Abstract
Six patients submitted to paroxysmal atrial fibrillation (AF) ablation presented with long post-reversion sinus pauses between a few hours to 2 months after their procedures, causing recurrent syncope or pre-syncope. Five patients required urgent pacemaker implantation. None of these patients had previous symptoms suggestive of sick sinus syndrome (SSS) or a history of symptomatic bradycardia. Acute or sub-acute sinus node dysfunction (SND) has only recently been suggested as a potential complication of AF ablation. In three of our patients, the sinus node artery (SNA) was exclusively left-sided, running along the high anterior left atrium in close proximity to the ostia of the left and right superior pulmonary veins. In a fourth case, the SNA originated from the right coronary artery and coursed along the high anterior left atrium close to the ostium of the right superior pulmonary vein. In the remaining two cases, a pre-procedural assessment of the SNA was not possible, although a post-procedural CT scan performed in one of these did not reveal any signs of the SNA. Overdrive suppression of the sinus node exacerbated by thermal injury to the SNA may have been implicated. This was supported by (i) the lack of symptoms/signs suggestive of SSS pre-ablation, (ii) post-ablation acute/sub-acute pronounced post-AF reversion sinus pauses, and (iii) the observation that the SNA coursed along areas typically ablated during an AF ablation. Although this case series is hypothesis-generating only, we hope it will raise the awareness for the occurrence of acute/sub-acute SND as a potential complication of AF ablation.
Keywords: Ablation, Atrial fibrillation, Case series, Pacemaker, Sinus node artery, Sick sinus syndrome
Learning points
Acute or sub-acute sinus node dysfunction (SND) is an unrecognized complication of atrial fibrillation (AF) ablation. This should be mentioned to the patient during the informed consent process.
The cause of acute or sub-acute SND after an AF ablation is still a matter of debate, but may include overdrive suppression exacerbated by thermal injury of the sinus node artery. Further prospective studies will be required to provide additional insight.
Introduction
Catheter ablation of atrial fibrillation (AF) is superior to antiarrhythmic drug therapy alone in reducing the burden of AF, prolonging the time in sinus rhythm and improving quality of life. Whether ablation per se prevents hard outcomes such as stroke and mortality remains a matter of debate, but recent data in the specific setting of heart failure is encouraging.1,2 Nevertheless, although acute complication rates have decreased significantly in recent years, AF ablation is still associated with a non-negligible risk of periprocedural complications.3 Vascular access complications constitute the majority, but AF ablation can also associate with cerebrovascular events, cardiac tamponade, phrenic nerve injury, pulmonary vein stenosis, or atrio-oesophageal fistula. Recently, some authors have proposed that ablation in the left atrium can also result in sinus node dysfunction (SND) requiring pacing.4–6 However, to the best of our knowledge, none of the larger studies assessing complication rates of AF ablation have made a reference to this potential complication thus far.3,7,8
Timeline
| Timing | Relevant data |
|---|---|
| Pre-ablation |
|
| Ablation |
|
| Post-ablation (between a few hours to 2 months) |
|
| Retrospective assessment of sinus node artery course |
|
Case series
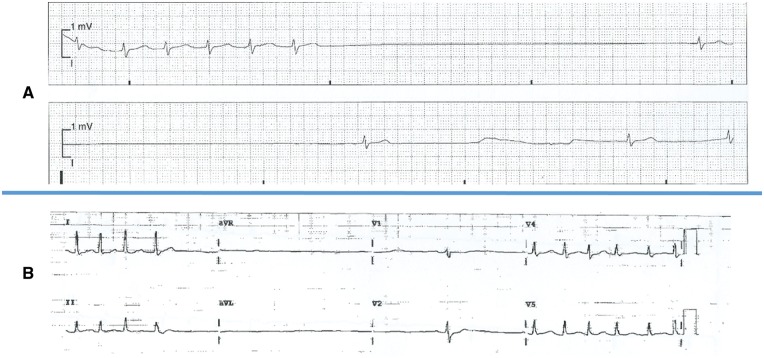
We assessed all patients submitted to ablation of paroxysmal AF in our high-volume department during a period of 40 months (n = 896) and who required or were considered for urgent unanticipated pacemaker implantation soon after the procedure. A previous history of persistent AF was an exclusion criteria, regardless of whether the patient was in sinus rhythm at the time of the ablation. Six patients fulfilled these criteria. All patients consented to the publication of data regarding their clinical history, AF ablation and computed tomographic (CT) scan. None of them had any signs or symptoms suggestive of SND or a history of symptomatic bradycardia prior to their ablation despite taking antiarrhythmic medication. Table 1 describes the six patients and their procedures. All had a normal baseline ECG and structurally normal hearts apart from one patient with an atrial septal defect with mild right ventricular systolic dysfunction. One patient was having a second procedure. Successful pulmonary vein isolation (PVI) was performed in all, using radiofrequency ablation in five cases and the cryoballoon in one. Table 2 describes the events following the ablation and the outcome. All of the patients presented with long sinus pauses (e.g. Figure 1A and B) following short runs of atrial arrhythmia between a few hours to 2 months after their procedures, causing recurrent syncope and/or pre-syncope. Five patients required urgent pacemaker implantation, while the 6th patient was considered for a pacemaker but his symptoms eventually settled once his paroxysmal tachyarrhythmia was controlled.
Table 1.
Baseline characteristics of the six patients included in this case series
| Patient | Age | Type of atrial fibrillation | Relevant comorbidities | Pre-procedural ECG | Pre-procedural echocardiogram | Medication prior to and after procedure | Procedure | Results of CT scan (retrospectively assessed) |
|---|---|---|---|---|---|---|---|---|
| 1 | 74 | Paroxysmal | ASD (ostium secundum) | SR, 55 b.p.m., borderline 1st degree AV block | Mild RV dysfunction, ASD, no other significant abnormalities |
|
PVI | Post-procedure. SNA not visualized. |
| 2 | 66 | Paroxysmal | Hypertension, typical atrial flutter | SR, 58 b.p.m., normal | No significant abnormalities |
|
PVI plus CTI line | Pre-procedure. Right-sided SNA arising from the RCA and coursing along the high anterior left atrium close to the ostium of the RSPV |
| 3 | 78 | Paroxysmal | Hypertension, previous pulmonary embolism | SR, 60 b.p.m., normal | No significant abnormalities |
|
PVI | Pre-procedure. Left-sided SNA arising from the Circumflex artery running along the high anterior left atrium in close proximity to the ostia of the left and right superior PVs |
| 4 | 80 | Paroxysmal | Hypertension | SR, 51 b.p.m., borderline 1st degree AV block | No significant abnormalities |
|
PVI | Pre-procedure. Left-sided SNA arising from the Left Main artery/proximal Circumflex artery and running along the high anterior left atrium in close proximity to the ostia of the left and right superior PVs |
| 5 | 68 | Paroxysmal | Hypertension, previous AF ablation | SR, 55 b.p.m., normal | No significant abnormalities |
|
Re-isolation of right-sided PVs and LIPV | Pre-procedure. Low quality examination as patient was in AF at ∼150 b.p.m. SNA not visualized. |
| 6 | 50 | Paroxysmal | None | SR, 50 b.p.m., normal | No significant abnormalities |
|
PVI with the cryoballoon | Pre-procedure. Left-sided SNA arising from the Circumflex artery running along the high anterior left atrium in close proximity to the ostia of the left and right superior PVs |
AF, atrial fibrillation; ASD, atrial septal defect; AV, atrioventricular; CTI, cavotricuspid isthmus; LIPV, left inferior pulmonary vein; PV, pulmonary vein; PVI, pulmonary vein isolation; RCA, right coronary artery; RSPV, right superior pulmonary vein; RV, right ventricular; SNA, sinus node artery; SR, sinus rhythm.
Table 2.
Outcome of the six patients included in this case series
| Patient | Age | Post-procedure symptoms | ECG findings | Treatment | Percentage of atrial pacing during follow-up | Symptoms during follow-up |
|---|---|---|---|---|---|---|
| 1 | 74 | Pre-syncope and syncope 24 h after AF ablation | Long sinus pauses following short runs of atrial arrhythmia | Dual-chamber PM implantation | ∼92–98% |
|
| 2 | 66 | Palpitations, dizziness and pre-syncope 15 days after AF ablation | Long sinus pauses following short runs of atrial arrhythmia | Dual-chamber PM implantation | ∼90–99% |
|
| 3 | 78 | Palpitations and dizziness a few hours after AF ablation, with complete initial resolution. Recurrent pre-syncope 1 month later. | Long sinus pauses following short runs of atrial arrhythmia | Dual-chamber PM implantation | <1% |
|
| 4 | 80 | Dizziness and pre- syncope a few hours after AF ablation | Long sinus pauses following short runs of atrial arrhythmia | Dual-chamber PM implantation | ∼47–58% |
|
| 5 | 68 | Palpitations and syncope 2 months after AF ablation | Long sinus pauses following short runs of atrial arrhythmia | Dual-chamber PM implantation | ∼18% |
|
| 6 | 50 | Palpitations and pre- syncope 1 week after AF ablation | Long sinus pauses following short runs of atrial arrhythmia | Dual-chamber PM implantation considered | – |
|
AF, atrial fibrillation; AV, atrioventricular; PM, pacemaker.
Figure 1.
(A, B) Electrocardiograms revealing significant post-reversion sinus pauses.
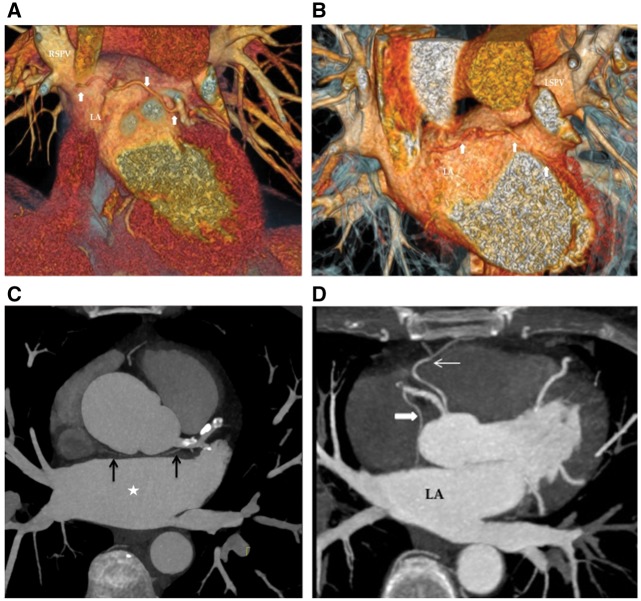
All patients except Patient 1 had had a CT scan prior to the AF ablation for an assessment of their left atrial and pulmonary vein morphology. In three cases, the sinus node artery (SNA) was exclusively left-sided (Figure 2A–C), running along the high anterior left atrium in close proximity to the ostia of the left and right superior pulmonary veins. In a fourth case, the SNA originated from the right coronary artery and coursed along the high anterior left atrium close to the ostium of the right superior pulmonary vein (Figure 2D). All of these areas would have been ablated during a typical PVI procedure. Patient 5 was in AF with fast ventricular rate (∼150 b.p.m.) at the time of the CT scan, which precluded any quality assessment of the course of the SNA, while Patient 1 had a CT scan after her AF ablation (for suspected pulmonary embolism) which did not show any signs of the SNA despite good image quality.
Figure 2.
(A) Electrocardiogram gated cardiac computed tomography, volume rendered view. Thick white arrows: Sinus node artery arising from the circumflex artery, coursing close to the base of the left atrial appendage, anterior roof of the left atrium and close to the ostium of the right superior pulmonary vein. (B) Electrocardiogram gated cardiac computed tomography, volume rendered view. Thick white arrows: sinus node artery arising from the circumflex artery, coursing close to the base of the left atrial appendage and anterior roof of the left atrium. Note the close relation of the vessel to the ostium of the left superior pulmonary vein. (C) Axial view of an electrocardiogram gated cardiac computed tomography, maximum intensity projection. Left sided origin of sinus nodal artery (black arrows). The vessel courses along the roof of left atrium (white star). (D) Axial view of an electrocardiogram gated cardiac computed tomography, maximum intensity projection. Thick white arrow: sinus node artery arising from the right coronary artery. Thin white arrow: Conus branch. LA, left atrium.
Discussion
Prolonged sinus pauses after paroxysms of AF may result from tachycardia-mediated remodelling of the sinus node and can potentially be treated with an AF ablation and cessation of antiarrhythmic drugs.9 Regional atrial remodelling near the sinus node area has also been linked with SND.10 Whereas persistent SND requiring pacemaker implantation has been reported in 7% of patients following ablation of long-standing persistent AF,11 significant bradyarrhythmia following electrical cardioversion of acute AF has been seen to occur in only 1% of patients.12 Recently, Hai et al.4 have made the first report of acute SND following left atrial ablation. Although tachycardia-mediated suppression and remodelling of the sinus node may have contributed to their patient’s persistent sinus arrest, the most likely mechanism was direct thermal injury to the SNA.4 The authors highlighted the fact that the SNA is exclusively left-sided in up to 30–40% of cases, with more than half of these running along the high anterior left atrium and the remainder either passing behind the left atrial appendage before overlying the roof or rarely running along the posterior left atrium to reach the right atrium.13,14
Acute/sub-acute persistent SND has only very recently been acknowledged as a potential complication of AF ablation,5 despite previous isolated case reports.4,6 Thermal injury to the SNA with subsequent ischaemia of the sinus node seems to have been at least partly implicated in all of our cases. This hypothesis was supported by (i) the complete lack of symptoms suggestive of sick sinus syndrome prior to the ablation, in particular syncope or pre-syncope, (ii) the lack of iatrogenic symptomatic bradycardia prior to the ablation, (iii) the acute or sub-acute occurrence of pronounced sinus pauses following the ablation, all after short runs of atrial arrhythmia, without any other plausible explanation, and (iv) the observation that the SNA coursed along areas typically ablated during a PVI procedure in those cases where an assessment of the SNA origin and course was possible. Our case series supports the findings of Hai et al.4 in their first report of acute SND. In their patient, the SNA originated from the left circumflex artery, running along the anterior left atrial wall, as we have documented in three of our patients. Left-sided sinus node arteries are probably more likely to be injured in the context of radiofrequency AF ablation due to their longer course along the base of the appendage or ridge, anterior or posterior roof and close to the ostia of both superior pulmonary veins. Such injury is then more likely to result in SND if the vessel is the sole supply to the sinus node. However, injury to the SNA can still occur when this vessel originates from the right coronary artery, in which case the artery could be damaged when ablating close to the ostium of the right superior pulmonary vein, as shown in one of our patients and a previous report by Leftheriotis et al.6 Furthermore, one of our patients had an AF ablation with the cryoballoon, suggesting this complication is not confined to radiofrequency ablation. The fact that all of our patients presented with post-reversion pauses rather than transient sinus bradycardia or sinoatrial arrest not preceded by a tachyarrhythmia may be possibly suggestive of ischaemia of the sinus node triggered by a tachyarrhythmia in the context of a partly damaged/occluded SNA. We cannot exclude that ablation of ganglionated plexi in the left atrium may have also played a role by transiently modifying autonomic tone, yet it is unlikely that this would have manifested as long post-reversion pauses up to 2 months post-ablation. Overdrive suppression is often the mechanism underlying post-reversion pauses in AF patients, and it is possible this may have been exacerbated in the context of ischaemia of the sinus node.
The reader should take into account some limitations of this case series when interpreting its results. Firstly, the lack of symptoms prior to the ablation cannot exclude the occurrence of significant bradycardia or pauses. Indeed, SND is often coexisting with AF. Still, the severe presentation with recurrent syncope or pre-syncope in patients with no such symptoms prior to their ablation suggests a cause–effect mechanism. Secondly, we recognize the possibility that some (rare) cases with a presentation beyond the first 24 hours post-procedure and follow-up at a different hospital may have been missed. Thirdly, patients with de novo or exacerbated SND after their ablation but with a milder presentation would not have received urgent pacemaker implantation and would have been preferentially treated with repeat ablation. As such, the reader should be aware that post-AF ablation SND may be more frequent than our numbers (6 patients out of 896) may suggest. Fourthly, we did not routinely request any CT scan after the ablation to confirm the disappearance of the SNA, as the additional radiation exposure would not be easily justifiable unless it had an impact on the patients’ management (Patient 1 had a post-procedure CT scan requested for a different reason). Therefore, this single-centre case series is hypothesis-generating only, and further studies will be required to clarify the mechanism underlying the SND post-AF ablation. Nevertheless, we hope this article will raise the awareness for the occurrence of acute/sub-acute SND as a potential complication of AF ablation.
In summary, acute or sub-acute SND is an unrecognized complication of paroxysmal AF ablation. Its underlying causes are a matter of debate, potentially including, but not limited to, overdrive suppression exacerbated by thermal injury of the SNA, and further prospective studies will be required to provide additional insight.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
References
- 1. Di Biase L, Mohanty P, Mohanty S, Santangeli P, Trivedi C, Lakkireddy D, Reddy M, Jais P, Themistoclakis S, Dello Russo A, Casella M, Pelargonio G, Narducci ML, Schweikert R, Neuzil P, Sanchez J, Horton R, Beheiry S, Hongo R, Hao S, Rossillo A, Forleo G, Tondo C, Burkhardt JD, Haissaguerre M, Natale A.. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation 2016;133:1637–1644. [DOI] [PubMed] [Google Scholar]
- 2. Nassir M. Catheter ablation versus standard conventional treatment in patients with left ventricular dysfunction and atrial fibrillation: the CASTLE-AF trial. Study presented at European Society of Cardiology Congress 2017. Barcelona.
- 3. Gupta A, Perera T, Ganesan A, Sullivan T, Lau DH, Roberts-Thomson KC, Brooks AG, Sanders P.. Complications of catheter ablation of atrial fibrillation: a systematic review. Circ Arrhythm Electrophysiol 2013;6:1082–1088. [DOI] [PubMed] [Google Scholar]
- 4. Hai JJ, Mulpuru SK, Williamson EE, Foley TA, Brady PA.. Sinus nodal dysfunction after left atrial flutter ablation. A preventable complication. Circ Arrhythm Electrophysiol 2014;7:360–361. [DOI] [PubMed] [Google Scholar]
- 5. Killu AM, Fender EA, Deshmukh AJ, Munger TM, Araoz P, Brady PA, Cha Y-M, Packer DL, Friedman PA, Asirvatham SJ, Noseworthy PA, Mulpuru SK.. Acute sinus node dysfunction after atrial ablation: incidence, risk factors, and management. Pacing Clin Electrophysiol 2016;39:1116–1125. [DOI] [PubMed] [Google Scholar]
- 6. Leftheriotis D, Flevari P, Mademli M, Anastasiou-Nana M.. Permanent sinus dysfunction after pulmonary vein isolation ablation: observation and potential mechanisms. Can J Cardiol 2014;30:1249.e13-5. [DOI] [PubMed] [Google Scholar]
- 7. Baman TS, Jongnarangsin K, Chugh A, Suwanagool A, Guiot A, Madenci A, Walsh S, Ilg KJ, Gupta SK, Latchamsetty R, Bagwe S, Myles JD, Crawford T, Good E, Bogun F, Pelosi F Jr, Morady F, Oral H.. Prevalence and predictors of complications of radiofrequency catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol 2011;22:626–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Natale A, Packer D, Skanes A, Ambrogi F, Biganzoli E.. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol 2010;3:32–38. [DOI] [PubMed] [Google Scholar]
- 9. Hocini M, Sanders P, Deisenhofer I, Jaïs P, Hsu LF, Scavée C, Weerasoriya R, Raybaud F, Macle L, Shah DC, Garrigue S, Le Metayer P, Clémenty J, Haïssaguerre M.. Reverse remodeling of sinus node function after catheter ablation of atrial fibrillation in patients with prolonged sinus pauses. Circulation 2003;108:1172–1175. [DOI] [PubMed] [Google Scholar]
- 10. Sairaku A, Nakano Y, Oda N, Makita Y, Kajihara K, Tokuyama T, Motoda C, Fujiwara M, Kihara Y.. Prediction of sinus node dysfunction in patients with persistent atrial flutter using the flutter cycle length. Europace 2012;14:380–387. [DOI] [PubMed] [Google Scholar]
- 11. Chang HY, Lin YJ, Lo LW, Chang SL, Hu YF, Li CH, Chao TF, Yin WH, Chen SA.. Sinus node dysfunction in atrial fibrillation patients: the evidence of regional atrial substrate remodeling. Europace 2013;15:205–211. [DOI] [PubMed] [Google Scholar]
- 12. Grönberg T, Nuotio I, Nikkinen M, Ylitalo A, Vasankari T, Hartikainen JE, Airaksinen KE.. Arrhythmic complications after electrical cardioversion of acute atrial fibrillation: the FinCV study. Europace 2013;15:1432–1435. [DOI] [PubMed] [Google Scholar]
- 13. Yokokawa M, Sundaram B, Oral H, Morady F, Chugh A.. The course of the sinus node artery and its impact on achieving linear block at the left atrial roof in patients with persistent atrial fibrillation. Heart Rhythm 2012;9:1395–1402. [DOI] [PubMed] [Google Scholar]
- 14. Ozturk E, Saglam M, Bozlar U, Kemal Sivrioglu A, Karaman B, Onat L, Cinar Basekim C.. Arterial supply of the sinoatrial node: a CT coronary angiographic study. Int J Cardiovasc Imaging 2011;27:619–627. [DOI] [PubMed] [Google Scholar]