have been included as ATA intermediate risk in recognition of their potential to be higher-risk tumors.
Within each ATA risk category, patients were analyzed based on two age groups: younger patients defined as <45 years old at diagnosis (corresponding to the <45-year-old age cutoff used in the AJCC seventh edition staging system), and older patients defined as 45–55 years old (corresponding to the cohort of patients moved into the younger patient group based on the <55-year-old age cutoff used in the AJCC eighth edition). Other tumor registry coded clinical variables were collected such as patient age at diagnosis, sex, histologic type, tumor size, presence or absence of minor or gross extrathyroidal extension, lymph node status, and presence or absence of distant metastases. The time to last follow-up or death, vital status, and cause of death for each patient were determined. For all deceased patients, the individual electronic medical records were reviewed to confirm the cause of death, AJCC stage, and ATA risk of recurrence. The primary outcome was DSS defined as the time from the date of tumor diagnosis to the date of death or last follow-up. DSS was stratified according to patients' AJCC stage, ATA risk of recurrence, and age group.
Statistical analysis was performed using IBM SPSS Statistics for Windows v24.0 (IBM Corp., Armonk, NY). Baseline characteristics are expressed as median and ranges for continuous variables and as proportions and frequencies for categorical variables. Categorical variables were compared using chi-square analysis. Survival was determined using the Kaplan–Meier method, and differences were compared using the log-rank test. A p-value of <0.05 was considered statistically significant.
Results
Baseline characteristics of 4881 patients with DTC aged <55 years at diagnosis with at least two years of follow-up by the tumor registry are outlined in Table 1. The majority of patients were female (73%) and had papillary thyroid cancer (96%). The median age at diagnosis was 40 years (range 18–54 years), and the median follow-up was 6.6 years (range 2–35 years). The vast majority of the patients were AJCC stage I (98%), while 37% (n = 1799) were ATA low risk, 55% (n = 2692) were ATA intermediate risk, and 8% (n = 390) were ATA high risk. When analyzed by age category, 3167 (65%) of patients were in the <45-year-old age group (3131 stage I, 36 stage II), and 1714 (35%) of patients were in the 45–55-year-old age group (1666 stage I, 48 stage II). The baseline characteristics of the study cohort were compared to those patients excluded for less than two years of follow-up. No differences were found in the median age, sex, histology, AJCC stage, or incidence of death from any cause between the two groups (data not shown).
Table 1.
Baseline Patient Characteristics
| Patient characteristic | n (%) |
|---|---|
| Patients | 4881 |
| Age at diagnosis, years, median (range) | 40 (18–54) |
| Age category | |
| 18–44 | 3167 (66%) |
| 45–54 | 1714 (35%) |
| Sex | |
| Female | 3568 (73%) |
| Male | 1313 (27%) |
| Histology | |
| Papillary | 4677 (96%) |
| Follicular (includes Hürthle cell carcinoma) | 204 (4%) |
| T stage | |
| T1 | 2181 (45%) |
| T2 | 999 (21%) |
| T3 | 1114 (23%) |
| T4 | 295 (6%) |
| Insufficient data | 292 (6%) |
| N stage | |
| N0/Nx | 3167 (65%) |
| N1 | 1571 (32%) |
| Insufficient data | 143 (3%) |
| M stage | |
| M0 | 4797 (98%) |
| M1 | 84 (2%) |
| Extrathyroidal extension | |
| None | 3224 (66%) |
| Microscopic | 1016 (21%) |
| Gross | 286 (6%) |
| Insufficient data | 355 (7%) |
| AJCC/TNM stage (< 55) | |
| I | 4797 (98%) |
| II | 84 (2%) |
| ATA risk of recurrence | |
| Low | 1799 (37%) |
| Intermediate | 2692 (55%) |
| High | 390 (8%) |
| Follow-up, years, median (range) | 6.6 (2–35) |
| Died of any cause | 229 (5%) |
| Disease-related death | 122 (2.5%) |
| Time to death, years, median (range) | 9.1 (2–34) |
AJCC, American Joint Committee on Cancer; TNM, tumor-node-metastasis; ATA, American Thyroid Association.
There were 229 (5%) overall deaths, and 122 (2.5%) thyroid cancer-related deaths during follow-up. The disease-specific deaths were associated with progressive distant metastases in 109 (89%) patients and with locoregional structural disease progression in 13 (11%) patients. Seventy-five percent of the disease-specific deaths occurred in patients diagnosed between 40 and 55 years of age (median age at diagnosis 47 years, range 22–54 years; see Table 2). Twenty-three percent of the disease-specific deaths occurred within five years of diagnosis, while 31% occurred 5–10 years after diagnosis and 46% occurred >10 years after diagnosis. The median time to disease-specific death was nine years (range 2–34 years). For stage I patients, the median time to disease-specific death was 10 years (range 2–34 years), and for stage II patients it was 6.5 years (range 2.5–23 years; p = 0.003). The majority of patients who died of disease had tumors of papillary histology (n = 79), while 43 were encoded as follicular carcinomas, of which 16 were reclassified as Hürthle cell carcinoma after review of pathology reports.
Table 2.
Characteristics of 122 Patients with Thyroid Cancer-Related Death
| Descriptor (number in entire cohort) | Number | Percentage |
|---|---|---|
| Median age at diagnosis (range) | 47 years (22–54 years) | — |
| Sex | ||
| Female (n = 3568) | 57 | 47% |
| Male (n = 1313) | 65 | 53% |
| Histology | ||
| Papillary (n = 4677) | 79 | 65% |
| Follicular (n = 204) | 43 | 35% |
| Age at diagnosis by decade | ||
| 18–19 (n = 57) | 0 | 0% |
| 20–29 (n = 736) | 8 | 7% |
| 30–39 (n = 1546) | 23 | 19% |
| 40–49 (n = 1710) | 55 | 45% |
| 50–54 (n = 832) | 37 | 30% |
| Time to death in years | ||
| <5 years | 28 | 23% |
| 5–10 years | 38 | 31% |
| >10 years | 56 | 46% |
DSS by AJCC stage and ATA risk category
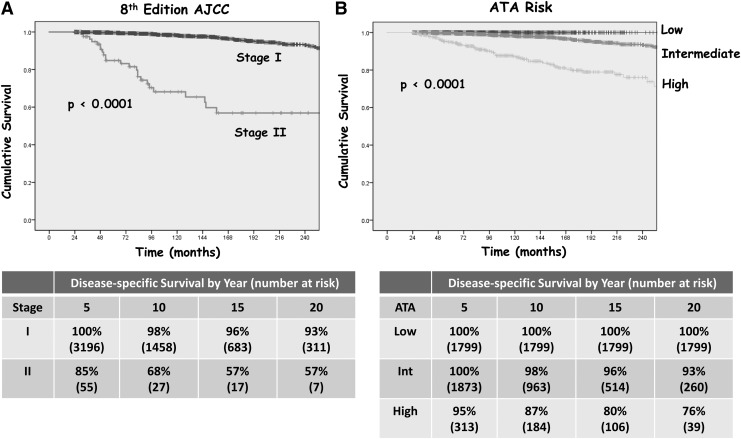
Of the 122 disease-specific deaths, 96 occurred in the 4797 stage I patients, and 26 occurred in the 84 stage II patients. While there were no disease-specific deaths in ATA low-risk patients, 59 disease-specific deaths were seen in the 2692 ATA intermediate-risk patients and 63 deaths occurred in the 390 ATA high-risk patients. Kaplan–Meier curves depicting DSS for the eighth edition AJCC prognostic stage groups and the ATA risk categories are shown in Figure 1. While AJCC stage I patients demonstrated an excellent 10-year DSS of 98%, the stage II patients had a worse than anticipated 10-year DSS of only 68% (p < 0.0001). As expected, the ATA low- and intermediate-risk patients had excellent 10-year DSS (100% and 98%, respectively), while the ATA high-risk patients demonstrated a lower 10-year DSS of 87% (p < 0.0001).
FIG. 1.
Twenty-year disease-specific survival (DSS) in 4881 differentiated thyroid carcinoma (DTC) patients aged <55 years at diagnosis by (A) eighth edition American Joint Committee on Cancer (AJCC) stage and (B) American Thyroid Association (ATA) risk category.
Integrating ATA risk categories with AJCC staging
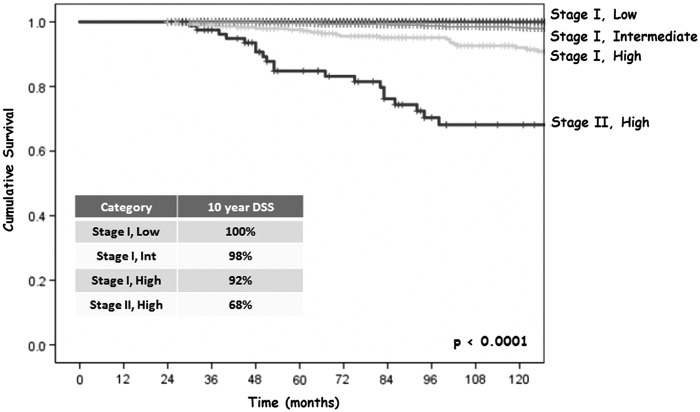
As shown in Table 3, AJCC stage I was composed of 38% ATA low-risk patients, 56% ATA intermediate-risk patients, and 6% ATA high-risk patients (on the basis of gross extrathyroidal extension, T3b/T4a/T4b, M0). By definition, all patients within AJCC stage II had distant metastases and were therefore classified as ATA high risk (any T, any N, M1). Thus, integration of the ATA risk-stratification system with the AJCC eighth edition staging system identified four potential subsets of patients (stage I low risk, stage I intermediate risk, stage I high risk, and stage II high risk). As shown in Figure 2, both stage I low- and intermediate-risk patients had excellent 10-year DSS (100% and 98%, respectively). However, stage I high-risk patients had a poorer 10-year DSS at 92%, and stage II high-risk patients had the worst 10-year DSS at 68% (p < 0.0001).
Table 3.
Distribution by AJCC Stage and ATA Risk Category in 4881 Differentiated Thyroid Cancer Patients <55 Years Old at Diagnosis
| ATA risk of recurrence category | ||||
|---|---|---|---|---|
| AJCC stage | Percent within AJCC stage | Low | Intermediate | High |
| I | 98% (4797/4881) | 38% (1799/4797) | 56% (2692/4797) | 6%a (306/4797) |
| II | 2% (84/4881) | — | — | 100%b (84/84) |
| Total (all stages) | 4881 | 1799 | 2692 | 390 |
Classified as ATA high risk on the basis of the presence of gross extrathyroidal extension (any N, T3b/T4a/T4b, M0).
Classified as ATA high risk on the basis of M1 disease (any T, any N, M1).
FIG. 2.
Ten-year DSS in 4881 DTC patients aged <55 years at diagnosis by eighth edition AJCC stage stratified by ATA risk category.
Evaluating the impact of age category on 10-year DSS within the four AJCC/ATA subgroups
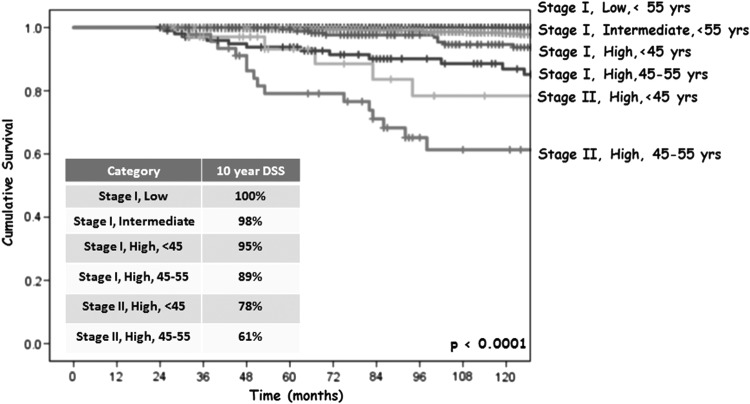
Because of the lower than expected 10-year DSS in the ATA high-risk patients in both AJCC stage I and stage II, the data were reanalyzed based on age groups (<45 vs. 45–55 years old) to evaluate the impact of the reclassification of patients in the eighth edition AJCC staging system and to determine whether there was a significant difference in DSS between the age groups within the respective AJCC/ATA subgroups. The corresponding Kaplan–Meier curves demonstrated six subgroups with progressively worse DSS as AJCC stage, ATA risk, and age increased (see Fig. 3): (i) stage I/ATA low risk, younger and older, 100% DSS; (ii) stage I/ATA intermediate risk, younger and older, 98% DSS; (iii) stage I/ATA high risk, younger, 95% DSS; (iv) stage I/ATA high risk, older, 89% DSS; (v) stage II/ATA high risk, younger, 78% DSS; and (vi) stage II/ATA high risk, older, 61% DSS.
FIG. 3.
Ten-year DSS in 4881 DTC patients aged <55 years at diagnosis by eighth edition AJCC stage stratified by ATA risk category and age group.
Specific 10-year DSS outcomes are presented in Figure 4 for each subgroup. Within the AJCC stage I cohort, ATA low- and intermediate-risk patients had the same excellent DSS (>98%) that varied little by age group. However, stage I ATA high-risk patients had a poorer 10-year DSS (92%) than the overall stage I group (98%; p < 0.001) with even worse outcomes seen in the older (45–55-year-old) cohort (89%; p = 0.002 compared to the younger stage I ATA high-risk patients with a 95% 10-year DSS).
FIG. 4.
Ten-year DSS in 4881 DTC patients aged <55 years at diagnosis by eighth edition AJCC stage stratified by ATA risk category and age group.
The largest difference in DSS was seen in the stage II, high-risk patients, where older patients had a substantially lower DSS compared to younger patients (61% vs. 78%, respectively; p = 0.04). Even though only 48 patients with distant metastases moved from seventh edition stage IV into the eighth edition stage II group, these patients represent 57% (48/84) of the eighth edition stage II cohort, thus accounting for the substantial impact of this small group on the DSS for the entire stage II cohort.
Discussion
This retrospective analysis of 4881 patients with DTC diagnosed prior to 55 years of age demonstrates that both the AJCC eighth edition and the ATA risk-stratification system can be used to estimate DSS for large cohorts of patients. However, since the AJCC stage groups include patients with a broad spectrum of disease, integration of AJCC stage with ATA risk moves from a “one size fits all” prognostic approach to a more refined and individualized DSS estimate than AJCC stage alone. From a practical standpoint, AJCC stage, ATA risk, and age at diagnosis are integrated to classify patients <55 years old at diagnosis into one of six discrete risk groups (see Fig. 4): (i) stage I, low risk: ∼100% 10-year DSS; (ii) stage I, intermediate risk: ∼98% 10-year DSS; (iii) stage I, high risk <45 years old: ∼95% 10-year DSS; (iv) stage I, high risk 45–55 years old: ∼89% 10-year DSS; (v) stage II, high risk <45 years old: ∼78% 10-year DSS; and (vi) stage II, high risk 45–55 years old: ∼61% 10-year DSS.
This simple six-category subclassification provides more realistic and personalized DSS risk estimates for individual patients than the overall DSS predicted for the full cohort of stage I and stage II patients. This is achieved without the need to gather any additional clinicopathologic information beyond that which is routinely collected for initial AJCC and ATA risk stratification of DTC patients. For practical purposes, the identification of low- and intermediate-risk patients within stage I does not significantly refine the 10-year DSS in these patients. Rather, it is the identification of high-risk patients within stage I and the further subdivision by age category that leads to the greatest refinement of risk of DSS compared to that predicted by the stage group alone. Further refinement of DSS estimates are important because a review of 14 staging systems for papillary thyroid cancer found that while all systems significantly predicted DSS, no existing staging system was able to identify accurately the small subset of patients who died of thyroid cancer in the lower stage groups (13). Future editions of the AJCC staging manual should consider using the ATA risk category to define additional prognostic stage subgroups or expand the number of possible stages in the <55-year-old age group. Alternatively, the next iteration of the AJCC staging manual should consider using age at diagnosis as a continuous variable, rather than an arbitrary single age cut point, to integrate age at diagnosis better across all prognostic stage groups (12,14,15).
Recent studies of DTC patients have reported an overall disease-related mortality rate of 1.0–2.6%, consistent with the observed rate of 2.5% (8,11,12,16,17). This figure is likely influenced by the degree of referral bias and length of follow-up in each study. The study included patients from the MSKCC tumor registry, which is comprised of patients referred at any point in their clinical course and follows their disease and vital status long term, regardless of where they continue their care. This allows disease-related deaths that occurred long after the initial tumor diagnosis to be captured, as evidenced by the fact that 77% of patients in the cohort died from disease more than five years after diagnosis.
While representing only 6% of the stage I prognostic group, patients classified as having ATA high-risk disease demonstrate a 10-year DSS of 92% (as opposed to the 98% 10-year DSS predicted for the entire stage I cohort). This is not surprising, since gross extrathyroidal extension (the primary reason that stage I patients would be classified as ATA high risk) is a known risk factor for disease-specific mortality (18–20). The DSS in stage I ATA high-risk patients can be further refined based on age group, with older patients (45–55 years old) having an even worse predicted 10-year DSS of only 89%. This is consistent with previous publications documenting worse DSS as age increases (12,17,21,22). Thus, integration of AJCC prognostic stage with ATA risk can better identify the small subgroup of AJCC stage I patients that die from thyroid cancer.
By definition, all young AJCC stage II patients (<55 years old) have ATA high-risk disease based on the presence of distant metastases. Thus, ATA risk cannot be used to individualize risk estimates in AJCC stage II patients. However, since increasing age is known to be a strong predictor of DSS, it is not unexpected that the older patients within this stage II cohort (45–55 years old) have a significantly worse 10-year DSS than the younger patients (61% vs. 78%, respectively; p = 0.004). The 78% 10-year DSS of <45-year-old stage II patients seen in the data is consistent with the approximately 80% 10-year overall survival for patients <45 years old with distant metastases previously reported (23).
Previous studies have noted that the number of patients with high-risk disease who are moved from seventh edition stage IVC to eighth edition stage II as a result of the change in age cutoff is small and therefore has minimal impact on the DSS of the stage II cohort (12,16). Indeed, in this study, only 48 patients aged between 45 and 55 years with distant metastases moved from seventh edition stage IVC to eighth edition stage II when reclassified, representing just 1.0% of the entire cohort. However, the migrated group had a 10-year DSS of 61% and, when moved to eighth edition stage II, comprised 57% of the stage II cohort (48/84). In this way, even the movement of a small number of patients at high risk of death significantly lowered the DSS of young stage II patients, resulting in the observed 68% 10-year DSS of AJCC stage II patients in the present study.
Prior studies examining the DSS after reclassification to AJCC eighth edition did not report DSS for “young” (18–55 years) versus “old” (>55 years) stage I and II groups separately (5–7). Rather, the DSS was reported by stage group, irrespective of age. In this way, the impact of the small number of high-risk patients aged 45–55 years that migrated to the young stage I and II groups may have been masked. For example, Kim et al. (5) described the migration of 611 patients into the stage I group (n = 2576) and 490 patients into the stage II group (n = 508). Only 10/490 patients downstaged into group II had distant metastases. By definition, these 10 patients must have been aged 45–55 years with distant metastases, and thus migrated from seventh edition >45 years stage IVC to eighth edition <55 years stage II. When considering the impact of these 10 patients in a cohort of 508 stage II patients of any age, it is not surprising that the 10-year DSS of the seventh and eighth edition stage II cohort remained similarly excellent (92.5% vs. 94.0%, respectively).
As with all retrospective studies, there are inherent limitations that must be acknowledged. All data analysis was based on tumor registry coded data (augmented by primary chart review only in patients who died during follow-up). Thus, some clinicopathologic factors now understood to have prognostic significance were not available for analysis (such as postoperative thyroglobulin levels, detailed histological evaluations classified using current nomenclature, vascular invasion, size and number of lymph node metastases, and detailed extent of gross extrathyroidal extension). As a result, ATA risk of recurrence was assigned using the previously validated 2009 ATA risk-stratification system using as much clinical information as was available. Although all follicular and Hürthle cell carcinomas were classified as intermediate risk in this study, it is recommended that clinicians classify these tumors according to the updated ATA risk-classification system using all available clinical information. Moreover, since MSKCC is a tertiary referral center, the DSS in this cohort may reflect a referral bias of more advanced thyroid disease. Lastly, it is possible that the median follow-up of 6.6 years fails to capture disease-related mortality in the very small number of low-risk patients who may have recurrence and disease progression decades after their original diagnosis (24). Conversely, the strength of this study include the large sample size, the relatively large number of disease-related deaths, and the meticulous follow-up of patients for survival endpoints, even if follow-up occurred outside of the center.
In summary, while the eighth edition provides meaningful risk stratification across all prognostic stage groups, the wide spectrum of disease included in the younger AJCC stage I/II patients requires an assessment of additional risk factors to refine and individualize these predictions for clinical use. A composite staging approach that integrates AJCC stage with ATA risk and age at diagnosis allows for the definition of six discrete risk cohorts that provide greater refinement of prognostic estimates for patients with DTC. While further studies are needed to validate this composite staging approach using the modified 2009 ATA risk-classification system, it represents another step forward in the movement toward individualized risk assessment and management for patients with DTC.
Acknowledgment
This work was supported by the Memorial Sloan Kettering Cancer Center Core Grant, P30 CA 008748 (Craig Thompson, PI) and the NCI SPORE in Thyroid Cancer, 5P50 CA172012-04 (James Fagin, PI).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2016. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26:1–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tuttle RM, Tala H, Shah J, Leboeuf R, Ghossein R, Gonen M, Brokhin M, Omry G, Fagin JA, Shaha A. 2010. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid 20:1341–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuttle RM, Haugen B, Perrier ND. 2017. Updated American Joint Committee on Cancer/tumor-node-metastasis staging system for differentiated and anaplastic thyroid cancer (eighth edition): what changed and why? Thyroid 27:751–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perrier ND, Brierley JD, Tuttle RM. 2018. Differentiated and anaplastic thyroid carcinoma: major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 68:55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim TH, Kim YN, Kim HI, Park SY, Choe JH, Kim JH, Kim JS, Oh YL, Hahn SY, Shin JH, Kim K, Jeong JG, Kim SW, Chung JH. 2017. Prognostic value of the eighth edition AJCC TNM classification for differentiated thyroid carcinoma. Oral Oncol 71:81–86 [DOI] [PubMed] [Google Scholar]
- 6.Kim M, Kim WG, Oh H-S, Park S, Kwon H, Song DE, Kim TY, Shong YK, Kim WB, Sung T-Y, Jeon MJ. 2017. Comparison of the seventh and eighth editions of the American Joint Committee on Cancer/Union for International Cancer Control tumor-node-metastasis staging system for differentiated thyroid cancer. Thyroid 27:1149–1155 [DOI] [PubMed] [Google Scholar]
- 7.Pontius LN, Oyekunle TO, Thomas SM, Stang MT, Scheri RP, Roman SA, Sosa JA. 2017. Projecting survival in papillary thyroid cancer: a comparison of the seventh and eighth editions of the American Joint Commission on Cancer/Union for International Cancer Control staging systems in two contemporary national patient cohorts. Thyroid 27:1408–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shteinshnaider M, Muallem Kalmovich L, Koren S, Or K, Cantrell D, Benbassat C. 2018. Reassessment of differentiated thyroid cancer patients using the eighth TNM/AJCC classification system: a comparative study. Thyroid 28:201–209 [DOI] [PubMed] [Google Scholar]
- 9.Rosario PW. 2017. Eighth edition of AJCC staging for differentiated thyroid cancer: is stage I appropriate for T4/N1b patients aged 45–55 years? Endocrine 56:679–680 [DOI] [PubMed] [Google Scholar]
- 10.Byrd DR, Greene FL. 2018. The eighth edition of TNM: implications for the surgical oncologist. Ann Surg Oncol 25:10–12 [DOI] [PubMed] [Google Scholar]
- 11.Nixon IJ, Kuk D, Wreesmann V, Morris L, Palmer FL, Ganly I, Patel SG, Singh B, Tuttle RM, Shaha AR, Gönen M, Shah JP. 2016. Defining a valid age cutoff in staging of well-differentiated thyroid cancer. Ann Surg Oncol 23:410–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganly I, Nixon IJ, Wang LY, Palmer FL, Migliacci JC, Aniss A, Sywak M, Eskander AE, Freeman JL, Campbell MJ, Shen WT, Vaisman F, Momesso D, Corbo R, Vaisman M, Shaha A, Tuttle RM, Shah JP, Patel SG. 2015. Survival from differentiated thyroid cancer: what has age got to do with it? Thyroid 25:1106–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang BH-H, Lo C-Y, Chan W-F, Lam K-Y, Wan K-Y. 2007. Staging systems for papillary thyroid carcinoma. Ann Surg 245:366–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jonklaas J, Nogueras-Gonzalez G, Munsell M, Litofsky D, Ain KB, Bigos ST, Brierley JD, Cooper DS, Haugen BR, Ladenson PW, Magner J, Robbins J, Ross DS, Skarulis MC, Steward DL, Maxon HR, Sherman SI. 2012. The impact of age and gender on papillary thyroid cancer survival. J Clin Endocrinol Metab 97:E878–E887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pathak KA, Mazurat A, Lambert P, Klonisch T, Nason RW. 2013. Prognostic nomograms to predict oncological outcome of thyroid cancers. J Clin Endocrinol Metab 98:4768–4775 [DOI] [PubMed] [Google Scholar]
- 16.Nixon IJ, Wang LY, Migliacci JC, Eskander A, Campbell MJ, Aniss A, Morris L, Vaisman F, Corbo R, Momesso D, Vaisman M, Carvalho A, Learoyd D, Leslie WD, Nason RW, Kuk D, Wreesmann V, Morris L, Palmer FL, Ganly I, Patel SG, Singh B, Tuttle RM, Shaha AR, Gönen M, Pathak KA, Shen WT, Sywak M, Kowalski L, Freeman J, Perrier N, Shah JP. 2016. An international multi-institutional validation of age 55 years as a cutoff for risk stratification in the AJCC/UICC staging system for well-differentiated thyroid cancer. Thyroid 26:373–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adam MA, Thomas S, Hyslop T, Scheri RP, Roman SA, Sosa JA. 2016. Exploring the relationship between patient age and cancer-specific survival in papillary thyroid cancer: rethinking current staging systems. J Clin Oncol 34:4415–4420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radowsky JS, Howard RS, Burch HB, Stojadinovic A. 2014. Impact of degree of extrathyroidal extension of disease on papillary thyroid cancer outcome. Thyroid 24:241–244 [DOI] [PubMed] [Google Scholar]
- 19.Kim SJ, Myong JP, Suh H, Lee KE, Youn YK. 2015. Optimal cutoff age for predicting mortality associated with differentiated thyroid cancer. PLoS One 10:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Youngwirth LM, Adam MA, Scheri RP, Roman SA, Sosa JA. 2017. Extrathyroidal extension is associated with compromised survival in patients with thyroid cancer. Thyroid 27:626–631 [DOI] [PubMed] [Google Scholar]
- 21.Bischoff L, Curry J, Ahmed I, Pribitkin E, Miller J. 2013. Is above age 45 appropriate for upstaging well-differentiated papillary thyroid cancer? Endocr Pract 19:995–997 [DOI] [PubMed] [Google Scholar]
- 22.Oyer SL, Smith VA, Lentsch EJ. 2012. Reevaluating the prognostic significance of age in differentiated thyroid cancer. Otolaryngol Head Neck Surg 147:221–226 [DOI] [PubMed] [Google Scholar]
- 23.Tran Cao HS, Johnston LE, Chang DC, Bouvet M. 2012. A critical analysis of the American Joint Committee on Cancer (AJCC)staging system for differentiated thyroid carcinoma in young patients on the basis of the Surveillance, Epidemiology, and End Results (SEER) registry. Surgery 152:145–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grogan RH, Kaplan SP, Cao H, Weiss RE, Degroot LJ, Simon CA, Embia OMA, Angelos P, Kaplan EL, Schechter RB. 2013. A study of recurrence and death from papillary thyroid cancer with 27 years of median follow-up. Surgery 154:1436–1447 [DOI] [PubMed] [Google Scholar]