Abstract
Introduction
Embolic myocardial infarction is an uncommon but increasingly recognized complication of infective endocarditis (IE). Its incidence ranges between 1% and 10%, but it has a high mortality rate. A high index of suspicion is required to diagnose it. Only case reports and small studies on this condition have been published; thus, it is unknown what the ideal treatment is. We review the challenges to diagnosing this disease and the most effective treatments for it.
Case presentation
We report a case series of three patients with acute coronary syndrome (ACS) in IE. The first patient presented with non-ST-elevation ACS. He underwent a stent placement for late-diagnosed embolic myocardial infarction, after which he was treated conservatively without valve replacement, with good results. The second patient with ST-elevation presented with ACS, for which conventional balloon angioplasty and successful double valve replacement were performed. ST-elevation ACS was also observed in the last patient, who experienced periannular complications, which necessitated surgery.
Discussion
Acute coronary syndrome is a rare complication of IE but is associated with an increased risk of heart failure and high mortality rate. Its management is complicated and cannot be standardized. Because each situation is unique, a multidisciplinary discussion is required to choose the best treatment.
Keywords: Embolic myocardial infarction, Endocarditis, Embolization, Angioplasty, Thrombolytic therapy , Case series
Learning points
Embolic myocardial infarction is an uncommon complication of infective endocarditis, however, it has increased risk of heart failure and a high mortality rate.
The diagnosis of this complication requires a high index of suspicion in patients who present with acute coronary syndrome and history of fever.
Transoesophageal echocardiography could be the initial diagnostic procedure.
The management of the embolic myocardial infarction is complicated and cannot be standardized. Because each situation is unique, a multidisciplinary discussion is required to choose the best treatment.
Fibrinolytic therapy is generally contraindicated and has sometimes resulted in severe intracranial haemorrhage.
Introduction
Embolic myocardial infarction is an uncommon but increasingly recognized complication of infective endocarditis (IE). Its incidence ranges between 1% and 10%1–3 but it has a high mortality rate (64%).1 The optimal treatment remains unknown due to the lack of additional information. Here, we report a case series and review this disease and the most appropriate treatments for it. Table 1 summarises the clinical, echocardiographic and microbiological characteristics of the patients.
Table 1.
Individual patient’s baseline characteristics
| Patient | Age (years) | Sex | ECG | Clinical presentation | Ecocardiography | Blood cultures | Coronariography | Management after CA | Other complications of IE | Follow-up and outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 71 | Male | SR + LBBB | Fever, heart failure, chest pain. | LVEF 20% hypokinesia inferior–posterior and lateral. Severe MR. Large mitral vegetation (11 m) | Negatives |
|
|
Splenic and renal infarction due to septic embolism | LVEF 30% at discharge. Stable at FC II. |
| 2 | 67 | Male | AF + mild ST- segment elevation in V5–V6 and aVL leads with ST-segment depression in leads V2–V3 | Fever, chest pain | LVEF 45%, akinetic motion of the inferior–posterior wall, small mitral valve vegetation causing severe MR. Periaortic abscess | Streptococcus sanguinis |
|
|
None |
|
| 3 | 59 | Male | ST-segment elevation in leads II, III, aVF, and V5 | Chest pain, fever. |
|
Staphylococcus epidermidis |
|
|
Spondylodiscitis in the C5–C6–C7 discs | Stable at FC I |
AF, atrial fibrillation; AR, aortic regurgitation; BMS, bare metal stent; CA, coronary angiography; CX, left circumflex coronary artery; FC, functional class; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; OM1, first obtuse marginal branch; SR, sinus rhythm.
Timeline
| Patient 1 | |
|---|---|
| Sept/2014 | Dental extraction |
| Nov/2014 | Fever, general malaise, dyspnoea and episodes of chest pain |
| 15/11/14 | Acute heart failure and hospital admission. TTE: LVEF 20% hypokinesia inferior-posterior and severe MR. Empiric antibiotics therapy (Cloxacillin 2g/4hours, gentamycin 60mg/8hours and Doxycycline 100mg/12hours for 4 weeks) |
| 17/11/14 | CA: Thrombus of the proximal CX and occlusion of the OM1. BMS in OM1 |
| 20/11/14 | TOE: severe MR and vegetation. TC: Splenic and renal infarction due to septic embolism |
| 22/11/14 | Blood cultures and serologies negative |
| 12/12/14 | Control TOE: disappearance of vegetation. LVEF 30% |
| 14/12/14 | Discharge home. Stable at FC II. |
|
| |
|
Patient 2
| |
| 1/08/2015 | Fever, anorexia, asthenia and weight loss |
| 20/08/2015 | NSTEACS and hospital admission. TTE: LVEF 45% akinesia inferior-posterior and mitral vegetation causing severe MR. TOE: aortic abscess. Empiric antibiotics therapy (Ampicillin 4g/8hours and gentamycin 240mg/24hours for 4 weeks) |
| 22/08/2015 | CA: thrombotic occlusion of the proximal CX. Thrombus aspiration and balloon angioplasty |
| 25/08/2015 | Blood cultures: Streptococcu Sanguinis |
| 06/09/2015 | Control blood cultures negatives. Double valve replacement with mechanical prosthesis |
| 20/09/2015 | Discharge home. |
|
| |
|
Patient 3
| |
| April/2016 | Fever, general malaise and dyspnea admission. |
| 26/06/2016 | STEACS and hospital admission. Urgent CA: occlusion of the PL. Implantation 2 BMS. TTE LVEF 55% inferior-basal akynesia |
| 08/07/2016 | Neck pain. MRI: spondylodisci C5-C7. TOE: Vegetations at mitral and aortic leaflet, causing severe AR. Empiric antibiotics therapy (Cloxacillin 3g/6hours and Rifampin 1200mg/24hours for 6 weeks) Blood cultures: Staphylococco epidermidis |
| 24/07/2016 | Blood cultures negatives. Replace mitral and aortic valves with mechanical prosthesis. |
| 14/08/2016 | Discharge home. |
AR: Aortic regurgitation; BMS: Bare metal stent; CA: coronary angiography; CX: left circumflex coronary artery; FC: Functional class; LBBB: left bundle branch block; LVEF: Left ventricular ejection fraction; MR: Mitral regurgitation; MRI: Magnetic resonance imagen; NSTEACS: Non ST Elevation acute coronary sindorme; OM1: first obtuse marginal branch; PL: posterolateral coronary artery; STEACS: ST elevation acute coronary síndrome; TOE: Transoesophageal echocardiogram; TTE: Transthoracic echocardiogram.
Case report
Patient 1
Patient information
A 71-year-old man had a history of hypertrophic cardiomyopathy and systolic anterior motion of the mitral valve, resulting in moderate mitral regurgitation (MR). He had a 3-week history of fever, general malaise, dyspnoea, and episodes of chest pain. He had undergone a dental extraction 2 months ago.
Physical examination
On examination, he presented with severe respiratory distress, his respiratory rate was 40 breaths/min, his pulse oximetry was 85% on room air, and he had a clearly audible pansystolic murmur.
Diagnostic assessment
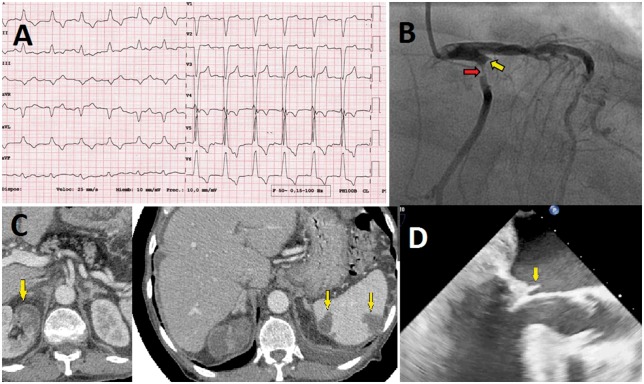
The electrocardiogram (ECG) on admission revealed a left bundle branch block (Figure 1A). The chest X-ray showed signs of acute cardiogenic pulmonary oedema. Laboratory tests detected the following: an elevation in high-sensitivity cardiac troponin T (hs-cTnT) at 4266 µg/L (normal <14 µg/L); creatine kinase (CK) 1705 U/L (normal 38–174 U/L); and acute phase reactants (APR): leucocytes 29, 130 mm3 (normal 4.5–11.5 mm3) with 95% neutrophils (normal 55–70%), erythrocyte sedimentation rate (ESR) 103 mm/h (normal 1–20 mm/h), C-reactive protein (CRP) 151 mg/L (normal <5 mg/L), and procalcitonine 2 g/L (normal <0.1 g/L), and mild renal insufficiency Cr 1.5 mg/dL (normal 0.7–1.2 mg/dL).
Figure 1.
(A) Electrocardiogram on admission showed a left bundle branch block. (B) Coronary angiography: severe stenosis with thrombus of the proximal left circumflex coronary artery (red arrow) and occlusion in the first obtuse marginal branch (yellow arrow). (C) Computed tomography that shows splenic hypodense images and areas of hypocaptation in right renal parenchyma, compatible with infarct areas. (D) Transoesophageal echocardiogram in mid-oesophageal three-chamber view with large vegetation on the posterior mitral leaflet (arrow).
Transthoracic echocardiography (TTE) showed a left ventricular ejection fraction (LVEF) of 20% secondary to hypokinesis of the inferior–posterior and lateral segments and a large eccentric jet of MR. Subsequent transoesophageal echocardiography (TOE) confirmed severe MR and showed an independently oscillating mobile vegetation, measuring 11 mm (Figure 1D and see Supplementary material online, Videos S1 and S2).
Interventions
On suspicion of IE that was complicated with acute myocardial infarction (AMI), the patient was admitted to the coronary care unit (CCU) and treated with antithrombotic therapy and intravenous empiric antibiotics (cloxacillin 2 g/4 h and gentamycin 60 mg/8 h). A coronary angiogram, performed 1 day after presentation, revealed a thrombus of the proximal circumflex coronary artery (CX), causing an abrupt occlusion of the first obtuse marginal branch (OM1) (Figure 1B and see Supplementary material online, Video S3).
The patient underwent implantation of a bare metal stent (BMS) in the OM1 and balloon angioplasty in the CX. Computed tomography showed splenic and renal infarction due to septic embolism (Figure 1C). Blood cultures were negative. The patient recovered uneventfully (without heart failure or abscess). Conservative attempts were maintained by adding doxycycline 100 mg/12 h due to the already known moderate-severe MR and in the light of the recent percutaneous coronary intervention.
Follow-up and outcomes
The evolution was favourable, with a decrease in APR, and a new batch of blood cultures and serological tests for Brucella, Coxiella, Legionella, Bartonella, Leishmania, Echinococcus, and Rickettsia were negative. A control TOE on his discharge home showed the disappearance of vegetation but the persistence of moderate-severe MR and an LVEF of 30%. Six months after discharge, the patient was stable at New York Heart Association (NYHA) functional class II.
Patient 2
Patient information
A 67-year-old man with a history of rheumatic fever and atrial fibrillation presented with acute chest pain and a 2-week history of fever, anorexia, asthenia, and weight loss.
Physical examination
The patient was haemodynamically stable but in mild distress as the result of ongoing chest pain. On auscultation, a loud pansystolic murmur could be heard.
Diagnostic assessment
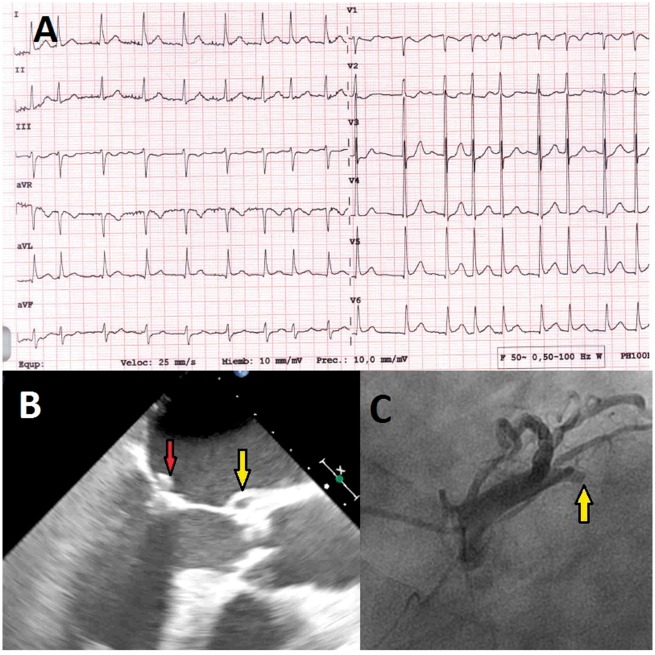
The ECG showed mild ST-segment elevation in the V5–V6 and aVL leads, with ST-segment depression in leads V2–V3 (Figure 2A). Laboratory tests were positive for biomarkers: hs-cTnT 1272 µg/L and CK 1049 U/L; and APR (CRP 104 mg/L, ESR 111 mm/h, leucocytes 9550 mm3 with 89% neutrophils). Transthoracic echocardiography demonstrated an LVEF of 45% with akinetic motion of the inferior–posterior wall and a small mitral valve vegetation that was causing severe MR. The patient was admitted to the CCU and diagnosed as having complicated IE with AMI. A TOE showed a cavity with systolic expansion at the mitral-aortic junction that was compatible with an abscess (Figure 2B and see Supplementary material online, Videos S4 and S5). Coronary angiography revealed a thrombotic occlusion of the proximal CX, likely due to a septic embolism (Figure 2C and see Supplementary material online, Video S6).
Figure 2.
(A) Electrocardiogram on admission showed atrial fibrillation at 110 b.p.m., mild ST-segment elevation in V5–V6 and aVL leads with ST-segment depression in leads V2–V3. (B) Transoesophageal echocardiogram in mid-oesophageal three-chamber view with 2 × 6 mm vegetation on the anterior mitral leaflet (red arrow) and a cavity with systolic expansion at the mitro-aortic junction compatible with an abscess (yellow arrow). (C) Coronary angiography showing an occlusion of the proximal left circumflex coronary artery (arrow).
Interventions
Percutaneous coronary intervention with thrombus aspiration and conventional balloon angioplasty were performed. The blood cultures grew Streptococcus sanguinis; thus, the patient was treated with ampicillin 4 g/8 h and gentamycin 240 mg/24 h for 4 weeks.
The patient underwent a successful double valve replacement with a mitral-aortic mechanical prosthesis due to the presence of an abscess and severe MR. The surgical pathology report described aggregates of Gram-positive cocci, consistent with IE.
Follow-up and outcomes
A repeat TTE before discharge showed improved ventricular function (LVEF 55%) and mild MR, without any vegetation at the leaflets. After 6 months of follow-up, the patient remains stable.
Patient 3
Patient information and physical examination
A 59-year-old male was referred to our emergency department for sudden-onset central chest pain. He was haemodynamically stable and dyspnoeic, with a Grade I–II systolic murmur at the mitral and aortic area.
Diagnostic assessment
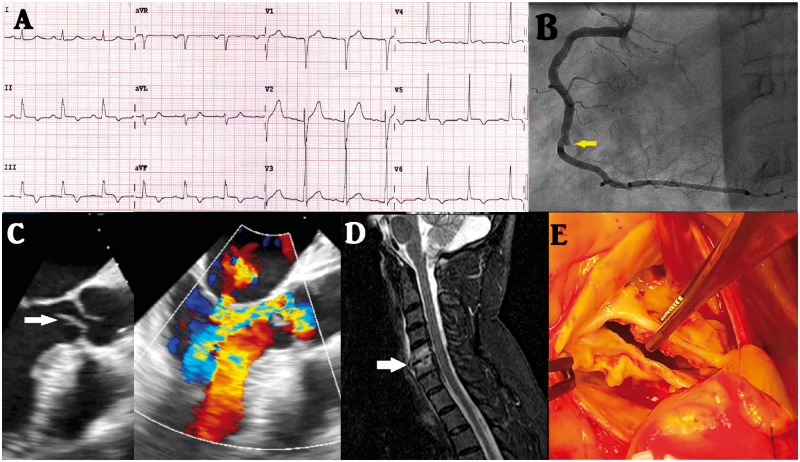
The ECG showed ST-segment elevation up to 1.5 mm in leads II, III, aVF, and V5 (Figure 3A). Laboratory tests detected an elevation in biomarkers hs-cTnT 841 µg/and CK 945 U/L. An urgent coronary angiography revealed an abrupt occlusion of the posterolateral artery (Figure 3B and see Supplementary material online, Video S7) that was treated successfully with implantation of two BMSs after an ineffective thrombectomy. The patient was admitted to the CCU with a diagnosis of inferioposterior AMI. The evolution was satisfactory, although he complained of severe neck pain that did not respond to analgesics or muscle relaxants. Cervical magnetic resonance imaging showed spondylodiscitis in the C5–C6–C7 discs (Figure 3D). In a second interview, the patient claimed to have had a persistent fever, general malaise, and dyspnoea 3 months ago, which were treated as pneumonia with empiric antibiotics.
Figure 3.
(A) Electrocardiogram on admission showing ST-segment elevation up to 1.5 mm and T-wave inversion in leads II, III, aVF, and V5. (B) Coronary angiography showing an abrupt occlusion of the posterolateral artery (arrow). (C) Grey-scale transoesophageal echocardiogram in mid-oesophageal three-chamber view with large vegetation on the aortic leaflet (arrow), and colour doppler transoesophageal echocardiogram in the same view with severe aortic regurgitation and moderate mitral regurgitation. (D) Magnetic resonance imaging showing cervical spondylodiscitis in the C5–C6–C7. (E) Intraoperative image of the aortic valve, showing perforation of the right ronary leaflet.
The TTE showed an LVEF of 55% with inferior-basal segment akinesia and imaging that was compatible with vegetation. The TOE confirmed the presence of vegetation at the auricular side of the anterior mitral leaflet (9 × 3 mm) and the ventricular side of the aortic right coronary cusp (15 × 6 mm), causing severe aortic regurgitation and moderate MR, respectively (Figure 3C and see Supplementary material online, Videos S8 and S9). The blood cultures grew Staphylococcus epidermidis ×2, and the patient was subsequently treated with cloxacillin 3 g/6 h and rifampin 1200 mg/24 h. Laboratory tests showed elevated levels of APR (CRP 53 mg/L, ESR 50 mm/h, leucocytes 12 780 mm3 with 89% neutrophils) although the patient remained afebrile throughout his hospitalization.
Interventions
After 6 weeks of antibiotic therapy and negative blood cultures, the heart team decided to replace the mitral and aortic valves with mechanical prostheses. The surgical pathology report showed several perforations in both valves (Figure 3E).
Follow-up and outcomes
The patient was discharged home, and over the 8-month follow-up, he has been stable at NYHA functional class II.
Discussion
Acute coronary syndrome is usually an early and uncommon complication of IE; 2 weeks is the most common delay for most emboli in IE,2 and the risk of embolism decreases after the commencement of antibiotic therapy. The following factors increase the risk of embolism: the presence of a mobile vegetation, vegetation length >10 mm, infection with staphylococci or non-viridans streptococci as the causative organism, and a previous embolism.3,4 In the largest series of 1210 patients,2 coronary embolism was located indifferently in the left anterior descending artery, right coronary artery, and circumflex artery. Coronary embolisms are most frequently observed in patients with aortic valve endocarditis, which can explained by the proximity between the aortic vegetations and coronary ostia.1,2,4
In previous series, such as a Spanish series of 586 patients,1 the most common cause of acute coronary syndrome (ACS) during the course of IE has been extrinsic coronary compression due to periannular complications (pseudoaneurysm, abscesses), followed by septic coronary embolism. But, in more recent series,2 the predominant mechanism that has been causing ACS is the embolization of vegetations in the coronary arteries. Other causes of ischaemia are occlusion of the coronary artery ostium by vegetation that arises from the aortic valve,5 severe AR that decreases coronary perfusion pressure, and ischaemia that is precipitated by the systemic inflammatory state in coronary arteries with previous atherosclerotic disease.1,2
Infective endocarditis should be suspected in patients who have fever and embolic myocardial infarction; in such cases, TTE must be performed. Echocardiographic findings in IE require initial empirical antibiotic treatment after blood culture extraction. Choosing the appropriate empirical treatment depends on several factors, such as if the patient has had previous antibiotic therapy, whether the infection affects the native or prosthetic valves, and the area of the infection (community, nosocomial, or non-nosocomial healthcare-associated IE). Regimens for empirical treatment should be the recommended in the 2015 ESC Guidelines for the management of IE4 and must be considered by an infectious disease specialist for further evaluation and treatment.
Due to the low incidence of coronary events in IE, only case reports and small studies have been published6–9; thus, the current literature on the management of these patients does not give clear recommendations. Bacteraemia impairs haemostasis; consequently, the value of anticoagulation in the prevention of embolism is limited in IE patients and can raise the risk of bleeding.5 Moreover, fibrinolytic therapy is generally contraindicated and sometimes results in severe intracranial haemorrhage,10,11 which is believed to be attributed to the high prevalence of silent cerebral infarctions and mycotic aneurysms due to septic embolisms12, with the bleeding risk that is associated with bacteraemia. There appears to be a trend towards the use of primary percutaneous coronary intervention (PPCI), but the efficacy and evidence of this approach are limited. Balloon inflation at the site of the occlusion can result in displacement of the vegetation and a greater risk of further embolic phenomena. A stent implantation might be helpful,8 but the risk of coronary artery mycotic aneurysm or stent infection should be taken into account before its use.4,9,13 Routine thrombus aspiration is not recommended during PPCI, according to the 2017 ESC Clinical Practice Guidelines,14 but in patients with IE that is complicated by embolic AMI, there could be benefits from manual thrombus aspiration due to the removal of infected material, although no data on this approach are available.
Although the management of IE that is complicated by embolic AMI cannot be standardized, several groups recommend TOE as one of the initial diagnostic procedures that should be performed in patients with non-ST-elevation ACS. Because the ischaemia is more likely to be secondary to paravalvular complications,1,15 in this case, the patient should be referred for valvular surgery without having a coronary angiography. Conversely, if there is ST-segment elevation, diagnostic coronary angiography is performed; if a coronary occlusion is demonstrated, thromboaspiration is recommended as the initial strategy, and if successful, the only strategy for these patients.6,16
Conclusion
Embolic myocardial infarction is an uncommon but increasingly recognized complication of IE that requires a high index of suspicion in patients with ACS and a history of fever. Its management is complicated and cannot be standardized. Because each situation is unique, a multidisciplinary discussion is required to choose the best treatment. In patients who can be stabilized with conservative medical management, early valvular surgery may be the safest option. Additional guidance for those who are likely to encounter this condition in clinical practice is welcomed.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Manzano MC, Vilacosta I, San Román JA.. Acute coronary syndrome in infective endocarditis. Rev Esp Cardiol 2007;60:24–31. [PubMed] [Google Scholar]
- 2. Roux V, Salaun E, Tribouilloy C, Hubert S, Bohbot Y, Casalta J-P, Barral PA, Rusinaru D, Gouriet F, Lavoute C, Haentjens J, Di Biscegli M, Dehaene A, Renard S, Casalta AC, Pradier J, Avierinos JF, Riberi A, Lambert M, Collart F, Jacquier A, Thuny F, Camoin-Jau L, Lepidi H, Raoult D, Habib G.. Coronary events complicating infective endocarditis. Heart 2017;103:1906–1910. [DOI] [PubMed] [Google Scholar]
- 3. Garvey GJ, Neu HC.. Infective endocarditis-an evolving disease. A review of endocarditis al Columbia-Presbyterian Medical Center, 1968-1973. Medicine 1978;57:105–127. [PubMed] [Google Scholar]
- 4. Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta J-P, Del Zotti F, Dulgheru R, El Khoury G, Erba PA, Iung B, Miro JM, Mulder BJ, Plonska-Gosciniak E, Price S, Roos-Hesselink J, Snygg-Martin U, Thuny F, Tornos Mas P, Vilacosta I, Zamorano JL.. ESC guidelines for the management of infective endocarditis. Eur Heart J 2015;36:3075–3128. [DOI] [PubMed] [Google Scholar]
- 5. Overend L, Rose E.. Uncertaintis in managing myocardial infarction associadted with infective endocarditis. Exp Clin Cardiol 2012;17:144–145. [PMC free article] [PubMed] [Google Scholar]
- 6. Gamaza S, Camacho SJ, León J, Agarrado A, Daroca T, Oneto MJ.. Acute coronary syndrome due to septic embolism secondary to infective endocarditis in a prosthetic mitral valve. Rev Esp Cardiol 2017;70:501–514. [DOI] [PubMed] [Google Scholar]
- 7. Herzog CA, Henry TD, Zimmer SD.. Bacterial endocarditis presenting as acute myocardial infarction: a cautionary note for the era of reperfusion. Am J Med 1991;90:392–397. [PubMed] [Google Scholar]
- 8. Glazier JJ, Mcginnity JG, Spears JR.. Coronary embolism complicating aortic valve endocarditis: treatment with placement of an intracoronary stent. Clin Cardiol 1997;20:885–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. García-Izquierdo E, Jiménez-Blanco M, Parra C, Ortega J.. Coronary septic embolism: an unusual presentation of acute myocardial infarction. Kardiol Pol 2017;75:616. [DOI] [PubMed] [Google Scholar]
- 10. Kleczynski P, Dziewierz A, Legutko J, Kmita A, Sorysz D, Bagienski M.. Acute myocardial infarction in a patient with chronic renal failure and endocarditis. Kardiol Pol 2013;71:650–652. [DOI] [PubMed] [Google Scholar]
- 11. Di Salvo TG, Tatter SB, O'Gara PT, Nielsen GP, Desanctis RW.. Fatal intracerebral hemorrhage following thrombolytic therapy of embolic myocardial infarction in unsuspected infective endocarditis. Clin Cardiol 1994;17:340–344. [DOI] [PubMed] [Google Scholar]
- 12. Hunter AJ, Girard DE.. Thrombolytics in infectious endocarditis associated myocardial infarction. J Emerg Med 2001;21:401–406. [DOI] [PubMed] [Google Scholar]
- 13. Matsumoto M, Konishi Y, Miwa S, Minakata K.. Mycotic aneurysm of the left coronary artery. Ann Thorac Surg 1998;65:841–842. [DOI] [PubMed] [Google Scholar]
- 14. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarctionin patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119–177. [DOI] [PubMed] [Google Scholar]
- 15. Schlaifer JD, Martin TD, Hill JA, Kerensky RA.. Coronary artery obstruction caused by perivalvular abscess in aortic valve endocarditis. Am Heart J 1996;131:413–416. [DOI] [PubMed] [Google Scholar]
- 16. Whitaker J, Saha M, Fulmali R, Perera D.. Successful treatment of ST elevation myocardial infarction caused by septic embolus with the use of a thrombectomy catheter in infective endocarditis. BMJ Case Rep 2011;2011. doi:10.1136/bcr.03.2011.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.