Abstract
Introduction
Transcatheter aortic valve implantation (TAVI) is a well-accepted alternative treatment for intermediate or high-risk patients with symptomatic severe native aortic valve stenosis. As the use of TAVI increases, there is a continuous growing insight into in the technical possibilities of the procedure and a parallel decrease in complications. A serious but rare complication of TAVI is a ventricular septal defect (VSD).
Case presentation
We report a case of a 90-year-old woman who underwent an uncomplicated TAVI procedure. She was readmitted within 2 weeks because of dyspnoea and oedema in the legs caused by acute left- and right-sided heart failure. Echocardiography showed a VSD of 1 cm, and mild to moderate paravalvular aortic regurgitation (PAR).
Discussion
This is the first report in which post-TAVI both a VSD and PAR are successfully repaired via a single percutaneous procedure.
Keywords: Case report, TAVI, Ventricular septal defect, Paravalvular aortic regurgitation, Percutaneous closure
Learning points
Successfully percutaneous closure of a ventricular septal defect and paravalvular aortic regurgitation after transcatheter aortic valve implantation in a single procedure.
Haemolysis due to the high-velocity blood flow through the devices can be self-limiting due to endothelialization.
Introduction
Transcatheter aortic valve implantation (TAVI) is a well-accepted alternative treatment for patients with symptomatic severe native aortic valve stenosis, who are at intermediate or high risk for surgical aortic valve replacement (SAVR).1 As the use of TAVI increases, there is a continuous growing insight into the technical possibilities of the procedure and a parallel decrease in complications. A rare but severe complication of TAVI is a procedure-related ventricular septal defect (VSD). This is due to perforation of the interventricular septum, because of a shift of calcified subvalvular tissue after implantation of the rigid stent frame in the aortic annulus. Due to the low occurrence of this complication, no standardized management or treatment recommendation exists. Paravalvular aortic regurgitation (PAR) is decreasing in incidence in recent years because of improved valve designs and implanting techniques. Occurrence of only a trace or mild PAR has no clinical consequences, so no treatment is needed. However, moderate or severe PAR is associated with increased mortality risk, and should therefore be avoided or treated if possible.2
Here, we describe the percutaneous treatment of a patient with a combined VSD and significant PAR after TAVI.
Timeline
| Day | Events |
|---|---|
| 1 | Transcatheter aortic valve implantation (TAVI) procedure, complicated by complete atrioventricular block. |
| 4 | Pacemaker implantation due to complete atrioventricular block. |
| 8 | Discharged. |
| 13 | Readmission: dyspnoea and oedema caused by acute left- and right-sided heart failure. |
| Transthoracic echocardiogram (TTE) and transoesophageal echocardiogram (TOE) showed mild to moderate paravalvular aortic regurgitation (PAR), and a ventricular septal defect (VSD). | |
| 15 | Percutaneous closure: atrial septal occluder (ASO) was placed to close VSD and Amplatzer vascular plug II was placed for closure of PAR in a single procedure. |
| 19 | TTE showed no PAR, and mild flow over VSD. |
| 20 | Discharged. |
| 27 | Readmission with fatigue and Hb of 3.5 mmol/L—most likely by haemolysis due to high-velocity blood flow through the devices. Red cell transfusions and iron suppletion were given. Patient could be discharged after Hb was stabilized. |
| TOE: mild PAR and mild flow over ASO. | |
| 80 | Still red cell transfusion depended—stop use of phenprocoumon, to reduce contributing factors for anaemia. |
| 6 months and 1 year | TTE: stable mild PAR and mild flow over ASO. No need for red cell transfusion. Doing clinically well. |
Case presentation
Initial procedure
A 90-year-old woman with a medical history of atrial fibrillation, transient ischaemic attack, hypertension, type 2 diabetes, and chronic renal dysfunction after nephrectomy was referred to our clinic with Class II heart failure symptoms due to severe aortic stenosis. She used a vitamin K antagonist for atrial fibrillation (CHA2DS2-VASc Score 7). On physical examination, a crescendo–decrescendo grade 3/6 systolic murmur was heard parasternal in the second right intercostal space with radiation to the carotid arteries. Coronary angiography showed no significant stenosis, and her transthoracic echocardiogram (TTE) showed good left ventricular function with severe aortic stenosis (peak gradient 92 mmHg, mean gradient 60 mmHg, valve area of 0.42 cm2). Multislice cardiac computed tomography (MSCT) showed an aortic valve annulus of 22.9 × 26.5 mm, area of 480 mm2, and perimeter of 77 mm. Therefore, a 29 mm of Medtronic CoreValve prosthesis was chosen. Based on her age and an increased operative risk demonstrated by a logistic EuroSCORE-II of 3.74%, our multidisciplinary valve team decided to treat the patient with TAVI.
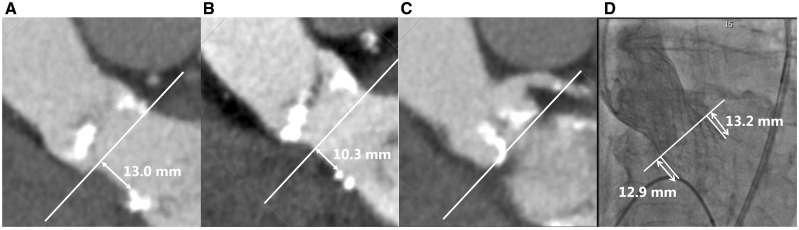
We performed the TAVI with the self-expandable CoreValve according to standard practice, with pre-dilation with a balloon of 25 mm. The final angiography showed only mild regurgitation and an implantation depth of 12.9 mm on non-coronary cusp and 13.2 mm on coronary cusp side. No post-dilation was performed. The procedure was complicated by a peri-procedural complete atrioventricular (AV) block, for which a permanent DDDR pacemaker was implanted after 3 days. One week post-TAVI, the patient was discharged in a clinically good condition. TTE at discharged showed a trace of PAR.
Readmission
However, 12 days post-TAVI, the patient presented to our emergency department with dyspnoea and oedema in the legs caused by acute left- and right-sided heart failure. On examination, a harsh holosystolic grade 3/6 murmur was heard along the sternal border and on the apex.
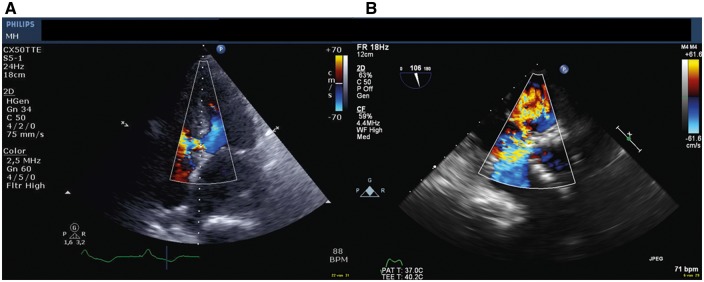
Transoesophageal echocardiogram (TOE) and TTE showed a mild to moderate PAR near the non-coronary cusp, and a VSD of 1 cm, just under the pericardial skirt of the CoreValve stent, with a flow from the left ventricular outflow tract (LVOT) to the right ventricular outflow tract [Vmax 4 m/s, diastolic vena contracta (VC) width 5 mm], and systolic pulmonary artery pressure of 44 mmHg. The rupture did not extend to the aortic root or to the right atrium (Figure 1). Retrospectively, on echocardiography 4 days after TAVI a small left to right shunt was already visible.
Figure 1.
(A) Transthoracic echocardiogram: Apical view of the severe left to right shunt through the ventricular septal defect. (B) Transoesophageal echocardiogram aortic short-axis view of the ventricular septal defect at the lower edge of the CoreValve stent.
During admission, the patient was haemodynamically and respiratory stable. However, kidney function and diuresis were deteriorating by left-sided heart failure. Since conservative treatment would mean a low chance of survival, decision was made to close the VSD and the PAR with a transcatheter closure device.
Closure procedure
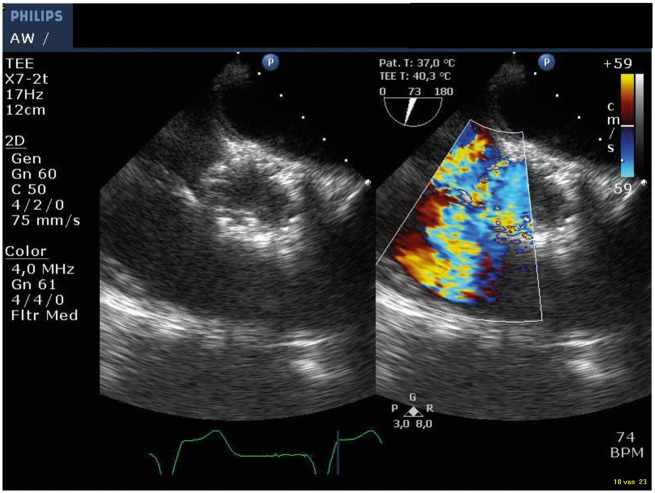
Ventricular septal defect and PAR closure was performed under general anaesthesia through the right femoral artery. At the start of the procedure, TOE showed an increased VSD flow (Vmax 2.9 m/s, diastolic VC width 5 mm) and increased moderate PAR, compared to the previous TOE 2 days earlier (Figure 2). Angiography showed a significant flow from the right coronary sinus to the right ventricle (Figure 3A).
Figure 2.
Transoesophageal echocardiogram aortic short axis with colour compare, showing the increased left to right shunt through the ventricular septal defect.
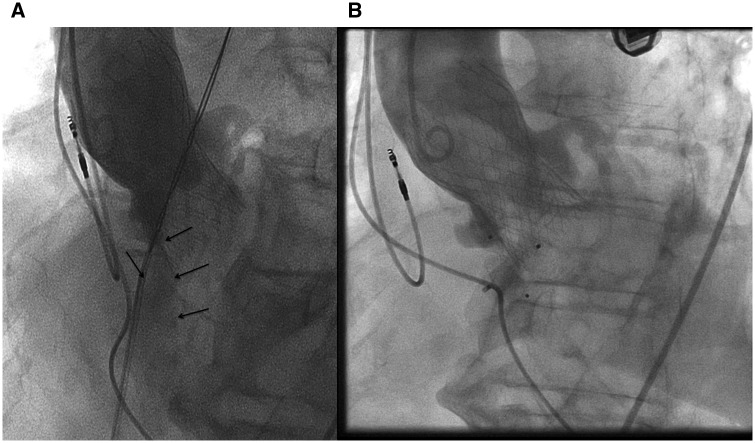
Figure 3.
Angiography. (A) Before closing ventricular septal defect and PAR: flow of contrast (arrows) from the right coronary sinus to the right ventricle. (B) After placement of atrial septal occluder and vascular plug, showing only mild flow through the devices.
At first, a 5 French catheter of Cordis JR was used to pass the VSD from the aortic side with a 35 softwire. The wire was advanced to the left pulmonary artery where it was snared. A loop was created by pulling back the softwire to the femoral vein. At second, the VSD was sized at 9 mm with a 20 mm PTS® sizing balloon. Subsequently, a 10-mm Amplatzer® atrial septal occluder (ASO) was positioned through the VSD by the use of an Amplatzer® patent ductus arteriosus (PDA) ductus delivery catheter of 6 French. The disc was on the left ventricle side located half inside the LVOT-part of the CoreValve and half on the muscular intraventricular septum and on the right ventricular side just above the tricuspid valve.
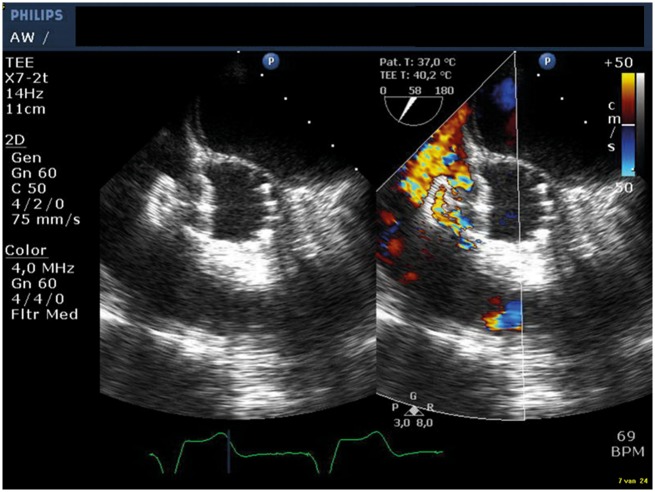
After ASO implantation, angiography showed a further increase of regurgitation through the paravalvular opening at the right coronary sinus and through the struts of the ASO to the right ventricle. To address the regurgitation, an Amplatzer vascular plug II was placed: a 6 French long-sheath braided of Cook was used to approach the valve from the aortic side. The CoreValve stent was passed through the stent struts just above the valve. Next the paravalvular opening was passed with a PT2 0.014″ 300 cm wire over which a Cordis Adroit MP1 of 5 French followed by a Cook CXI 4 French support catheter of 125 cm were advanced into the right ventricle using the telescope technique. An Amplatzer vascular plug II of 8 mm was placed in the paravalvular regurgitation trajectory from the VSD to the right coronary sinus. The final angiography showed no obstruction of the right coronary artery and only trace regurgitation through the vascular plug and septal occluder (Figure 3B). TOE showed a good position of the ASO and a marked reduction of both systolic and diastolic flow through the VSD, with a systolic VC width of 3 mm (Figure 4), without a sign of LVOT obstruction.
Figure 4.
Transoesophageal echocardiogram showing the atrial septal occluder in the ventricular septal defect. It shows a marked reduction of the flow through the ventricular septal defect after placement of the atrial septal occluder.
After the procedure, the patient was immediately asymptomatic and kidney function and diuresis improved within a few days. Four days after the procedure, TTE showed no PAR and a further decrease of the VSD flow (Vmax 3.7–4.4 m/s) and lower systolic pulmonary artery pressures (28 mmHg) (Figure 5). The following morning, the patient could be discharged without any symptoms.
Figure 5.
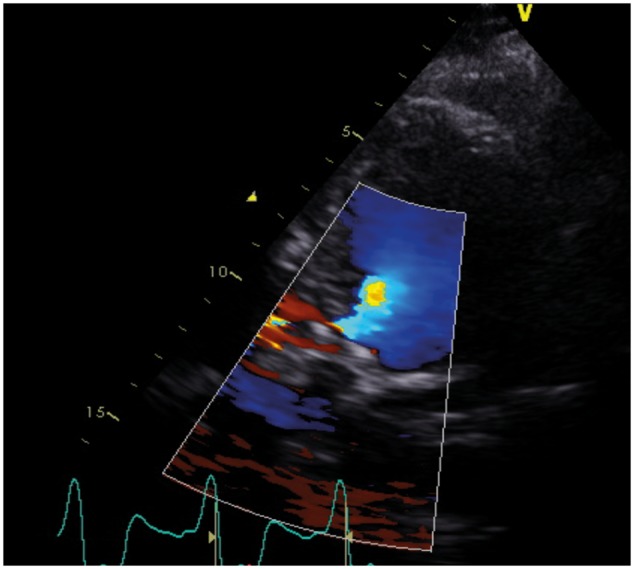
Transthoracic echocardiogram showing mild flow over the ventricular septal defect, 4 days after placement of the atrial septal occluder.
Follow-up
Seven days after discharge, patient presented to our emergency department with severe fatigue and possible melena. She appeared to have a severe anaemia with an Hb level of 3.5 mmol/L (reference value 7.4–9.6 mmol/L), LD 2563 U/L (reference value 0–250 U/L), and haptoglobin <0.05 g/L (reference value 0.3–2 g/L). Prothrombin time-international normalized ratio (PT-INR) was 4.42 (reference value 0.9–1.15). TOE showed mild PAR through the Amplatzer device and a mild left to right shunt, both through and past the ASO (Vmax > 4 m/s), and normal left ventricle function. Working diagnosis for the cause of anaemia was haemolysis due to the high-velocity blood flow through the devices. Gastrointestinal causes were excluded, since gastrointestinal endoscopy showed no abnormalities. After six units red cell transfusion, patient was discharged with oral iron suppletion. The following weeks, she received red cell transfusions in outpatient setting twice (first time 3 units, second time 8 units). Furthermore, darbepoetin alfa was started and the phenprocoumon was stopped completely. Cessation of anticoagulation does not improve endothelialization, and therefore not directly influences haemolytic anaemia in a positive way. However, we still decided to interrupt the anticoagulation use in order to minimize other possible contributing factors to a worsening of the anaemia. One year after the complicated TAVI procedure, patient is doing well, living at home without the need of red cell transfusions, and without any antiplatelet/anticoagulant treatment or embolization problems. The mild PAR and mild left to right shunt across the VSD has remained stable, without any haemodynamic or clinical consequences or haemolysis.
Discussion
Ventricular septal defect is a known but rare complication of TAVI. To our best knowledge, only 20 patients with acquired VSD post-TAVI have been reported in literature. Only six of these patients were treated with a percutaneous intervention for closure.3 Ando et al.3 describes in his systematic review the reports of those 20 patients with VSD post-TAVI. The main findings of this review are that in 85% of the cases a balloon expandable valve was used, and that the proposed potential risks for VSD post-TAVI are; severe, asymmetric calcification of the native valve; elliptic aortic annulus; oversizing of the valve; or higher placement of the valve, since a common factor in many of the cases is a direct trauma to the septum from the implanted valve, compounded by further annulus dilation. Noteworthy, none of these potential risk factors were present in our case. On the pre-procedural MSCT-scan, a small calcification is seen on the septum side of the LVOT about 10 and 13 mm of the annulus (Figure 6). Although the calcification is positioned quite a distance from the annulus, the implantation depth of the valve on the non-coronary cusp side was 12.9 mm (Figure 6). Therefore, we cannot exclude that this calcification might have contributed to the occurrence of the VSD during balloon or stent expansion. To find out the exact mechanism of injury of the ventricular septum, a post-operative MSCT-scan could have been of additive value. However, this scan is not routinely performed and therefore not available.
Figure 6.
(A–C) Pre-procedural multislice cardiac computed tomography from different angles, showing small calcification on the septum side of the left ventricular outflow tract about 10 and 13 mm of the annulus. (D) Valve implantation depth on peri-procedural angiography.
According to the ACC guidelines,4 closure of VSD via percutaneous or surgical procedures in these high-risk patients should only be performed in case conservative treatment has failed. As reported by Ando et al.,3 of the known patients with a VSD following TAVI, only one-third of the patients underwent closure of the VSD, mostly by a percutaneous intervention. Also in our case, a percutaneous intervention was performed, because the patient was symptomatic with deterioration of kidney function and decreasing diuresis due to left-sided heart failure, as well as the size of her shunt (1 cm) being too large for conservative treatment. The percutaneous technique is an attractive approach compared to a surgical closure technique, since TAVI patients are high-surgical risk patients. However, a known concern of this percutaneous approach is that the effect of the VSD closure device on the valve function is unpredictable, since the VSD is often located proximal to the implanted valve. Furthermore, the implanted valve might dislodge during implantation of the VSD closure device. Despite these possible complications, a low mortality rate after VSD closure and no technical difficulties have been reported for VSD closure post-TAVI.3
Traumatic intravascular haemolysis is a known complication in patients with prosthetic device implantations. This haemolysis is mainly caused by mechanical trauma to red blood cells from turbulent blood flow due periprosthetic leakage.5,6 This turbulence creates high shear stress forces on red blood cells resulting in their destruction.7 This shear stress is most severe directly post-implantation, and decreases gradually when endothelialization progresses. In animal models of valve or non-valvular device implantation, the process of endothelialization starts about 1 month after implantation, and is completed within 3–6 months after implantation.8 Therefore, haemolysis caused by mild periprosthetic leakage can be self-limiting due to endothelialization. In general, downsizing antiplatelet/anticoagulant therapy is advisable in similar cases; a patient-tailored regiment is advised, depending on the severity of the anaemia and the pro-thrombogenic risk of each individual patient.
In contrast to VSD, PAR post-TAVI is a more common complication, although decreasing in recent years. A trace or mild PAR has no clinical consequences. However, our patient had at least moderate PAR. We had two reasons to treat this paravalvular regurgitation. First, it has been shown that moderate PAR is associated with increased risk of mortality, and should therefore be avoided or treated if possible.2 Second, both the VSD and the PAR may cause haemolytic anaemia, which also occured in our patient. Therefore, pragmatically we have treated both. If significant PAR is present peri-procedural, post-dilation of the prosthetic valve is often performed. If it occurs post-procedural and there are no contra-indications for interventional treatment, the procedure is often performed by closure of the defect with vascular plugs.9
Conclusion
This is the first report in which post-TAVI both a VSD and PAR are successfully repaired via a single percutaneous procedure. Furthermore, this case shows that haemolysis due to high-velocity blood flow through the devices can be self-limiting due to endothelialization.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: Dr Stella serves as Physician Proctor for Edwards Lifesciences. Dr Krings is a Member of the Advisory Board of Siemens and serves as Consultant for Edwards Lifesciences.
References
- 1. Holmes DR, Mack MJ, Kaul S, Agnihotri A, Alexander KP, Bailey SR, Calhoon JH, Carabello BA, Desai MY, Edwards FH, Francis GS, Gardner TJ, Kappetein AP, Linderbaum JA, Mukherjee C, Mukherjee D, Otto CM, Ruiz CE, Sacco RL, Smith D, Thomas JD.. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol 2012;59:1200–1254. [DOI] [PubMed] [Google Scholar]
- 2. Généreux P, Head SJ, Hahn R, Daneault B, Kodali S, Williams MR, van Mieghem NM, Alu MC, Serruys PW, Kappetein AP, Leon MB.. Paravalvular leak after transcatheter aortic valve replacement: the new achilles’ heel? A comprehensive review of the literature. J Am Coll Cardiol 2013;61:1125–1136. [DOI] [PubMed] [Google Scholar]
- 3. Ando T, Holmes AA, Taub CC, Slovut DP, Derose JJ.. Iatrogenic ventricular septal defect following transcatheter aortic valve replacement: a systematic review. Heart Lung Circ 2016;25:968–974. [DOI] [PubMed] [Google Scholar]
- 4. Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, del Nido P, Fasules JW, Graham TP, Hijazi ZM, Hunt SA, King ME, Landzberg MJ, Miner PD, Radford MJ, Walsh EP, Webb GD.. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease. J Am Coll Cardiol 2008;52:1890–1947. [DOI] [PubMed] [Google Scholar]
- 5. Nevaril CG, Lynch EC, Alfrey CP, Hellums JD.. Erythrocyte damage and destruction induced by shearing stress. J Lab Clin Med 1968;71:784–790. [PubMed] [Google Scholar]
- 6. Lam BK, Cosgrove DM, Bhudia SK, Gillinov A. M.. Hemolysis after mitral valve repair: Mechanisms and treatment. Ann Thorac Surg 2004;77:191–195. [DOI] [PubMed] [Google Scholar]
- 7. Liu JS, Lu PC, Chu SH.. Turbulence characteristics downstream of bileaflet aortic valve prostheses. J Biomech Eng 2000;122:118–124. [DOI] [PubMed] [Google Scholar]
- 8. Han YM, Gu X, Titus JL, Rickers C, Bass JL, Urness M, Amplatz K.. New self-expanding patent foramen ovale occlusion device. Catheter Cardiovasc Interv 1999;47:370–376. [DOI] [PubMed] [Google Scholar]
- 9. Gafoor S, Steinberg DH, Franke J, Bertog S, Vaskelyte L, Hofmann I, Sievert H.. Tools and techniques—clinical: paravalvular leak closure. EuroIntervention 2014;9:1359–1363. [DOI] [PubMed] [Google Scholar]