Abstract
Objective
To examine associations between early developmental exposure to ambient pesticides and autism spectrum disorder.
Design
Population based case-control study.
Setting
California’s main agricultural region, Central Valley, using 1998-2010 birth data from the Office of Vital Statistics.
Population
2961 individuals with a diagnosis of autism spectrum disorder based on the Diagnostic and Statistical Manual of Mental Disorders, fourth edition, revised (up to 31 December 2013), including 445 with intellectual disability comorbidity, were identified through records maintained at the California Department of Developmental Services and linked to their birth records. Controls derived from birth records were matched to cases 10:1 by sex and birth year.
Exposure
Data from California state mandated Pesticide Use Reporting were integrated into a geographic information system tool to estimate prenatal and infant exposures to pesticides (measured as pounds of pesticides applied per acre/month within 2000 m from the maternal residence). 11 high use pesticides were selected for examination a priori according to previous evidence of neurodevelopmental toxicity in vivo or in vitro (exposure defined as ever v never for each pesticide during specific developmental periods).
Main outcome measure
Odds ratios and 95% confidence intervals using multivariable logistic regression were used to assess associations between pesticide exposure and autism spectrum disorder (with or without intellectual disabilities) in offspring, adjusting for confounders.
Results
Risk of autism spectrum disorder was associated with prenatal exposure to glyphosate (odds ratio 1.16, 95% confidence interval 1.06 to 1.27), chlorpyrifos (1.13, 1.05 to 1.23), diazinon (1.11, 1.01 to 1.21), malathion (1.11, 1.01 to 1.22), avermectin (1.12, 1.04 to 1.22), and permethrin (1.10, 1.01 to 1.20). For autism spectrum disorder with intellectual disability, estimated odds ratios were higher (by about 30%) for prenatal exposure to glyphosate (1.33, 1.05 to 1.69), chlorpyrifos (1.27, 1.04 to 1.56), diazinon (1.41, 1.15 to 1.73), permethrin (1.46, 1.20 to 1.78), methyl bromide (1.33, 1.07 to 1.64), and myclobutanil (1.32, 1.09 to 1.60); exposure in the first year of life increased the odds for the disorder with comorbid intellectual disability by up to 50% for some pesticide substances.
Conclusion
Findings suggest that an offspring’s risk of autism spectrum disorder increases following prenatal exposure to ambient pesticides within 2000 m of their mother’s residence during pregnancy, compared with offspring of women from the same agricultural region without such exposure. Infant exposure could further increase risks for autism spectrum disorder with comorbid intellectual disability.
Introduction
Autism spectrum disorder comprises severe developmental disorders characterized by atypical socialization, and restricted and repetitive behaviors and interests. Genetics have a role,1 2 with heritability estimates of 38%3 to 83%,4 but more information is needed about environmental factors operating in early development.3 Prenatal exposures to several types of pesticides have been associated with impaired neurodevelopment,5 6 7 8 and the few studies that have considered autism spectrum disorder have suggested that organophosphates9 and organochlorines10 11 could increase risk.
Experimental in vivo and in vitro studies of autism12 13 14 suggested changes in neuroprotein levels, altered gene expression, and neurobehavioral abnormalities after exposure to certain pesticides.12 14 For example, when the organophosphate chlorpyrifos was administered prenatally at subtoxic levels to a mouse model that displays several behavioral traits related to the autism spectrum, male offspring showed delayed motor function maturation and enhanced behavioral features associated with autism spectrum disorder.13
So far, knowledge about pesticide exposure in the real world and risk of autism spectrum disorder is scarce. In this large population based study, we assess prenatal and infant exposure to high use pesticides, which have been a priori selected on the basis of previous evidence for their experimental neurodevelopmental toxicity. Use of these pesticides in an agriculturally intensive region of California, United States, were recorded in the California state mandated Pesticide Use Reporting (CA-PUR) program. These records were integrated in our geographic information system tool, which links exposure records to addresses from birth records of the study population.
Methods
Study design and population
Records of autism spectrum disorder cases were retrieved from the registry maintained at the California Department of Developmental Services (DDS), based on diagnostic data collected by contracted regional centers (https://www.dds.ca.gov/RC/RCList.cfm). We included all individuals with a primary diagnosis of autistic disorder (code 299.00) reported on the DDS client development evaluation report, which implements criteria of the Diagnostic and Statistical Manual of Mental Disorders, fourth edition, revised (DSM-IV-R)15 up to 31 December 2013 (“autistic disorder” is the most severe diagnosis of autism spectrum disorder under DSM-IV criteria).16 Validation studies have established the reliability and validity of the DDS client development evaluation report in California.17 Eligibility for DDS services does not depend on citizenship or financial status, and services are available to all children. We used California birth records data from the Office of Vital Statistics to create a statewide case-control sample of 1998-2010 births. We matched DDS case records to birth records using a probabilistic linkage18 based on child and parental identifiers including first and last name, birth date, and sex. We estimated the probability that two records were for the same person by assigning total linkage scores generated for matches with the National Program of Cancer Registries Link Plus software (linkage rate 86.3%).19 We manually checked cases with borderline scores; the main reason for non-linkage was missing information on birth or DDS records.
Randomly selected controls from birth records were matched to each case 10:1 by birth year and sex. From the statewide sample (n=33 921 cases, n=339 210 controls), we excluded 3401 (10%) case records and 42 519 (12.5%) control records with missing, implausible, or non-viable gestational ages (included range 147-322 days) or birth weights (included range 500-6800 g), and non-singleton births. We also excluded 1296 (0.4%) controls who died before age 6 (identified by linkage to the California death registry).18 We restricted our sample to the eight major agricultural counties (San Joaquin, Stanislaus, Merced, Madera, Fresno, Kings, Tulare, and Kern); 38 331 participants (2961 cases and 35 370 controls) resided here at the time of birth and diagnosis. Although the CA-PUR covers the state of California, the mandatory reporting reflects agricultural use pesticides (see supplemental eMethods), which has a different spatial resolution from other pesticide use recorded in the Pesticide Use Reporting system. In urban areas (such as on structures and right of way applications or near roadway applications), non-agricultural pesticide use is most common but this is only reportable to the Pesticide Use Reporting at the county level (low spatial resolution); thus variables that estimate pesticide exposure for urban areas would be expected to result in markedly higher exposure misclassification.
We distinguished cases according to comorbid intellectual disability (in our study period recorded as “mental retardation” and diagnosed according to DSM-IV criteria corresponding to ICD-9 (international classification of diseases, 9th revision)). Information on pregnancy characteristics including gestational age, birth weight, pregnancy complications, and sociodemographics (maternal/paternal age, race/ethnicity, education) was retrieved from birth records.
Pesticide exposure
Residential birth addresses, as listed on birth certificates, were geocoded by our open source geocoder (historical address information was not available).20 CA-PUR21 includes information on all agricultural pesticide applications with the date, location, and amount of active ingredient applied (see supplemental eMethods). CA-PUR reports were combined with land use survey information from the California Department of Water Resources, which provides the location of specific crops, in a geographic information system-based computer model to estimate pesticide exposure from agricultural applications (technical details published elsewhere22). Briefly, for each pesticide, we summed pounds applied per acre (1 acre=4046.9 m2) per month within a 2000 m radius of each residential address. Our geographic information system tool generated calendar month averages, which we then used to generate developmental period-specific averages (for the three months before gestation, each month of gestation/gestation, and the first year of life) using weights according to the developmental period/gestational days covered by a calendar month. For sensitivity analyses, we also used a 2500 m radius in the same manner. The length of the gestational period for controls was truncated to the length of the matched cases to ensure comparable exposure periods. We defined exposure as any versus none to a specific substance during a specific developmental period; we chose this method to avoid making assumptions about the relative toxicity of agents, shape of the association, or the exposure potential due to presence at the time of application. It is, however, possible that this approach generates non-differential exposure error and underestimates effects.
We a priori decided to select from among 25 most used pesticide substances with peer reviewed published reports of neurodevelopmental interference, leaving 11 pesticides for analysis (classifications shown in eTable 1). These substances included glyphosate,23 24 25 26 chlorpyrifos,9 27 diazinon,28 29 30 acephate,31 32 33 malathion,33 34 35 permethrin,6 9 bifenthrin,9 33 36 methyl bromide,37 38 imidacloprid,39 40 avermectin,41 42 and myclobutanil.14 43
Statistical analysis
Tetrachoric/Spearman correlations (binary/continuous) of pesticide exposures were examined within and between developmental periods. Pesticide use over time was plotted; maps were drawn using ArcGIS 10.4 (ESRI). Odds ratios and 95% confidence intervals were estimated for associations between developmental period-specific pesticide exposures and autism spectrum disorder with unconditional logistic regression. We adjusted all models for the matching variables sex and year of birth, and selected potential confounders on the basis of previous knowledge.10 44 These potential confounders included maternal age, indicators of socioeconomic status (that is, maternal race/ethnicity and education), and nitrogen oxides44 (NOx; pregnancy average) as a marker of traffic related air pollution. For air pollution assessment, we used the California Line Source (CALINE4) emissions model, a modified Gaussian dispersion model of local gasoline and diesel vehicles emissions estimated for 1500 m distance from the residential address based on traffic volume, roadway geometry, vehicle emission rates, and meteorological conditions (wind speed/direction, temperature, atmospheric stability, and mixing heights).45 46 47
While we estimated parameters for each pesticide in separate models because of collinearities, we also explored multi-pesticide models for two or three selected pesticides for substances that showed associations with autism spectrum disorder in single pesticide models and belonged to different chemical classes. For those pesticides with more than one substance per class (organophosphates, pyrethroids), we selected a representative chemical (eg, chlorpyrifos for organophosphates) based on the strongest previous evidence for neurodevelopmental toxicity.48 To further adjust for coexposure, we adjusted for 11 pesticides in logistic models; in sensitivity analyses, a semi-Bayesian approach was used as described elsewhere.49 There was little difference in effect estimates between the fully adjusted conventional logistic and the hierarchical modeling approach, so we present the logistic modeling results only.49 50 51
We also stratified analyses by autism spectrum disorder with or without comorbid intellectual disability to assess risk in more severely impaired individuals separately. We conducted sensitivity analyses adjusting for additional variables including maternal birth place (US v non-US); residence in urban or rural areas52; socioeconomic status categories based on census data related to income, education, and occupation53; source of payment for delivery (indicator of socioeconomic status); and preterm birth. None of these variables changed the estimates of interest by more than 5%, thus they were not retained in final models.54 Sensitivity analyses also included restricting to term births, and stratifying by sex. Analyses were conducted with SAS 9.3.
Patient and public involvement
No patients were directly involved in setting the research question or the outcome measures, nor were they involved in developing plans for design or implementation of the study. However, the study responds to concerns by the families of patients with autism that environmental toxic exposures in early life are suspected to contribute to risks for autism spectrum disorder. There are plans to disseminate the results of the research to the relevant patient community. Affected families are thanked in the acknowledgments.
Results
Baseline characteristics and exposure
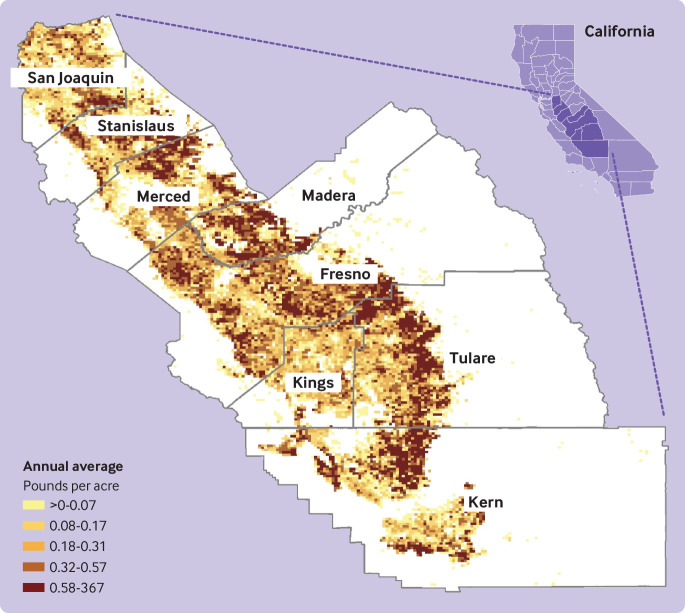
In our sample, individuals with autism spectrum disorder were mainly male (>80%), had older mothers, and had mothers who had completed more years of education than control mothers (table 1). Correlations between several pesticides in the same or across developmental periods were moderate to high (rt=0.45-0.85; eTable 2). In figure 1, we present a map of the study area showing pesticide applications for the most used substance glyphosate as an example.
Table 1.
Study population characteristics by autism spectrum disorder status and population controls in the Central Valley, CA*
| ASD (all; n=2961) | ASD with ID (n=445) | ASD without ID (n=2516) | Controls (n=35 370) | |
|---|---|---|---|---|
| Sex | ||||
| Males | 2403 (81.2) | 354 (79.6) | 2049 (81.4) | 29 225 (82.6) |
| Females | 558 (18.8) | 91 (20.4) | 467 (18.6) | 6145 (17.4) |
| Year of birth | ||||
| 1998-2004 | 1033 (34.9) | 243 (54.6) | 790 (31.4) | 14 390 (40.7) |
| 2005-2010 | 1928 (65.1) | 202 (45.4) | 1726 (68.6) | 20 980 (59.3) |
| Maternal age | ||||
| ≤19 | 233 (7.9) | 38 (8.5) | 195 (7.8) | 4931 (13.9) |
| 20-24 | 808 (27.3) | 136 (30.6) | 672 (26.7) | 10 253 (29.0) |
| 25-29 | 884 (29.9) | 117 (26.3) | 767 (30.5) | 9779 (27.6) |
| 30-34 | 592 (20.0) | 96 (21.6) | 496 (19.7) | 6712 (19.0) |
| ≥35 | 443 (15.0) | 58 (13.0) | 385 (15.3) | 3694 (10.4) |
| Missing data | 1 (0.0) | — | 1 (0.0) | 1 (0.0) |
| Maternal race or ethnicity | ||||
| White to non-Hispanic | 1100 (37.1) | 166 (37.3) | 934 (37.1) | 9943 (28.1) |
| Hispanic to any race | 1424 (48.1) | 205 (46.1) | 1219 (48.4) | 20 802 (58.8) |
| Black | 149 (5.0) | 33 (7.4) | 116 (4.6) | 1562 (4.4) |
| Asian/Pacific Islanders | 214 (7.2) | 35 (7.9) | 179 (7.1) | 2155 (6.1) |
| Others | 74 (2.5) | 6 (1.3) | 68 (2.7) | 908 (2.6) |
| US born | ||||
| Yes | 2133 (72.0) | 314 (70.6) | 1819 (72.3) | 22 152 (62.6) |
| No | 828 (28.0) | 131 (29.4) | 697 (27.7) | 13 209 (37.3) |
| Missing data | — | — | — | 9 (0.0) |
| Maternal education | ||||
| Less than 8th grade | 174 (5.9) | 34 (7.6) | 140 (5.6) | 4518 (12.8) |
| 9th to 12th grade | 435 (14.7) | 69 (15.5) | 366 (14.5) | 7583 (21.4) |
| High school graduate | 937 (31.6) | 152 (34.2) | 785 (31.2) | 10 909 (30.8) |
| Some degree less than college | 923 (31.2) | 131 (29.4) | 792 (31.5) | 7854 (22.2) |
| College or more | 455 (15.4) | 48 (10.8) | 407 (16.2) | 3925 (11.1) |
| Missing data | 37 (1.2) | 11 (2.5) | 26 (1.0) | 581 (1.6) |
ASD=autism spectrum disorder; ID=intellectual disability. Data are number (%) or individuals.
Central Valley counties include San Joaquin, Stanislaus, Merced, Madera, Fresno, Kings, Tulare, and Kern.
Fig 1.
Pesticide application of glyphosate in Central Valley, CA, 1998-2010
Association between autism spectrum disorder and exposure to pesticides, coadjusted for developmental period exposures
For all cases of autism spectrum disorder combined, coadjusted for developmental period-specific exposures (three months before pregnancy, during pregnancy, and during the first year of life), odds ratios were increased for pregnancy exposure to most substances. Associations were strongest for chlorpyrifos (1.15; 95% confidence interval 1.02 to 1.29), diazinon (1.14; 1.02 to 1.28), and avermectin (1.14; 1.03 to 1.26). Related to first year of life exposure, most odds ratios were close to one, and only the odds ratios for bifenthrin, malathion, and glyphosates were slightly raised (table 2). For autism spectrum disorder with intellectual disability comorbidity, coadjustment for the exposures in all three periods resulted in attenuated effect estimates during and before pregnancy, while odds ratios became more pronounced for exposures in the first year of life, particularly for glyphosate (1.60; 1.09 to 2.34), diazinon (1.45; 1.11 to 1.89), malathion (1.29; 1.00 to 1.65), and bifenthrin (1.33; 1.03 to 1.72; table 2). Exposure in the three months before pregnancy (indicating exposure just before or around conception) had weaker associations with autism spectrum disorder than exposure during pregnancy or the first year of life, after exposure period coadjustment (table 2, eTable 3). We saw variation in exposure between developmental periods to each pesticide considered, likely due to annual and seasonal changes in application rates (eg, for permethrin, among the controls, 1.5% were solely exposed in the three months before pregnancy, 4.8% were exposed only during pregnancy, 7.6% were exposed only in the first year of life, and 12.1% were exposed in all three periods; eTable 4). For exposures by trimester, no clear patterns were identified (data not shown).
Table 2.
Odds ratios and 95% confidence intervals* for association between pesticide exposure and all cases of autism spectrum disorder (ASD) combined and those with intellectual disability comorbidity, coadjusted for developmental period of pesticide exposure, by pesticide substance
| Pesticide substance | ASD (all) | ASD with intellectual disability | |||||
|---|---|---|---|---|---|---|---|
| 3 months before pregnancy | Pregnancy | First year of life | 3 months before pregnancy | Pregnancy | First year of life | ||
| Glyphosate† | 0.97 (0.87 to 1.08) | 1.11 (0.96 to 1.28) | 1.09 (0.94 to 1.26) | 0.96 (0.74 to 1.26) | 0.96 (0.66 to 1.40) | 1.60 (1.09 to 2.34) | |
| Chlorpyrifos | 0.92 (0.84 to 1.01) | 1.15 (1.02 to 1.29) | 1.04 (0.93 to 1.18) | 0.80 (0.64 to 1.00) | 1.18 (0.88 to 1.59) | 1.30 (0.96 to 1.76) | |
| Diazinon | 0.92 (0.81 to 1.05) | 1.14 (1.02 to 1.28) | 0.99 (0.88 to 1.11) | 0.86 (0.65 to 1.13) | 1.18 (0.90 to 1.54) | 1.45 (1.11 to 1.89) | |
| Acephate | 1.09 (0.92 to 1.30) | 1.10 (0.95 to 1.27) | 0.94 (0.82 to 1.08) | 1.40 (0.97 to 2.03) | 1.07 (0.77 to 1.50) | 1.10 (0.80 to 1.50) | |
| Malathion | 0.94 (0.82 to 1.08) | 1.08 (0.97 to 1.20) | 1.09 (0.98 to 1.20) | 0.72 (0.50 to 1.03) | 1.05 (0.81 to 1.37) | 1.29 (1.00 to 1.65) | |
| Permethrin | 1.07 (0.95 to 1.20) | 1.04 (0.94 to 1.16) | 1.04 (0.94 to 1.16) | 1.07 (0.82 to 1.39) | 1.24 (0.95 to 1.62) | 1.23 (0.94 to 1.59) | |
| Bifenthrin | 1.10 (0.97 to 1.26) | 0.96 (0.86 to 1.07) | 1.09 (0.98 to 1.20) | 1.18 (0.85 to 1.64) | 0.92 (0.69 to 1.22) | 1.33 (1.03 to 1.72) | |
| Methyl bromide | 1.15 (1.01 to 1.31) | 0.95 (0.85 to 1.07) | 1.06 (0.95 to 1.19) | 0.97 (0.72 to 1.31) | 1.20 (0.92 to 1.57) | 1.21 (0.94 to 1.57) | |
| Imidacloprid | 1.09 (0.98 to 1.21) | 0.92 (0.83 to 1.02) | 0.97 (0.87 to 1.07) | 1.02 (0.79 to 1.33) | 0.88 (0.68 to 1.14) | 1.08 (0.84 to 1.39) | |
| Avermectin | 1.03 (0.94 to 1.13) | 1.14 (1.03 to 1.26) | 0.97 (0.87 to 1.08) | 1.08 (0.85 to 1.38) | 0.98 (0.75 to 1.26) | 1.08 (0.83 to 1.41) | |
| Myclobutanil | 0.97 (0.87 to 1.07) | 1.06 (0.96 to 1.17) | 0.98 (0.89 to 1.09) | 1.06 (0.83 to 1.35) | 1.23 (0.95 to 1.58) | 1.09 (0.84 to 1.41) | |
Logistic regression; adjusted for year of birth, sex, maternal race or ethnicity, maternal age, maternal education, and NOx (CALINE4) as a marker of traffic related air pollution, and simultaneously for exposures three months before pregnancy (just before or around conception), during pregnancy, and during the first year of life; pesticide exposure defined as ever versus never to specific substance in considered developmental period.
Glyphosate compounds include glyphosate isopropylamine salt, glyphosate potassium salt, glyphosate monoammonium salt, glyphosate diammonium salt, glyphosate trimesium, and glyphosate dimethylamine salt.
Association between prenatal or infant exposure to pesticides and autism spectrum disorder
For all cases of autism spectrum disorder, considering the pregnancy and infant exposures separately, exposure during pregnancy was associated with about a 10% increase in adjusted odds ratios for glyphosate (1.16; 95% confidence interval 1.06 to 1.27), chlorpyrifos (1.13; 1.05 to 1.23), diazinon (1.11; 1.01 to 1.21), malathion (1.11; 1.01 to 1.22), avermectin (1.12; 1.04 to 1.22), and permethrin (1.10; 1.01 to 1.20). Also adjusting for all 11 pesticides resulted in attenuation of associations. However, odds ratios for glyphosate and avermectin remained elevated for exposure during pregnancy, while odds ratios for the remaining pesticides were close to one, and the odds ratio for imidacloprid fell below one (table 3).
Table 3.
Odds ratios and 95% confidence intervals for association between all cases of autism spectrum disorder combined and pesticide exposure during pregnancy and first year of life in logistic regression models, by pesticide substance
| Pesticide substance | Pregnancy | First year of life | |||||
|---|---|---|---|---|---|---|---|
| No of exposed cases/controls | Model 1* | Model 2† | No of exposed cases/controls | Model 1* | Model 2† | ||
| Glyphosate‡ | 2293/26 660 | 1.16 (1.06 to 1.27) | 1.12 (0.99 to 1.27) | 2256/26 390 | 1.15 (1.05 to 1.26) | 1.17 (1.04 to 1.32) | |
| Chlorpyrifos | 1799/20 914 | 1.13 (1.05 to 1.23) | 1.07 (0.96 to 1.19) | 1876/22 125 | 1.10 (1.02 to 1.20) | 1.04 (0.93 to 1.16) | |
| Diazinon | 764/9176 | 1.11 (1.01 to 1.21) | 1.09 (0.98 to 1.20) | 787/9890 | 1.04 (0.95 to 1.14) | 1.00 (0.90 to 1.11) | |
| Acephate | 341/4047 | 1.09 (0.97 to 1.23) | 1.06 (0.93 to 1.19) | 381/4783 | 1.00 (0.90 to 1.13) | 0.96 (0.85 to 1.08) | |
| Malathion | 642/7277 | 1.11 (1.01 to 1.22) | 1.05 (0.95 to 1.16) | 784/8911 | 1.11 (1.02 to 1.21) | 1.07 (0.97 to 1.18) | |
| Permethrin | 930/10 773 | 1.10 (1.01 to 1.20) | 1.04 (0.94 to 1.14) | 1047/12 129 | 1.10 (1.01 to 1.19) | 1.05 (0.96 to 1.16) | |
| Bifenthrin | 638/7300 | 1.03 (0.94 to 1.13) | 0.96 (0.87 to 1.07) | 886/9671 | 1.09 (1.00 to 1.19) | 1.05 (0.95 to 1.15) | |
| Methyl bromide | 657/8085 | 1.03 (0.94 to 1.13) | 0.95 (0.86 to 1.05) | 761/8986 | 1.08 (0.99 to 1.18) | 1.04 (0.94 to 1.15) | |
| Imidacloprid | 1123/14 490 | 0.93 (0.86 to 1.00) | 0.81 (0.74 to 0.89) | 1323/16 771 | 0.95 (0.88 to 1.02) | 0.86 (0.78 to 0.95) | |
| Avermectin | 1513/17 212 | 1.12 (1.04 to 1.22) | 1.10 (1.00 to 1.22) | 1719/20 100 | 1.07 (0.99 to 1.15) | 1.01 (0.90 to 1.12) | |
| Myclobutanil | 1254/15 222 | 1.04 (0.96 to 1.12) | 0.99 (0.90 to 1.09) | 1375/16 871 | 1.01 (0.93 to 1.09) | 0.95 (0.86 to 1.05) | |
Logistic regression; adjusted for year of birth, sex, maternal race or ethnicity, maternal age, maternal education, and NOx (CALINE4) as a marker of traffic related air pollution; pesticide exposure defined as ever versus never to specific substance in considered developmental period.
Logistic regression; adjusted as model 1; pesticide exposure defined as ever versus never to specific substance in considered developmental period with all considered pesticides in the model.
Glyphosate compounds include glyphosate isopropylamine salt, glyphosate potassium salt, glyphosate monoammonium salt, glyphosate diammonium salt, glyphosate trimesium, and glyphosate dimethylamine salt.
Association between prenatal or infant exposure to pesticide and autism spectrum disorder with intellectual disability
Among cases of autism spectrum disorder with intellectual disability, odds ratios had greater increases (by 30-40%) in pregnancy and infancy for glyphosate, chlorpyrifos, diazinon, permethrin, methyl bromide, and myclobutanil when considering the pregnancy and infant periods separately (table 4). Among cases without intellectual disability (about 85% of cases), estimated odds ratios were similar to those reported for the models analyzing all cases of autism spectrum disorder (eTable 5).
Table 4.
Odds ratios and 95% confidence intervals for association between autism spectrum disorder with intellectual disability comorbidity and exposure to pesticides during pregnancy and first year of life in logistic regression models
| Pesticide substance | Pregnancy | First year of life | |||||
|---|---|---|---|---|---|---|---|
| No of exposed cases/controls | Model 1* | Model 2† | No of exposed cases/controls | Model 1* | Model 2† | ||
| Glyphosate‡ | 351/26 660 | 1.33 (1.05 to 1.69) | 1.12 (0.82 to 1.53) | 360/26 390 | 1.51 (1.18 to 1.92) | 1.37 (0.99 to 1.89) | |
| Chlorpyrifos | 284/20 914 | 1.27 (1.04 to 1.56) | 1.07 (0.82 to 1.41) | 301/22 125 | 1.31 (1.07 to 1.61) | 1.00 (0.75 to 1.34) | |
| Diazinon | 152/9176 | 1.41 (1.15 to 1.73) | 1.24 (0.97 to 1.58) | 169/9890 | 1.51 (1.23 to 1.85) | 1.37 (1.07 to 1.74) | |
| Acephate | 65/4047 | 1.26 (0.95 to 1.65) | 1.17 (0.88 to 1.56) | 74/4783 | 1.25 (0.96 to 1.62) | 1.12 (0.85 to 1.47) | |
| Malathion | 99/7277 | 1.12 (0.89 to 1.41) | 0.94 (0.74 to 1.21) | 125/8911 | 1.23 (0.99 to 1.52) | 1.02 (0.80 to 1.29) | |
| Permethrin | 175/10 773 | 1.46 (1.20 to 1.78) | 1.36 (1.08 to 1.71) | 191/12 129 | 1.44 (1.19 to 1.75) | 1.27 (1.01 to 1.60) | |
| Bifenthrin | 91/7300 | 1.13 (0.89 to 1.43) | 1.05 (0.81 to 1.35) | 134/9671 | 1.33 (1.08 to 1.64) | 1.22 (0.97 to 1.54) | |
| Methyl bromide | 133/8085 | 1.33 (1.07 to 1.64) | 1.12 (0.88 to 1.42) | 148/8986 | 1.33 (1.08 to 1.63) | 1.09 (0.86 to 1.39) | |
| Imidacloprid | 163/14 490 | 0.93 (0.76 to 1.13) | 0.73 (0.58 to 0.92) | 198/16 771 | 1.01 (0.83 to 1.22) | 0.78 (0.62 to 0.99) | |
| Avermectin | 209/17 212 | 1.05 (0.87 to 1.28) | 0.84 (0.66 to 1.07) | 247/20 100 | 1.09 (0.90 to 1.33) | 0.78 (0.60 to 1.01) | |
| Myclobutanil | 217/15 222 | 1.32 (1.09 to 1.60) | 1.20 (0.94 to 1.53) | 232/16 871 | 1.27 (1.05 to 1.54) | 1.04 (0.81 to 1.33) | |
Logistic regression; adjusted for year of birth, sex, maternal race or ethnicity, maternal age, maternal education, and NOx (CALINE4) as a marker of traffic related air pollution; pesticide exposure defined as ever versus never to specific substance in considered developmental period.
Logistic regression; adjusted as model 1; pesticide exposure defined as ever versus never to specific substance in considered developmental period with all considered pesticides in the model.
Glyphosate compounds include glyphosate isopropylamine salt, glyphosate potassium salt, glyphosate monoammonium salt, glyphosate diammonium salt, glyphosate trimesium, and glyphosate dimethylamine salt.
Multi-pesticide models
In multi-pesticide models with two or three pesticides, most odds ratios were above one for all cases of autism spectrum disorder combined even though several confidence intervals widened (table 5). For autism spectrum disorder with intellectual disability and pesticide exposure during the first year of life, estimated associations were pronounced for glyphosate (odds ratio 1.34; 95% confidence interval 1.03 to 1.74) and permethrin (1.31; 1.07 to 1.62); also including chlorpyrifos or myclobutanil changed little in the associations for glyphosate and permethrin, whereas the estimated odds ratios for chlorpyrifos or myclobutanil were null (table 5).
Table 5.
Multi-pesticide models of association among all cases of autism spectrum disorder combined and those with intellectual disability comorbidity, and exposure of selected pesticides from different chemical classes during pregnancy and the first year of life*
| Mutually adjusted odds ratio (95% CI) | |||||
|---|---|---|---|---|---|
| All cases of autism spectrum disorder (exposure during pregnancy) | |||||
| Permethrin | 1.06 (0.97 to 1.16) | 1.06 (0.97 to 1.15) | 1.05 (0.96 to 1.14) | 1.06 (0.97 to 1.16) | 1.06 (0.97 to 1.16) |
| Chlorpyrifos | 1.11 (1.02 to 1.21) | — | 1.07 (0.97 to 1.18) | — | — |
| Glyphosate† | — | 1.14 (1.03 to 1.26) | 1.09 (0.98 to 1.23) | 1.16 (1.04 to 1.29) | 1.14 (1.04 to 1.26) |
| Myclobutanil | — | — | — | 0.96 (0.88 to 1.05) | — |
| Methyl bromide | — | — | — | — | 0.98 (0.89 to 1.08) |
| Autism spectrum disorder with intellectual disability (exposure during pregnancy) | |||||
| Permethrin | 1.40 (1.13 to 1.73) | 1.39 (1.13 to 1.72) | 1.37 (1.10 to 1.70) | 1.35 (1.09 to 1.67) | 1.35 (1.09 to 1.68) |
| Chlorpyrifos | 1.12 (0.90 to 1.40) | — | 1.07 (0.83 to 1.37) | — | — |
| Glyphosate† | — | 1.17 (0.91 to 1.50) | 1.12 (0.84 to 1.51) | 1.08 (0.82 to 1.43) | 1.12 (0.86 to 1.45) |
| Myclobutanil | — | — | — | 1.17 (0.94 to 1.46) | — |
| Methyl bromide | — | — | — | — | 1.18 (0.94 to 1.48) |
| Autism spectrum disorder with intellectual disability (exposure in the first year of life) | |||||
| Permethrin | 1.36 (1.10 to 1.69) | 1.31 (1.07 to 1.62) | 1.31 (1.06 to 1.63) | 1.30 (1.05 to 1.61) | 1.29 (1.04 to 1.59) |
| Chlorpyrifos | 1.15 (0.92 to 1.44) | — | 1.00 (0.77 to 1.31) | — | — |
| Glyphosate† | — | 1.34 (1.03 to 1.74) | 1.33 (0.98 to 1.81) | 1.31 (0.99 to 1.74) | 1.29 (0.98 to 1.68) |
| Myclobutanil | — | — | — | 1.04 (0.84 to 1.30) | — |
| Methyl bromide | — | — | — | — | 1.15 (0.92 to 1.44) |
Adjusted for year of birth, sex, maternal race or ethnicity, maternal age, maternal education, and NOx (CALINE4) as a marker of traffic related air pollution; pesticide exposure defined as ever versus never to specific substance in considered developmental period.
Glyphosate compounds include glyphosate isopropylamine salt, glyphosate potassium salt, glyphosate monoammonium salt, glyphosate diammonium salt, glyphosate trimesium, and glyphosate dimethylamine salt.
Sensitivity analyses: buffer size, sex stratification, area type, and term birth restriction
In sensitivity analyses, we examined associations between autism spectrum disorder and pesticide exposure within a 2500 m distance from home; findings were similar or slightly stronger than those for the 2000 m distance (eTable 6). Stratifying by sex, associations among male individuals were similar as seen for the entire sample, with increased odds ratios for glyphosate, chlorpyrifos, diazinon, permethrin, and avermectin. Among female individuals, the findings were similar but the 95% confidence intervals were wider due to the smaller number of cases (eTable 7). Restricting to term births only or adjusting for area type (urban, rural) did not change our findings appreciably (data not shown).
Discussion
To our knowledge, this study is the largest to investigate pesticide exposure and autism spectrum disorder so far, and the first to also consider the disorder with intellectual disability comorbidity. Our results indicate small to moderately increased risks for the disorder in offspring with prenatal exposure to the organophosphates chlorpyrifos, diazinon, and malathion, the pyrethroids permethrin and bifenthrin, as well as to glyphosate, avermectin, and methyl bromide compared with offspring of women without such exposure within 2000 m of their residence. For autism spectrum disorder with comorbid intellectual disability, risks were more pronounced for exposures during the first year of life. Importantly, the pesticides considered for analysis were selected a priori on the basis of experimental evidence indicating neurodevelopmental toxicity. Thus, our findings support the hypotheses that prenatal and infant pesticide exposures to these substances increase the risks for autism spectrum disorder, and exposures in infancy could contribute to risks for more severely impaired phenotypes with comorbid intellectual disability.
Comparison with other studies
Environmental toxicants have been suspected to increase the risk of autism spectrum disorder, with available research suggesting associations between air pollution and the disorder.44 55 56 57 Studies examining pesticides and the disorder are rare. In a California study of DDS case records (n=465) linked to birth records from 1996-98, researchers assigned exposures during pregnancy using CA-PUR, similar to our approach; findings suggested that grouped organochlorines were strongly associated with risks of pregnancy (odds ratio 6.1 (95% confidence interval 2.4 to 15.3)).10 Another study included 486 cases of autism spectrum disorder and assigned pounds per active ingredient in aggregated chemical classes (organophosphates, organochlorines, pyrethroids, carbamates), also derived from CA-PUR data for applications within 1250-1750 m from the home address9; findings suggested a 60% increased risk for the disorder related to organophosphate exposures during pregnancy. Children of mothers living near agricultural pyrethroid applications just before conception or during their third trimester also were at greater risk for autism spectrum disorder and general developmental disability (odds ratios ranging from 1.7 to 2.3).9 In a smaller case-control study measuring organochlorines and polychlorinated biphenyls in banked mid-pregnancy serum (from 2000 to 2003), higher concentrations for several compounds in cases than in general population controls were seen.11
We did not consider organochlorines because many have been banned from use in California for decades. In a high risk, mother-child study of 46 cases of autism spectrum disorder, prenatal urinary dimethylthiophosphate was associated with the disorder in girls but not in boys58; in our study, we saw little evidence of a sex difference in effects. Overall, the few earlier studies corroborate our findings for most of the pesticides we examined. While all the 11 pesticides were a priori selected among high use substances, based on prior evidence for neurodevelopmental toxicity, odds ratios were increased for several but not all substances in our analyses. Possible explanations could include different mechanisms related to the development of autism spectrum disorder, bioavailability of the chemical (eg, in homes resulting from ambient applications and based on chemical properties), and the application practices in these real world scenarios. Different combinations of substances or mixture exposures might also result in synergistic effects, including those leading to a selective survival of the fetus.59
Although environmental exposure studies considering autism spectrum disorder are rare, organophosphates and pyrethroids have been related to neurodevelopmental and cognitive impairments in children in previous studies.5 7 60 61 Decrements in IQ scores at age 7 have been associated with prenatal residential proximity to agricultural use of organophosphates and pyrethroids, acephate, chlorpyrifos, and diazinon,5 in line with our findings. Pyrethroid metabolites in maternal urine during pregnancy and in child urine were associated with worse behavioral scores assessed in 6 year old children.62 Thus, human studies corroborate the adverse effect of early developmental exposure to ambient pesticides on child neurodevelopment, consistent with our findings.
Additional evidence is provided by experimental studies. Mice exposed in utero to chlorpyrifos showed postnatal deficits in social behavior and restricted interests while the behavior of the dams (maternal mice) was not affected.63 Prenatal exposure to chlorpyrifos enhanced brain oxidative stress and prostaglandin E2 synthesis in a mouse model of autism.64 Oxidative stress and dysregulated immune responses are implicated in organophosphate related toxicity and pathogenesis of autism spectrum disorder, suggesting a possible mode of action.13 Coexposing mice shortly after birth to cypermethrin (a pyrethroid) and endosulfan altered levels of neuroproteins and resulted in neurobehavioral abnormalities.12 Gene expression of mouse cortical neurons was altered by certain fungicides and resembled transcriptional changes thought to underlie development of autism spectrum disorder.2 14 Translational research connecting toxicological and animal studies with findings from epidemiological studies is needed to identify the specific modes of action of pesticides relevant for the pathogenesis of autism spectrum disorder.65 66 67 68 69
Residential proximity to pesticide applications during pregnancy has been shown to be a valid indicator of prenatal exposure.70 71 72 73 Pesticides, including organophosphates, have been identified in serum, indoor air, and dust in homes in agricultural areas in California.74 75 Elevated levels in five of seven pesticides applied within 1250 m of homes according to Pesticide Use Reporting records were also measured in dust from such homes.76 Our exposure assessment method using the geographic information system tool has been validated against serum concentrations of organochlorines,77 and specific methylation patterns found among those with organophosphate exposure,78 and can be considered a valid proxy for prenatal exposures.
Strengths and limitations of our study
A strength of our study was our pesticide exposure assessment tool; it can estimate exposures for multiple substances with short half-lives for which frequent measurements of metabolites would be necessary but not feasible in a population based study of the size needed to investigate the risk of autism spectrum disorder. California’s mandatory Pesticide Use Reporting program is recognized as the most detailed and comprehensive worldwide. Thus, we were able to rely on agricultural application records of specific pesticides with high spatial and temporal resolution, which we believe is a strength that could have reduced exposure misclassification, because we relied on Pesticide Use Reporting information based on the date of application using a relatively fine spatial scale (a buffer of 2000 m) around the residential address. We also relied on the gestational age and birth date to construct individual exposure estimates corresponding to different developmental periods. We still have to assume that individuals were present at their residences around the application dates and that these applications resulted in exposures in the targeted periods only and did not get trapped in or around homes over extended periods of time. Our registry based design avoided participation bias due to self selection and recall bias of parents (which is an issue in case-control studies that rely on self reports of past exposures).
Although our ability to pinpoint one or more specific substances was limited by the collinearity of pesticide exposure owing to agricultural practices, we could capture the real life scenario of populations living in agricultural areas; typically, a variety of substances are used over several weeks or months. Sensitivity analyses using the 2500 m radius buffer further corroborated and even strengthened our results. Simultaneous exposures to frequently used pesticides are likely in residences near agricultural applications, and some of our findings could reflect adverse effects of typical exposure mixtures or coexposures. Multi-pesticide models coadjusted for all pesticides or for two or three substances were generally consistent with our single pesticide models. We present results from real world exposure scenarios while being cognizant of issues of collinearity, sparse data, or overly restrictive modeling assumptions.
A limitation was that we only had birth addresses available and that 9-30% of families could have moved during pregnancy.79 However, most moves in pregnancy have been found to be local (<10 km), and misclassification would be expected to be non-differential because moving residence would happen before diagnosis; thus any bias would likely be toward the null. We also lacked exposure information on pesticides from other sources such as diet or occupation, potentially resulting in underestimation of total exposure if these were associated with residential exposures (eg, women who work and live on farms); however, this would have been similar for cases and controls and most likely to have resulted in attenuation of risk estimates toward the null.54 We also lacked information about passive and active smoking. However, pregnancy smoking rates are very low in California (<2%),80 and smoking in public places has been banned since the 1990s. Even though we had detailed information on potential confounders, and sensitivity analyses did not change our findings, uncontrolled residual confounding always remains a concern.
Conclusions
Our findings suggest that risk of autism spectrum disorder increases with prenatal and infant exposure to several common ambient pesticides that have been shown to affect neurodevelopment in experimental studies. Further research should be translational and integrate experimental and epidemiological approaches to further elucidate underlying mechanisms in the development of the disorder. However, from a public health and preventive medicine perspective, our findings support the need to avoid prenatal and infant exposure to pesticides to protect early brain development.
What is already known on this topic
Common pesticides have been previously shown to cause neurodevelopmental impairment in experimental research
Environmental exposures during early brain development are suspected to increase risk of autism spectrum disorders in children
What this study adds
Prenatal or infant exposure to a priori selected pesticides—including glyphosate, chlorpyrifos, diazinon, and permethrin—were associated with increased odds of developing autism spectrum disorder
Exposure of pregnant women and infants to ambient pesticides with a potential neurodevelopmental toxicity mode of action should be avoided as a preventive measure against autism spectrum disorder
Acknowledgments
We thank the patients with autism spectrum disorder and their families and people throughout California involved in providing and processing information, including affected families, birth registration professionals, medical record clerks, and other registry staff.
Web extra.
Extra material supplied by authors
Web appendix: Online materials
Contributors: OSvE directed the analysis and drafted the manuscript. OSvE and BR contributed to design of the study and interpretation of the results. CL carried out the main statistical analysis. XC contributed to setting up the database and quality control. MC was responsible for the geographical information system methods. FY contributed to the statistical analyses. CL, MC, and ASP contributed to exposure estimation. JW estimated the air pollution exposure. All authors commented on drafts and read and approved the final manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. OSvE is the guarantor.
Funding: This work was supported by the US National Institute of Environmental Health Sciences (grants R21ES022389; R21ES025558). The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the funders.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the National Institute of Environmental Health Sciences for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; BR has been acting as an expert witness/consultant in a lawsuit for the plaintiffs against Monsanto in non-Hodgkins lymphoma litigation concerning glyphosate; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: This research was approved by the University of California, Los Angeles Office of the Human Research Protection Program and the California Committee for the Protection of Human Subjects, and was exempt from informed consent requirements as there was no contact with the study population.
Data sharing: No additional data are available.
The lead author affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
References
- 1. Geschwind DH. Genetics of autism spectrum disorders. Trends Cogn Sci 2011;15:409-16. 10.1016/j.tics.2011.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gandal MJ, Haney JR, Parikshak NN, et al. CommonMind Consortium. PsychENCODE Consortium. iPSYCH-BROAD Working Group Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science 2018;359:693-7. 10.1126/science.aad6469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hallmayer J, Cleveland S, Torres A, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry 2011;68:1095-102. 10.1001/archgenpsychiatry.2011.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sandin S, Lichtenstein P, Kuja-Halkola R, Hultman C, Larsson H, Reichenberg A. The heritability of autism spectrum disorder. JAMA 2017;318:1182-4. 10.1001/jama.2017.12141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gunier RB, Bradman A, Harley KG, Kogut K, Eskenazi B. Prenatal residential proximity to agricultural pesticide use and IQ in 7-year-old children. Environ Health Perspect 2017;125:057002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Horton MK, Rundle A, Camann DE, Boyd Barr D, Rauh VA, Whyatt RM. Impact of prenatal exposure to piperonyl butoxide and permethrin on 36-month neurodevelopment. Pediatrics 2011;127:e699-706. 10.1542/peds.2010-0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rauh V, Arunajadai S, Horton M, et al. Seven-year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environ Health Perspect 2011;119:1196-201. 10.1289/ehp.1003160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol 2014;13:330-8. 10.1016/S1474-4422(13)70278-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shelton JF, Geraghty EM, Tancredi DJ, et al. Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: the CHARGE study. Environ Health Perspect 2014;122:1103-9. 10.1289/ehp.1307044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Roberts EM, English PB, Grether JK, Windham GC, Somberg L, Wolff C. Maternal residence near agricultural pesticide applications and autism spectrum disorders among children in the California Central Valley. Environ Health Perspect 2007;115:1482-9. 10.1289/ehp.10168 10.1289/ehp.10168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lyall K, Croen LA, Sjödin A, et al. Polychlorinated biphenyl and organochlorine pesticide concentrations in maternal mid-pregnancy serum samples: association with autism spectrum disorder and intellectual disability. Environ Health Perspect 2017;125:474-80. 10.1289/EHP277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee I, Eriksson P, Fredriksson A, Buratovic S, Viberg H. Developmental neurotoxic effects of two pesticides: behavior and neuroprotein studies on endosulfan and cypermethrin. Toxicology 2015;335:1-10. 10.1016/j.tox.2015.06.010 [DOI] [PubMed] [Google Scholar]
- 13. De Felice A, Greco A, Calamandrei G, Minghetti L. Prenatal exposure to the organophosphate insecticide chlorpyrifos enhances brain oxidative stress and prostaglandin E2 synthesis in a mouse model of idiopathic autism. J Neuroinflammation 2016;13:149. 10.1186/s12974-016-0617-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pearson BL, Simon JM, McCoy ES, Salazar G, Fragola G, Zylka MJ. Identification of chemicals that mimic transcriptional changes associated with autism, brain aging and neurodegeneration. Nat Commun 2016;7:11173. 10.1038/ncomms11173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. American Psychiatric Association Diagnostic and statistical manual of mental disorders. 4th ed, text revision American Psychiatric Association, 2000. [Google Scholar]
- 16. American Psychiatric Association Diagnostic and statistical manual of mental disorders. 5th ed, text revision American Psychiatric Association, 2015. [Google Scholar]
- 17. Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, van de Water J, Pessah IN. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environ Health Perspect 2006;114:1119-25. 10.1289/ehp.8483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sandhu KS. Systems properties of proteins encoded by imprinted genes. Epigenetics 2010;5:627-36. 10.4161/epi.5.7.12883 [DOI] [PubMed] [Google Scholar]
- 19.US Department of Health and Human Services, National Center for Chronic Disease Prevention and Health Promotion. Registry Plus, a suite of publicly available software programs for collecting and processing cancer registry data. 2010. https://www.cdc.gov/cancer/npcr/tools/registryplus/lp.htm
- 20. Goldberg DW, Jacquez GM. Advances in geocoding for the health sciences. Spat Spatiotemporal Epidemiol 2012;3:1-5. 10.1016/j.sste.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 21. Regulation CDoP Summary of pesticide use report data—2008. California Environmental Protection Agency, 2009. [Google Scholar]
- 22. Cockburn M, Mills P, Zhang X, Zadnick J, Goldberg D, Ritz B. Prostate cancer and ambient pesticide exposure in agriculturally intensive areas in California. Am J Epidemiol 2011;173:1280-8. 10.1093/aje/kwr003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fiorino E, Sehonova P, Plhalova L, Blahova J, Svobodova Z, Faggio C. Effects of glyphosate on early life stages: comparison between Cyprinus carpio and Danio rerio. Environ Sci Pollut Res Int 2018;25:8542-9. 10.1007/s11356-017-1141-5 [DOI] [PubMed] [Google Scholar]
- 24. Gallegos CE, Bartos M, Bras C, Gumilar F, Antonelli MC, Minetti A. Exposure to a glyphosate-based herbicide during pregnancy and lactation induces neurobehavioral alterations in rat offspring. Neurotoxicology 2016;53:20-8. 10.1016/j.neuro.2015.11.015 [DOI] [PubMed] [Google Scholar]
- 25. Cattani D, Cesconetto PA, Tavares MK, et al. Developmental exposure to glyphosate-based herbicide and depressive-like behavior in adult offspring: Implication of glutamate excitotoxicity and oxidative stress. Toxicology 2017;387:67-80. 10.1016/j.tox.2017.06.001 [DOI] [PubMed] [Google Scholar]
- 26. Nielsen LN, Roager HM, Casas ME, et al. Glyphosate has limited short-term effects on commensal bacterial community composition in the gut environment due to sufficient aromatic amino acid levels. Environ Pollut 2018;233:364-76. 10.1016/j.envpol.2017.10.016 [DOI] [PubMed] [Google Scholar]
- 27. Eskenazi B, Huen K, Marks A, et al. PON1 and neurodevelopment in children from the CHAMACOS study exposed to organophosphate pesticides in utero. Environ Health Perspect 2010;118:1775-81. 10.1289/ehp.1002234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Slotkin TA, Bodwell BE, Levin ED, Seidler FJ. Neonatal exposure to low doses of diazinon: long-term effects on neural cell development and acetylcholine systems. Environ Health Perspect 2008;116:340-8. 10.1289/ehp.11005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Timofeeva OA, Roegge CS, Seidler FJ, Slotkin TA, Levin ED. Persistent cognitive alterations in rats after early postnatal exposure to low doses of the organophosphate pesticide, diazinon. Neurotoxicol Teratol 2008;30:38-45. 10.1016/j.ntt.2007.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yen J, Donerly S, Levin ED, Linney EA. Differential acetylcholinesterase inhibition of chlorpyrifos, diazinon and parathion in larval zebrafish. Neurotoxicol Teratol 2011;33:735-41. 10.1016/j.ntt.2011.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fernandes LS, Emerick GL, dos Santos NA, de Paula ES, Barbosa F, Jr, dos Santos AC. In vitro study of the neuropathic potential of the organophosphorus compounds trichlorfon and acephate. Toxicol In Vitro 2015;29:522-8. 10.1016/j.tiv.2015.01.001 [DOI] [PubMed] [Google Scholar]
- 32. Liu X, Zhang Q, Li S, et al. Developmental toxicity and neurotoxicity of synthetic organic insecticides in zebrafish (Danio rerio): A comparative study of deltamethrin, acephate, and thiamethoxam. Chemosphere 2018;199:16-25. 10.1016/j.chemosphere.2018.01.176 [DOI] [PubMed] [Google Scholar]
- 33. Gunier RB, Bradman A, Harley KG, Kogut K, Eskenazi B. Prenatal residential proximity to agricultural pesticide use and IQ in 7-year-old children. Environ Health Perspect 2017;125:057002. 10.1289/EHP504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. dos Santos AA, Naime AA, de Oliveira J, et al. Long-term and low-dose malathion exposure causes cognitive impairment in adult mice: evidence of hippocampal mitochondrial dysfunction, astrogliosis and apoptotic events. Arch Toxicol 2016;90:647-60. 10.1007/s00204-015-1466-0 [DOI] [PubMed] [Google Scholar]
- 35. Karmakar S, Patra K, Jana S, Mandal DP, Bhattacharjee S. Exposure to environmentally relevant concentrations of malathion induces significant cellular, biochemical and histological alterations in Labeo rohita. Pestic Biochem Physiol 2016;126:49-57. 10.1016/j.pestbp.2015.07.006 [DOI] [PubMed] [Google Scholar]
- 36. Beghoul A, Kebieche M, Gasmi S, et al. Impairment of mitochondrial integrity and redox status in brain regions during a low-dose long-term exposition of rats to pyrethrinoïds: the preventive effect of quercetin. Environ Sci Pollut Res Int 2017;24:19714-22. 10.1007/s11356-017-9675-0 [DOI] [PubMed] [Google Scholar]
- 37. de Souza A, Narvencar KP, Sindhoora KV. The neurological effects of methyl bromide intoxication. J Neurol Sci 2013;335:36-41. 10.1016/j.jns.2013.09.022 [DOI] [PubMed] [Google Scholar]
- 38. Gunier RB, Bradman A, Castorina R, et al. Residential proximity to agricultural fumigant use and IQ, attention and hyperactivity in 7-year old children. Environ Res 2017;158:358-65. 10.1016/j.envres.2017.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim S, Lee HS, Park Y. Perinatal exposure to low-dose imidacloprid causes ADHD-like symptoms: Evidences from an invertebrate model study. Food Chem Toxicol 2017;110:402-7. 10.1016/j.fct.2017.10.007 [DOI] [PubMed] [Google Scholar]
- 40. Pandey SP, Mohanty B. Disruption of the hypothalamic-pituitary-thyroid axis on co-exposures to dithiocarbamate and neonicotinoid pesticides: Study in a wildlife bird, Amandava amandava. Neurotoxicology 2017;60:16-22. 10.1016/j.neuro.2017.02.010 [DOI] [PubMed] [Google Scholar]
- 41. Sun YJ, Long DX, Li W, Hou WY, Wu YJ, Shen JZ. Effects of avermectins on neurite outgrowth in differentiating mouse neuroblastoma N2a cells. Toxicol Lett 2010;192:206-11. 10.1016/j.toxlet.2009.10.021 [DOI] [PubMed] [Google Scholar]
- 42. Weichert FG, Floeter C, Meza Artmann AS, Kammann U. Assessing the ecotoxicity of potentially neurotoxic substances - evaluation of a behavioural parameter in the embryogenesis of Danio rerio. Chemosphere 2017;186:43-50. 10.1016/j.chemosphere.2017.07.136 [DOI] [PubMed] [Google Scholar]
- 43. Zhu B, Liu L, Gong YX, Ling F, Wang GX. Triazole-induced toxicity in developing rare minnow (Gobiocypris rarus) embryos. Environ Sci Pollut Res Int 2014;21:13625-35. 10.1007/s11356-014-3317-6 [DOI] [PubMed] [Google Scholar]
- 44. Becerra TA, Wilhelm M, Olsen J, Cockburn M, Ritz B. Ambient air pollution and autism in Los Angeles county, California. Environ Health Perspect 2013;121:380-6. 10.1289/ehp.1205827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Levitin J, Harkonen J, Kakkonen J, Nikmo J. Evaluation of the CALINE4 and CAR-FMI models against measurements near a major road. Atmos Environ 2013;39:4439-52 10.1016/j.atmosenv.2005.03.046 . [DOI] [Google Scholar]
- 46. Benson PE. A review of the development and application of the Caline3 and Caline4 models. Atmospheric Environment Part B 1992;26:379-90 10.1016/0957-1272(92)90013-I . [DOI] [Google Scholar]
- 47. Wu J, Laurent O, Li L, Hu J, Kleeman M. Adverse reproductive health outcomes and exposure to gaseous and particulate-matter air pollution in pregnant women. Research report 188. Health Effects Institute, 2016. [PMC free article] [PubMed] [Google Scholar]
- 48. Cao F, Souders CL, 2nd, Li P, Pang S, Qiu L, Martyniuk CJ. Biological impacts of organophosphates chlorpyrifos and diazinon on development, mitochondrial bioenergetics, and locomotor activity in zebrafish (Danio rerio). Neurotoxicol Teratol 2018;70:18-27. 10.1016/j.ntt.2018.10.001 [DOI] [PubMed] [Google Scholar]
- 49. Greenland S. A semi-Bayes approach to the analysis of correlated multiple associations, with an application to an occupational cancer-mortality study. Stat Med 1992;11:219-30. 10.1002/sim.4780110208 [DOI] [PubMed] [Google Scholar]
- 50. Greenland S, Robins JM. Empirical-Bayes adjustments for multiple comparisons are sometimes useful. Epidemiology 1991;2:244-51. 10.1097/00001648-199107000-00002 [DOI] [PubMed] [Google Scholar]
- 51. Sullivan SG, Greenland S. Bayesian regression in SAS software. Int J Epidemiol 2013;42:308-17. 10.1093/ije/dys213 [DOI] [PubMed] [Google Scholar]
- 52.US Census Buero. Census 2000 Gateway. Accessed online 3 January 2018. https://www.census.gov/main/www/cen2000.html
- 53. Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control 2001;12:703-11. 10.1023/A:1011240019516 [DOI] [PubMed] [Google Scholar]
- 54. Rothman KJGS, Lash TL. Modern epidemiology. 3rd ed 2008. [Google Scholar]
- 55. von Ehrenstein OS, Aralis H, Cockburn M, Ritz B. In utero exposure to toxic air pollutants and risk of childhood autism. Epidemiology 2014;25:851-8. 10.1097/EDE.0000000000000150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kalkbrenner AE, Daniels JL, Chen JC, Poole C, Emch M, Morrissey J. Perinatal exposure to hazardous air pollutants and autism spectrum disorders at age 8. Epidemiology 2010;21:631-41. 10.1097/EDE.0b013e3181e65d76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Roberts AL, Lyall K, Hart JE, et al. Perinatal air pollutant exposures and autism spectrum disorder in the children of Nurses’ Health Study II participants. Environ Health Perspect 2013;121:978-84. 10.1289/ehp.1206187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Philippat C, Barkoski J, Tancredi DJ, et al. Prenatal exposure to organophosphate pesticides and risk of autism spectrum disorders and other non-typical development at 3 years in a high-risk cohort. Int J Hyg Environ Health 2018;221:548-55. 10.1016/j.ijheh.2018.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kioumourtzoglou MA, Raz R, Wilson A, et al. Traffic-related air pollution and pregnancy loss. Epidemiology 2019;30:4-10. 10.1097/EDE.0000000000000918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wagner-Schuman M, Richardson JR, Auinger P, et al. Association of pyrethroid pesticide exposure with attention-deficit/hyperactivity disorder in a nationally representative sample of U.S. children. Environ Health 2015;14:44. 10.1186/s12940-015-0030-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rauh VA, Garfinkel R, Perera FP, et al. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics 2006;118:e1845-59. 10.1542/peds.2006-0338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Viel JF, Rouget F, Warembourg C, et al. Behavioural disorders in 6-year-old children and pyrethroid insecticide exposure: the PELAGIE mother-child cohort. Occup Environ Med 2017;74:275-81. 10.1136/oemed-2016-104035 [DOI] [PubMed] [Google Scholar]
- 63. Lan A, Kalimian M, Amram B, Kofman O. Prenatal chlorpyrifos leads to autism-like deficits in C57Bl6/J mice. Environmental health: a global access science source. 2017;16:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. De Felice A, Scattoni ML, Ricceri L, Calamandrei G. Prenatal exposure to a common organophosphate insecticide delays motor development in a mouse model of idiopathic autism. PLoS One 2015;10:e0121663. 10.1371/journal.pone.0121663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. De Coster S, van Larebeke N. Endocrine-disrupting chemicals: associated disorders and mechanisms of action. J Environ Public Health 2012;2012:713696. 10.1155/2012/713696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. de Souza JS, Kizys MM, da Conceição RR, et al. Perinatal exposure to glyphosate-based herbicide alters the thyrotrophic axis and causes thyroid hormone homeostasis imbalance in male rats. Toxicology 2017;377:25-37. 10.1016/j.tox.2016.11.005 [DOI] [PubMed] [Google Scholar]
- 67. Defarge N, Takács E, Lozano VL, et al. Co-formulants in glyphosate-based herbicides disrupt aromatase activity in human cells below toxic levels. Int J Environ Res Public Health 2016;13:13. 10.3390/ijerph13030264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pandey SP, Tsutsui K, Mohanty B. Endocrine disrupting pesticides impair the neuroendocrine regulation of reproductive behaviors and secondary sexual characters of red munia (Amandava amandava). Physiol Behav 2017;173:15-22. 10.1016/j.physbeh.2017.01.030 [DOI] [PubMed] [Google Scholar]
- 69. Saillenfait AM, Ndiaye D, Sabaté JP. Pyrethroids: exposure and health effects--an update. Int J Hyg Environ Health 2015;218:281-92. 10.1016/j.ijheh.2015.01.002 [DOI] [PubMed] [Google Scholar]
- 70. Lu C, Fenske RA, Simcox NJ, Kalman D. Pesticide exposure of children in an agricultural community: evidence of household proximity to farmland and take home exposure pathways. Environ Res 2000;84:290-302. 10.1006/enrs.2000.4076 [DOI] [PubMed] [Google Scholar]
- 71. Ward MH, Lubin J, Giglierano J, et al. Proximity to crops and residential exposure to agricultural herbicides in iowa. Environ Health Perspect 2006;114:893-7. 10.1289/ehp.8770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Curwin BD, Hein MJ, Sanderson WT, et al. Urinary pesticide concentrations among children, mothers and fathers living in farm and non-farm households in iowa. Ann Occup Hyg 2007;51:53-65. 10.1093/annhyg/mel062 [DOI] [PubMed] [Google Scholar]
- 73. Fenske RA, Lu C, Curl CL, Shirai JH, Kissel JC. Biologic monitoring to characterize organophosphorus pesticide exposure among children and workers: an analysis of recent studies in Washington State. Environ Health Perspect 2005;113:1651-7. 10.1289/ehp.8022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Whitehead TP, Crispo Smith S, Park JS, Petreas MX, Rappaport SM, Metayer C. Concentrations of Persistent organic pollutants in California children’s whole blood and residential dust. Environ Sci Technol 2015;49:9331-40. 10.1021/acs.est.5b02078 [DOI] [PubMed] [Google Scholar]
- 75. Quirós-Alcalá L, Bradman A, Nishioka M, et al. Pesticides in house dust from urban and farmworker households in California: an observational measurement study. Environ Health 2011;10:19. 10.1186/1476-069X-10-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gunier RB, Ward MH, Airola M, et al. Determinants of agricultural pesticide concentrations in carpet dust. Environ Health Perspect 2011;119:970-6. 10.1289/ehp.1002532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ritz B, Costello S. Geographic model and biomarker-derived measures of pesticide exposure and Parkinson’s disease. Ann N Y Acad Sci 2006;1076:378-87. 10.1196/annals.1371.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Paul KC, Chuang YH, Cockburn M, Bronstein JM, Horvath S, Ritz B. Organophosphate pesticide exposure and differential genome-wide DNA methylation. Sci Total Environ 2018;645:1135-43. 10.1016/j.scitotenv.2018.07.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bell ML, Belanger K. Review of research on residential mobility during pregnancy: consequences for assessment of prenatal environmental exposures. J Expo Sci Environ Epidemiol 2012;22:429-38. 10.1038/jes.2012.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tong VT, Dietz PM, Morrow B, et al. Centers for Disease Control and Prevention (CDC) Trends in smoking before, during, and after pregnancy--Pregnancy Risk Assessment Monitoring System, United States, 40 sites, 2000-2010. MMWR Surveill Summ 2013;62:1-19. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Online materials