Abstract
Background
Contrast-induced encephalopathy (CIE) is a rare complication of coronary angiography (CAG) caused by a direct neurotoxic reaction to iodinated contrast medium. Contrast-induced encephalopathy can result in a variety of neurological symptoms following within minutes to hours after contrast injection. It manifests most frequently as transient cortical blindness, headache, or confusion. In the majority of known cases, symptoms completely resolve solely with supportive care. We present a case where CIE takes a more dramatic course.
Case summary
A 67-year-old woman was scheduled for elective CAG, due to progressive typical chest pain. Within minutes after injection of iso-osmolar iodinated contrast medium, the patient showed a sudden decline in consciousness while all other vital functions remained normal. Shortly, after the patient was admitted to the intensive care unit due to acute-onset coma and respiratory insufficiency. A computed tomography scan of the brain showed bilateral cerebral oedema, which in combination with the development of symptoms after contrast injection led to the diagnosis of CIE. Remarkable decrease of cerebral oedema was observed 1 day later and slowly clinical recovery ensued. After 23 days, the patient was discharged from the cardiology ward. Follow-up at the outpatient clinic showed no lasting neurological deficits.
Discussion
While most symptoms of CIE are relatively mild and transient in nature, we describe a more devastating course that occurred with the use of only a low quantity of iso-osmolar contrast medium. We emphasize that even the more severe manifestations of CIE can develop at any dosage, and with all types of iodinated contrast medium.
Keywords: Iodinated contrast medium, Neurotoxicity, Encephalopathy, Coronary angiography, Case report
Learning points
Contrast-induced encephalopathy (CIE) most commonly manifests with relatively benign conditions (e.g. confusion, transient cortical blindness), but as shown in our case report, it can also lead to life-threatening complications as acute-onset coma.
Severe manifestations of CIE can develop at small quantities, and with all types of iodinated contrast medium.
Introduction
Contrast-induced encephalopathy (CIE) is a rare complication of coronary angiography (CAG) caused by a direct neurotoxic reaction to iodinated contrast medium. Contrast-induced encephalopathy can result in a variety of neurological symptoms following contrast injection, spanning from confusion and headache to more serious manifestations such as transient cortical blindness, which is the most frequent symptom of CIE. Now, we present a case where CIE takes a far more devastating course, resulting in a sudden decrease in the level of consciousness during CAG after only a small amount of contrast injected.
Timeline
| Timeline | Events |
|---|---|
| 2 months before presentation | Admittance to emergency cardiac care department with increase of typical chest pain. There was no acute coronary syndrome, thus further ischaemia detection using perfusion cardiac magnetic resonance (CMR) was scheduled. |
| 1 month before presentation | Perfusion CMR: perfusion defect in five segments (left anterior descending artery territory). Coronary angiography was scheduled. |
| Day 1 | Coronary angiography was performed. Decline in level of consciousness shortly after iodinated contrast injection. |
| 0–4 h later | Glasgow Coma Scale score 3 and respiratory insufficiency requiring intubation. Patient is admitted to intensive care unit. Computed tomography scan showed bilateral cerebral oedema. Treatment with intravenous dexamethasone and deep sedation is initiated. |
| Day 4 | Marked neurological recovery. Deep sedation was terminated and patient was transferred to cardiology ward. |
| Day 23 | Discharge from cardiology ward to neurological rehabilitation centre. |
| 3 weeks after discharge | Magnetic resonance imaging of the brain: resolution of all previous abnormalities. |
| 6 weeks after discharge | In the outpatient, clinic no lasting focal neurological deficits were observed. |
Case presentation
A 67-year-old woman presented to our hospital with symptoms of progressive angina pectoris on exertion, which relieved with rest and with the use of sublingual nitroglycerine tablets. She was planned for elective CAG. She had a medical history of stable coronary artery disease. Seventeen years earlier a chronic total occlusion (CTO) of the intermediate branch, and generalized atheroma without substantial stenosis in the left anterior descending (LAD) artery were found during CAG. The right coronary artery (RCA) and the circumflex artery (CX) did not show abnormalities. The CTO was left untreated after dobutamine stress-echo was not suggestive for ischaemia. Furthermore, the patient had a history of Type 1 diabetes, hypertension, and adenocarcinoma of the left breast—adequately treated with subsequent lumpectomy, axillary node dissection, and chemo radiation. She was allergic to beta-lactam antibiotics and intolerant to statins. Neither the patient nor her family had a history of adverse reactions to iodinated contrast medium. Upon admission her prescribed medication included acetylsalicylic acid, atenolol, sublingual nitroglycerine, metformin, insulin, and anastrozole.
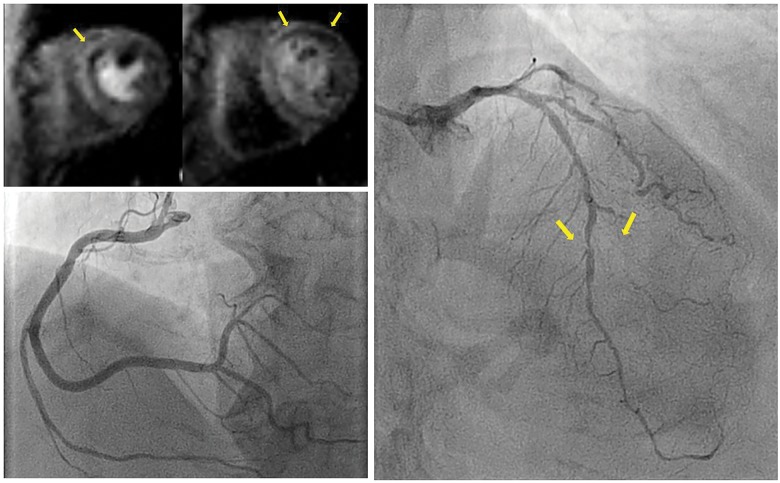
Prior to the current admission, a perfusion cardiac magnetic resonance (CMR) scan was made to assess the potential amount of myocardial ischaemia. Left and right ventricular function and dimensions were normal with a left ventricular ejection fraction of 61% and mild hypokinesia at the apex and anterior wall. A perfusion defect in five segments of LAD artery was observed, without late-gadolinium-enhancement (Figure 1; Supplementary material online, Videos S1–S6). Therefore, the patient was scheduled for elective CAG with the option for percutaneous coronary intervention (PCI).
Figure 1.
Perfusion cardiac magnetic resonance and coronary angiography prior to event. (A) Perfusion cardiac magnetic resonance short axis showed perfusion defects (arrows) in mid (left) and basal (right) anteroseptal segments, which suggests stress-induced ischaemia of left anterior descending artery. (B) A normal large dominant right coronary artery. (C) A serial stenosis of the left anterior descending artery, including a 90% stenosis of the mid left anterior descending artery and an occluded second diagonal branch (arrows). Furthermore, a small circumflex artery with wall-irregularities can be observed. The previously known chronic total occlusion of the intermediate branch is not depicted.
Upon entering the catheterization laboratory, the patient had a normal level of consciousness. Physical examination included a pulse rate of 84 b.p.m., blood pressure of 114/75 mmHg, a respiratory rate of 15/min, and 97% oxygen saturation. Cardiac and pulmonary auscultation was unremarkable. Pre-procedural electrocardiogram and laboratory results were normal. Estimated glomerular filtration ratio at baseline was 66 mL/min. A loading dose of 300 mg clopidogrel, 5000 international units of unfractioned heparin, and because of anxiety prior to the procedure, 2.5 mg of diazepam were administered prior to CAG. Contrast medium used during CAG was iodixanol, an iso-osmolar type of iodinated contrast (290 mOsm/kg H2O). CAG revealed a normal dominant RCA and a normal CX. There were serial severe stenoses of the proximal, mid- and distal LAD artery with an occluded second diagonal branch. The previously known CTO of the anterolateral branch could be observed with modest collateral filling from a small CX.
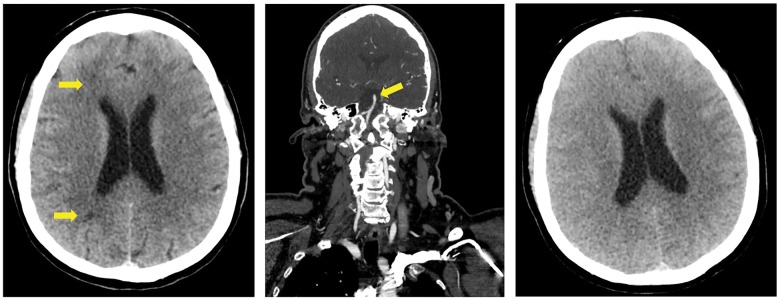
During the switch from diagnostic to guiding catheter, the patient showed a sudden decline in her level of consciousness. At that moment a total of 100 mL of contrast medium had been injected. No air embolus could be observed on CAG. She was unresponsive to verbal commands and painful stimuli, left-sided hypertonia of the arm was observed and there was a bilateral pathological plantar reflex. There were no jerks or twitches and the brainstem reflexes were unremarkable. Her blood pressure was 160/85 mmHg, and all other vital functions were normal, as well as the blood glucose level. An adverse reaction to diazepam could not be excluded, and therefore, 0.5 mg of flumazenil was administered, which did not improve the neurological state. An initial computed tomography (CT) scan of the brain showed right-sided hypodensities in the watershed regions, suggestive of ischaemia, which did not fully explain the neurological state (Figure 2). Computed tomography cerebral angiography showed a normal basilar artery, excluding brain stem stroke. For further observation and treatment, the patient was transferred to the intensive care unit (ICU). The snoring respiration continued at a rate of 20/min and with a decline in level of consciousness to a Glasgow Coma Scale score of 3 a secure airway could not be guaranteed, and therefore, the patient was subsequently intubated. Four hours after the initial CT scan of the brain, a repeat scan now interestingly showed diffuse bilateral cerebral oedema with complete loss of gyral patterns. The acute onset of neurological symptoms after contrast medium administration and the diffuse cerebral oedema as seen on the CT scan led to the diagnosis of CIE.
Figure 2.
Computed tomography scans after decline in consciousness of patient. (A) Computed tomography scan of the brain after coronary angiography: hypodensities in the anterior and posterior watershed regions on the right side, suggestive for ischaemia, which did not completely explain the neurological state. (B) A computed tomography angiography of neck and head showed a normal basilar artery and thus excluded brain stem stroke. (C) Computed tomography scan of the brain, 4 h later when patient was admitted to the intensive care unit: marked bilateral cortical oedema with loss of gyral pattern.
At the ICU, the patient was deeply sedated with propofol and treated with loading dose of 50 mg of intravenous dexamethasone, followed by 8 mg of dexamethasone twice a day. Neurosurgical intervention was not deemed useful because the oedema was bilateral. Due to atypical jerking of the arms, brief treatment with levetiracetam was initiated, but the electroencephalography did not suggest epilepsy, so it was discontinued. One day later, a notable improvement with a decrease of cerebral oedema and no signs of ischaemia was seen on a repeat CT scan. Consequently, treatment with dexamethasone was no longer required, and therefore, discontinued.
After 58 h of coma, sedation was terminated and the patient showed marked neurological recovery. She remained somewhat bradyphrenic and disorientated and had a mild left-sided hemiparesis. She was transferred back to the cardiology ward.
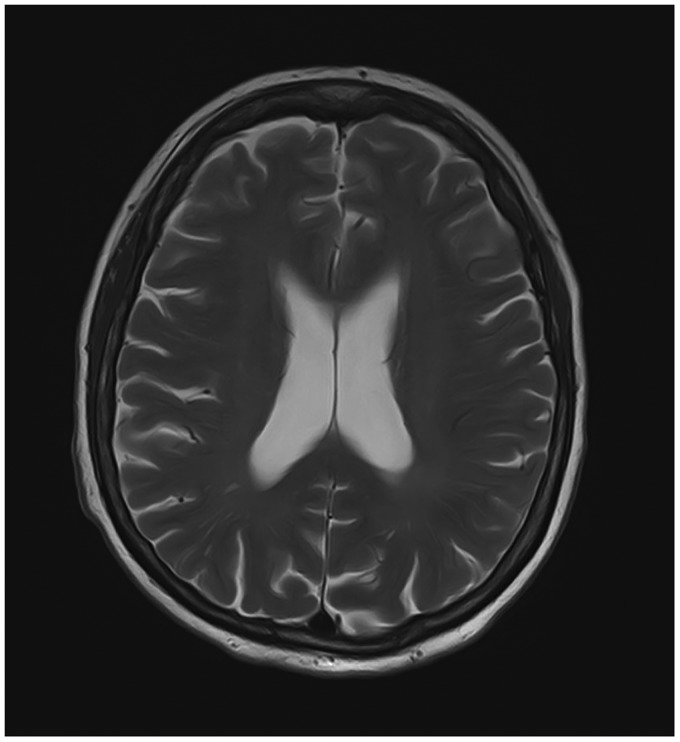
In a multidisciplinary meeting with cardiologists and cardiac surgeons, a novel CAG or PCI was abandoned due to the preceding event with iodine-containing contrast media. Coronary artery bypass grafting was considered, but it was concluded that surgical intervention would not lead to complete revascularization and total relief of symptoms. Optimal medical treatment with dual antiplatelet therapy, captopril, metoprolol, isosorbide mononitrate, and ivabradine was continued. Contrast-induced nephropathy did not occur during hospital stay. The patient was discharged 23 days after the incident—including 4 days in the ICU—to a neurological rehabilitation centre. A follow-up magnetic resonance imaging (MRI) scan of the brain after 3 weeks showed complete resolution of all previous abnormalities, including the right-sided hypodensities, thereby excluding ischaemia (Figure 3). Upon follow-up in the outpatient clinic, there were no focal neurological deficits.
Figure 3.
Magnetic resonance imaging scan at follow-up. Three weeks after discharge from cardiology ward, a follow-up magnetic resonance imaging scan of the brain (T2 weighted) showed complete resolution of all previous abnormalities and no sign of ischaemia.
Discussion
Contrast-induced encephalopathy is a very rare complication of CAG, and is a consequence of intra-arterial injection of iodinated contrast medium CIE in patients undergoing CAG. It has been documented in merely 50 case reports involving CAG and is more often a complication of invasive cerebral angiography, where it is estimated to occur in 1–2% of procedures.1,2 Furthermore there have been few cases where iodinated contrast injection led to a decline in level of consciousness.3–5 What is notable about this case is the acute onset of severe neurological symptoms after the administration of only a low quantity of an iso-osmolar contrast compound (100 mL, 290 mOsm/kg H2O). This enforces the conception that even the more severe manifestations of CIE can occur at any injected dosage of any type of iodinated contrast medium. By definition symptoms are reversible, and prognosis is usually good solely with supportive care.2 Direct neurotoxicity to the cerebral cortex by iodinated contrast medium when integrity of the blood–brain barrier (BBB) is compromised is regarded as the pathophysiological mechanism behind CIE.6 High-osmolar ionic iodinated contrast media is associated with enhanced degradation of the BBB in rabbits than their non-ionic low-osmolar counterparts.7 In theory this makes sense, because a high-osmolar compound would exert greater osmotic force on the vessel walls, forcing tight junctions and cells apart. However, CIE has been reported in humans with both high-osmolar and iso-osmolar iodinated contrast media.2 Our case suggests that changing from a hyperosmolar type of contrast to an iso-osmolar one is not an adequate strategy to avoid CIE, as severe CIE still occurred at low quantities of an iso-osmolar contrast compound. Potential patient-related factors that might have attributed to BBB-disruption in this specific case are hypertension and diabetes. Studies with experimentally induced hypertension in rats have shown to increase microvascular permeability and tight junction degradation in the BBB.8,9 Furthermore, diabetics have a higher permeability of the BBB compared with healthy controls as assessed by MRI of the brain.10
There is a large diversity in neurological symptoms and radiological findings when CIE occurs. Transient cortical blindness is the most prevalent manifestation of CIE, reported in half of all described cases. It has been proposed that cortical blindness is caused by a relatively high permeability of the BBB in the occipital cortex. Other symptoms include headache, confusion, and convulsions.2 The reported radiological findings on cerebral CT-imaging include (sub)cortical enhancement, mimicking of subarachnoid haemorrhage, and cerebral parenchymal swelling.3,11,12 Also absence of radiological findings has been reported.13
The occurrence of CIE is difficult to predict. For example, our patient previously underwent CAG without an adverse reaction to contrast. Conversely there are case reports of patients who after being diagnosed with CIE, did not experience recurrence when a subsequent CAG was performed.14 In patients with CIE, the prognosis is relatively good with supportive care only, and in most cases complete resolution of symptoms is established.2 However, the heterogeneous and often mild nature of CIE might lead to mis- and underdiagnosis of this condition. While this complication of iodinated contrast injection might not have led to lasting deficits, the patient was denied for revascularization therapy, which illustrates another harmful consequence of CIE.
If, in the future, a procedure with iodinated contrast is medically indicated—e.g. invasive cerebral angiography or CT angiography of brain—it is important to weigh in the potential risk of recurrent CIE and the diagnostic necessity of the procedure.
Conclusion
In conclusion, we reported a rare case of CIE following CAG with the use of a low quantity of iso-osmolar contrast medium, which resulted in bilateral cerebral oedema and coma. Fortunately it did not lead to any permanent neurological deficits.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Lantos G. Cortical blindness due to osmotic disruption of the blood-brain barrier by angiographic contrast material: CT and MRI studies. Neurology 1989;39:567–571. [DOI] [PubMed] [Google Scholar]
- 2. Spina R, Simon N, Markus R, Muller DWM, Kathir K.. Core curriculum contrast-induced encephalopathy following cardiac catheterization. Catheter Cardiovasc Interv 2017;90:257–268. [DOI] [PubMed] [Google Scholar]
- 3. Sharp S, Stone J, Beach R.. Contrast agent neurotoxicity presenting as subarachnoid hemorrhage. Neurology 1999;52:1503–1505. [DOI] [PubMed] [Google Scholar]
- 4. Liao M-T, Lin T-T, Lin L-Y, Hwang J-J, Tseng C-D.. Contrast-induced encephalopathy after percutaneous coronary intervention. Acta Cardiol Sin 2013;29:277–280. [PMC free article] [PubMed] [Google Scholar]
- 5. Obermann M, Moeller-Hartmann C, Naegel S.. Neurotoxic cerebral oedema following coronary angiography. J Neurol Neurosurg Psychiatry 2013;84:458–459. [DOI] [PubMed] [Google Scholar]
- 6. Caille JM, Allard M.. Neurotoxicity of hydrosoluble iodine contrast media. Invest Radiol 1988;23(Suppl 1):1290–1298. [DOI] [PubMed] [Google Scholar]
- 7. Velaj R, Drayer B, Albright R, Fram E.. Comparative neurotoxicity of angiographic contrast media. Neurology 1985;35:1290. [DOI] [PubMed] [Google Scholar]
- 8. Mohammadi M, Golam D.. Acute hypertension induces brain injury and blood-brain barrier disruption through reduction of claudins mRNA expression in rat. Pathol Res Pract 2014;210:985–990. [DOI] [PubMed] [Google Scholar]
- 9. Fan Y, Yang X, Tao Y, Lan L, Zheng L, Sun J.. Tight junction disruption of blood—brain barrier in white matter lesions in chronic hypertensive rats. Neuroreport 2015;26:1039–1043. [DOI] [PubMed] [Google Scholar]
- 10. Starr JM, Wardlaw J, Ferguson K, Maclullich A, Deary IJ, Marshall I.. Increased blood–brain barrier permeability in type II diabetes demonstrated by gadolinium magnetic resonance imaging. J Neurol Neurosurg Psychiatry 2003;74:70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paul LJ, Vicente JM, Pastorin R, Alfredo CA.. A case of temporary nonthrombotic hemiplegia and aphasia due to neurotoxicity from angiographic contrast material? Radiologia 2009;51:614–617. [DOI] [PubMed] [Google Scholar]
- 12. Eleftheriou A, Rashid AS, Lundin F.. Late transient contrast-induced encephalopathy after percutaneous coronary intervention. J Stroke Cerebrovasc Dis 2018;27:140–106. [DOI] [PubMed] [Google Scholar]
- 13. Dattani A, Au L, Tay KH, Davey P.. Contrast-induced encephalopathy following coronary angiography with no radiological features: a case report and literature review. Cardiology 2018;139:197–201. [DOI] [PubMed] [Google Scholar]
- 14. Law S, Panichpisal K, Demede M, John S, Marmur JD, Nath J, Baird AE.. Case report contrast-induced neurotoxicity following cardiac catheterization. Case Rep Med 2012;2012:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.