Abstract
BACKGROUND
Since 2004, a regimen of 6 months of treatment with oxaliplatin plus a fluoropyrimidine has been standard adjuvant therapy in patients with stage III colon cancer. However, since oxaliplatin is associated with cumulative neurotoxicity, a shorter duration of therapy could spare toxic effects and health expenditures.
METHODS
We performed a prospective, preplanned, pooled analysis of six randomized, phase 3 trials that were conducted concurrently to evaluate the noninferiority of adjuvant therapy with either FOLFOX (fluorouracil, leucovorin, and oxaliplatin) or CAPOX (capecitabine and oxaliplatin) administered for 3 months, as compared with 6 months. The primary end point was the rate of disease-free survival at 3 years. Noninferiority of 3 months versus 6 months of therapy could be claimed if the upper limit of the two-sided 95% confidence interval of the hazard ratio did not exceed 1.12.
RESULTS
After 3263 events of disease recurrence or death had been reported in 12,834 patients, the noninferiority of 3 months of treatment versus 6 months was not confirmed in the overall study population (hazard ratio, 1.07; 95% confidence interval [CI], 1.00 to 1.15). Noninferiority of the shorter regimen was seen for CAPOX (hazard ratio, 0.95; 95% CI, 0.85 to 1.06) but not for FOLFOX (hazard ratio, 1.16; 95% CI, 1.06 to 1.26). In an exploratory analysis of the combined regimens, among the patients with T1, T2, or T3 and N1 cancers, 3 months of therapy was noninferior to 6 months, with a 3-year rate of disease-free survival of 83.1% and 83.3%, respectively (hazard ratio, 1.01; 95% CI, 0.90 to 1.12). Among patients with cancers that were classified as T4, N2, or both, the disease-free survival rate for a 6-month duration of therapy was superior to that for a 3-month duration (64.4% vs. 62.7%) for the combined treatments (hazard ratio, 1.12; 95% CI, 1.03 to 1.23; P = 0.01 for superiority).
CONCLUSIONS
Among patients with stage III colon cancer receiving adjuvant therapy with FOLFOX or CAPOX, noninferiority of 3 months of therapy, as compared with 6 months, was not confirmed in the overall population. However, in patients treated with CAPOX, 3 months of therapy was as effective as 6 months, particularly in the lower-risk subgroup. (Funded by the National Cancer Institute and others.)
Since 2004, oxaliplatin with a fluo-ropyrimidine has been standard adjuvant chemotherapy in patients with stage III colon cancer. Three phase 3 trials convincingly showed that the addition of oxaliplatin improved disease-free survival; with longer follow-up, these findings were extended to overall survival.1–5 Accordingly, a 6-month regimen of FOLFOX (fluorouracil, leucovorin, and oxaliplatin) or CAPOX (capecitabine and oxaliplatin) became the standard adjuvant therapy in stage III disease.
The risk of oxaliplatin-based sensory neurotoxicity depends on the cumulatively administered dose of the drug. Neurotoxicity often peaks several months after the last oxaliplatin exposure, which makes empirical dose individualization difficult. Such toxic effects can be severe and persist long beyond the actual treatment, which potentially affects patients’ activities of daily living for the rest of their lives.6
Given the cumulative nature of oxaliplatin-mediated neurotoxicity, shorter adjuvant therapy would be beneficial for patients and reduce the use of health care resources if efficacy were maintained. A prospective investigation with a sufficiently powered noninferiority design would require the enrollment of a large number of patients and a small noninferiority margin to reliably conclude that the clinical outcome was not meaningfully compromised. Thus, the International Duration Evaluation of Adjuvant Therapy (IDEA) collaboration was formed with the goal of prospectively pooling data from six clinical trials of adjuvant therapy involving patients with stage III colon cancer to evaluate the primary hypothesis that 3 months of FOLFOX or CAPOX therapy would be noninferior to 6 months of therapy in the rate of disease-free survival at 3 years. Noninferiority of 3 months versus 6 months of therapy could be claimed if the upper limit of the two-sided 95% confidence interval of the hazard ratio did not exceed 1.12. This margin was chosen on the basis of clinical acceptability, since it corresponded to a worsening of 2.7 percentage points in the 3-year rate of disease-free survival (from 72% to 69.3%), as determined by consensus among the IDEA collaborators.7
METHODS
Clinical Trials and Patients
Established in 2006, IDEA was an academic collaboration of clinicians and statisticians who were involved in six randomized, phase 3 clinical trials enrolling patients with stage III colon cancer in 12 countries (Table 1).7 The trials were CALGB/SWOG (Cancer and Leukemia Group B/Southwest Oncology Group) 80702 (ClinicalTrials.gov number, NCT01150045), IDEA France (EudraCT number, 2009-010384-16), SCOT (Short Course Oncology Treatment) (NCT00749450; Current Controlled Trials number, ISRCTN59757862, and EudraCT number, 2007-003957-10), ACHIEVE (Adjuvant Chemotherapy for Colon Cancer with High Evidence) (UMIN Clinical Trials Registry number, UMIN000008543), TOSCA (Three or Six Colon Adjuvant) (OsSC number, 2007-000354-31), and HORG (Hellenic Oncology Research Group) (NCT01308086). The research protocol was approved by the relevant institutional review board or ethics committee at each site and is available with the full text of this article at NEJM.org. All the patients provided written informed consent.
Table 1.
Characteristics of the Study Patients (Modified Intention-to-Treat Population).*
| Characteristic | TOSCA (N = 2402) | SCOT (N =3983) | IDEA France (N =2010) | CALGB/SWOG 80702 (N = 2440) | HORG (N = 708) |
ACHIEVE (N = 1291) | All Patients (N = 12,834) |
|---|---|---|---|---|---|---|---|
| Countries | Italy | U.K., Denmark, Spain, Australia, Sweden, New Zealand | France | U.S., Canada | Greece | Japan | |
| Median age (range) — yr | 64 (20–83) | 65 (20–84) | 64 (18–85) | 61 (19–88) | 67 (20–75) | 66 (28–85) | 64 (18–88) |
| Male sex — no. (%) | 1348 (56.1) | 2356 (59.2) | 1144 (56.9) | 1348 (55.3) | 398 (56.2) | 649 (50.3) | 7,243 (56.4) |
| ECOG performance status — no. (%) | |||||||
| 0 | 2268 (94.4) | 2827 (71.0) | 1479 (73.6) | 1734 (71.1) | 579 (81.8) | 1245 (96.4) | 10,132 (79.0) |
| 1 | 130 (5.4) | 1156 (29.0) | 502 (25.0) | 680 (27.9) | 128 (18.1) | 46 (3.6) | 2,642 (20.6) |
| 2 | 1 (<0.1) | 0 | 29 (1.4) | 26 (1.1) | 1 (0.1) | 0 | 57 (0.4) |
| Missing data | 3 (0.1) | 0 | 0 | 0 | 0 | 0 | 3 (<0.1) |
| Tumor stage — no. (%) | |||||||
| T1 | 76 (3.2) | 128 (3.2) | 78 (3.9) | 135 (5.5) | 1 (0.1) | 75 (5.8) | 493 (3.8) |
| T2 | 236 (9.8) | 333 (8.4) | 161 (8.0) | 288 (11.8) | 60 (8.5) | 119 (9.2) | 1,197 (9.3) |
| T3 | 1773 (73.8) | 2347 (58.9) | 1399 (69.6) | 1598 (65.5) | 549 (77.5) | 734 (56.9) | 8,400 (65.5) |
| T4 | 291 (12.1) | 1174 (29.5) | 372 (18.5) | 359 (14.7) | 96 (13.6) | 363 (28.1) | 2,655 (20.7) |
| Missing data | 26 (1.1) | 1 (<0.1) | 0 | 60 (2.5) | 2 (0.3) | 0 | 89 (0.7) |
| Nodal stage — no. (%) | |||||||
| N1 | 1748 (72.8) | 2749 (69.0) | 1501 (74.7) | 1739 (71.3) | 472 (66.7) | 959 (74.3) | 9,168 (71.4) |
| N2 | 636 (26.5) | 1233 (31.0) | 506 (25.2) | 630 (25.8) | 230 (32.5) | 332 (25.7) | 3,567 (27.8) |
| Missing data | 18 (0.7) | 1 (<0.1) | 3 (0.1) | 71 (2.9) | 6 (0.8) | 0 | 99 (0.8) |
| Risk group — no. (%) | |||||||
| T1,T2, or T3 N1 | 1553 (65.5) | 2032 (51.0) | 1245 (62.0) | 1507 (63.6) | 416 (59.1) | 718 (55.6) | 7,471 (58.7) |
| T4, N2, or both | 817 (34.5) | 1950 (49.0) | 764 (38.0) | 864 (36.4) | 288 (40.9) | 573 (44.4) | 5,256 (41.3) |
| Median no. of lymph nodes examined (range) | 18 (0–85) | Not recorded | 20 (1–99) | 20 (1–132) | 18 (10–85) | 21 (1–123) | 19 (0–132) |
| Chemotherapy regimen — no. (%) | |||||||
| CAPOX | 840 (35.0) | 2649 (66.5) | 201 (10.0) | 0 | 412 (58.2) | 969 (75.1) | 5,071 (39.5) |
| FOLFOX | 1562 (65.0)† | 1334 (33.5) | 1809 (90.0) | 2440 (100) | 296 (41.8)† | 322 (24.9) | 7,763 (60.5) |
| Median follow-up time — mo | 61.7 | 36.8 | 51.3 | 34.9 | 47.5 | 36.7 | 41.8 |
Percentages may not total 100 because of rounding. ACHIEVE denotes Adjuvant Chemotherapy for Colon Cancer with High Evidence, CALGB/SWOG Cancer and Leukemia Group B/Southwest Oncology Group, HORG Hellenic Oncology Research Group, IDEA International Duration Evaluation of Adjuvant Therapy, SCOT Short Course Oncology Treatment, and TOSCA Three or Six Colon Adjuvant.
Patients in this trial received FOLFOX4; those in the other trials received modified FOLFOX6.
Each trial investigated the effect of the duration of adjuvant oxaliplatin-based therapy on disease-free survival, with patients randomly assigned to receive 3 months or 6 months of therapy. Several trials included additional features (e.g., the inclusion of patients with stage II or rectal cancers) or the use of other adjuvant therapies (e.g., celecoxib or bevacizumab) (Table S1 in the Supplementary Appendix, available at NEJM.org). However, this report includes only the findings with respect to patients with stage III colon cancer. Five of the six trials allowed the use of either FOLFOX41 or modified FOLFOX68,9 (fluorouracil, leucovorin, and oxaliplatin administered in different doses and methods in the two regimens) or CAPOX.3,9 The trial conducted in the United States and Canada allowed the use of only modified FOLFOX6 and not CAPOX. The nonrandomized choice of therapy was made by the treating physicians.
Each trial provided individual patient data to the independent statistical center at Mayo Clinic Rochester. Three trials (TOSCA, IDEA France, and HORG) were inadvertently registered in databases that were not compliant with the criteria of the International Committee of Medical Journal Editors because of an administrative error, which was corrected after 212 patients (1.7% of the study population) had been enrolled in the trials.
Primary End Point and Treatment
The primary end point of the six trials was disease-free survival, which was defined as the time from the date of randomization to the date of a first relapse, the diagnosis of a secondary colorectal cancer after the initial diagnosis, or death from any cause, whichever occurred first. The doses and delivery schedules of the oxaliplatin-based adjuvant treatment options have been described previously.7 The reference duration of 6 months was chosen in accordance with pivotal trials in which the efficacy of the duration had been established.1,3,8–11
Statistical Analysis
We used a modified intention-to-treat method to conduct the primary analysis, which included all the patients who had undergone randomization and had received at least one dose of a trial drug (Fig. S1 in the Supplementary Appendix). The analysis was conducted according to the patients’ original randomization assignments. We determined that 3390 events of disease recurrence or death would provide a power of 90% to declare noninferiority for 3 months versus 6 months of therapy, given the predefined noninferiority margin at a one-sided type I error rate of 0.025 and assuming a 3-year disease-free survival rate of 72% in the 6-month therapy group. An independent statistician planned and conducted one interim analysis for futility (i.e., to determine inferiority of 3 months of therapy) using the Lan–DeMets implementation of the O’Brien–Fleming stopping boundaries. For disease-free survival, we used a Cox regression model stratified according to each trial to estimate hazard ratios and two-sided 95% confidence intervals for the comparison of 3 months versus 6 months of therapy.
Preplanned analyses included assessments of noninferiority of 3 months versus 6 months of therapy within subgroups that were defined according to the stage of tumor penetration (T) and nodal status (N) (Table S2 in the Supplementary Appendix) and chemotherapy regimen (FOLFOX or CAPOX). The proportional-hazards assumption for the stratified Cox model was examined with the use of scaled Schoenfeld residuals.12 Q statistics and I2 values were used to assess the potential heterogeneity of trial-specific hazard ratios comparing disease-free survival between 3 months and 6 months of therapy. A P value that was associated with a Q statistic of less than 0.05 was considered to indicate significant heterogeneity, and an I2 value of close to 1 was considered to indicate an increased degree of heterogeneity. Preplanned analyses were not affected by interim analyses of the individual trials. Adjustment for multiple testing was performed if interaction results were significant.
RESULTS
Patients
From June 2007 through December 2015, a total of 13,025 patients with stage III colon cancer were enrolled in six concurrently conducted phase 3 trials. Of these patients, 12,834 met the criteria for the modified intention-to-treat analysis. Table 1 lists pertinent characteristics of the patients in each trial and in the modified intention-to-treat analysis. Although most of the characteristics of the patients and their tumors were similar, some differences were notable among the trials. The percentage of patients with T4 tumors (in which the lesion penetrates to the surface of the visceral peritoneum or is adherent to adjacent organs) varied from 12.1% (in TOSCA) to 29.5% (in SCOT). The percentage of patients with N2 tumors (involving ≥4 nodes) varied from 25.2% (in IDEA France) to 32.5% (in HORG). Perhaps most important, the use of CAPOX or FOLFOX varied greatly. Although CALGB/SWOG restricted treatment to FOLFOX and only 10% of the patients in IDEA France received CAPOX, the majority of patients in SCOT (66.5%) and ACHIEVE (75.1%) received CAPOX. Overall, about 40% of patients received CAPOX, and 60% FOLFOX. Table S3 in the Supplementary Appendix lists the characteristics of the patients and the tumors according to treatment. At the time of the data cutoff (February 2017), the median follow-up varied from 34.9 months in CALGB/SWOG to 61.7 months in TOSCA. Each trial defined individual follow-up intervals.
Treatment Adherence
As expected, treatment adherence (i.e., the percentage of patients who received all planned therapy) was lower in the 6-month therapy group than in the 3-month therapy group (Table 2). For fluorouracil and capecitabine, the mean percentage of doses that were delivered was 92.4% and 91.2%, respectively, in the 3-month therapy group, as compared with 81.6% and 78.0% in the 6-month therapy group. For oxaliplatin, the mean percentage of FOLFOX and CAPOX doses were 91.4% and 89.8%, respectively, in the 3-month therapy group, as compared with 72.8% and 69.3% in the 6-month therapy group (P<0.001).
Table 2.
Treatment Adherence, According to Chemotherapy Regimen and Duration of Therapy.*
| Measurement of Treatment Adherence | FOLFOX | CAPOX | ||||
|---|---|---|---|---|---|---|
| 3 Mo (N = 3870) | 6 Mo (N = 3893) | P Value | 3 Mo (N = 2554) | 6 Mo (N = 2517) | P Value | |
| Extent of therapy | ||||||
| Therapy duration | ||||||
| Median (IQR) — wk | 12 (12–12) | 24 (20–24) | 12 (12–12) | 24 (18–24) | ||
| Simple range — wk | 2–24 | 2–44 | 3–24 | 3–36 | ||
| Missing data — no. (%) | 2 (0.1) | 6 (0.2) | 0 | 1 (<0.1) | ||
| Completion of cycles — no. (%) | ||||||
| Received planned no. of cycles | 3459 (89.4) | 2731 (70.2) | <0.001† | 2167 (84.8) | 1612 (64.0) | <0.001† |
| Did not receive planned no. of cycles | 345 (8.9) | 1123 (28.8) | 321 (12.6) | 858 (34.1) | ||
| Received more than planned no. of cycles | 42 (1.1) | 8 (0.2) | 21 (0.8) | 1 (<0.1) | ||
| Missing data | 24 (0.6) | 31 (0.8) | 45 (1.8) | 46 (1.8) | ||
| Percentage of dose delivered | ||||||
| Fluorouracil‡ | <0.001§ | NA | NA | |||
| Mean — % | 92.4±22.7 | 81.6±26.6 | ||||
| Median (IQR) — % | 99.8 (89.2–100) | 89.9 (73.8–100) | ||||
| Simple range — %¶ | 9.4–362.7 | 1.2–398.1 | ||||
| Missing data — no. (%) | 28 (0.7) | 40 (1.0) | ||||
| Capecitabine | NA | NA | <0.001§ | |||
| Mean — % | 91.2±23.5 | 78.0±29.4 | ||||
| Median (IQR) — % | 100 (87.5–100) | 87.5 (62.5–100) | ||||
| Simple range — %¶ | 25.0–393.8 | 0.4–339.2 | ||||
| Missing data — no. (%) | 56 (2.2) | 58 (2.3) | ||||
| Oxaliplatin | <0.001§ | <0.001§ | ||||
| Mean — % | 91.4±19.9 | 72.8±25.6 | 89.8±21.7 | 69.3±28.3 | ||
| Median (IQR) — % | 99.5 (88.6–100) | 78.9 (56.1–93.7) | 100 (87.7–100) | 75.0 (50.0–95.2) | ||
| Simple range — %¶ | 14.7–284.4 | 4.9–249.1 | 25.0–200.0 | 10.9–146.3 | ||
| Missing data — no. (%) | 4 (0.1) | 6 (0.2) | 8 (0.3) | 2 (0.1) | ||
| Dose intensity | ||||||
| Difference between capecitabine or fluorouracil and oxaliplatin in percentage of dose delivered — no. (%)‖ | <0.001† | <0.001† | ||||
| <10 percentage points | 3601 (93.0) | 2701 (69.4) | 2242 (87.8) | 1761 (70.0) | ||
| 10 to <20 percentage points | 127 (3.3) | 404 (10.4) | 131 (5.2) | 230 (9.1) | ||
| 20 to 30 percentage points | 39 (1.0) | 287 (7.4) | 47 (1.8) | 146 (5.8) | ||
| >30 percentage points | 71 (1.8) | 459 (11.8) | 70 (2.7) | 321 (12.8) | ||
| Missing data | 32 (0.8) | 42 (1.1) | 64 (2.5) | 59 (2.3) | ||
Plus–minus values are means ±SD. Percentages may not total 100 because of rounding. IQR denotes interquartile range, and NA not applicable.
This P value was calculated by means of the chi-square test.
The listed values are for both infusion and bolus of fluorouracil.
This P value was calculated by means of the Kruskal–Wallis test.
A total of 30 patients (0.2%) underwent longer treatment than the assigned duration, which resulted in an upper-range percentage of more than 150%.
For each patient, the absolute difference was calculated as percentage of the dose of capecitabine or fluorouracil that was delivered minus the percentage of the dose of oxaliplatin that was delivered, as measured in percentage points. A larger between-group difference indicates that a patient discontinued oxaliplatin earlier or had a greater dose reduction in oxaliplatin than in capecitabine or fluorouracil.
Primary End Point
At the time of the database lock, 3263 events of disease recurrence or death (96.3% of the estimated events) had occurred. This occurrence resulted in a retained statistical power of 89% for the noninferiority analysis. At a median follow-up of 41.8 months, noninferiority of 3 months of therapy versus 6 months was not confirmed in the modified intention-to-treat population (hazard ratio, 1.07; 95% confidence interval [CI], 1.00 to 1.15; P = 0.11 for noninferiority of 3-month therapy; P = 0.045 for superiority of 6-month therapy) (Fig. 1A). The 3-year rates of disease-free survival were 74.6% (95% CI, 73.5 to 75.7) in the 3-month therapy group as compared with 75.5% (95% CI, 74.4 to 76.7) in the 6-month therapy group. No violation of the proportional-hazards assumption (P>0.10) and no meaningful heterogeneities (I2<0.26, P>0.17 in Q statistics after adjustment for false discovery rate) in hazard ratios across individual trials were detected in the overall population or in key subgroups.
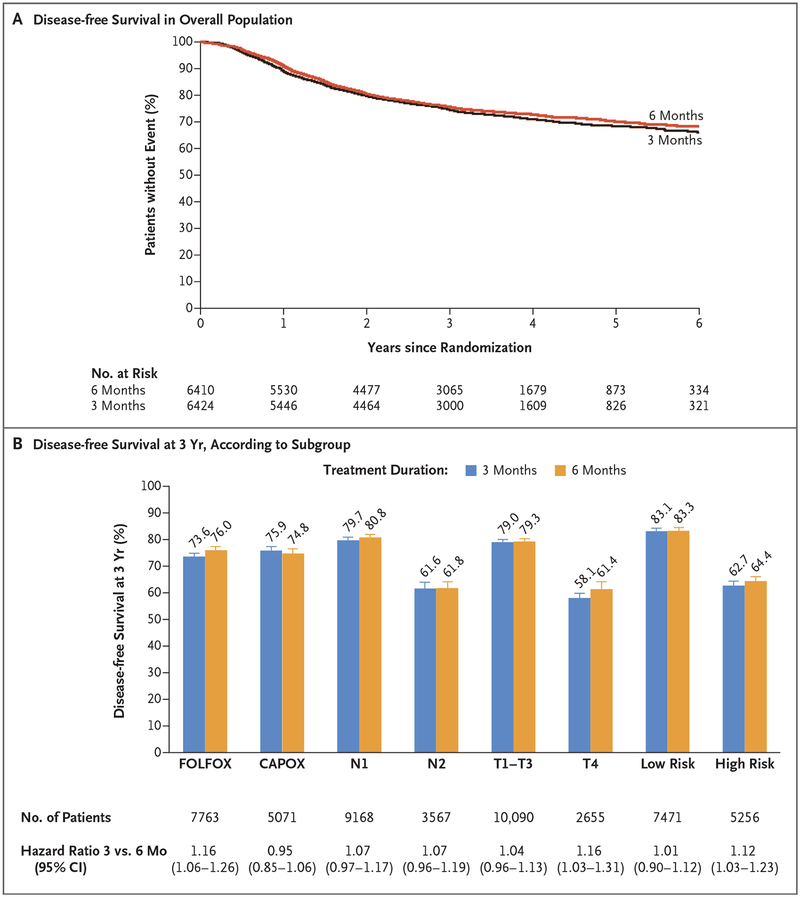
Figure 1. Disease-free Survival with 3 Months versus 6 Months of Adjuvant Therapy.
Panel A shows the distribution of disease-free survival in the overall modified intention-to-treat population. At a median follow-up of 41.8 months, noninferiority of 3 months of treatment versus 6 months was not confirmed (hazard ratio, 1.07; 95% confidence interval [CI], 1.00 to 1.15; P = 0.11 for noninferiority of 3-month therapy; P = 0.045 for superiority of 6-month therapy). The 3-year rate of disease-free survival was 74.6% (95% CI, 73.5 to 75.7) in the 3-month therapy group, as compared with 75.5% (95% CI, 74.4 to 76.7) in the 6-month therapy group. Panel B shows the 3-year rate of disease-free survival according to subgroup, including treatment, tumor and nodal status, and risk.
Adverse Events
A shorter duration of adjuvant therapy was associated with significantly lower rates of adverse events than a longer duration, independent of the chemotherapy regimen (Table 3, and Table S4 in the Supplementary Appendix). Neurotoxicity of grade 2 or higher during active therapy and in the month after cessation of treatment was substantially lower in the 3-month therapy group (16.6% with FOLFOX and 14.2% with CAPOX) than in the 6-month therapy group (47.7% with FOLFOX and 44.9% with CAPOX). In addition, rates of diarrhea, neutropenia, thrombocytopenia, nausea, mucositis, fatigue, and the hand–foot syndrome were also substantially lower with a shorter treatment duration.
Table 3.
Selected Adverse Events, According to Treatment and Duration of Therapy.*
| Adverse Event | FOLFOX | CAPOX | ||||||
|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 or 4 | P Value | Grade 1 | Grade 2 | Grade 3 or 4 | P Value | |
| number (percent) | number (percent) | |||||||
| Any adverse event | <0.001 | <0.001 | ||||||
| 3 mo | 1008 (30.7) | 1039 (31.6) | 1236 (37.6) | 496 (35.0) | 578 (40.8) | 342 (24.2) | ||
| 6 mo | 363 (11.0) | 1056 (32.1) | 1874 (56.9) | 203 (14.6) | 674 (48.5) | 512 (36.9) | ||
| Peripheral sensory neurotoxicity† | <0.001 | <0.001 | ||||||
| 3 mo | 2661 (83.4) | 450 (14.1) | 80 (2.5) | 1211 (85.8) | 164 (11.6) | 37 (2.6) | ||
| 6 mo | 1700 (52.2) | 1036 (31.8) | 519 (15.9) | 763 (55.0) | 500 (36.0) | 124 (8.9) | ||
| Diarrhea | <0.001 | 0.01 | ||||||
| 3 mo | 2611 (83.8) | 356 (11.4) | 147 (4.7) | 1171 (82.8) | 139 (9.8) | 104 (7.4) | ||
| 6 mo | 2525 (79.8) | 411 (13.0) | 227 (7.2) | 1090 (78.5) | 176 (12.7) | 122 (8.8) | ||
| Febrile neutropenia | 0.33 | 0.04 | ||||||
| 3 mo | 2897 (97.7) | 7 (0.2) | 62 (2.1) | 1407 (99.4) | 6 (0.4) | 2 (0.1) | ||
| 6 mo | 2933 (97.1) | 20 (0.7) | 68 (2.3) | 1373 (98.8) | 9 (0.6) | 8 (0.6) | ||
| Neutropenia | <0.001 | <0.001 | ||||||
| 3 mo | 1310 (66.4) | 264 (13.4) | 400 (20.3) | 898 (73.4) | 231 (18.9) | 94 (7.7) | ||
| 6 mo | 1087 (54.1) | 389 (19.4) | 534 (26.6) | 733 (61.2) | 321 (26.8) | 143 (11.9) | ||
| Thrombocytopenia | <0.001 | <0.001 | ||||||
| 3 mo | 1812 (92.0) | 139 (7.1) | 19 (1.0) | 1104 (90.3) | 93 (7.6) | 26 (2.1) | ||
| 6 mo | 1703 (85.0) | 264 (13.2) | 37 (1.8) | 966 (80.7) | 181 (15.1) | 50 (4.2) | ||
| Nausea | <0.001 | 0.02 | ||||||
| 3 mo | 1729 (87.6) | 213 (10.8) | 31(1.6) | 1070 (87.4) | 117 (9.6) | 37 (3.0) | ||
| 6 mo | 1636 (81.5) | 327 (16.3) | 45 (2.2 | 997 (83.3) | 163 (13.6) | 37 (3.1) | ||
| Vomiting | 0.29 | 0.91 | ||||||
| 3 mo | 1863 (94.6) | 82 (4.2) | 25 (1.3) | 1151 (94.0) | 48 (3.9) | 25 (2.0) | ||
| 6 mo | 1878 (93.6) | 101 (5.0) | 27 (1.3) | 1119 (93.5) | 62 (5.2) | 16 (1.3) | ||
| Mucositis | <0.001 | 0.007 | ||||||
| 3 mo | 1029 (95.2) | 44 (4.1) | 8 (0.7) | 1085 (97.1) | 29 (2.6) | 3 (0.3) | ||
| 6 mo | 1005 (91.4) | 76 (6.9) | 18 (1.6) | 1050 (95.1) | 44 (4.0) | 10 (0.9) | ||
| Fatigue | <0.001 | <0.001 | ||||||
| 3 mo | 1722 (87.4) | 215 (10.9) | 34 (1.7) | 1130 (92.3) | 82 (6.7) | 12 (1.0) | ||
| 6 mo | 1594 (79.6) | 327 (16.3) | 82 (4.1) | 1034 (86.4) | 129 (10.8) | 34 (2.8) | ||
| Hand-foot syndrome | 0.03 | <0.001 | ||||||
| 3 mo | 307 (98.7) | 4 (1.3) | 0 | 654 (94.4) | 34 (4.9) | 5 (0.7) | ||
| 6 mo | 294 (96.1) | 11 (3.6) | 1 (0.3) | 593 (86.2) | 77 (11.2) | 18 (2.6) | ||
Listed are the maximal grades of adverse events that were reported during the treatment period. In addition to the listed grades of adverse events, 19 patients had grade 5 events. In the SCOT trial, data regarding adverse events were collected only for the first 617 patients who were enrolled. P values are for the overall comparison of the three grade levels. All the P values of patients for each adverse event is provided in Table were calculated by means of the chi-square test for trend. An expanded version of this table that includes the denominators S4 in the Supplementary Appendix.
The listed grades of peripheral sensory neurotoxicity represent the maximal levels at any time after randomization.
Subgroup Analysis According to Treatment
Although noninferiority of 3 months, as compared with 6 months, of therapy could not be confirmed in the overall population, prespecified subgroup analyses revealed clinically relevant findings according to treatment. Among the patients who received FOLFOX, 6 months of adjuvant therapy was superior to 3 months (hazard ratio, 1.16; 95% CI, 1.06 to 1.26; P = 0.001 for superiority of 6-month therapy) (Fig. 1B, and Fig. S5A in the Supplementary Appendix), with a difference in 3-year disease-free survival rate of 2.4 percentage points for all stages combined (73.6% vs. 76.0%). However, among the patients who received CAPOX, the hazard ratio for disease-free survival for 3 months versus 6 months was 0.95 (95% CI, 0.85 to 1.06), which met the prespecified margin for noninferiority (Fig. 1B, and Fig. S5B in the Supplementary Appendix). The 3-year rates of disease-free survival were 75.9% and 74.8% for 3 months and 6 months of therapy with CAPOX, respectively. The interaction test according to treatment was highly significant (P = 0.006) (Fig. 2), with a P value of 0.02 after adjustment for multiple comparisons. Patients who received CAPOX versus FOLFOX had a higher rate of T4 disease (24.3% vs. 18.6%, P<0.001), but no significant differences were seen in the nodal stage, sex, or the number of lymph nodes that were examined.
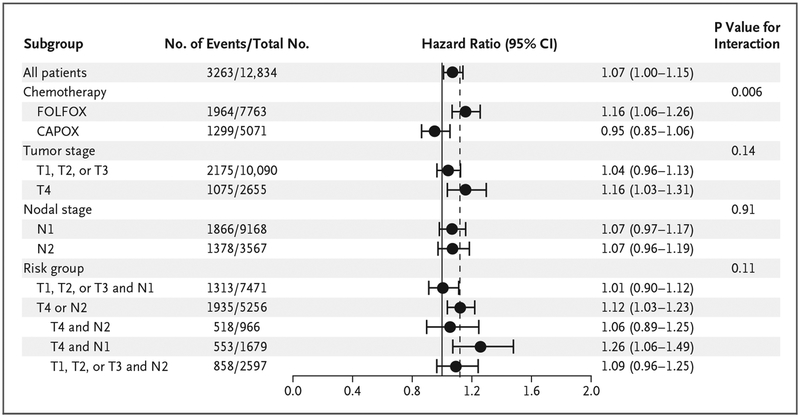
Figure 2. Disease-free Survival with 3 Months versus 6 Months of Therapy, According to Subgroup.
Among the subgroups of patients who were evaluated for disease-free survival, noninferiority of 3 months of therapy versus 6 months was confirmed only in the patients who had received CAPOX (hazard ratio, 0.95; 95% CI, 0.85 to 1.06) in the main analysis and in the patients at low risk (T1, T2, or T3 N1 disease) (hazard ratio, 1.01; 95% CI, 0.90 to 1.12) in an exploratory analysis. The test for interaction according to treatment was highly significant (P = 0.006), but the overall test for interaction according to risk group was not (P = 0.11). The interaction with therapy duration was not significant for tumor stage (P = 0.14) or for nodal stage (P = 0.91). The dashed vertical line indicates the noninferiority margin of 1.12 for the upper boundary of the two-sided 95% confidence interval.
Subgroup Analysis According to Tumor and Nodal Stage
There was no significant difference in the observed hazard ratio for 3 months versus 6 months of therapy between patients with N1 tumors (involving ≤3 positive nodes) (hazard ratio, 1.07; 95% CI, 0.97 to 1.17) and those with N2 tumors (involving ≥4 nodes) (hazard ratio, 1.07; 95% CI, 0.96 to 1.19; P = 0.91 for interaction) (Fig. 2). In patients with T4 cancers, a therapy duration of 3 months was inferior to a duration of 6 months (hazard ratio, 1.16; 95% CI, 1.03 to 1.31). Among all the patients with T1, T2, or T3 cancers, the upper boundary of the two-sided 95% confidence interval for 3 months of therapy was 0.01 higher than the prespecified noninferiority margin (hazard ratio, 1.04; 95% CI, 0.96 to 1.13) (Fig. 1B, and Fig. S5C and S5D in the Supplementary Appendix). The interaction between the duration of therapy and T stage was not significant (P = 0.14 for interaction).
Since patients with T4 or N2 cancers had a very similar, poor prognosis (3-year disease-free survival of approximately 60% vs. 80% for other stages), in an exploratory analysis, we investigated the effect of a shorter duration of therapy among patients at low risk (T1, T2, or T3 and N1 cancers, in 58.7% of patients) and among those at high risk (T4, N2, or both, in 41.3%) (Fig. 1B). Among the patients with low-risk cancers, 3 months of therapy was noninferior to 6 months of therapy (hazard ratio, 1.01; 95% CI, 0.90 to 1.12), with 3-year rates of disease-free survival of 83.1% and 83.3%, respectively. However, among the patients with high-risk cancers, 6 months of therapy was superior to 3 months (hazard ratio, 1.12; 95% CI, 1.03 to 1.23; P = 0.01 for superiority), even though the absolute difference in the 3-year rate of disease-free survival was 1.7 percentage points between 3 months (62.7%; 95% CI, 60.8 to 64.6) and 6 months (64.4%; 95% CI, 62.6 to 66.4) (Fig. 1B, and Fig. S5E in the Supplementary Appendix). Of note, the interaction between therapy duration and risk group was not significant (P = 0.11 for interaction) (Fig. 2).
Subgroup Analysis According to Treatment and Risk Group
Among the patients with low-risk tumors, 3 months of therapy with CAPOX was noninferior to 6 months, with a 3-year rate of disease-free survival of 85.0% versus 83.1% (hazard ratio, 0.85; 95% CI, 0.71 to 1.01). Even among the patients with high-risk tumors, 3 months of therapy with CAPOX compared favorably with 6 months but missed the noninferiority margin, possibly owing to the number of patients in the group, with a 3-year rate of disease-free survival of 64.1% versus 64.0% (hazard ratio, 1.02; 95% CI, 0.89 to 1.17) (Table S5 and Fig. S2 in the Supplementary Appendix). Independent of risk group, outcomes after 3 months of FOLFOX therapy were worse than those after 6 months. Among the patients with high-risk tumors, 6 months of therapy with FOLFOX was superior to 3 months, with a 3-year rate of disease-free survival of 61.5% versus 64.7% (hazard ratio, 1.20; 95% CI, 1.07 to 1.3). The results were largely consistent among the individual trials (Fig. S3 in the Supplementary Appendix).
DISCUSSION
The IDEA collaboration was a prospectively conducted study involving 12,834 patients with stage III colon cancer who were enrolled in six individual trials and randomly assigned to receive either 3 months or 6 months of adjuvant therapy with oxaliplatin and a fluoropyrimidine. Robust data were generated regarding benefits and risks according to the duration of therapy in these patients. As expected, a shorter duration of adjuvant therapy was associated with a significantly lower incidence and severity of adverse events, especially neurotoxicity, but also symptomatic side effects such as the hand–foot syndrome, mucositis, nausea, fatigue, and diarrhea. These benefits have to be counterbalanced against any potential loss of efficacy. In this regard, although noninferiority was not confirmed for 3 months of therapy in the overall cohort, the difference in the 3-year rate of disease-free survival between 3 months and 6 months of therapy was 0.9 percentage points (95% CI, −2.4 to 0.6) in favor of longer therapy, which is a difference of limited clinical relevance. The definitive body of evidence provided by IDEA allows for the individualization of treatment duration on the basis of treatment, patient preference, and disease characteristics.
Noninferiority of a 3-month therapy duration was documented in subgroups on the basis of treatment (CAPOX or FOLFOX) and underlying risk. For all the patients who received CAPOX, a therapy duration of 3 months of adjuvant therapy was noninferior to a duration of 6 months, independent of disease stage and risk group. For FOLFOX, a therapy duration of 3 months was inferior to a duration of 6 months when all stages and risk groups were combined (Table S5 in the Supplementary Appendix).
The difference in the performance of the CAPOX and FOLFOX treatment regimens was an unexpected finding. Since no randomization according to treatment was performed, no clear statement can be made about whether either adjuvant treatment was superior. However, in consistent findings across the trials and tumor stages, an additional 3 months of CAPOX did not improve survival outcomes. For patients receiving FOLFOX, a longer duration of therapy increased the rate of disease-free survival, particularly among patients with high-risk cancers. One hypothesis is that adherence and the overall dose intensity of a 6-month duration of an oral therapy might attenuate over time, so that the absence of difference between 3 months and 6 months of CAPOX might be due to a reduced overall dose intensity in the 6-month therapy group. In IDEA, the documented dose intensity of capecitabine in the 6-month therapy group did not differ significantly from that of fluorouracil, but the data were not based on rigorous diary-based documentation. An alternative explanation relates to the specific dosing schedule of oxaliplatin (130 mg per square meter of body-surface area every 3 weeks with CAPOX versus 85 mg per square meter every 2 weeks with FOLFOX) and especially the fluoropyrimidine regimen (capecitabine twice daily for 2 of every 3 weeks with CAPOX vs. a 46-hour infusion of fluorouracil every 2 weeks with FOLFOX). The protracted delivery of a fluoropyrimidine with CAPOX might have been more effective than the twice-monthly infusions with FOLFOX as an adjuvant therapy, a hypothesis that has been supported in previous prospective trials.13,14 Further propensity analyses will address potential selection biases regarding the choice of adjuvant therapy regimen. Of note, none of the patients who were treated at centers in the United States received CAPOX. Given the documented side effects of oral fluoropyrimidines among U.S. patients,15 the use of CAPOX in these patients will require careful monitoring.
Our exploratory analysis suggests that a risk-based approach toward determining the duration of adjuvant therapy may be warranted. In a lower-risk group (defined as patients with T1, T2, or T3 N1 disease), 3 months of adjuvant therapy appeared to be sufficient, especially when CAPOX was chosen. In a higher-risk group (patients with T4, N2, or both), longer treatment may be appropriate, especially when FOLFOX is the chosen regimen.
Our study has several limitations. The six trials were conducted in heterogeneous settings, in different countries, and by independent clinical trial groups. Subgroup analyses were performed without adjustment for multiplicity. According to the study design, we did not test the discontinuation of oxaliplatin at 3 months while continuing a fluoropyrimidine, a common clinical practice. The study design did not call for patients to undergo a secondary randomization to CAPOX or FOLFOX, since no significant difference in effect was expected. This lack of randomization resulted in substantial differences in the use of the two treatments among the trials. Also, in the six trials, there were no standardized follow-up procedures, including intervals of imaging and laboratory assessments. In addition, there were substantial differences in the duration of follow-up, so that the contribution of events to the overall analysis were not homogeneously distributed among the trials over time. Since every trial met a median 3-year follow-up time for disease-free survival, the 3-year rate of disease-free survival can be used as a reasonable estimate for the performance of the drugs in the overall study group. Although data on overall survival are not yet mature, the 3-year rate of disease-free survival has been shown to be a surrogate for the 5-year rate of overall survival in a pooled analysis of adjuvant trials and is accepted as a regulatory end point.16
The data presented here provide a framework for individual discussions between patients and oncologists regarding potential trade-offs between side effects and efficacy of adjuvant therapy. IDEA represents an academic collaboration with an independent data center, without commercial support. The results are relevant for the 400,000 patients worldwide in whom stage III colon cancer is diagnosed annually and for whom adjuvant oxaliplatin-based therapy can be considered.
In conclusion, among patients with stage III colon cancer who were receiving adjuvant therapy with FOLFOX or CAPOX, the noninferiority of a 3-month duration of therapy, as compared with a 6-month duration, was not confirmed. However, the results were strongly affected by the selected treatment and risk group. In patients treated with CAPOX, 3 months of therapy was as effective as 6 months, particularly in the lower-risk subgroup. In patients treated with FOLFOX, 6 months of therapy resulted in a higher rate of disease-free survival, particularly in the high-risk subgroup. These data suggest that the choice of treatment regimen, duration of therapy, and characteristics of the patients may be balanced against the substantial risk of increased toxicity of longer oxaliplatin-based therapy, including persistent neurotoxicity.
Supplementary Material
Acknowledgments
CALGB/SWOG 80702 was supported by grants (U10CA180821, U10CA180835, U10CA180882, and U10CA180888) from the National Cancer Institute; IDEA France by Institut National du Cancer and a grant (PHRC2009) from Programme Hospitalier de Recherche Clinique en Cancérologie; SCOT by a grant (EME 09/800/34) from the National Institute for Health Research, Efficacy and Mechanism Evaluation, the National Institute for Health Research, Health Technology Assessment, and a grant (C1348/A15960) from Cancer Research United Kingdom; ACHIEVE by the Japanese Foundation for Multidisciplinary Cancer Treatment; TOSCA by a grant (FARM 5RWTWZ) from L’Agenzia Italiana del Farmaco; and HORG by the HORG Foundation.
Dr. Grothey reports receiving grant support and honoraria, paid to the Mayo Clinic, from Genentech/Roche, Boston Biomedical, Bayer, Amgen, Merck/MSD, and Boehringer Ingelheim and grant support, paid to the Mayo Clinic, from Eisai; Dr. Yoshino, receiving grant support from GlaxoSmithKline and Boeh-ringer Ingelheim; Dr. Taieb, receiving lecture fees, advisory board fees, and reimbursement for attending meetings from Amgen, Roche, Merck, and Servier and lecture fees and advisory board fees from Baxalta, Eli Lilly, Sanofi, Celgene, and Sirtex; Dr. Souglakos, receiving grant support, consulting fees, lecture fees, and advisory board fees, paid to his institution, from Amgen, Roche, and Merck, grant support, consulting fees, and lecture fees from Sanofi, consulting fees from Servier, and lecture fees from Merck/MSD; Dr. Shi, receiving consulting fees and honoraria from Bayer HealthCare Pharmaceuticals; Dr. Labianca, receiving consulting fees from Roche, Sanofi Aventis, and Merck Serono; Dr. Meyerhardt, receiving consulting fees and advisory board fees from Genentech and honoraria from Chugai; Dr. Vernerey, receiving advisory board fees from HalioDx; Dr. Yamanaka, receiving honoraria from Chugai Pharma and Boehringer Ingelheim, grant support and honoraria from Takeda Pharma, and consulting fees from Gilead Sciences; Dr. Renfro, receiving consulting fees from Ignyta and advisory board fees from Bayer; Dr. Saunders, receiving advisory board fees and lecture fees from Roche, Merck, Sanofi, Amgen, and Servier and advisory board fees from Eisai; Dr. Andre, receiving lecture fees and consulting fees from Roche and Sanofi Aventis and lecture fees from Yakult and Baxter; and Dr. Iveson, receiving honoraria from Eli Lilly, advisory board fees and travel support from Servier, advisory board fees from Roche and Celgene, and travel support from Bayer. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We acknowledge the contributions of the late Irene Floriani and late Franck Bonnetain to this international collaboration.
References
- 1.André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004; 350: 2343–51. [DOI] [PubMed] [Google Scholar]
- 2.André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009; 27: 3109–16. [DOI] [PubMed] [Google Scholar]
- 3.Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011; 29: 1465–71. [DOI] [PubMed] [Google Scholar]
- 4.Kuebler JP, Wieand HS, O’Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol 2007; 25:2198–204. [DOI] [PubMed] [Google Scholar]
- 5.André T, de Gramont A, Vernerey D, et al. Adjuvant fluorouracil, leucovorin, and oxaliplatin in stage II to III colon cancer: updated 10-year survival and outcomes according to BRAF mutation and mismatch repair status of the MOSAIC study. J Clin Oncol 2015; 33: 4176–87. [DOI] [PubMed] [Google Scholar]
- 6.Grothey A Oxaliplatin-safety profile: neurotoxicity. Semin Oncol 2003; 30: Suppl 15: 5–13. [DOI] [PubMed] [Google Scholar]
- 7.André T, Iveson T, Labianca R, et al. The IDEA (International Duration Evaluation of Adjuvant Chemotherapy) collaboration: prospective combined analysis of phase III trials investigating duration of adjuvant therapy with the FOLFOX (FOLFOX4 or modified FOLFOX6) or XELOX (3 versus 6 months) regimen for patients with stage III colon cancer: trial design and current status. Curr Colorectal Cancer Rep 2013; 9: 261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alberts SR, Sargent DJ, Nair S, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA 2012; 307: 1383–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Gramont A, Van Cutsem E, Schmoll HJ, et al. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncol 2012; 13: 1225–33. [DOI] [PubMed] [Google Scholar]
- 10.Allegra CJ, Yothers G, O’Connell MJ, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J Clin Oncol 2011; 29: 11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haller DG, Catalano PJ, Macdonald JS, et al. Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: final report of Intergroup 0089. J Clin Oncol 2005; 23: 8671–8. [DOI] [PubMed] [Google Scholar]
- 12.Therneau T, Grambsch P. Modeling survival data: extending the Cox model. New York: Springer, 2000. [Google Scholar]
- 13.Chau I, Norman AR, Cunningham D, et al. A randomised comparison between 6 months of bolus fluorouracil/leucovorin and 12 weeks of protracted venous infusion fluorouracil as adjuvant treatment in colorectal cancer. Ann Oncol 2005; 16: 549–57. [DOI] [PubMed] [Google Scholar]
- 14.Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 2005; 352: 2696–704. [DOI] [PubMed] [Google Scholar]
- 15.Haller DG, Cassidy J, Clarke SJ, et al. Potential regional differences for the tolerability profiles of fluoropyrimidines. J Clin Oncol 2008; 26: 2118–23. [DOI] [PubMed] [Google Scholar]
- 16.Sargent DJ, Wieand HS, Haller DG, et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol 2005; 23: 8664–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.