Abstract
Bisphenol A (BPA) is one of the most produced chemicals in the world and has been widely employed in the food industry. Continuous and widespread exposure to BPA through drinking water and food leads to health concerns for humans. This study evaluated the effects of boron (B) on BPA-mediated oxidative stress in male Wistar albino rats. Rats were equally divided into 5 groups; corn oil was given orally to the control group; 25 mg kg–1 of BPA dissolved in corn oil was given orally to the second group. All other groups received the same dose of BPA and different doses of B (5, 10, and 20 mg kg–1 per day, respectively) orally for 30 days. The administration of BPA significantly decreased glutathione levels and increased malondialdehyde levels in rat tissues. Furthermore, BPA treatment reduced the catalase and superoxide dismutase activities in tissues and erythrocytes. Also, mRNA expression levels of TNF-α, IL-1β, and IL-6 in the brain, liver, and testes of rats were augmented, whereas IL-10 was decreased with BPA treatment. Besides, BPA treatment adversely altered biochemical parameters and caused damage to the cell integrity of rat tissues. However, B administration reversed BPA-induced alterations in rat tissues in a dose-dependent manner. Furthermore, B exhibited antioxidant and anti-inflammatory effects and regulated metabolic and histopathological alterations in male Wistar albino rats exposed to BPA.
1. Introduction
Bisphenol A (BPA) is a monomer widely found in polycarbonate plastics including food storage containers, baby bottles and water bottles.1,2 It is also employed in the production of internal coatings for beverage and food cans to protect the food content from direct contact with the metal. However, BPA residues can migrate into foods due to high temperatures. Exposure to BPA occurs mainly by ingestion, inhalation, and dermal routes.1,3 This substance causes various adverse health effects such as cancer, and endocrine, reproductive, metabolic and cardiovascular disorders.4,5 BPA also induces ROS-mediated oxidative stress by decreasing antioxidant enzymes (especially, superoxide dismutase, catalase, GSH reductase and GSH peroxidase) and by increasing the level of thiobarbituric acid-reactive substances. In addition, ROS generation caused by BPA significantly compromises mitochondrial function.6 Some reports have indicated that enhanced inflammation plays important roles in BPA-induced disorders.4,7–9 Fischer et al.4 showed that BPA altered the production of inflammatory cytokines and subsequently caused immune dysfunction, which may promote the development of breast cancer. Aboul Ezz et al.8 reported that BPA caused cardiotoxic effects by the overproduction of free radicals. Besides, Yin et al.9 suggested that ROS plays a pivotal role in BPA-caused male reproductive toxicity.
Boron (B) is an element of the metalloid group and possesses properties between nonmetals and metals.10 It is found only in compounds because it does not occur in free form in nature. The most common compounds of boron include boric acid, borax, borax-pentahydrate, sodium perborates, ulexite, and colemanite. In addition, boric acid is the most probable form of boron after ingestion by the organism.11,12 Boron compounds are employed in the food industry as food preservatives, emulsifiers, water softeners, stabilizers, and pH adjusters. They are also used in various applications, including glass fiber, insulation, laundry bleach, chemical fertilizer, herbicides, frit, enamel coating, and ceramic glazes.10,13,14 Boron is an essential element found in plants, and high levels of this element are provided by several foodstuffs, including beans, peanuts, avocado, grape juice, table wine, and chocolate powder. A single adult diet contains up to 40 mg of B.15,16 Also, B plays a significant role in metabolism by regulating enzymes and hormones.17–19 Moreover, B exhibits antioxidant activity through inhibiting the generation of ROS and has antigenotoxic and hepatoprotective effects.20–22 Based on the well-known positive effects of B, the consumption of foodstuffs rich in B may ameliorate the negative health outcomes of risky chemicals such as BPA.
This study aimed to investigate the possible protective role of B in BPA-induced oxidative stress, alterations in biochemical parameters and histopathological alterations in rat tissues. In addition, the expression levels of IL-1β, IL-10, IL-6, and TNF-α genes were evaluated in the brain, liver, and testis tissues.
2. Materials and methods
2.1. Materials
BPA and boric acid (H3BO3) were purchased from Alfa Aesar (Karlsruhe, Germany) and Sigma-Aldrich Co. (St Louis, Missouri, USA), respectively. Boric acid is employed in this study as the boron source due to its high purity (>99%) and good absorption features in the gastrointestinal tract. Besides, B was dissolved in physiological saline and BPA was dissolved in corn oil. All other reagents and chemicals were of analytical grade and obtained from various commercial sources.
2.2. Study design
2.2.1. Animals and groups
Thirty-five male Wistar albino rats (250–300 g) were purchased from the Experimental Animal Application and Research Center of Afyon Kocatepe University, Turkey. All experimental protocols were confirmed by the Afyon Kocatepe University Ethics Committee (permit number: 49533702-31). Before starting the experiment, the rats were adapted to the animal facility circumstances for seven days. Throughout the experimental period, all animals were kept in a well-ventilated room at 25 °C with 50–55% relative humidity in a 12 h light/dark cycle. Also, they were maintained on fresh deionized water and a diet containing a low level of B23ad libitum. To determine the level of B in the rat diet, ICP-OES (Optima 8300 DV, Waltham, MA, USA) was employed. The rats were allocated into five groups (n = 7). All groups were treated with the related compounds for 30 days. Group I served as the control group and was treated orally with corn oil (0.5 ml). Group II was given 25 mg kg–1 day–1 of BPA by gastric gavage.7 Moreover, the same dose of BPA concomitantly was given with different doses of B (5, 10 and 20 mg kg–1 day–1, respectively)21 to Groups III, IV and V.
2.2.2. Sample collection and processing
The male Wistar albino rats were fasted overnight and on the following day they were anesthetized with ketamine/xylazine. After the collection of blood samples by cardiac puncture, the rats were sacrificed and their tissues including the lung, liver, testis, kidney, heart, and brain were obtained. Firstly, plasma samples were obtained from blood samples within 30 min by means of centrifugation (3500 rpm, 15 min, 4 °C). Then, the precipitated erythrocytes were washed 3 times in isotonic saline (pH 7.4) to obtain a 10% erythrocyte suspension. The above-mentioned rat tissues were directly washed with ice-cold 0.9% NaCl buffer. Afterwards, in chilled Tris-HCl buffer (pH 7.4), tissue samples were trimmed and rinsed. In the same buffer, the tissues were homogenized and then centrifuged (3500 rpm, 10 min, 4 °C). The prepared supernatants were kept at –20 °C until the measurement.
2.3. Measurement of oxidative stress markers
The level of malondialdehyde (MDA), an important lipid peroxidation biomarker, was measured in whole tissue homogenates and blood samples according to the spectrophotometric methods of Ohkawa et al.24 and Draper and Hadley,25 respectively. The concentration of MDA was determined at 532 nm by using a UV-Visible spectrophotometer (Shimadzu 1601, Tokyo, Japan) and expressed as nmol per g in wet tissue and nmol per ml in blood.
The level of reduced glutathione (GSH), an important substance of the non-enzymatic defence system, was spectrophotometrically measured in tissue and blood samples.26 The method determines the reaction between glutathione and 5,5′-dithiobis-2-nitrobenzoic acid resulting in thiolate formation at 412 nm. The GSH level was expressed as nmol per g wet tissue and nmol per ml blood.
Superoxide dismutase (SOD) and catalase (CAT) are antioxidant enzymes that play an essential role against oxidative stress. The SOD activity of the erythrocyte lysate and the tissue homogenate was spectrophotometrically measured at 560 nm (ref. 27) and expressed as U per g protein in tissue and U per g hemoglobin (Hb) in erythrocyte. The activity of CAT in the erythrocyte lysate28 and the tissue homogenate29 was spectrophotometrically determined through monitoring the decomposition of H2O2 at 240 nm for 45 s at room temperature and expressed as k per μg protein in tissue and k per gHb in erythrocyte (k; nmol min–1). The hemoglobin content of erythrocytes and the protein content of tissues were required for the calculation of CAT and SOD activities and measured according to the methods of Drabkin and Austin30 and Lowry et al.,31 respectively.
2.4. Histopathology of tissues
After the dissection of the rats, their tissues were collected and fixed with formaldehyde solution (10% w/v). Afterwards, histopathological analysis of the tissues including the brain, lung, heart, liver, testis and kidney was performed by employing follow-up procedures. At the end of this procedure, the tissues were embedded in paraffin blocks. Then, they were cut as paraffin sections (5–6 μm thick) and stained with haematoxylin–eosin. Histopathological changes of the tissues were analyzed using a light microscope (Olympus Bx51) which was combined with an Olympus DP20 camera and the results are presented in Table 6.
Table 6. Effects of BPA treatment and concomitant treatment of BPA with B5, B10, and B20 on histopathological changes in the tissues of rats.
| Tissue | Histopathological changes | Control | BPA | B5 + BPA | B10 + BPA | B20 + BPA |
| Brain | Degenerative changes of neurons and focal gliosis | –(7/7) | +(3/7) | +(4/7) | +(6/7) | –(5/7) |
| ++(3/7) | –(3/7) | –(1/7) | +(2/7) | |||
| +++(1/7) | ||||||
| Lung | Thickening in the interalveolar septal tissue | –(7/7) | +(5/7) | +(4/7) | –(7/7) | –(7/7) |
| ++(2/7) | –(3/7) | |||||
| Alveolar edema | –(7/7) | +(7/7) | –(4/7) | –(5/7) | –(6/7) | |
| +(3/7) | +(2/7) | +(1/7) | ||||
| Heart | Hyaline degeneration of the myocardium | –(7/7) | –(1/7) | –(2/7) | –(6/7) | –(7/7) |
| +(4/7) | +(3/7) | +(1/7) | ||||
| ++(1/7) | ++(2/7) | |||||
| +++(1/7) | ||||||
| Kidney | Focal mononuclear cell infiltration in the glomerulus | –(7/7) | +(1/7) | –(4/7) | –(5/7) | –(7/7) |
| ++(6/7) | +(3/7) | +(2/7) | ||||
| Degenerative changes in tubular epithelial cells | –(7/7) | –(1/7) | –(5/7) | –(6/7) | –(7/7) | |
| +(5/7) | +(2/7) | +(1/7) | ||||
| ++(1/7) | ||||||
| Liver | Sinusoidal dilatation and hyperemia | –(7/7) | +(6/7) | –(5/7) | –(4/7) | –(7/7) |
| ++(1/7) | ++(2/7) | +(3/7) | ||||
| Degenerative changes in hepatocytes | –(7/7) | +(7/7) | –(2/7) | –(4/7) | –(6/7) | |
| +(3/7) | +(3/7) | +(1/7) | ||||
| ++(2/7) | ||||||
| Testis | Reduced spermatogenic density in tubulus seminiferus contortus | –(7/7) | +(7/7) | –(6/7) | –(6/7) | –(6/7) |
| ++(1/7) | +(1/7) | +(1/7) | ||||
| Degenerative changes in Sertoli cells | –(7/7) | +(7/7) | –(5/7) | –(4/7) | –(6/7) | |
| +(3/7) | +(4/7) | +(1/7) |
2.5. Determination of biochemical parameters
The plasma concentrations of aspartate transaminase (AST), alkaline phosphatase (ALP), alanine transaminase (ALT), glucose, triglyceride, low-density lipoprotein (LDL), high-density lipoprotein (HDL), cholesterol, creatinine and blood urea nitrogen (BUN) were determined by using commercial diagnostic kits (Biolabo Laboratories, Maizy, Picardy, France) according to the manufacturer's recommendation.
2.6. Determination of the expression levels of IL-1β, IL-6, TNF-α, and IL-10 genes
Real-time PCR was employed to determine the expression levels of IL-1β, IL-6, TNF-α, and IL-10. Tri-Pure Reagent (Roche, Germany) was used to extract total RNA of the tissues (testis, liver and brain). The quantification of the isolated RNA was performed by using Nanodrop ND-1000 (Thermo, USA) and the RNA purity was determined at OD260/280. An RT2 HT First Strand kit (Qiagen, Germany) was used for the reverse transcription of total RNA. Specific primers for IL-1β (sense: CACCTCTCAAGCAGAGCACAG, anti-sense: GGGTTCCATGGTGAAGTCAAC), IL6: (sense: CCTGGAGTTTGTGAAGAACAACT anti-sense: GGAAGTTGGGGTAGGAAGGA), IL 10: (sense: AGTGGAGCAGGTGAAGAATGA, anti-sense: TCATGGCCTTGTAGACACCTT), TNF-α (sense: CTTCTGTCTACTGAACTTCGG, anti-sense: GTGCTTGATCTGT-TGTTTCC), and GAPDH (GAPDH sense: ACCACAGTCCATGCCATCAC, anti-sense: TCCACCACCCTGTTGCTGTA) were acquired from Ella Biotech GmbH (Martinsried, Germany). The qRT-PCR analysis was carried out through a Rotor-Gene Q with RT2 SYBR-Green ROX master mix (Qiagen, Germany). The gene expression level was analyzed in triplicate for each sample. The normalization of the data was performed against GAPDH. The results were given as relative gene expression by the 2–ΔΔCt method.
2.7. Data analysis
The data from the study were analyzed using SPSS (20.0) software and expressed as means with their standard deviations (±SD). One-way analysis of variance was performed followed by the post hoc test of Duncan. p < 0.05 was accepted as statistically significant.
3. Results
3.1. Boron content analysis of animal feed
The B content of the prepared rodent diet was analyzed and the level of B was found to be less than 0.018 mg kg–1. The influence of this concentration of B is insignificant; therefore, the diet was found to be suitable for the present study.
3.2. Effect on LPO and GSH
Treatment with BPA significantly increased MDA levels of blood, testis, liver, brain, and kidney, (p < 0.001); lung (p = 0.001); and heart (p < 0.05) tissues when compared to the control group. In contrast, B dose-dependently ameliorated the changes in whole blood and tissues (Table 1). Administration of BPA decreased GSH levels in whole blood and lung (p < 0.001), heart and kidney (p = 0.001), liver (p < 0.01), and brain and testis (p < 0.05) tissues compared to the control group. However, B application yielded higher levels of GSH in blood and other tissues in the control group than in the BPA group (Table 2).
Table 1. Effects of BPA treatment and concomitant treatment of BPA with B5, B10, and B20 on malondialdehyde levels in rat tissues.
| Treatment design | Blood (nmol ml–1) | Kidney (nmol g–1 tissue) | Liver (nmol g–1 tissue) | Heart (nmol g–1 tissue) | Brain (nmol g–1 tissue) | Lung (nmol g–1 tissue) | Testis (nmol g–1 tissue) |
| Control | 6.71 ± 0.77d | 5.14 ± 0.39c | 6.22 ± 0.81c | 3.53 ± 0.26b | 3.36 ± 0.84b | 4.36 ± 0.66c | 4.41 ± 0.90b |
| BPA | 12.16 ± 1.21a | 9.25 ± 0.92a | 11.10 ± 1.22a | 4.64 ± 0.81a | 6.01 ± 0.90a | 6.14 ± 0.87a | 7.04 ± 1.07a |
| B5 + BPA | 8.45 ± 1.43bc | 9.07 ± 1.10a | 7.36 ± 0.91b | 4.12 ± 0.77ab | 5.22 ± 0.86a | 5.79 ± 0.59ab | 4.93 ± 0.44b |
| B10 + BPA | 9.16 ± 1.31b | 7.65 ± 0.85b | 7.26 ± 0.69bc | 4.23 ± 0.80ab | 5.38 ± 1.03a | 5.23 ± 0.80b | 4.52 ± 0.78b |
| B20 + BPA | 7.47 ± 1.09cd | 6.05 ± 1.00c | 7.16 ± 0.86bc | 3.86 ± 0.43b | 5.13 ± 1.17a | 5.20 ± 0.63b | 4.58 ± 0.47b |
Table 2. Effects of BPA treatment and concomitant treatment of BPA with B5, B10, and B20 on glutathione levels in rat tissues.
| Treatment design | Blood (nmol ml–1) | Kidney (nmol g–1 tissue) | Liver (nmol g–1 tissue) | Heart (nmol g–1 tissue) | Brain (nmol g–1 tissue) | Lung (nmol g–1 tissue) | Testis (nmol g–1 tissue) |
| Control | 62.84 ± 7.30a | 27.80 ± 3.52a | 29.32 ± 4.17a | 22.77 ± 3.46a | 22.13 ± 2.90a | 27.94 ± 2.76a | 24.75 ± 3.40a |
| BPA | 42.18 ± 5.03c | 19.09 ± 2.35b | 22.85 ± 2.00c | 16.93 ± 1.11c | 18.01 ± 2.57b | 21.93 ± 1.91c | 21.36 ± 1.62b |
| B5 + BPA | 47.91 ± 6.20b | 24.14 ± 4.18a | 23.42 ± 2.44bc | 18.74 ± 1.50bc | 20.30 ± 1.87ab | 23.25 ± 1.25bc | 21.69 ± 2.140b |
| B10 + BPA | 51.48 ± 5.45b | 24.91 ± 2.25a | 24.94 ± 3.44bc | 18.88 ± 1.32bc | 21.74 ± 1.92a | 24.43 ± 1.56b | 22.31 ± 1.43ab |
| B20 + BPA | 61.77 ± 6.64a | 25.04 ± 4.34a | 27.17 ± 3.88ab | 19.79 ± 2.74b | 21.91 ± 2.87a | 24.49 ± 1.98b | 23.45 ± 1.33ab |
3.3. Effect on antioxidant enzymes
SOD activities in the BPA group were found to be lower in erythrocytes, lung and liver (p < 0.001); brain and testis (p < 0.01); and heart and kidney (p < 0.05) tissues compared to the control group (Table 3). Similarly, the CAT activities of the BPA group were also reduced in erythrocytes, brain, lung, kidney, heart, and testis (p < 0.001); and liver (p = 0.001) tissues compared to the control group (Table 4). However, B treatment reversed the changes in the activities of SOD and CAT.
Table 3. Effects of BPA treatment and concomitant treatment of BPA with B5, B10, and B20 on superoxide dismutase activities in erythrocytes and tissues of rats.
| Treatment design | Erythrocyte (U gHb–1) | Kidney (U μg–1 protein) | Liver (U μg–1 protein) | Heart (U μg–1 protein) | Brain (U μg–1 protein) | Lung (U μg–1 protein) | Testis (U μg–1 protein) |
| Control | 21.57 ± 1.75a | 8.14 ± 1.48a | 8.54 ± 1.20a | 7.13 ± 0.96a | 7.40 ± 1.38a | 5.58 ± 0.89a | 5.90 ± 1.15a |
| BPA | 14.89 ± 1.48b | 5.58 ± 1.73b | 5.52 ± 0.76c | 5.68 ± 0.98b | 4.37 ± 0.96c | 3.64 ± 0.87c | 4.01 ± 1.04b |
| B5 + BPA | 15.25 ± 2.28b | 5.76 ± 1.53b | 6.26 ± 1.23bc | 6.42 ± 0.67ab | 5.26 ± 1.23bc | 4.12 ± 0.79bc | 4.65 ± 0.82b |
| B10 + BPA | 16.06 ± 2.91b | 7.11 ± 1.42ab | 7.32 ± 1.10ab | 6.44 ± 0.72ab | 6.18 ± 1.34ab | 4.88 ± 0.7ab | 4.13 ± 0.92b |
| B20 + BPA | 21.56 ± 3.35a | 6.94 ± 1.27ab | 7.45 ± 1.24ab | 7.08 ± 1.03a | 6.3 ± 1.46ab | 5.12 ± 0.63a | 4.50 ± 0.77b |
Table 4. Effects of BPA treatment and concomitant treatment of BPA with B5, B10, and B20 on catalase activities in erythrocytes and tissues of rats.
| Treatment design | Erythrocyte (k per gHb) | Kidney (k per μg protein) | Liver (k per μg protein) | Heart (k per μg protein) | Brain (k per μg protein | Lung (k per μg protein) | Testis (k per μg protein) |
| Control | 6.34 ± 1.3a | 1.71 ± 0.39a | 6.68 ± 1.28a | 1.12 ± 0.38a | 1.08 ± 0.17a | 1.31 ± 0.19a | 0.43 ± 0.07a |
| BPA | 3.08 ± 0.71c | 0.68 ± 0.12c | 4.23 ± 1.03b | 0.46 ± 0.11b | 0.48 ± 0.15c | 0.61 ± 0.10c | 0.13 ± 0.03c |
| B5 + BPA | 3.18 ± 0.97c | 0.87 ± 0.16c | 4.33 ± 0.75b | 0.88 ± 0.19a | 0.66 ± 0.19b | 0.68 ± 0.17c | 0.15 ± 0.04c |
| B10 + BPA | 4.04 ± 0.66c | 1.54 ± 0.33ab | 4.61 ± 1.20b | 0.87 ± 0.23a | 0.75 ± 0.13b | 0.85 ± 0.13b | 0.17 ± 0.03c |
| B20 + BPA | 5.19 ± 1.06b | 1.35 ± 0.26b | 5.06 ± 0.89b | 0.93 ± 0.15a | 1.03 ± 0.16a | 1.27 ± 0.17a | 0.25 ± 0.05b |
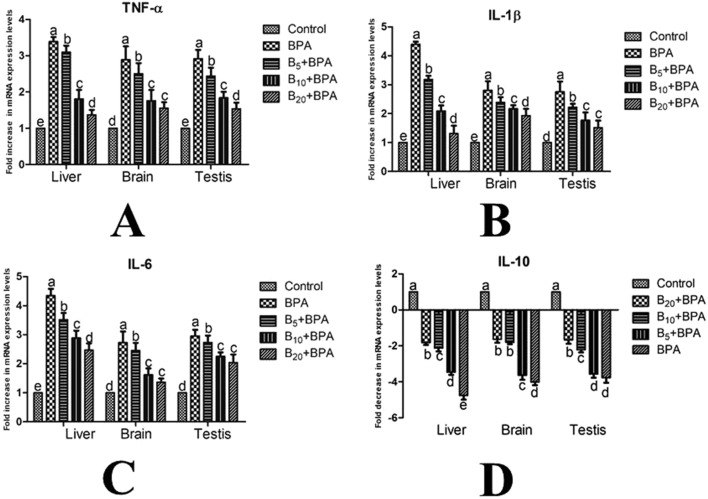
3.4. Effects on gene expression levels
Treatment with BPA increased the expression levels of TNF-α (Fig. 1A), IL-1β (Fig. 1B), and IL-6 (Fig. 1C) genes, whereas it decreased the expression level of the IL-10 (Fig. 1D) gene in the brain, liver, and testis tissues compared to the control group (p < 0.001). However, application of B ameliorated BPA-induced changes in the expression levels of these inflammation-related genes dose-dependently.
Fig. 1. Effects of BPA treatment and concomitant treatment of BPA with B5, B10, and B20 on TNF-α (A), IL-1β (B), IL-6 (C), and IL-10 (D) gene expression levels in the liver, brain and testis tissues of male rats by RT-PCR. BPA: bisphenol-A at a dose of 25 mg kg–1; B5: boron at 5 mg kg–1 dose; B10: boron at 10 mg kg–1 dose; B20: boron at 20 mg kg–1 dose.
3.5. Effects on biochemical parameters
Administration of BPA (25 mg kg–1, p.o.) significantly increased the plasma levels of AST, ALT, ALP, glucose, LDL, cholesterol, BUN, creatinine (p < 0.001), and triglyceride (p < 0.01), whereas it decreased the HDL level (p < 0.001). Boron administration reversed BPA-induced changes in the biochemical parameters of rats (Table 5).
Table 5. Effects of BPA treatment and concomitant treatment of BPA with B5, B10, and B20 on biochemical parameters of rats.
| Treatment design | ALP (IU L–1) | ALT (IU L–1) | AST (IU L–1) | Glucose (mg dL–1) | Cholesterol (mg dL–1) | HDL (mg dL–1) | LDL (mg dL–1) | Triglyceride (mg dL–1) | Creatinine (mg dL–1) | BUN (mg dL–1) |
| Control | 93.15 ± 6.94d | 35.57 ± 4.07c | 106.75 ± 11.63d | 87.2 ± 8.23d | 78.89 ± 9.98c | 46.58 ± 8.75a | 27.82 ± 3.47d | 74.45 ± 7.05c | 0.64 ± 0.07e | 28.76 ± 3.11d |
| BPA | 131.42 ± 5.61a | 62.83 ± 6.64a | 239.08 ± 23.7a | 196.23 ± 14.05a | 113.14 ± 12.37a | 15.84 ± 4.6d | 75.69 ± 9.54a | 93.46 ± 11.98a | 2.84 ± 0.32a | 57.93 ± 6.56a |
| B5 + BPA | 119.02 ± 6.44b | 55.88 ± 6.76b | 166.37 ± 20.03b | 149.04 ± 13.58b | 108.76 ± 11.06ab | 19.12 ± 4.55d | 55.22 ± 7.09b | 88.01 ± 11.12ab | 2.02 ± 0.22b | 48.25 ± 4.46b |
| B10 + BPA | 113.13 ± 5.88b | 50.69 ± 5.37b | 141.78 ± 12.3c | 136.8 ± 10.82b | 100.12 ± 12.93b | 29.44 ± 4.46c | 41.21 ± 5.63c | 83.99 ± 7.23abc | 1.54 ± 0.1c | 40.05 ± 3.63c |
| B20 + BPA | 103.68 ± 7.05c | 40.89 ± 3.77c | 125.71 ± 11.39c | 114.05 ± 9.19c | 86.38 ± 9.83c | 37.32 ± 7.51b | 33.5 ± 4.84d | 80.37 ± 7.85bc | 1.15 ± 0.13d | 35.34 ± 3.6c |
3.6. Effects on histopathology
Table 6 and Fig. S1–S6 in the ESI† demonstrate the histopathological alterations of tissues. In the BPA group, neuronal degeneration and focal gliosis were observed in the brain tissue (Fig. S1-B†), and thickening of the interalveolar septal tissue and alveolar edema (Fig. S2-B and C†) were observed in the lung tissue. Hyaline degeneration areas in the myocardium (Fig. S3-B and C†) were also observed. In the kidney, focal mononuclear cell infiltration in the glomerulus and degenerative changes in tubular epithelial cells were seen (Fig. S4-B and C†). In the liver, sinusoidal dilatation, hyperemia and degenerative changes in hepatocytes were observed (Fig. S5-B and C†). Reduced spermatogenic density and degenerative changes in Sertoli cells (Fig. S6-B and C†) in the testis tissue were also observed. In the groups that received B, moderate histopathological changes were seen in all of the above-mentioned tissues in a dose dependent manner compared to the BPA group (Fig. S1–6C, D and E†). No significant changes were observed in the rat tissues of the control group (Fig. S1–6A†).
4. Discussion
The present study showed that oral exposure to BPA caused lipid peroxidation whereas it decreased SOD and CAT enzyme activities and GSH levels in Wistar albino rats. In accordance with our study, recent studies about BPA have also demonstrated that the administration of this substance at different doses adversely affected oxidative stress parameters by increasing MDA levels, decreasing GSH levels, and decreasing SOD and CAT activities in experimental animals.8,32–34 Also, several antioxidant substances have been evaluated for their protective or preventive effects against BPA induced oxidative stress. For instance, Poormoosavi et al.35 reported that BPA treatment (10 mg kg–1, 8 weeks) increased MDA levels and decreased GSH levels and total antioxidant capacity in the serum of rats. Furthermore, concomitant treatment with an Asparagus officinalis extract (400 mg kg–1) exhibited protective effects against BPA-induced oxidative damage. Similarly, Morgan et al.36 observed that BPA administration to rats at a dose of 25 mg kg–1 for 50 days increased MDA levels and decreased GSH levels and the enzyme activities of SOD and CAT in the kidney, brain, and testis tissues. However, cinnamon treatment (200 mg kg–1) ameliorated BPA induced oxidative damage. Also, Jain et al.37 investigated the effect of concomitant administration of BPA (2 and 20 μg kg–1 for 28 days) and N-acetylcysteine (100 mg kg–1) on oxidative stress parameters in rat brains and concluded that N-acetylcysteine successfully reversed BPA-induced alterations. Consistent with these studies, B, which is a potent antioxidant substance,38 showed protective effects on BPA-induced oxidative damage in the blood and brain, heart, lung, kidney, testis, and liver tissues of rats in our study. This protective effect of B may be associated with its scavenger effects on free radicals.20,21
Oxidative stress plays an essential role in the emergence of a number of chronic disorders such as diabetes and cancer by inducing inflammation. This situation can activate several transcription factors such as p53 and NFκB, which might demonstrate the relation of BPA with the inflammatory processes.34,39 Elswefy et al.40 gave BPA (50 mg kg–1) to rats orally for 8 weeks and reported that BPA administration significantly increased the serum level of IL-1β and reduced the level of IL-10. Also, Hussein and Eid41 administered BPA to Swiss Albino male mice via drinking water after dissolving in 1% ethanol for 3 weeks at doses of 48, 60, 80, 120 and 240 mg kg–1 which correspond to 1/50, 1/40, 1/30, 1/20 and 1/10 LD50 of BPA, respectively. They concluded that IL-1β expression levels were significantly increased in the liver at all selected doses. Moreover, the expression level of IL-6 was augmented significantly at higher doses (1/20 and 1/10 LD50). Similarly, we determined that BPA treatment at a dose of 25 mg kg–1 increased the expression of TNF-α, IL-6, and IL-1β, while it reduced the expression level of IL-10. In contrast, B treatment reversed BPA-induced alterations in a dose dependent manner based on its anti-inflammatory effect.38,42
Different doses and durations of BPA treatment have affected biochemical parameters in experimental studies.33,35,43,44 The intraperitoneal application of BPA to mice at doses of 0.5 and 2 mg kg–1 for 4 weeks dose-dependently increased the levels of blood glucose, triglycerides, total cholesterol and LDL, and significantly reduced the levels of HDL.43 Poormoosavi et al.35 reported that exposure of 10 mg kg–1 of BPA to rats for 8 weeks significantly increased the bilirubin, urea and creatinine levels and the activities of AST, ALT, and ALP. Moreover, Asparagus officinalis extract (400 mg kg–1) supplementation significantly normalized these parameters compared to BPA-treated rats. Also, Abdel-Wahab44 determined that 10 mg kg–1 of BPA treatment in rats elevated the level of total bilirubin and the activity of AST and ALP in the serum, and the levels of markers are reversed by thymoquinone treatment at a dose of 10 mg kg–1. Our results are in agreement with the above-mentioned studies. Administration of BPA increased plasma ALT, ALP, AST, triglyceride, LDL, cholesterol, glucose, BUN and creatinine levels and decreased HDL levels. Concomitant treatment of BPA and B brought the levels of these markers close to that of the control group dose-dependently.
The present study suggests that oral administration of BPA (25 mg kg–1) induces obvious histopathological alterations in various rat tissues including focal gliosis and neuronal degeneration in the brain; hyaline degeneration in myocardial cells; interalveolar septal thickening and alveolar edema formation in the lungs; focal glomerular mononuclear cell infiltration and degenerative changes in tubular cells in the kidney; degenerative changes in hepatocytes around the vena centralis in the liver; and degenerative changes in Sertoli cells and decrease of spermatogenic density in the testis. Morgan et al.36 investigated the effects of BPA (25 mg kg–1) on the kidney, brain, and testis tissues of male Wistar albino rats and found that BPA treatment induced hypercellularity and glomerular congestion in the kidney; pyknosis, neuronophagia and perineuronal satellitosis in the mid brain; and perivascular edema, hyperemia, and degeneration in the testis. Comparably, BPA treatment (25 mg kg–1) of rats caused congestion areas in the kidney,45 necrotic lesions and congestion areas in the liver7 and the formation of many hyperchromatic cells in the brain.46 Similarly, Kamel et al.34 reported that oral treatment with BPA at a dose of 20 mg kg–1 for 30 days induced degenerative changes of parenchymal cells, sinusoidal congestion, multifocal vacuolar degeneration and lymphoid aggregates in the liver of rats. Moreover, an irregular outline of seminiferous tubules and detached degenerative primary spermatogonia from the wall of seminiferous tubules were seen in the testes. Also, Kattaia and Abdel Baset47 reported that a 50 mg kg–1 BPA treatment resulted in mild thickening of the interalveolar septa and inflammatory cell infiltration in focal areas, and extravasated red blood cells in the alveolar and bronchiolar lumen of the lung tissue of male rats. Poormoosavi et al.35 observed that the treatment of rats with 10 mg kg–1 BPA induced necrotic alterations of hepatocytes, and increased lymphocytic infiltration and proliferation of Kupffer cells. Besides, glomerular congestion, swelling in proximal tubules and acute tubular necrosis were seen in the kidney. Also, Kanwal et al.48 indicated that application of a high dose of BPA (100 mg kg–1) induced hepatic toxicity, illustrated by the pyknotic nucleus, necrosis, vacuolization, and inflammation around the portal and central vein of male Wistar albino rats. The results of the current study regarding histopathological changes are in accordance with previously reported studies and B ameliorated BPA induced changes in rat tissues due to its antioxidant and cell protective effects.21,38
5. Conclusion
To the best of our knowledge, this work is the first to research the effects of B on BPA-induced toxicity in rat tissues by evaluating the changes in antioxidant, biochemical, inflammatory, and histopathological parameters. The present study demonstrated that B successfully reversed BPA-induced alterations in oxidative stress, alterations in biochemical parameters and inflammation. Also, B prevented tissue damage by inhibiting lipid peroxidation and activating antioxidant enzymes.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
The Scientific Research Council of Afyon Kocatepe University, Turkey (Project no: 17.KARIYER.46) financially supported this study. The summary of the present work was partially presented as an oral presentation at the 3rd International Turkic World Conference on Chemical Sciences and Technologies, 10–13 September 2017, Baku, Azerbaijan.
Footnotes
†Electronic supplementary information (ESI) available. See DOI: 10.1039/c8tx00312b
References
- Schecter A., Malik N., Haffner D., Smith S., Harris T. R., Paepke O., Birnbaum L. Environ. Sci. Technol. 2010;44:9425–9430. doi: 10.1021/es102785d. [DOI] [PubMed] [Google Scholar]
- Esplugas R., LLovet M. I., Bellés M., Serra N., Vallvé J. C., Domingo J. L., Linares V. Food Chem. Toxicol. 2018;114:270–277. doi: 10.1016/j.fct.2018.02.046. [DOI] [PubMed] [Google Scholar]
- Cao X.-L., Corriveau J., Popovic S. J. Food Prot. 2010;73:1085–1089. doi: 10.4315/0362-028x-73.6.1085. [DOI] [PubMed] [Google Scholar]
- Fischer C., Mamillapalli R., Goetz L. G., Jorgenson E., Ilagan Y., Taylor H. S. Horm. Cancer. 2016;7:241–251. doi: 10.1007/s12672-016-0254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjum S., Rahman S., Kaur M., Ahmad F., Rashid H., Ansari R. A., Raisuddin S. Food Chem. Toxicol. 2011;49:2849–2854. doi: 10.1016/j.fct.2011.07.062. [DOI] [PubMed] [Google Scholar]
- Kovacic P. Med. Hypotheses. 2010;75:1–4. doi: 10.1016/j.mehy.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Korkmaz A., Ahbab M. A., Kolankaya D., Barlas N. Food Chem. Toxicol. 2010;48:2865–2871. doi: 10.1016/j.fct.2010.07.019. [DOI] [PubMed] [Google Scholar]
- Aboul Ezz H. S., Khadrawy Y. A., Mourad I. M. Cytotechnology. 2013;67:145–155. doi: 10.1007/s10616-013-9672-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L., Dai Y., Cui Z., Jiang X., Liu W., Han F., Lin A., Cao J., Liu J. Toxicol. Appl. Pharmacol. 2017;314:98–108. doi: 10.1016/j.taap.2016.11.013. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Fujiwara T. Pflugers Arch. 2008;456:671–677. doi: 10.1007/s00424-007-0370-8. [DOI] [PubMed] [Google Scholar]
- Hunt C. D. J. Trace Elem. Exp. Med. 2003;16:291–306. [Google Scholar]
- Hunt C. D. Environ. Health Perspect. 1994;102:35–43. doi: 10.1289/ehp.94102s735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorei R. Origins Life Evol. Biospheres. 2012;42:3–17. doi: 10.1007/s11084-012-9269-2. [DOI] [PubMed] [Google Scholar]
- Beyer K. H., Bergfeld W. F., Berndt W. O., Boutwell R. K., Carlton W. W., Hoffmann D. K., Schroeter A. L. J. Am. Coll. Toxicol. 1983;2:87–125. [Google Scholar]
- Devirian T. A., Volpe S. L. Crit. Rev. Food Sci. Nutr. 2003;43:219–231. doi: 10.1080/10408690390826491. [DOI] [PubMed] [Google Scholar]
- Hunt C. D., in Encyclopedia of Dietary Supplements, ed. P. M. Coates, M. Blackman, G. M. Cragg, M. A. Levine, J. Moss and J. D. White, Marcel Dekker, New York, 2005, pp. 55–63. [Google Scholar]
- Nielsen F., Hunt C., Mullen L., Hunt J. FASEB J. 1987;1:394–397. [PubMed] [Google Scholar]
- Hunt C. D. J. Trace Elem. Exp. Med. 1996;9:185–213. [Google Scholar]
- Armstrong T. A., Spears J. W., Lloyd K. E. J. Anim. Sci. 2001;79:1549. doi: 10.2527/2001.7961549x. [DOI] [PubMed] [Google Scholar]
- Coban F. K., Liman R., Cigerci I. H., Ince S., Hazman O., Bozkurt M. F. Fresenius Environ. Bull. 2015;24:4059–4066. [Google Scholar]
- Ince S., Kucukkurt I., Demirel H. H., Acaroz D. A., Akbel E., Cigerci I. H. Chemosphere. 2014;108:197–204. doi: 10.1016/j.chemosphere.2014.01.038. [DOI] [PubMed] [Google Scholar]
- Türkez H., Geyikoglu F., Tatar A., Keleş S., Özkan A. Z. Naturforsch., C: J. Biosci. 2007;62:889–896. doi: 10.1515/znc-2007-11-1218. [DOI] [PubMed] [Google Scholar]
- Bourgeois A.-C., Scott M. E., Sabally K., Koski K. G. J. Nutr. 2007;137:2080–2086. doi: 10.1093/jn/137.9.2080. [DOI] [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Draper H. H., Hadley M. Methods Enzymol. 1990;186:421–431. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- Beutler E., Duron O., Kelly B. M. J. Lab. Clin. Med. 1963;61:882–888. [PubMed] [Google Scholar]
- Sun Y., Oberley L. W., Li Y. Clin. Chem. 1988;34:497–500. [PubMed] [Google Scholar]
- Luck H., Catalase, in Methods in Analysis, ed. H. U. Bergmeyer, Academy Press, London., 1955. [Google Scholar]
- Aebi H., in Methods of enzymatic analysis, ed. H.-U. Bergmeyer, Academic Press, New York and London, 1974, pp. 673–677. [Google Scholar]
- Drabkin D. L., Austin J. H. J. Biol. Chem. 1935;112:51–65. [Google Scholar]
- Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Hassan Z. K., Elobeid M. A., Virk P., Omer S. A., Elamin M., Daghestani M. H., Alolayan E. M. Oxid. Med. Cell. Longevity. 2012;2012:194829. doi: 10.1155/2012/194829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaydın T., Oznurlu Y., Sur E., Celik I., Uluısık D., Dayan M. Biotech. Histochem. 2018:1–8. doi: 10.1080/10520295.2017.1420821. [DOI] [PubMed] [Google Scholar]
- Kamel A. H., Foaud M. A., Moussa H. M. J. Basic Appl. Zool. 2018;79:6. [Google Scholar]
- Poormoosavi S. M., Najafzadehvarzi H., Behmanesh M. A., Amirgholami R. Toxicol. Rep. 2018;5:427–433. doi: 10.1016/j.toxrep.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan A. M., El-Ballal S. S., El-Bialy B. E., El-Borai N. B. Toxicol. Rep. 2014;1:92–101. doi: 10.1016/j.toxrep.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S., Mahendra Kumar C. H., Suranagi U. D., Mediratta P. K. Food Chem. Toxicol. 2011;49:1404–1409. doi: 10.1016/j.fct.2011.03.032. [DOI] [PubMed] [Google Scholar]
- Acaroz U., Ince S., Arslan-Acaroz D., Gurler Z., Kucukkurt I., Demirel H. H., Arslan H. O., Varol N., Zhu K. Food Chem. Toxicol. 2018;118:745–752. doi: 10.1016/j.fct.2018.06.029. [DOI] [PubMed] [Google Scholar]
- Ince S., Arslan-Acaroz D., Demirel H. H., Varol N., Ozyurek H. A., Zemheri F., Kucukkurt I. Biomed. Pharmacother. 2017;96:263–268. doi: 10.1016/j.biopha.2017.09.141. [DOI] [PubMed] [Google Scholar]
- Elswefy S. E. S., Abdallah F. R., Atteia H. H., Wahba A. S., Hasan R. A. Int. J. Exp. Pathol. 2016;97:369–379. doi: 10.1111/iep.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein R. M., Eid J. I. Life Sci. J. 2013;10:1050–1059. [Google Scholar]
- Turkez H. J. Appl. Toxicol. 2008;28:658–664. doi: 10.1002/jat.1318. [DOI] [PubMed] [Google Scholar]
- Moghaddam H. S., Samarghandian S., Farkhondeh T. Toxicol. Mech. Methods. 2015;25:507–513. doi: 10.3109/15376516.2015.1056395. [DOI] [PubMed] [Google Scholar]
- Abdel-Wahab W. M. Pak. J. Biol. Sci. 2014;17:1152–1160. doi: 10.3923/pjbs.2014.1152.1160. [DOI] [PubMed] [Google Scholar]
- Korkmaz A., Aydoğan M., Kolankaya D., Barlas N. Environ. Toxicol. 2011;26:325–337. doi: 10.1002/tox.20556. [DOI] [PubMed] [Google Scholar]
- Aydogan M., Korkmaz A., Barlas N., Kolankaya D. Toxicology. 2008;249:35–39. doi: 10.1016/j.tox.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Kattaia A. A. A., Abdel Baset S. A. Egypt. J. Histol. 2014;37:24–35. [Google Scholar]
- Kanwal Q., Qadir A., Amina A., Iqbal H. H., Munir B. Environ. Sci. Pollut. Res. 2018;25:11884–11892. doi: 10.1007/s11356-018-1211-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.