Abstract
Intracerebral hemorrhage (ICH) is a stroke subtype associated with significant morbidity and mortality. The purpose of this review is to provide an update on important research on ICH over the past three years. Topics covered include risk factors, imaging predictors of hematoma expansion, scoring schema to predict hematoma expansion, hemostatic therapies, acute blood pressure lowering, intraventricular administration of alteplase for intraventricular hemorrhage, and the current status of surgical therapies.
Keywords: intracerebral hemorrhage, hematoma expansion, blood pressure lowering, antithrombotic reversal, deompressive hemicraniectomy, intraventricular alteplase
Introduction
Intracerebral hemorrhage (ICH) accounts for about 10% to 20% of all strokes and is associated with greater morbidity and mortality than ischemic strokes 1. Many modifiable risk factors, including prescription medications, have been identified for ICH. Cranial computed tomography (CT) is the imaging modality of choice for the diagnosis of acute ICH. Several CT parameters may predict hematoma expansion and neurologic worsening. These CT parameters, along with clinical criteria, have been used to create practical scoring schema to predict hematoma expansion. Despite high morbidity and mortality, there are no specific interventions that have been shown to improve clinical outcome after ICH. Over the last three years, important research has been carried out on the reversal of antiplatelet and oral anticoagulant (OAC)-associated ICH (OAC-ICH), acute blood pressure lowering, clearance of intraventricular hemorrhage (IVH) with intraventricular administration of alteplase, and decompressive craniectomy.
Risk factors for intracerebral hemorrhage
Modifiable risk factors for ICH include arterial hypertension ( Figure 1), excessive alcohol consumption, decreased low-density lipoprotein cholesterol, low serum triglyceride levels, prescription medications, current cigarette smoking, and drugs of abuse (for example, cocaine, heroin, amphetamines, and ephedrine) 1. Arterial hypertension is one of the most important modifiable risk factors as the crude prevalence among adults in the US has been estimated to be 45.6%; this is an increase from 31.9% based on prior definitions of arterial hypertension 2. Certain prescription medications, such as cyclooxygenase (COX) inhibitors, P2Y12 inhibitors, OACs, selective serotonin reuptake inhibitors (SSRIs), and statins, have also been associated with an increased risk of ICH.
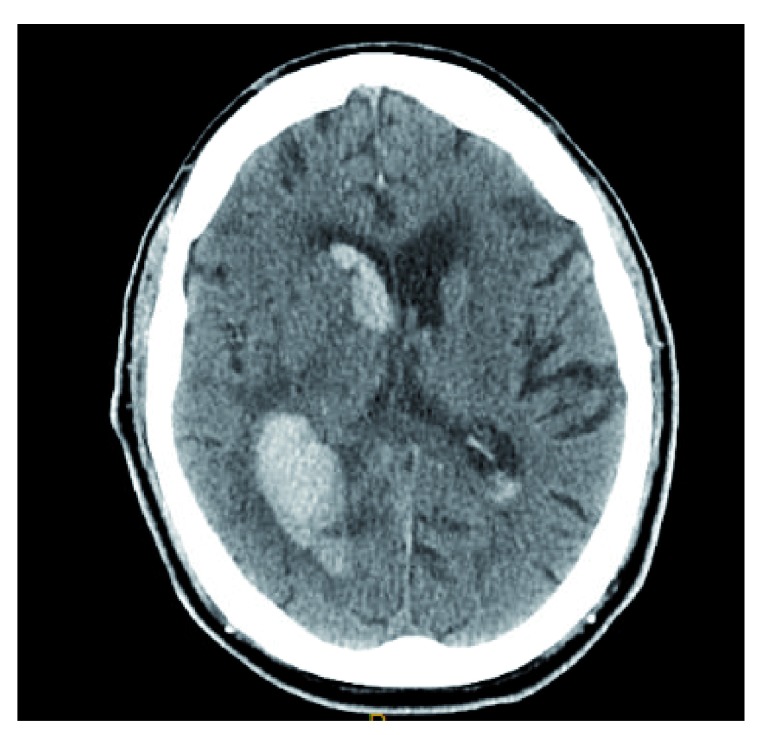
Figure 1. Intracerebral Hemorrhage due to arterial hypertension.
( A) Hyper-attenuating hemorrhage visualized in the pons. ( B) Hyper-attenuating hemorrhage centered in the left medial thalamus. Both locations are typical for hemorrhage related to long-standing hypertensive arteriopathy. These images constitute original, unpublished data obtained by the authors.
Platelet inhibition related to COX-1 enzyme inhibitors (for example, aspirin) and P2Y12 purinoreceptor antagonists (for example, clopidogrel, ticagrelor, prasugrel, and cangrelor) is associated with increased ICH volume growth and worse clinical outcome 3. In a retrospective study of 82,576 patients, in-hospital mortality was higher among patients with ICH on dual antiplatelet therapy compared with no antiplatelet therapy 3.
A weak association between SSRIs/statins and ICH has been made. SSRIs cause antiplatelet effects by inhibiting serotonin reuptake into platelets 4. In a large retrospective cohort study, SSRI use was associated with an increased risk of ICH. This effect was most notable within the first 30 days of use and when used concomitantly with OACs 4.
The “Stroke Prevention by Aggressive Reduction in Cholesterol Levels” (SPARCL) trial suggested that statins potentially increase the risk for future ICH among patients with previous ischemic stroke or ICH 5. Since publication of the SPARCL study, data derived primarily from retrospective studies have not confirmed that statin use is a risk factor for ICH, and some have suggested that statin use is associated with improved mortality 5. In a meta-analysis of patients with prior ischemic stroke or ICH, there was a statistically significant risk reduction in poor functional outcome and all-cause mortality in both groups 6. Lobar localization of ICH, multiple bleeds, or multiple micro-hemorrhages may portend a high risk for statin use in patients with ICH. However, the posited association between statins/SSRIs and ICH requires more robust prospective evaluation.
ICH is the most feared complication of OAC use. The CROMIS-2 (“Cerebral microbleeds and intracranial hemorrhage risk in patients anticoagulated for atrial fibrillation after acute ischemic stroke or transient ischemic attack”) study was a recent prospective observational multicenter study of 1409 patients starting OAC following transient ischemic attack or ischemic stroke and identified risk factors for OAC-ICH 7. The presence of cerebral microbleeds and that of diabetes mellitus were the only two variables associated with ICH during the follow-up period. The authors noted that cerebral microbleeds may be a neuroimaging biomarker of a bleeding-prone arteriopathy. Interestingly, the commonly used “HAS-BLED” (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio [INR], elderly [age of at least 65 years], and drugs/alcohol concomitantly) score did not independently differentiate between patients with ICH and those without ICH. Precise identification of these risk factors and their magnitude of risk will promote patient-tailored management for secondary ischemic stroke prevention in the setting of atrial fibrillation. For example, isolation of the left atrial appendage may be an option for patients for whom the risk of ICH exceeds the risk of ischemic stroke 8. Cerebral amyloid angiopathy (CAA) is a non-modifiable risk factor for ICH and is associated with a higher risk of recurrent ICH than arterial hypertension–associated ICH. Apolipoprotein E (ApoE) alleles are important genetic risk factors for the development of sporadic CAA 9. For example, the number of ε4 alleles relates to clinical severity of CAA-related lobar ICH 9. The ε2 is also associated with an increased risk of CAA-related lobar ICH 9.
In patients with lobar ICH, pathologically graded moderate or severe CAA was independently associated with CT findings of subarachnoid hemorrhage and “finger-like” projections and an ApoE genotype. These diagnostic criteria may be helpful in determining CAA-associated ICH 10.
Imaging predictors of hematoma enlargement
Hematoma enlargement is a known risk factor for poor outcome in patients with ICH 11, 12. Therefore, imaging predictors of hematoma expansion continue to be an important area of research. The computed tomography angiography (CTA) “spot sign” has been described as a predictor for hematoma expansion and poor functional outcome 13. One limitation of relying on CTA to predict hematoma expansion is the generalizability to medical centers where early CTA is not readily available. Importantly, the CTA “spot sign” is inversely related to ICH onset-to-CTA time 14. Additionally, in a large patient-level meta-analysis, the presence of a CTA spot sign did not significantly add to a prediction model of hematoma expansion based on established predictors 15.
Several recent studies have examined specific CT parameters to predict hematoma expansion. Li et al. described a CT finding called the “blend sign” to predict hematoma expansion 16. The “blend sign” was defined as blending of a hypo-attenuating area within the hyper-attenuated ICH with a well-defined margin. In their cohort, time to baseline CT scan, initial hematoma volume, and presence of “blend sign” on baseline CT were independent predictors of hematoma growth 16. The “blend sign” also has comparative positive predictive value to the spot sign for predicting neurologic deterioration, making it a useful CT marker when CTA is not available 17.
Analogous to the “blend sign”, the “black hole sign” is another CT sign to predict hematoma expansion. The “black hole sign” is defined as a hypo-attenuating area encapsulated within the hyper-attenuating ICH with a clearly defined border ( Figure 2). Similar to the “blend sign”, time to baseline CT scan, initial hematoma volume, and presence of a “black hole sign” on baseline CT were independent predictors of hematoma growth 12. Notably, both signs are reflections of heterogeneity within the ICH bed. The presence of these signs may represent bleeding at two distinct time periods 5. Additionally, Morotti et al. have shown that using a combination of the CTA spot sign and identification of any hypodensity within the hematoma on CT is superior to predicting hematoma expansion than either sign alone 18.
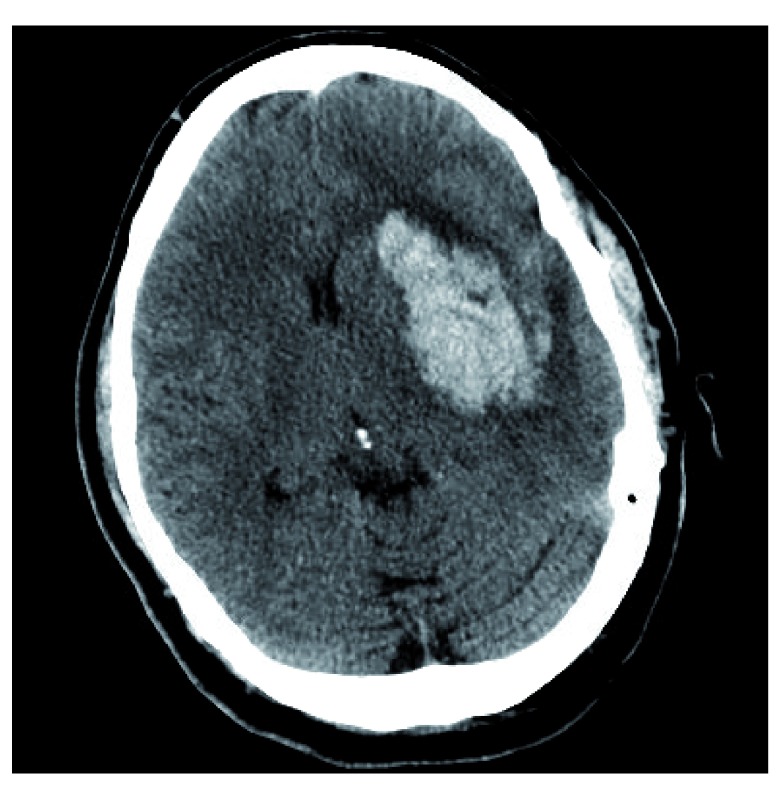
Figure 2. Left putaminal hemorrhage with positive “black hole sign”.
A well-delineated margin is appreciated between the hypo-attenuating, round black hole and the hyper-attenuating hemorrhage. This image constitutes original, unpublished data obtained by the authors.
Scores to predict hematoma expansion
Scoring schema that can accurately predict hematoma expansion are practical tools for future research. Interventions that do not show efficacy in an indiscriminate sample of patients with ICH may show efficacy in patients at high risk for hematoma expansion. Previous studies 19, 20 did not discriminate enrollment for high and low risk of ICH expansion; therefore, this may be an important source of residual confounding despite randomization. A practical baseline score may alleviate this source of confounding or allow post-hoc adjusted analysis. Three scoring systems that have been introduced to predict hematoma expansion are the “BRAIN” score 21 ( Table 1), the “BAT” score 18 ( Table 2), and a 9-point prediction score by Brouwers et al. 22 ( Table 3).
Table 1. “BRAIN” score.
| Components | Points |
|---|---|
| Baseline intracerebral
hemorrhage (ICH) volume |
Milliliters per score: ≤10 = 0;
10–20 = 5; >20 = 7 |
| Recurrent ICH | Yes = 4 |
| Anticoagulation with
warfarin at onset |
Yes = 6 |
| Intraventricular extension | No = 0; Yes = 2 |
| Number of hours to
baseline computed tomography |
≤1 = 5; 1–2 = 4; 2–3 = 3;
3–4 = 2; 4–5 = 1; >5 = 0 |
| Total score | 0–24 |
Table 2. “BAT” score.
| Components | Points |
|---|---|
| Blend sign | Yes = 1 |
| Any hypodensity | Yes = 2 |
| Time from onset to
non-contrast computed tomography (<2.5 hours, ≥2.5 hours) |
<2.5 hours = 2 |
| Total score | 0–5 |
Table 3. Nine-point prediction score for hematoma expansion by Brouwers et al. 22.
| Components | Points |
|---|---|
| Warfarin use | Yes = 2 |
| Time to initial computed
tomography (CT) |
≤6 hours = 2 |
| Baseline intracerebral
hemorrhage volume |
<30 mL = 0; 30–60 mL
= 1; >60 mL = 2 |
| CT angiography spot sign | Absent = 0; present = 3;
unavailable = 1 |
| Total score | 0–9 |
The “BRAIN” score is 24-point score based on baseline ICH volume, recurrent ICH, anticoagulation with warfarin at symptom onset, intraventricular extension, and number of hours to baseline CT from symptom onset. The maximum score, 24, retrospectively predicted hematoma expansion with an 85.8% probability in a pilot randomized controlled trial 21. The “BAT” score is composed of three components: presence of a blend sign, any hypodensity within the hyper-attenuating hematoma, and time from onset to non-contrast CT. A dichotomized score (of 3 or more than 3) predicted hematoma expansion with 50% sensitivity and 89% specificity 18. Brouwers et al. have proposed a 9-point score derived from a multivariate analysis based on variables from a large retrospective cohort 22. Components associated with hematoma expansion include warfarin use, time to initial CT, baseline ICH volume, and presence of the CTA spot sign. A dichotomized score showed strong association with hematoma expansion 22.
Hemostatic therapies
Given the poor outcome associated with hematoma expansion, a considerable amount of research has been dedicated to pharmacological hemostatic therapies. Importantly, pharmacological therapies that reduce hematoma growth do not always translate into improved functional outcome or survival. For example, recombinant activated factor VII (rFVIIa) is one such agent 23.
Recently, tranexamic acid (TXA) has been assessed as a hemostatic therapy in a phase 3 randomized controlled trial in patients with spontaneous ICH. The premise of this study was based on favorable data for the use of TXA in patients with traumatic intracranial hemorrhage. There was a smaller mean increase in hematoma volume in the TXA group compared with the placebo group. Similar to rFVIIa, there was no improvement in functional status or mortality at 90 days 24.
Compared with patients with spontaneous ICH, those on antithrombotic therapy have a greater likelihood of secondary hematoma expansion and an increased risk of death or poor functional outcome. Reversal agents play a major role in the management of these patients 25.
Platelet transfusion compared with placebo after primary ICH in patients on antiplatelet agents was assessed in the PATCH (“Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral hemorrhage associated with antiplatelet therapy”) phase 3 randomized controlled trial 26. The majority of patients enrolled in the study used COX inhibitors only. The odds of death or dependence at 3 months was higher in the platelet transfusion group compared with the standard care group. The authors hypothesized that collateral perfusion around the ICH may be impaired and that platelet transfusion may increase the risk of thrombosis and subsequent lesion expansion. Additionally, platelet transfusions have pro-inflammatory effects and may enhance vascular permeability associated with inflammation and platelet consumption 26. The results of this study may not be generalizable to patients taking P2Y12 inhibitors, as only 5 such patients were enrolled in the study.
Anticoagulant reversal is the mainstay of therapy for patients with OAC-ICH 25, 27, 28. In a large retrospective study composed of 16 registries, patients with vitamin K antagonist (VKA)-associated ICH (VKA-ICH) who did not undergo reversal had the highest crude case fatality rate compared with those who received reversal agents 28. VKAs deplete coagulation factors II, VII, IX, and X. Repletion of factors may be accomplished by using either fresh frozen plasma (FFP) or prothrombin complex concentrate (PCC). The INCH (“Fresh frozen plasma versus prothrombin complex concentrate in patients with intracranial hemorrhage related to vitamin K antagonists”) study compared the safety and efficacy of FFP versus PCC in patients with VKA-ICH 26. 9% of patients in the FFP group compared with 67% of patients in PCC arm achieved the primary endpoint of an INR of not more than 1.2 within 3 hours of treatment initiation. PCC was also superior for secondary imaging endpoints, including hematoma expansion. Thromboembolic events occurred in seven patients in the PCC arm compared with one patient in the FFP arm. Importantly, the study did not find any statistical difference between clinical endpoints, although these were considered secondary endpoints and this study was inadequately powered to detect differences in clinical endpoints. This study highlights the importance of future adequately powered studies to compare clinical outcomes of PCC and FFP.
Direct oral anticoagulants (DOACs) are being increasingly used in patients with non-valvular atrial fibrillation (NVAF) because of an improved efficacy-to-safety ratio and fewer food and drug interactions compared with VKAs. Furthermore, DOACs have a substantially lower risk of ICH compared with VKAs; apixaban is associated with the lowest risk of ICH among the DOACs 29. In a prospective observational study, DOAC-ICH was analogous to VKA-ICH in regard to baseline hematoma volume and intraventricular extension ( Figure 3) 30. Three antidotes have been studied for the reversal of DOAC-ICH: idarucizumab, andexanet alfa, and ciraparantag.
Figure 3. Rivaroxaban-related intracerebral hemorrhage.
Hyper-attenuating hemorrhage is visualized in the left posterior temporal-parietal region with intraventricular extension. This image constitutes original, unpublished data obtained by the authors.
A prospective cohort study evaluated the efficacy of idarucizumab, a monoclonal antibody fragment that binds dabigatran, in patients with dabigatran-related serious bleeding and those required to undergo an urgent procedure. This study enrolled 18 patients with ICH. This study showed a promising reversal effect. In the group of patients with serious bleeding, the dilute thrombin time normalized in 98% of patients 31. This study did not compare idarucizumab with alternative reversal agents and it remains unknown whether this reversal agent improves clinical outcomes. Interestingly, 34% of enrolled patients had a normal dilute thrombin time at the time of enrollment, highlighting the importance of well-designed randomized controlled trials to determine clinical effectiveness.
Andexanet alfa has also been assessed in patients with acute major bleeding 32. In total, 20 patients with ICH were enrolled in the efficacy population of the study. Following andexanet bolus, median anti-factor Xa activity decreased by 89% from baseline. However, median anti-factor Xa rose to 39% of baseline in the rivaroxaban group and 30% of baseline in the apixaban group 4 hours following an andexanet alfa infusion. Importantly, thrombotic events occurred in 18% of patients; stroke and deep venous thrombosis were the most common thrombotic events 32.
Ciraparantag is small synthetic water-soluble molecular entity that binds to heparin and oral direct factor Xa and factor II inhibitors by charge interaction and uncouples these drugs from their targets. In a phase 1 study of healthy subjects, a single intravenous dose of ciraparantag following 60 mg of edoxaban demonstrated full reversal of anticoagulation within 10 minutes; reversal was sustained for 24 hours 33.
Acute blood pressure lowering
Blood pressure lowering is another potential target to prevent hematoma growth. Acute elevation in blood pressure after ICH is common and is a predictor of outcome 34. Hematoma expansion may be the means by which elevation of blood pressure portends early mortality and poor clinical outcome 34. The “Intensive Blood-Pressure Lowering in Patients with Acute Cerebral Hemorrhage” (ATACH-2) trial was a randomized controlled trial comparing intensive blood pressure lowering (defined as a systolic blood pressure goal of 110 to 139 mm Hg) with standard treatment (defined as a systolic blood pressure goal of 140 to 179 mm Hg) within 4.5 hours after symptom onset using intravenous nicardipine 19. Intensive blood pressure lowering did not result in a lower rate of death or disability compared with the standard treatment arm. Importantly, there was a statistically significant higher rate of renal adverse events in patients who were randomly assigned to the intensive treatment group. The ATACH-2 trial was consistent with an earlier randomized controlled trial by Anderson et al. which also did not find a reduction in death or disability in patients who underwent intensive blood pressure lowering 20. Furthermore, neither study showed a significant reduction in hematoma expansion—the presumed mechanism by which acute blood pressure lowering exerts its therapeutic action.
Subgroups from the ATACH-2 trial with imaging predictors of hematoma expansion have also been assessed. In a preplanned analysis of the ATACH-2 trial, patients who had a spot sign did not benefit from intensive blood pressure reduction similar to the entire study population 35. An additional post-hoc subgroup analysis of the same study found no benefit of intensive blood pressure lowering in patients with several CT markers of hematoma expansion 36.
In a prospective multicenter study of 600 patients with ICH and baseline magnetic resonance imaging, the authors found that changes in mean arterial pressure by 10 mm Hg were associated with diffusion-weighted imaging (DWI) lesions 37. These results are consistent with a prior study by Garg et al., who found an association between DWI lesions and greater acute blood pressure reductions 38. The authors note that patients with a greater burden of small-vessel disease may be deficient of normal cerebral autoregulation at the time of ICH and that acute blood pressure lowering may precipitate small-vessel ischemia 37. Importantly, there was an independent association between DWI lesions and 90-day outcomes 37.
The data on the efficacy of combined blood pressure lowering and anticoagulant reversal for patients with OAC-ICH remain sparse. A retrospective study of 1176 patients found that the combination of target INR reversal of less than 1.3 and systolic blood pressure lowering to less than 160 mm Hg was associated with lower rates of hematoma enlargement and in-hospital mortality 11. Although their data are biologically plausible, conclusions are limited by the confines of retrospective analysis without prospective confirmation. In summary, the optimal management of acute hypertension in patients with spontaneous non-traumatic ICH remains a therapeutic dilemma and an important area of future research.
Intraventricular alteplase for intraventricular hemorrhage
In patients with spontaneous ICH, the presence of IVH portends a higher mortality compared with those without IVH. Disruption of IVH may relieve acute obstructive hydrocephalus and neurotoxicity associated with hemorrhage. The CLEAR III (“Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Hemorrhage”) trial was a randomized controlled trial that assessed whether pharmacological disruption of IVH via intraventricular alteplase improves outcomes 39. The vast majority of patients represented in the trial were patients with thalamic hemorrhages with secondary intraventricular rupture. Patients who received alteplase had smaller IVH volumes and a shorter duration to ventricular patency. Despite these radiographic improvements, there was no improvement in 90-day functional outcomes in the patients who received intraventricular alteplase.
Staykov et al. have studied adjuvant lumbar drainage in patients with IVH treated with intraventricular alteplase 40. In an open-label, parallel-group study, patients were randomly assigned to either adjuvant lumbar drainage or no lumbar drainage after IVH resolution through intraventricular alteplase. There was a statistically significant reduction in shunt dependency in the lumbar drainage arm 40. This pilot study supports further prospective assessment of this intervention in a larger randomized controlled trial using patient-centered clinical endpoints.
Surgical therapies for intracerebral hemorrhage
Two landmark randomized controlled trials did not show benefit of surgical intervention compared with medical management in patients with ICH. Patients with large ICH may develop significant delayed edema and this may cause secondary neurological injury due to mass effect. Decompressive craniectomy may mitigate the effects of delayed edema. In a retrospective study of 73 ICH patients who underwent decompressive craniectomy, 29% of patients had a favorable neurological outcome. This was noted to be superior to a historical cohort with lower ICH severity. Meaningful conclusions, however, are confined to retrospective analysis and support the need for further prospective data 41. The SWITCH (“Swiss Trial of Decompressive Craniectomy Versus Best Medical Treatment of Spontaneous Supratentorial Intracerebral Hemorrhage”) trial is an ongoing randomized controlled trial that will attempt to further define the role of decompressive hemicraniectomy in patients with supratentorial ICH.
There are ongoing studies of minimally invasive surgical techniques to alleviate surgical trauma that may offset the benefit of hematoma evacuation. The MISTIE (“Safety and efficacy of minimally invasive surgery plus alteplase in intracerebral hemorrhage evacuation”) study was a phase 2, open-label trial that evaluated catheter-based removal of ICH in patients with spontaneous, non-traumatic, supratentorial ICH 42. Catheters were placed under direct imaging guidance and clot aspiration was performed; alteplase then was administered through the catheters, allowed to dwell, and then re-opened for gravitational drainage. The minimally invasive surgery (MIS)-with-alteplase group had significantly smaller hemorrhage volumes compared with the medical management arms. Of note, for a composite safety endpoint of any cranial bleeding (symptomatic or asymptomatic) of more than 5 mL 72 hours after the last alteplase dose, there were significantly more events in the MIS-with-alteplase group. The landmark phase 3 study, recently published online, showed no benefit in the primary efficacy endpoint (mRS 0–3 at 365 days) 43.
In a meta-analysis of 14 studies, MIS was significantly associated with reduced death or significant functional impairment compared with both medical treatment and conventional craniotomy. This meta-analysis included multiple types of MIS and had relatively low heterogeneity 44.
Future directions and conclusions
ICH remains an important cause of morbidity and mortality. To date, no specific therapies directed toward ICH have been shown to improve functional outcome on the basis of randomized controlled trials. Despite this, patients who underwent maximal medical and surgical management have better outcomes than would be predicted on the basis of current prognostic scores 45, 46.
A lack of specific therapies may be reflective of either currently ineffective therapies or effective therapies that may only be beneficial in certain subgroups of patients with ICH. Therefore, further research into the identification of these subgroups may be an important step forward. For example, study groups composed of only patients with a high risk of hematoma expansion or exclusion of patients with a low risk of hematoma expansion may be beneficial. The use of clinical and radiographic parameters that have been established may allow for this.
Further emphasis on more translational research for acute ICH therapies may be warranted. For example, pioglitazone, deferoxamine, and stem cell replacement have been assessed in translational research settings and led to clinical safety studies 47. Perihematomal edema (PHE) is associated with short-term functional outcome and one such potential therapeutic target 48. Prior studies employing dexamethasone and therapeutic hypothermia to reduce PHE have revealed excess harm, and an ongoing study using deferoxamine, an iron chelator, has suspended enrollment because of an increased incidence of acute respiratory distress syndrome 49.
Complementary to research for acute ICH therapies, further research into risk factors for ICH may be necessary to lower the prevalence of this devastating stroke subtype. As prescriptions for COX-1 inhibitors, P2Y12 purinoreceptor antagonists, SSRIs, statins, VKAs, and DOACs are common, a more precise knowledge of the risk-to-benefit ratio of these medications and ICH is warranted for patient-tailored care. Additionally, optimal selection of patients for OAC for secondary stroke prevention will likely be further modified as further research is carried out on patients with high-risk features for ICH.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
David J Werring, Department of Brain Repair and Rehabilitation, UCL, Stroke Research Centre, London, UK
Daniel Hanley, Department of Neurology, Johns Hopkins University School of Medicine, Divisions of Neurosciences Critical Care and Brain Injury Outcomes, 600 N Wolfe Street, Phipps 455, Baltimore, MD, USA
Aaron Gusdon, Divisions of Neurosciences Critical Care and Brain Injury Outcomes, Department of Neurology, Johns Hopkins University School of Medicine, 600 N Wolfe Street, Phipps 455, Baltimore, MD, USA
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 2 approved]
References
- 1. An SJ, Kim TJ, Yoon BW: Epidemiology, Risk Factors, and Clinical Features of Intracerebral Hemorrhage: An Update. J Stroke. 2017;19(1):3–10. 10.5853/jos.2016.00864 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 2. Muntner P, Carey RM, Gidding S, et al. : Potential US Population Impact of the 2017 ACC/AHA High Blood Pressure Guideline. Circulation. 2018;137(2):109–18. 10.1161/CIRCULATIONAHA.117.032582 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 3. Khan NI, Siddiqui FM, Goldstein JN, et al. : Association Between Previous Use of Antiplatelet Therapy and Intracerebral Hemorrhage Outcomes. Stroke. 2017;48(7):1810–7. 10.1161/STROKEAHA.117.016290 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 4. Renoux C, Vahey S, Dell’Aniello S, et al. : Association of Selective Serotonin Reuptake Inhibitors With the Risk for Spontaneous Intracranial Hemorrhage. JAMA Neurol. 2017;74(2):173–180. 10.1001/jamaneurol.2016.4529 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 5. Endres M, Nolte CH, Scheitz JF: Statin Treatment in Patients With Intracerebral Hemorrhage. Stroke. 2018;49(1):240–6. 10.1161/STROKEAHA.117.019322 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 6. Ziff OJ, Banerjee G, Ambler G, et al. : Statins and the risk of intracerebral haemorrhage in patients with stroke: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2019;90(1):75–83. 10.1136/jnnp-2018-318483 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 7. Wilson D, Ambler G, Shakeshaft C, et al. : Cerebral microbleeds and intracranial haemorrhage risk in patients anticoagulated for atrial fibrillation after acute ischaemic stroke or transient ischaemic attack (CROMIS-2): a multicentre observational cohort study. Lancet Neurol. 2018;17(6):539–47. 10.1016/S1474-4422(18)30145-5 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 8. Holmes DR, Reddy VY, Turi ZG, et al. : Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374(9689):534–42. 10.1016/S0140-6736(09)61343-X [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 9. Charidimou A, Gang Q, Werring DJ: Sporadic cerebral amyloid angiopathy revisited: recent insights into pathophysiology and clinical spectrum. J Neurol Neurosurg Psychiatry. 2012;83(2):124–37. 10.1136/jnnp-2011-301308 [DOI] [PubMed] [Google Scholar]
- 10. Rodrigues MA, Samarasekera N, Lerpiniere C, et al. : The Edinburgh CT and genetic diagnostic criteria for lobar intracerebral haemorrhage associated with cerebral amyloid angiopathy: model development and diagnostic test accuracy study. Lancet Neurol. 2018;17(3):232–40. 10.1016/S1474-4422(18)30006-1 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 11. Kuramatsu JB, Gerner ST, Schellinger PD, et al. : Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. JAMA. 2015;313(8):824–36. 10.1001/jama.2015.0846 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Li Q, Zhang G, Xiong X, et al. : Black Hole Sign: Novel Imaging Marker That Predicts Hematoma Growth in Patients With Intracerebral Hemorrhage. Stroke. 2016;47(7):1777–81. 10.1161/STROKEAHA.116.013186 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 13. Demchuk AM, Dowlatshahi D, Rodriguez-Luna D, et al. : Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the CT-angiography spot sign (PREDICT): a prospective observational study. Lancet Neurol. 2012;11(4):307–14. 10.1016/S1474-4422(12)70038-8 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. Dowlatshahi D, Brouwers HB, Demchuk AM, et al. : Predicting Intracerebral Hemorrhage Growth With the Spot Sign: The Effect of Onset-to-Scan Time. Stroke. 2016;47(3):695–700. 10.1161/STROKEAHA.115.012012 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 15. Al-Shahi Salman R, Frantzias J, Lee RJ, et al. : Absolute risk and predictors of the growth of acute spontaneous intracerebral haemorrhage: a systematic review and meta-analysis of individual patient data. Lancet Neurol. 2018;17(10):885–94. 10.1016/S1474-4422(18)30253-9 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. Li Q, Zhang G, Huang YJ, et al. : Blend Sign on Computed Tomography: Novel and Reliable Predictor for Early Hematoma Growth in Patients With Intracerebral Hemorrhage. Stroke. 2015;46(8):2119–23. 10.1161/STROKEAHA.115.009185 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 17. Sporns PB, Schwake M, Schmidt R, et al. : Computed Tomographic Blend Sign Is Associated With Computed Tomographic Angiography Spot Sign and Predicts Secondary Neurological Deterioration After Intracerebral Hemorrhage. Stroke. 2017;48(1):131–5. 10.1161/STROKEAHA.116.014068 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Morotti A, Dowlatshahi D, Boulouis G, et al. : Predicting Intracerebral Hemorrhage Expansion With Noncontrast Computed Tomography: The BAT Score. Stroke. 2018;49(5):1163–9. 10.1161/STROKEAHA.117.020138 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 19. Qureshi AI, Palesch YY, Barsan WG, et al. : Intensive Blood-Pressure Lowering in Patients with Acute Cerebral Hemorrhage. N Engl J Med. 2016;375(11):1033–43. 10.1056/NEJMoa1603460 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Anderson CS, Heeley E, Huang Y, et al. : Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med. 2013;368(25):2355–65. 10.1056/NEJMoa1214609 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Wang X, Arima H, Al-Shahi Salman R, et al. : Clinical prediction algorithm (BRAIN) to determine risk of hematoma growth in acute intracerebral hemorrhage. Stroke. 2015;46(2):376–81. 10.1161/STROKEAHA.114.006910 [DOI] [PubMed] [Google Scholar]
- 22. Brouwers HB, Chang Y, Falcone GJ, et al. : Predicting hematoma expansion after primary intracerebral hemorrhage. JAMA Neurol. 2014;71(2):158–64. 10.1001/jamaneurol.2013.5433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mayer SA, Brun NC, Begtrup K, et al. : Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358(20):2127–37. 10.1056/NEJMoa0707534 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 24. Sprigg N, Flaherty K, Appleton JP, et al. : Tranexamic acid for hyperacute primary IntraCerebral Haemorrhage (TICH-2): an international randomised, placebo-controlled, phase 3 superiority trial. Lancet. 2018;391(10135):2107–15. 10.1016/S0140-6736(18)31033-X [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Frontera JA, Lewin JJ, 3rd, Rabinstein AA, et al. : Guideline for Reversal of Antithrombotics in Intracranial Hemorrhage: Executive Summary. A Statement for Healthcare Professionals From the Neurocritical Care Society and the Society of Critical Care Medicine. Crit Care Med. 2016;44(12):2251–7. 10.1097/CCM.0000000000002057 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Baharoglu MI, Cordonnier C, Al-Shahi Salman R, et al. : Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): a randomised, open-label, phase 3 trial. Lancet. 2016;387(10038):2605–13. 10.1016/S0140-6736(16)30392-0 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 27. Steiner T, Poli S, Griebe M, et al. : Fresh frozen plasma versus prothrombin complex concentrate in patients with intracranial haemorrhage related to vitamin K antagonists (INCH): a randomised trial. Lancet Neurol. 2016;15(6):566–73. 10.1016/S1474-4422(16)00110-1 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 28. Parry-Jones AR, Di Napoli M, Goldstein JN, et al. : Reversal strategies for vitamin K antagonists in acute intracerebral hemorrhage. Ann Neurol. 2015;78(1):54–62. 10.1002/ana.24416 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Granger CB, Alexander JH, McMurray JJ, et al. : Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–92. 10.1056/NEJMoa1107039 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Purrucker JC, Haas K, Rizos T, et al. : Early Clinical and Radiological Course, Management, and Outcome of Intracerebral Hemorrhage Related to New Oral Anticoagulants. JAMA Neurol. 2016;73(2):169–77. 10.1001/jamaneurol.2015.3682 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. Pollack CV, Jr, Reilly PA, Eikelboom J, et al. : Idarucizumab for Dabigatran Reversal. N Engl J Med. 2015;373(6):511–20. 10.1056/NEJMoa1502000 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Connolly SJ, Milling TJ, Jr, Eikelboom JW, et al. : Andexanet Alfa for Acute Major Bleeding Associated with Factor Xa Inhibitors. N Engl J Med. 2016;375(12):1131–41. 10.1056/NEJMoa1607887 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. Ansell JE, Bakhru SH, Laulicht BE, et al. : Single-dose ciraparantag safely and completely reverses anticoagulant effects of edoxaban. Thromb Haemost. 2017;117(2):238–45. 10.1160/TH16-03-0224 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Butcher KS, Jeerakathil T, Hill M, et al. : The Intracerebral Hemorrhage Acutely Decreasing Arterial Pressure Trial. Stroke. 2013;44(3):620–6. 10.1161/STROKEAHA.111.000188 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Morotti A, Brouwers HB, Romero JM, et al. : Intensive Blood Pressure Reduction and Spot Sign in Intracerebral Hemorrhage: A Secondary Analysis of a Randomized Clinical Trial. JAMA Neurol. 2017;74(8):950–60. 10.1001/jamaneurol.2017.1014 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Morotti A, Boulouis G, Romero JM, et al. : Blood pressure reduction and noncontrast CT markers of intracerebral hemorrhage expansion. Neurology. 2017;89(6):548–54. 10.1212/WNL.0000000000004210 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 37. Kidwell CS, Rosand J, Norato G, et al. : Ischemic lesions, blood pressure dysregulation, and poor outcomes in intracerebral hemorrhage. Neurology. 2017;88(8):782–8. 10.1212/WNL.0000000000003630 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Garg RK, Liebling SM, Maas MB, et al. : Blood pressure reduction, decreased diffusion on MRI, and outcomes after intracerebral hemorrhage. Stroke. 2012;43(1):67–71. 10.1161/STROKEAHA.111.629493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hanley DF, Lane K, McBee N, et al. : Thrombolytic removal of intraventricular haemorrhage in treatment of severe stroke: results of the randomised, multicentre, multiregion, placebo-controlled CLEAR III trial. Lancet. 2017;389(10069):603–11. 10.1016/S0140-6736(16)32410-2 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 40. Staykov D, Kuramatsu JB, Bardutzky J, et al. : Efficacy and safety of combined intraventricular fibrinolysis with lumbar drainage for prevention of permanent shunt dependency after intracerebral hemorrhage with severe ventricular involvement: A randomized trial and individual patient data meta-analysis. Ann Neurol. 2017;81(1):93–103. 10.1002/ana.24834 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 41. Esquenazi Y, Savitz SI, El Khoury R, et al. : Decompressive hemicraniectomy with or without clot evacuation for large spontaneous supratentorial intracerebral hemorrhages. Clin Neurol Neurosurg. 2015;128:117–22. 10.1016/j.clineuro.2014.11.015 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Hanley DF, Thompson RE, Muschelli J, et al. : Safety and efficacy of minimally invasive surgery plus alteplase in intracerebral haemorrhage evacuation (MISTIE): a randomised, controlled, open-label, phase 2 trial. Lancet Neurol. 2016;15(12):1228–37. 10.1016/S1474-4422(16)30234-4 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Hanley DF, Thompson RE, Rosenblum M, et al. : Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): a randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet. 2019;393(10175):1021–1032. 10.1016/S0140-6736(19)30195-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Scaggiante J, Zhang X, Mocco J, et al. : Minimally Invasive Surgery for Intracerebral Hemorrhage. Stroke. 2018;49(11):2612–20. 10.1161/STROKEAHA.118.020688 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Morgenstern LB, Zahuranec DB, Sánchez BN, et al. : Full medical support for intracerebral hemorrhage. Neurology. 2015;84(17):1739–44. 10.1212/WNL.0000000000001525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sembill JA, Gerner ST, Volbers B, et al. : Severity assessment in maximally treated ICH patients: The max-ICH score. Neurology. 2017;89(5):423–31. 10.1212/WNL.0000000000004174 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Zhou Y, Wang Y, Wang J, et al. : Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Prog Neurobiol. 2014;115:25–44. 10.1016/j.pneurobio.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 48. Volbers B, Giede-Jeppe A, Gerner ST, et al. : Peak perihemorrhagic edema correlates with functional outcome in intracerebral hemorrhage. Neurology. 2018;90(12):e1005–e1012. 10.1212/WNL.0000000000005167 [DOI] [PubMed] [Google Scholar]
- 49. Kim H, Edwards NJ, Choi HA, et al. : Treatment Strategies to Attenuate Perihematomal Edema in Patients With Intracerebral Hemorrhage. World Neurosurg. 2016;94:32–41. 10.1016/j.wneu.2016.06.093 [DOI] [PubMed] [Google Scholar]