Abstract
Background
Although left ventricular aneurysm (LVA) is the most common mechanical complication of myocardial infarction (MI), it rarely involves the inferior or posterior left ventricular wall. Ventricular septal rupture (VSR) may be a fatal mechanical complication of MI but rarely occurs in the posterior or inferior portion of the interventricular septum. Thus, LVA and VSR as two mechanical complications of MI in the same patient are extremely rare.
Case summary
A 65-year-old woman, who had inferior ST-segment elevation myocardial infarction 2 months before without reperfusion therapy, was admitted with exertional dyspnoea for 1 month. Echocardiography and computed tomography revealed true inferoposterior LVA and VSR as concurrent complications of MI. These imaging findings were confirmed during cardiac surgery. After successful coronary bypass grafting and ventriculoplasty, the patient recovered quickly and was discharged from the hospital.
Discussion
A rare case of post-infarction inferoposterior LVA with concurrent interventricular septal rupture was reported. Transthoracic and transoesophageal echocardiography and cardiac computed tomography were valuable tools for the diagnosis of this rare condition. Combined coronary bypass grafting and ventriculoplasty were effective in treating this often fatal complication of inferior MI.
Keywords: Inferoposterior left ventricular aneurysm, Ventricular septal rupture, Myocardial infarction, Echocardiography, Case report
Learning points
Concurrent true inferoposterior left ventricular aneurysm and interventricular septal rupture secondary to inferior myocardial infarction (MI) is extremely rare but does occur.
Transthoracic and transoesophageal echocardiography and cardiac computed tomography are valuable tools for the diagnosis of the concurrent mechanical complications. Combined coronary arterial bypass grafting and ventriculoplasty are effective in treating this often fatal complication of inferior MI.
Introduction
Left ventricular aneurysm (LVA), first reported by Fulton,1 is the most common mechanical complication of ST-segment elevation myocardial infarction (STEMI). Although the incidence of LVA has decreased dramatically due to an early revascularization strategy, its occurrence is still associated with a poor clinical outcome. Compared with the anterior wall, the most prevalent site of LVA, only 9% of LVA involve the inferior or posterior left ventricular wall.2 Thus, true LVA involving the inferoposterior wall is rare.
Ventricular septal rupture (VSR) is an often fatal mechanical complication of acute myocardial infarction (AMI) and the incidence of VSR has dropped to nearly 0.2% in the era of thrombolysis.3 About 60% of post-infarction VSR is located in the anterior or apical portion of the interventricular septum secondary to complete obstruction of the left anterior descending coronary artery,4 whereas only 20–40% of VSR is found in the posterior or inferior portion of interventricular septum as a result of an inferoposterior AMI.5 However, LVA and VSR as two mechanical complications of AMI in the same patient are extremely rare.
In this study, we report an unusual case of concurrent true inferoposterior LVA and VSR after inferoposterior AMI.
Timeline
| Day | Events |
|---|---|
| 2 months ago | The patient was admitted into a rural hospital due to acute inferior myocardial infarction (MI) without reperfusion therapy |
| 1 | The patient presented with dyspnoea for 1 month |
| Electrocardiogram: sinus rhythm, old inferior MI | |
| Laboratory tests: elevated NT-proBNP, cTnI within normal limit | |
| Transthoracic echocardiography: a remarkable inferoposterior left ventricular aneurysm (LVA) with ruptured ventricular septum | |
| 2 | Transoesophageal echocardiography: a wide neck, thin-walled large inferoposterior LVA was detected. The orifice between the aneurysm and right ventricle was located at the inferior septum and near the apex |
| 5 | Coronary angiography: multivessel disease and total occlusion in the proximal segment of the right coronary artery |
| 6 | Cardiac computed tomography: in agreement with echocardiographic findings |
| 7 | Cardiac surgery: the diagnosis of concurrent true inferoposterior LVA and ventricular septal rupture was confirmed. Combined coronary arterial bypass grafting with ventriculoplasty were performed |
| 8 | A 3.4 mm residual ventricular septal shunt was found by early bedside echocardiography. The patient’s haemodynamic status was stable |
| 14 | Transthoracic echocardiography: normalized left ventricular cavity and systolic function. A 4 mm residual shunt located at the mid-septal segment was detected |
| 16 | The patient was discharged from the hospital |
Case presentation
A 65-year-old woman was admitted to the Department of Cardiology of Qilu Hospital of Shandong University because of exertional dyspnoea for 1 month. The New York Heart Association (NYHA) functional class was III. Two months prior, she was admitted into a rural hospital with a 2-day history of severe chest pain and a diagnosis of late presentation inferior STEMI was established. Unfortunately, due to the time delay since her onset of chest pain and her refusal to receive invasive angiography, reperfusion therapy was not performed and only oral aspirin, clopidogrel, simvastatin, isosorbide dinitrate, and gliclazide were prescribed at the local hospital. She was a non-smoker but had a history of hypertension, diabetes, cerebral infarction, and angina pectoris.
On current admission, her heart rate and blood pressure were 82 b.p.m. and 99/60 mmHg, respectively, and her body mass index was 24 kg/m2. Physical examination revealed a distension of the jugular veins, moist rales at the basal lungs, and a harsh pansystolic murmur at the parasternal left fourth intercostal space. No oedema of the lower extremities was noted.
Laboratory tests revealed normal levels of creatine kinase isoenzyme MB (16 U/L, normal range 0–25 U/L) and cardiac troponin I (0.02 ng/mL, normal range 0–0.06 ng/mL). Serum low-density lipoprotein cholesterol (90 mg/dL) and glycosylated haemoglobin (11.1%) levels were not satisfactorily controlled, which should be lower than 70 mg/dL and 7% respectively, according to international guidelines on blood cholesterol and diabetes management for patients with clinical atherosclerotic cardiovascular disease. The level of N-terminal prohormone of brain natriuretic peptide was 7421 pg/mL (<125 pg/mL to rule out heart failure) and hypersensitive C-reactive protein level was 1.53 mg/L (normal range 0–3 mg/L). Results of routine tests of blood, urine and stool samples were normal. Hepatic and renal functions were not affected.
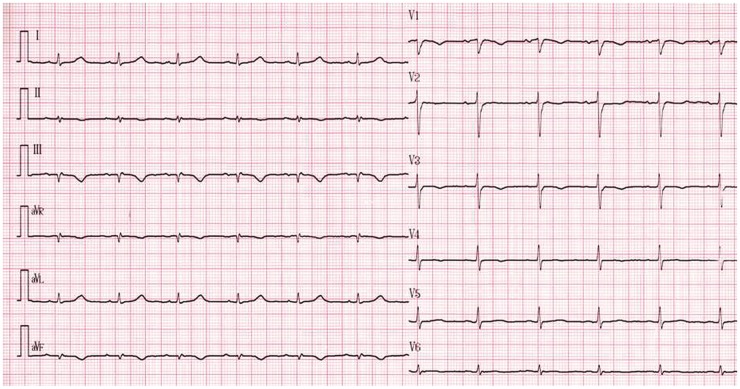
Electrocardiography showed abnormal Q waves and inverted T waves in leads III and aVF (Figure 1), suggesting an inferior myocardial infarction (MI). Transthoracic echocardiography (TTE) demonstrated that all four cardiac chambers were slightly enlarged. A giant (47 × 38 mm in diameter) and thin-walled (4 mm in thickness) inferoposterior LVA with a wide neck (25 mm in diameter) was observed in the oblique parasternal short-axis view (Figure 2A, Supplementary material online, Movie S1).The Gatewood and Nanda index6 was > 0.5. Although the aneurysmal wall was akinetic, the remaining left ventricular segments were hyperkinetic, resulting in an ejection fraction of 41% as measured by the modified biplane Simpson’s algorithm. There were also mild pericardial effusion, moderate tricuspid and mitral valve regurgitation, and an abnormal left ventricular (LV) filling pattern. The pulmonary systolic pressure estimated from the tricuspid regurgitant peak flow velocity was 53 mmHg. Colour Doppler imaging (Figure 2B) showed laminar flow between the aneurysm and the left ventricular cavity but a systolic shunt flow between the aneurysm and the right ventricle was identified, with a peak pressure gradient of 63 mmHg estimated by the simplified Bernoulli equation.
Figure 1.
Electrocardiogram suggesting inferoposterior myocardial infarction.
Figure 2.
Transthoracic echocardiogram showing a large left ventricular inferoposterior aneurysm (A) with a wide neck (arrow) and ventricular septal rupture in the mid septum (arrowhead) with a systolic turbulent flow (B). Transoesophageal echocardiogram showing a large aneurysm (C) with a wide neck (arrows) and the aperture of ventricular septal disruption (dashed line), as well as the shunt from the aneurysm to the right ventricle through the perforation (D). Ane, aneurysm; LV, left ventricle; RV, right ventricle.
Transoesophageal echocardiography (TOE) revealed a large inferoposterior aneurysm (54 × 40 mm in diameter) connected to the LV cavity via a wide neck (25 mm in diameter), and a systolic shunt flow originated from the disrupted aperture (8 mm in diameter) of the aneurysm near the right ventricular apex (Figure 2C and D, Supplementary material online, Movie S2).
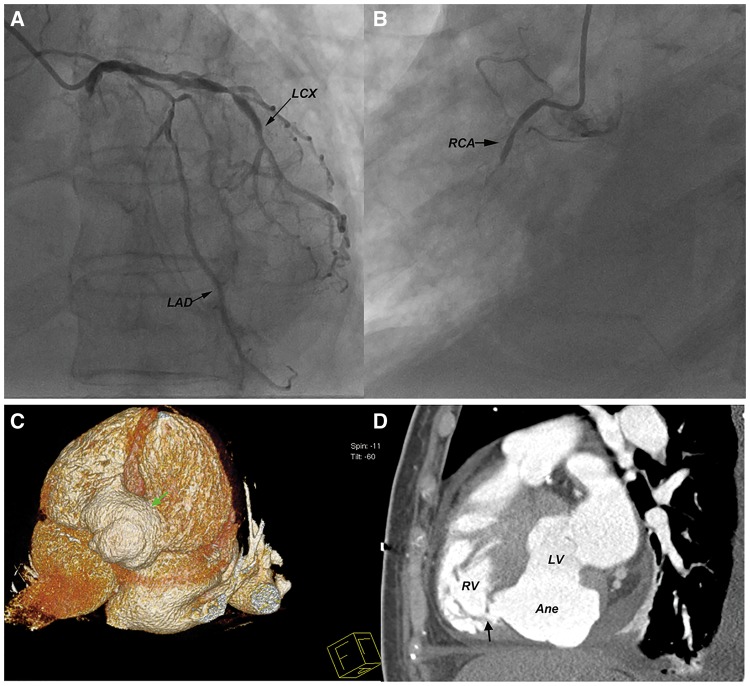
Coronary angiography performed 5 days after admission showed multivessel severe stenosis, especially a total occlusion in the proximal segment of the right coronary artery (Figure 3A and B). On three-dimensional computed tomography, the LVA was confirmed to extrude to the inferior and right of the left ventricle and cannulated with the right ventricle through a VSR near the apex. Viewed from the diaphragmatic surface, the LVA occupied the whole atrioventricular crux, bridging the inferior left and right ventricular walls, extending upward to the coronary sulcus and downward to the middle part of the interventricularis posterior sulcus. The whirl-shaped aneurysm arose from the infero-basal segment of left ventricle, extended to the mid-septal level, and leaked into the right ventricle though an orifice near the apex (Figure 3C and D).
Figure 3.
Coronary angiograms showing multiple stenotic lesions of the left anterior descending and circumflex arteries (A) and complete obstruction of the right coronary artery (B). Reconstructed three-dimensional computed tomogram showing a large inferoposterior ventricular aneurysm (green arrow) that occupied the whole atrioventricular crux (C) and communicated with the right ventricle through an aperture near the apex (black arrow) (D). LAD, left anterior descending artery; LCX, left circumflex arteries; RCA, right coronary artery.
The patient was transferred to the Department of Cardiac Surgery, where coronary bypass surgery and left ventriculoplasty were performed. During surgery, no pericardial adhesion or haemorrhage was found after opening the pericardial sac, excluding the possibility of pseudo-aneurysm. A large aneurysm was detected at the left ventricular inferior wall, extending to the left of the posterior descending artery, with a size of 40 × 60 mm. Ischaemic regions were observed around the aneurysm, and the myocardial continuity between the ventricles and the aneurismal body was confirmed. A diagnosis of true LVA was established. After the left anterior descending artery and the second obtuse marginal artery were bypassed with two saphenous vein grafts, the aneurysm was incised. No mural thrombus was found. A disrupted aperture (10 mm in diameter) was observed near the right ventricular apex. Ventriculoplasty was performed by placing a 40 × 60 mm bovine epicardial patch inside the aneurysm and suturing the patch to the nearby left ventricular wall along the border of the aneurysm, in an attempt to segregate the aneurysm from the left ventricle and preserve effective left ventricular volume (Figure 4).
Figure 4.
Surgical views demonstrating an inferoposterior left ventricular aneurysm (left panel) and the incised aneurysm (right panel) with a disrupted aperture (arrow). The patient’s head is to the right of the figures. Ane, aneurysm.
The patient was transferred to the cardiac care unit after the surgery where she received 1:1 intra-aortic balloon pump assistance in sinus rhythm. A 3.4-mm residual ventricular septal shunt was found with early bedside echocardiography, but the haemodynamic status was stable and the patient recovered quickly. There was no difficulty in weaning the intra-aortic balloon pump at the second day after the surgery and the patient was discharged from the cardiac care unit.
Echocardiography 1 week after surgery revealed the aneurysm was segregated from the left ventricle which resumed a normal shape, and the left ventricular ejection fraction increased to 57% with a normal left ventricular filling pattern. The right ventricular anterior–posterior dimension at end-diastole decreased to 21 mm from 25 mm pre-operatively. The E/A ratio of tricuspid flow, tricuspid annular plane systolic excursion and right ventricular fractional area change were 1.3, 2.3 cm, and 53%, respectively, which were comparable to those measured pre-operatively (1.2, 2.1 cm, and 51%, respectively). The pulmonary systolic pressure estimated from the tricuspid regurgitant peak flow velocity dropped to 38 mmHg. There was no flow inside the segregated aneurysm, but a residual shunt flow of 4 mm in diameter was detected along the inferior border of the patch (Figure 5). The patient recovered from surgery successfully and was discharged from hospital. Oral aspirin, clopidogrel, atorvastatin, valsartan, metoprolol, spirolactone, metformin, acarbose, and trimetazidine were prescribed before discharge. One month after discharge, the NYHA functional class improved to II as confirmed by a telephone follow-up.
Figure 5.
Transthoracic echocardiograms after surgery illustrating the restored left ventricular shape with thickened walls (A) and a segregated left ventricular aneurysm (B). A residual shunt along the inferior border of the patch was detected (C). Arrowhead: bovine epicardial patch. Ane, aneurysm; LA, left atrium; LV, left ventricle; RV, right ventricle.
Discussion
Historically, the term ‘ventricular aneurysm’ was based on the pathological finding of a dilated ventricle with a considerable portion of the wall markedly thinned and replaced by fibrous tissue.1 Aneurysms in the inferior wall are uncommon. Although the combination of subepicardial aneurysms and VSR have been reported, these aneurysms were pseudo-aneurysms.7,8 Recently, several cases of inferoposterior ventricular aneurysm complicating AMI have been reported, with no concurrent VSR.9–11 Davila et al.12 reported a case of combined inferior pseudo-aneurysm and VSR after a non-ST segment MI. Chan et al.13 reported two cases of right ventricular intramyocardial dissection with a left-to-right shunt post-MI. To our knowledge, concurrent true inferoposterior LVA and VSR after STEMI has never been reported.
Transoesophageal echocardiography is more accurate than TTE in identifying posterior or inferior wall aneurysms and has replaced contrast ventriculography as the diagnostic standard.14 Doppler echocardiography has 100% sensitivity and specificity for the diagnosis of VSR.3 In addition to the anatomical information, the ventricular wall motion evaluated by real-time echocardiography has important prognostic significance. In the current case, the anatomic details of the aneurysm and VSR identified by both TTE and TOE techniques agreed well with the surgical findings. The echocardiographic features of continuous myocardium, laminar blood flow through a wide neck and Gatewood and Nanda index > 0.5 conformed to the standard true aneurysm proposed by Gatewood.6
The differentiation of the infarction-related VSR from congenital ventricular septal defect should be made. We believe the following four points may lend support to the former diagnosis. First, the patient was a 65-year-old woman who were asymptomatic until the onset of chest pain. Second, the VSR was located inside the LVA with an orifice of 8 mm in diameter and clearly delineated rims, while the right ventricle was only mildly dilated and pulmonary hypertension was moderate on echocardiography. Third, ischaemic tissues and myocardial scars were seen around the aneurysm during surgery. Fourth, the wall motion in the infero-basal and infero-septal left ventricular segments was akinetic, in line with the coronary angiographic findings of multi-vessel disease and total occlusion of the right coronary artery.
Previous studies found that the in-hospital mortality with an inferior STEMI was substantially increased when combined with VSR.15 The present case was unusual in that the patient was in a stable haemodynamic condition albeit with symptoms of heart failure for more than 1 month. We believe the giant aneurysm may act as a buffer area to shunting flow, thus preventing the surge of pulmonary artery pressure, as evidenced by the peak systolic gradient of 63 mmHg across the septal rupture.
Once LVA or VSR was detected, cardiac surgery is the treatment of choice. However, the timing of surgery varies depending on the state of rupture and the presence of concomitant lesions.16 The aneurysm size does not affect the selection of patients who undergo anterior aneurysm resection, as long as the remaining myocardium shows adequate contraction. This criterion fits with the general aneurysmectomy for inferior or posterior LVA.2 We chose to reconstruct the left ventricle with a patch of bovine epicardium because ventriculoplasty may be superior to ventricular aneurysmectomy for preserving effective left ventricular volume. However, long-term follow-up is needed for evaluating the effect of the residual ventricular septal shunt, the risk of thrombosis in the quarantined aneurysm and the superiority of ventriculoplasty used in this case.
Conclusion
A rare case of concurrent true inferoposterior LVA and VSR secondary to inferior MI was reported. Transthoracic and transoesophageal echocardiography and cardiac computed tomography were valuable tools for the diagnosis of this rare condition. Combined coronary bypass grafting and ventriculoplasty were effective in treating this often fatal complication of inferior STEMI.
Funding
This work was supported by National Natural Science Foundation of China [81571689 to Z.P.F.].
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Fulton MN. Aneurysm of the ventricle of the heart. JAMA 1941;116:115. [Google Scholar]
- 2. DePace NL, Dowinsky S, Untereker W, Lemole GM, Spagna PM, Meister SG.. Giant inferior wall left ventricular aneurysm. Am Heart J 1990;119:400–402. [DOI] [PubMed] [Google Scholar]
- 3. Anderson DR, Adams S, Bhat A, Pepper JR.. Post-infarction ventricular septal defect: the importance of site of infarction and cardiogenic shock on outcome. Eur J Cardiothorac Surg 1989;3:554–557. [DOI] [PubMed] [Google Scholar]
- 4. Birnbaum Y, Fishbein MC, Blanche C, Siegel RJ.. Ventricular septal rupture after acute myocardial infarction. N Engl J Med 2002;347:1426–1432. [DOI] [PubMed] [Google Scholar]
- 5. Mishra A, Sanghi P, Batra R.. Post-infarction ventricular septal defect—a case report. Kardiol Pol 2008;66:551–554. [PubMed] [Google Scholar]
- 6. Gatewood RP, Nanda NC.. Differentiation of left ventricular pseudoaneurysm from true aneurysm with two dimensional echocardiography. Am J Cardiol 1980;46:869–878. [DOI] [PubMed] [Google Scholar]
- 7. Epstein JI, Hutchins GM.. Subepicardial aneurysm: a rare complication of myocardial infarction. Am J Med 1983;75:639–644. [DOI] [PubMed] [Google Scholar]
- 8. Hayashi T, Ichikawa M, Yutani C, Iwata A, Yamaguchi T, Matsuda N, Lim Y-J, Mishima M.. Echocardiographic progression of a subepicardial aneurysm after inferior myocardial infarction. J Cardiol 2009;54:339–343. [DOI] [PubMed] [Google Scholar]
- 9. Penkalla A, Solowjowa N, Dandel M, Knosalla C.. Giant true inferoposterior left ventricular aneurysm presenting with heart failure: insights from multimodality imaging. Eur J Cardiothorac Surg 2014;46:333.. [DOI] [PubMed] [Google Scholar]
- 10. Subban V, Makadia N, Rajaram RS, Ravikumar R, Kurian VM, Sankardas MA.. Giant left ventricular aneurysm complicating silent inferoposterior myocardial infarction. J Card Surg 2009;24:697–699. [DOI] [PubMed] [Google Scholar]
- 11. Ono R, Funabashi N, Takaoka H, Ozawa K, Ota S, Nakatani Y, Matsumiya G, Kobayashi Y.. Massive myocardial aneurysm due to inferior to posterior myocardial infarction complicated with right-sided heart failure in a 36-year-old male. Int J Cardiol 2016;209:98–102. [DOI] [PubMed] [Google Scholar]
- 12. Davila CD, Slipczuk L, Gupta S.. Simultaneous left ventricular pseudo-aneurysm and ventricular septal rupture after a non-ST segment myocardial infarction. J Med Cases 2015;6:55–58. [Google Scholar]
- 13. Chan W, Yan B, Warren R, Goldblatt J, Aggarwal A.. A rare complication of left ventricular rupture–right ventricular intramyocardial dissection with left-to-right shunting. Int J Cardiol 2007;121:e19–e21. [DOI] [PubMed] [Google Scholar]
- 14. Atik FA, Navia JL, Vega PR, Gonzalez-Stawinski GV, Alster JM, Gillinov AM, Svensson LG, Pettersson BG, Lytle BW, Blackstone EH.. Surgical treatment of postinfarction left ventricular pseudoaneurysm. Ann Thorac Surg 2007;83:526–531. [DOI] [PubMed] [Google Scholar]
- 15. Crenshaw BS, Granger CB, Birnbaum Y, Pieper KS, Morris DC, Kleiman NS, Vahanian A, Califf RM, Topol EJ.. Risk factors, angiographic patterns, and outcomes in patients with ventricular septal defect complicating acute myocardial infarction. Circulation 2000;101:27–32. [DOI] [PubMed] [Google Scholar]
- 16. Arsan S, Akgun S, Turkmen M, Kurtoglu N, Yildirim T.. Delayed rupture of a postinfarction left ventricular true aneurysm. Ann Thorac Surg 2004;77:1813–1815. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.