Abstract
Purpose of Review.
This review summarizes the recent epidemiologic literature examining environmental exposures and health outcomes in rural, minority populations in the southwestern and mountain west region of the United States identifying areas requiring further data and research.
Recent Findings.
Recent studies (2012–2017) in this region have primarily focused on arsenic exposure (n=10 studies) with similar results reported across populations in this region. Associations between arsenic and cadmium were reported for cardiovascular and kidney disease, type II diabetes, cognitive function, hypothyroidism, and increased prevalence and mortality for lung and other cancers. Also in this review are studies of exposure to particulate matter, environmental tobacco smoke, pesticides and fungicides, heat and ozone.
Summary.
Although small, the current literature identified in this review report consistent adverse health outcomes associated with particulate matter, arsenic, cadmium, and other exposures among rural, minority populations in the southwest/mountain west region of the U.S. This literature provides important insight into the environmental exposures and health effects experienced by the rural populations in these regions. Additional studies that identify sources of environmental exposure are needed. Greater representation of the rural and minority populations from this region into large health studies also remains a need.
Keywords: environmental epidemiology, environmental exposure, rural population, minority population, western United States, southwestern United States
Introduction
The southwestern/mountain west region of the United States, rich in minerals and other natural resources, played a significant role in the frontier history of the country. The population of this region includes a large proportion of Hispanics and Native Americans [1]. In fact, almost one-half of the US Native American population live in the western region [2]. Because the region is sparsely populated [3], it is the beneficiary of relatively few epidemiology studies assessing the association of environmental exposures and health outcomes. Our objective is to conduct a systemized review [4] of the environmental epidemiology studies conducted among rural, minority populations in southwestern/mountain west region of the U.S. published between mid 2012 and mid 2017.
Methods
Database search and eligibility criteria
We developed an a priori protocol which guided our search and inclusion/exclusion criteria by which we judged eligibility of study inclusion. We performed searches in PubMed, EMBASE, CINAHL, PsychINFO, Cochrane Library, Web of Science, Scopus, and the Native Health Database during August 2017 using controlled and keyword terms for Environmental Exposure, Epidemiology, Rural, and Minority. We also performed hand searching of reference lists, the National Institute of Environmental Health Sciences website, and Google Scholar. Similar to the other reviews in this issue, we limited our searches in each database to those studies published in the five years between July 2012 and June 2017 in English. Full search strategies for each database are available in supplemental information.
Studies were eligible for inclusion if they were environmental epidemiological studies in rural populations; conducted in the Southwest and Mountain West geographical locations of the U.S., in an area including the states of Arizona (AZ), Colorado (CO), Nevada (NV), New Mexico (NM), Texas (TX), and Utah (UT), and reported a clinical health outcome related to an environmental exposure. Studies that were conducted in urban locations were excluded, as were studies of pre-clinical biomarkers. After a pilot round to calibrate agreement, two investigators independently screened titles and abstracts against the exclusion criteria. Three investigators then screened full-text articles against the inclusion criteria. We managed this process with the systematic review application Rayyan [5].
Data Abstraction, evaluation, and synthesis
Data were abstracted from the records to capture exposure, study design, location and population, health outcome(s), covariates and main results. The main results during the article evaluation and selection phase were defined as the measures of association between exposure and health outcome reported in the abstract and along with the confidence interval or significance level. Records were classified by exposure type and the data organized in tables.
Results
Search Results
Our search identified 1413 studies via database searching and hand-searching relevant publications. After removing duplicates, 977 records remained to be screened by review of the title and abstract. This resulted in exclusion of 910 that with closer review did not meet our inclusion criteria of including an environmental exposure and a health outcome and a rural population from our study region. After screening the remaining studies by reading the full text (N=67), we further excluded 46 that did not fully fit the above inclusion criteria, as they were either not epidemiological studies (n=27), not a rural population (n=7), not a minority population (n=9), and were outside of the geographical region of interest (n=3). Twenty one studies were included in our final narrative synthesis. Figure 1 is a flowchart of the search, screening, and inclusion/exclusion process. Tables 1a-1d describe the data organized by exposure category: air, water, biomarker, and all other exposures.
Figure 1.
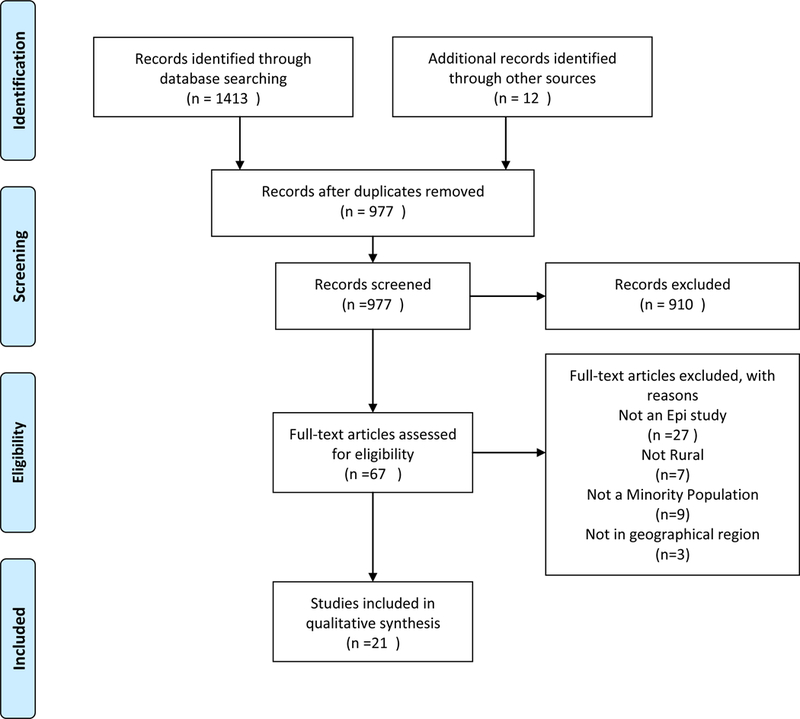
Flowchart of the search, screening, and inclusion/exclusion process. From [6]. For more information, visit www.prisma-statement.org
Table 1.
Summary of environmental epidemiology studies among rural, minority populations in the southwest/mountain west region of the United States published between 2012 and 2017 by environmental or biological media used to assess exposure.
| Table 1.a. Air. | |||||||
|---|---|---|---|---|---|---|---|
| Reference | Study Location | Sample size (source) | Sample Characteristics | Exposure | Health Outcome | Covariates | Results |
| Rajkumar, et al. 201710 | Arizona (AZ), Oklahoma (OK), South Dakota (SD), North Dakota (ND) | n=1843 (Strong Heart Study) | 1254 smokers, 1843 non-smokers, Adult Native Americans, age: 45– 74 years, 62.8% women non-smokers |
Environmental tobacco smoke (ETS), dietary factors (vitamin E, vitamin C, fiber, β-carotene, total polyunsaturated fatty acids) | Cardiovascular disease (CVD) | Age, sex, smoking history, albuminuria, total calorie intake. | Elevated hazard ratio for CVD among ETS exposed versus unexposed (HR 1.22; 95% Ci, 1.03 to 1.44). Stronger effects of ETS on CVD among those consuming diets low in vitamin E. |
| Rodopoulou, et al. 20149 | Las Cruces and Dona Ana Counties, New Mexico (NM) | n= 4739 Emergency room visits; n=2381 hospital admissions (Memorial Medical Center 2007–2010) | Adults > 18 years, 46.7% Hispanic | PM10; PM10–2.5; PM2.5; Ozone (with and without lag, by season and regional source of air pollution) | Respiratory and cardiovascular emergency room (ER) visits and hospital admissions | Season, long-term pollutant trends, daily temperature, influenza outbreaks | Significant increase in cardiovascular ER visits for PM10 (3.1%) and PM10-(2.5%) in warm season (April–Sept). When high PM10 (4150 μg/m3) was excluded, significant increased incidence of respiratory ER visits reported for PM10 (3.2%) and PM2.5 (5.2%) |
| Table 1.b. Water | |||||||
|---|---|---|---|---|---|---|---|
| Reference | Study Location | Sample size (source) | Sample Characteristics | Exposure | Health Outcome | Covariates | Results |
| Edwards et al. 20146 | 3 rural counties in west Texas (TX) | n=527 participants in the Facing Rural Obstacles Now Through Intervention, Education and Research (FRONTIER) cohort | Adults age 40–96 years, 42% Hispanic | Arsenic (As) and selenium (Se) in residential groundwater (used for drinking and cooking) estimated by inverse distance weighted interpolation | Cognitive function (language, delayed memory, executive functioning) | Age, ethnicity, gender, education, smoking status, AS3MT (rs10748835) polymorphism |
Low-level As exposure was negatively associated with cognitive functioning including language (p < 0.001) and executive functioning (p < 0.001). AS3MT gene modified the risk |
| Gong et al. 20127 | 3 rural counties in west Texas (TX) | N=499 participants (FRONTIER) | Adults from FRONTIER cohort (mean 61 years), 68% women, 41% Hispanic | Interpolated residential groundwater As | Coronary heart disease (CHD), hypertension, or hyperlipidemia | Age, ethnicity, gender, education, smoking, alcohol, anti-hyperlipidemia medication, AS3MT (rs10748835) | Higher As associated with CHD (p<0.05) and AS3MT genotype GG vs. AA (p<0.05). Hypertension associated with higher As. Hyperlipidemia associated with AS3MT genotype AG vs. AA (p<0.05). |
| Gong, G. 20158 | 3 rural counties in west Texas (TX) | n=726 participants in Facing rural obstacles now through intervention, education and research (FRONTIER) | Adults from FRONTIER cohort, 53% Hispanic, 69% female | Interpolated As and iodine levels in residential groundwater | Hypothyroidism | Age, gender, annual household income, and health insurance coverage | As concentrations >8 mg/L and cumulative As exposure significant predictors for hypothyroidism among Hispanics. Ethnic difference may be due to higher percentage of Hispanics (p=0.0622) living in areas with arsenic >8 mg/L compared to non-Hispanic Whites. Prevalence of hypothyroidism was significantly higher in this rural cohort than the US population |
| Table 1.c. Biomonitoring | |||||||
|---|---|---|---|---|---|---|---|
| Author (date) | Study Location | Sample size (source) | Sample Characteristics | Exposure | Health Outcome | Covariates | Results |
| Franceschini, et al. 201723 | Arizona (AZ), Oklahoma (OK), South Dakota (SD), North Dakota (ND) | N= 3714 (Strong Heart Study) | Adult Native Americans; age: 45–74 years; enrolled from 1989 to 1991; mean age 56 years, 59% female |
Urinary cadmium (Cd) | Hypertension | Age, sex, geographic area, body mass index, smoking, and kidney function | Urinary Cd significantly associated with higher systolic blood pressure (p=0.002). Significant association present among light- and never-smokers P=0.002), but not among never-smokers (p=0.18). Cd was also associated with diastolic blood pressure among light- and never-smokers (p=0.004). |
| Garcia-Esquinas, et al. 201320 | AZ, OK, SD, ND | n= 3932; 386 overall cancer deaths (Strong Heart Study) |
Adult Native Americans; age: 45–74 years; followed through 2008; 58.5% female |
Urinary inorganic arsenic (iAs) and total As (iAs and methylated As) | Cancer mortality | Sex, age, education, smoking, alcohol use, BMI, Breast was adjusted for menopausal status and parity, Kidney was adjusted for glomerular filtration rate | Comparing the 80th versus 20th percentiles of As, hazard ratio (HR, 95% CI) for overall cancer 1.14 (0.92–1.41), lung cancer HR 1.56 (1.02–2.39), liver cancer HR 1.34 (0.66, 2.72), prostate cancer HR 3.30 (1.28–8.48), kidney cancer HR 0.44 (0.14, 1.14), pancreatic cancer HR 2.46 (1.09–5.58), lymphatic and hematopoietic cancers HR 0.46 (0.22–0.96). No association between As and cancers of the esophagus and stomach, colon and rectum or breast. |
| Garcia-Esquinas, et al. 201424 | AZ, OK, SD, ND | n= 3792; (Strong Heart Study) | Adult Native Americans; age: 45–74 years; 59.4% female |
Urinary cadmium (Cd) | Cancer mortality | Sex, age, smoking status, cigarette pack-years, and body mass index | Comparing the 80th versus 20th percentiles of Cd, Hazard ratios (HR, 95% CI) for total cancer HR 1.30 (1.09, 1.55), lung cancer HR 2.27 (1.58, 3.27), pancreatic cancer HR 2.40 (1.39, 4.17), and for all smoking-related cancers combined, HR was 1.56 (95% CI: 1.24, 1.96). in mediation analysis, estimated % lung cancer deaths among smokers attributed to Cd was 9.0% (2.8%, 21.8%). |
| Gribble, et al. 201317 | AZ, OK, SD, ND | n= 3925 total; n= 1939 diabetics and n=1986 non-diabetics (Strong Heart Study) |
Adult Native Americans; age: 45–74 years; 58.9% female |
Percentages of urinary inorganic (iAs), methylarsonate (MMA) and dimethylarsinate (DMA) As species | Body mass index (BMI), percent body fat and fat free mass | Age, sex; education, alcohol use; smoking; BMI, geographical regions and urine creatinine | Higher mean %DMA and lower mean %MMA were associated with elevated BMI. %DMA was 2.4% (2.1, 2.6) higher per increase in BMI category, and %MMA was 1.6% (1.4, 1.7) lower. Similar patterns were observed for % body fat, fat free mass, and waist circumference, However these associations were attenuated or disappeared when adjusted for BMI. |
| Gribble, et al. 201216 | AZ, OK, SD, ND | n= 3925 total; n= 1939 diabetics and n=1,986 non-diabetics (Strong Heart Study) |
Adult Native Americans; age: 45–74 years; Diabetic n= 1939, 62.8% female; Non-diabetic n=1986, 55.7% female |
Urinary total As | Type II diabetes | Age, sex; education, alcohol use, smoking; BMI, geographic region, urinary creatinine | Comparing the 75th versus 25th percentiles of total As excretion, the prevalence ratio for diabetes was 1.14 (95% CI 1.08, 1.21). |
| Kim, et al. 201314 | AZ | n= 300; Type II Diabetes n= 150 (Strong Heart Study, AZ only) |
Adult Native Americans; cases diagnosed between 1982–2007: mean age 33.0 (SD ± 6.9) years; controls mean age 31.6 (SD ± 8.0) years | Urinary total arsenic (As) and inorganic As (iAs) | Type II diabetes | Age, sex, urine creatinine, BMI | Two-fold increase in total and iAs, elevated risk for incident diabetes OR 1.11 (95% CI 0.79 −1.57) and 1.16 (0.89 – 1.53) respectively. Positive relationship between quartiles of iAs and incident diabetes (p = 0.056); post-hoc comparison of quartiles 2–4 with quartile 1 revealed 2-fold higher odds of diabetes in the upper quartiles (OR = 2.14 (95% CI 1.19 to 3.85)). |
| Kuo, et al., 201515 | AZ, OK, SD, ND | n=1694 (Strong Heart Study) |
Adult Native Americans; age: 45–74 years; diabetes-free participants recruited in 1989–1991 and followed through 1998–1999, 53.0% female |
Total and speciated urinary As; and percent inorganic arsenic (iAs), monomethylarsonate (MMA), and dimethylarsinate (DMA) |
Type II diabetes, metabolic problem | Study center, age, sum of iAS and methylated arsenic concentrations, sex, education, smoking, alcohol consumption, BMI, and waist-to-hip ratio | Per 5% increase in MMA% the hazard ratio (95% CI) for diabetes incidence was 0.77(0.63–0.93) and 0.82 (0.73–0.92) when iAs% and DMA%, respectively, were not included. DMA% was associated with higher diabetes incidence only when MMA% decreased, but not when iAs% decreased. iAs% was associated with higher diabetes incidence when MMA% decreased. |
| Mateen, et al., 201713 | AZ, OK, SD, ND | n= 2402 baseline 1989–1991 cohort (Strong Heart Study) |
Adult Native Americans; age: 45–74 years; 63.1% female |
Total urinary arsenic (As) | Carotid intima media thickness (CIMT), atherosclerotic plaque in the carotid, and plaque score | Age, sex, education, smoking, body mass index, hypertension, diabetes, LDL cholesterol, and eGFR. | Comparing 80th vs. 20th percentile urinary As, the mean difference in CIMT was 0.01 mm (95%CI: 0.00, 0.02 mm), the relative risk of plaque presence was 1.04 (95%CI: 0.99, 1.09), and the geometric mean ratio of plaque score was 1.05 (95%CI: 1.01, 1.09). |
| Moon, et al. 201311 | AZ, OK, SD, ND | N=3575; CVD cases n=1184, fatal CVD n=439 (Strong Heart Study) |
Adult Native Americans; age: 45–74 years; 60.2% female | Urinary arsenic as a sum of inorganic arsenic (iAs), and methylated arsenic species at baseline enrollment | Incident CVD | Socio-demographic factors, smoking, body mass index, and lipids | Comparing the highest to lowest quartile As concentrations, the hazard ratios (95% CI) for cardiovascular disease, coronary heart disease, and stroke mortality after adjustment were 1.65 (1.20, 2.27; p trend< 0.001), 1.71 (1.19, 2.44; p-trend<0.001) and 3.03 (1.08, 8.50; p-trend=0.061). Hazard ratios for incident cardiovascular disease, coronary heart disease, and stroke were 1.32 (1.09, 1.59; p-trend=0.002), 1.30 (1.04, 1.62; p-trend=0.006), and 1.47 (0.97, 2.21; p-trend=0.032). |
| Newman, et al. 201612 | AZ, OK, SD, ND | N=2875 (Strong Heart Study) |
Adult Native Americans; age: 45–74 years; 48.6% female |
Sum of inorganic and methylated urinary arsenic (As) species at baseline enrollment | Peripheral arterial disease (PAD) measured by ankle brachial index (ABI) | Sex, age, education, smoking, body mass index, low-density lipoprotein cholesterol, diabetes, hypertension, and estimated glomerular filtration rate & study center | Comparing the highest to lowest tertiles of urinary As, hazard ratios (95% CI): were 0.57 (0.32, 1.01) for ABI <0.9 and 2.24 (1.01, 4.32) for ABI >1.4. Increased As methylation associated with 2-fold increased risk of ABI >1.4 (HR 2.04 (1.02, 3.41)). Long-term low-to-moderate total As and increased As methylation associated with ABI >1.4 but not ABI <0.9; where ABI< 0.9 indicative of atherosclerosis, and >1.4 associated with diabetes and chronic kidney disease |
| Tellez-Plaza, et al. 201323 | AZ, OK, SD, ND | n= 3348; CVD n=1084, and deaths n=400 (Strong Heart Study) |
Adult Native Americans; age: 45–74 years; 60% female |
Urinary cadmium (Cd) | Incident cardiovascular diseases and coronary heart disease (CHD) mortality |
Sex, postmenopausal status for women, education, body mass index (BMI), total cholesterol, estimated LDL cholesterol, hypertension, diabetes, and estimated GFR, smoking status | Comparing 80th to 20th percentile of Cd, Hazard Ratio (HR, 95% CI) was 1.43 (1.21–1.70) for cardiovascular mortality, 1.34 (1.10–1.63) for CHD mortality; 1.24 (1.11–1.38) for incident CVD, 1.22 (1.08–1.38) for coronary heart disease, 1.75 (1.17–2.59) for stroke and 1.39 (1.01–1.94) for heart failure. The associations were similar across subgroups, including never-smokers. |
| Tellez-Plaza, et al. 201322 | AZ, OK, SD, ND | n=2864; PAD Cases n=470 (Strong Heart Study) |
Adult Native Americans; age: 45–74 years; followed until 1999; 61.1% female | Urinary cadmium (Cd) | Incident peripheral arterial disease (PAD) measured by ankle brachial index (ABI) | Smoking status and pack-year | Hazard ratio (95% CI) comparing 80th to 20th percentile of Cd was 1.41 (1.05, 1.81) for PAD. Comparing the highest to lowest tertiles was HR1.96 (1.32, 2.81). |
| Zheng, et al. 201318 | AZ, OK, SD, ND | n=3821 (Strong Heart Study) |
Adult Native Americans; age: 45–74 years; 59.1% female | Total urinary arsenic (As) | Albuminuria defined as urinary albumin-creatinine ratio (ACR) | Sex, age, location, education, BMI, smoking, alcohol use, diabetes, systolic blood pressure, hypertension medication, eGFR | Comparing the three highest to lowest quartiles of As, Adjusted prevalence ratios for ACR were 1.16 (1.00–1.34), 1.24 (1.07–1.43), and 1.55 (1.35–1.78), respectively (p-value for trend <0.001). |
| Zheng, et al. 201519 | AZ, OK, SD, ND | N= 3119 (Strong Heart Study) |
Adult Native Americans; age: 45–74 years; no disease group - 57% female; prevalent CKD group - 77% female; incident CKD group - 68% female |
Total urinary arsenic (As) including inorganic (iAs), Monomethylarsonate (MMA) and dimethylarsinate (DMA) | Chronic kidney disease (CDK) defined as glomerular filtration rate ≤ 60, kidney transplant or on dialysis. |
Sex, age, location, education, smoking, BMI, hypertension medication, systolic blood pressure (SBP), diabetes status, and fasting glucose | The adjusted odds ratio (OR; 95% CI) of prevalent CKD for an interquartile range in total arsenic was 0.7 (0.6, 0.8), mostly due to an inverse association with iAs (OR: 0.4 [0.3, 0.4]) and MMA and DMA positively associated with prevalent CKD after adjustment for iAs (OR: 3.8 and 1.8). The adjusted hazard ratio (HR) of incident CKD for an IQR in total As was 1.2 (1.03, 1.41). The HR for iAs, MMA and DME were 1.0 (0.9, 1.2), 1.2 (1.00, 1.3), and 1.2 (1.0, 1.4) respectively. |
| Table 1.d. Other | |||||||
|---|---|---|---|---|---|---|---|
| Author (date) | Study Locaiton | Sample size (source) | Sample Characteristics | Exposure | Health Outcome | Covariates | Results |
| McKenzie, et al. 201425 | Rural Colorado (CO) | n=124842 birth records from Colorado Vital Birth Statistics | 73% Non-Hispanic White mothers, 49% female babies | Inverse distance weighting of natural gas wells found within a 10 mile buffer around maternal residence | Congenital heart defects (CHDs), neural tube defects (NTDs), oral clefts, preterm birth, and term low birth weight. | Adjusted for maternal ethnicity, infant sex, maternal smoking, maternal alcohol, parity, residential elevation, and maternal education | Compared with the absence of any gas wells within a 10-mile radius, exposure tertile associated with increased risk of CHD (OR=1.3 (1.2, 1.5)) and NTD (OR = 2.0 (1.0, 3.9)). Exposure was negatively associated with preterm birth and positively associated with fetal growth, although the magnitude of association was small. No association was found between exposure and oral clefts. |
| Zhang, et al. 201626 | Colorado (CO) Community and Migrant Health Centre (C/MHC) | n=14481 patient records including 150 migrant and 231 seasonal farm workers | 62% Hispanic for all patients, 87.7% and 86.6% Hispanic for migrant and seasonal farmworkers respectively | Daily mean apparent temperature (temperature and humidity), average daily ozone O3 | Daily heat versus all-cause or cardiovascular-specific clinic visits | Time trends and weekly patterns | Estimates of heat effects on average daily clinic visits among migrant farmworkers were positive (88.0%, 95% CI: 26.2% to 180.0%). For migrant farmworkers, heat had a stronger association for males, and their risk of going to a clinic increased by 118.1% on hot days compared to normal days. estimated per cent increases of excess risk on patient clinic visits were 88.0% without adjustment for O3 and 92.6% with adjustment for O3, respectively. |
Study Locations and Populations
The results included studies from eight states (AZ, CO, NM, TX, Oklahoma (OK), North Dakota (ND), and South Dakota (SD)). The study populations included 15 studies of Native Americans from AZ, OK, SD, and ND, and five studies of with large proportions of Hispanics from NM, CO, and TX (see Table 2). Five of these states were included among our original six target states, no studies in UT were found, and OK, ND and SD were included as these populations were aggregated with a large cohort from AZ and it was not possible to disaggregate the results. The population in that study cohort was Native American and rural, and shared many of the characteristics of our targets as well. Exposure and outcomes were evaluated with the same methods and the populations represented were all rural.
Table 2:
Summary of reviewed studies by gender, race/ethnicity, exposure, and health outcomes for adults and children
| Variable | Adults (18 years and older) | Children |
|---|---|---|
| Gender | Male and female6–26 | Male and female25 |
| Race/Ethnicity of Study Population | Native American10–24 Mixed6–9,25,26 |
Mixed25 |
| Exposure Media | Water6–8,11
Air9,10 Other22,27 |
Air26 Other25 |
| Exposure Measurement | Ambient Ozone26 Ambient PM9 Body composition17 Diet10 Drinking water7 GIS interpolation6,8 Daily Temperature26 Proximity to emission sources25 Environmental Tobacco Smoke10 Urinary metabolites11–16,18–26 |
Proximity to emission sources25 |
| Health Outcomes | Arsenic metabolism17 Blood pressure23 Cancer mortality20,24 Cardiovascular Disease7,9–13,21,22 Clinic visits26 Cognitive function6 Diabetes (Type II)14–16 Hypothyroidism8 Kidney disease19,26 Respiratory conditions9 |
Birth defects25 |
Seventeen studies represented the results of two population-based cohorts. Three of these 17 studies were included from the Facing Rural Obstacles Now Through Intervention, Education and Research (FRONTIER study), which was based in rural western TX and focused on Hispanics [6–8]. Fourteen of the 17 studies were included from the Strong Heart Study cohort of Native Americans living in AZ, OK, ND and SD.
Recent Findings
Particulate Matter and Environmental Tobacco Smoke
Mass concentration of ambient particulate matter (PM) less than or equal to 10 um in diameter (PM10) and PM10–2.5 (the mass difference between PM10 and PM2.5) were associated with an increase emergency room visits for cardiovascular events among adults during the warm season in a southern NM community adjacent to the U.S.-Mexico border [9]. Similar effects were noted for respiratory emergency visits when high PM10 (>4150 μg/m3) concentrations were excluded. Participants exposed to environmental tobacco smoke in the Strong Heart Study (n =1843 non-smoking Native Americans) were at increased risk for cardiovascular disease relative to unexposed participants as demonstrated in the hazard ratio of 1.22 (CI:1.03 – 1.44) [10]. Stronger effects were observed among participants who consumed low vitamin E diets compared to those consuming higher amounts, particularly on the additive scale.
Arsenic and Iodine
Cardiovascular Disease.
When analyzing urinary arsenic exposure markers among Strong Heart Study participants, Moon et al. [11] reported significant associations with cardiovascular disease incidence as well as mortality. Although long-term, low-to-moderate concentrations of urinary arsenic and increasing arsenic methylation did not impact atherosclerosis risk among Strong Heart Study participants [12], these species were associated with small increases in carotid intima media thickness and atherosclerotic plaque in the carotid [13]. In the FRONTIER study, arsenic exposure - quantified by interpolated residential groundwater concentrations - was associated with coronary heart disease and hypertension, while hyperlipidemia association was associated with an allelic variant of the AS3MT gene, but not water arsenic concentrations [7].
Kidney Disease and Metabolic Disorders.
Also in the Strong Heart Study, long-term, low-to-moderate urinary arsenic concentrations and increasing arsenic methylation significantly elevated the risk of diabetes and chronic kidney disease [12]. The sum of inorganic and methylated species combined also increased the risk for diabetes [14–16]. Higher relative levels of urinary dimethylated arsenic species (DMA) excretion and lower levels of monomethylated species (MMA) were associated with increased body mass index [17]. In the FRONTIER study, Gong et al. [8] investigated associations between arsenic and iodine groundwater concentrations and hypothyroidism among 726 participants. Arsenic in groundwater >8 µg/L and cumulative arsenic exposure were significant predictors for hypothyroidism among Hispanics.
Kidney Disease.
Independent findings of the inverse association of inorganic arsenic with the prevalence of chronic kidney disease as evidenced by elevated prevalence of albuminuria points toward the importance of the detoxication processes of accumulated body burden of arsenic [18]. In a follow up analysis, both MMA and DMA were associated with increased chronic kidney disease incidence [19].
Other Outcomes.
Comparing the 80th versus 20th percentiles of urinary arsenic concentrations in the Strong Heart Study cohort, concentrations of methylated and inorganic urinary arsenic were also shown to increase the risk for lung and prostate cancer mortality [20]. Edwards et al. [6] reported that exposure to low-level arsenic, based on estimated ground water concentrations in the FRONTIER study, was negatively associated with language (p < 0.001) and executive functioning (p < 0.001).
Cadmium
Urinary cadmium levels were associated with increased risk for cardiovascular and coronary heart disease mortality in the Strong Heart Study cohort when comparing the 80th to the 20th percentile of concentrations [21]. The associations were similar in most study subgroups, including never-smokers. Urinary cadmium concentrations were also associated with an increased risk for incident peripheral arterial disease in models adjusted for smoking [22], and elevated systolic blood pressure in models adjusted for kidney function [23]. Also in the Strong Heart Study, Garcia-Esquinas et al. [24] reported significant associations between urinary cadmium concentrations and total incidence of cancer, as well as increased mortality from lung cancer, pancreatic cancer, and all smoking-related cancers combined.
Other Exposures
Associations between residential proximity to natural gas development and birth outcomes were examined using 124,842 birth records from Colorado Vital Birth Statistics [25]. Prevalence of congenital heart defects increased with exposure tertile. Neural tube defect was associated with the highest tertile of exposure (>125 natural gas wells per mile) compared with no natural gas wells within a 10-mile radius. Exposure was negatively associated with preterm birth and positively associated with fetal growth and no association was found between exposure and oral clefts.
Zhang et al. [26] evaluated the effects of daily temperature, humidity and ozone on clinic visits in a retrospective electronic medical records review that included 380 clinic visits from Hispanic migrant and seasonal farmworkers to a community clinic in CO. Heat effects were associated with increased average daily clinic visits among migrant farmworkers, with stronger associations among male seasonal farmworkers. Increased excess risk on patient clinic visits were 88.0% without adjustment for ozone, and 92.6% with adjustment.
Conclusions
Although small, the current body of literature suggests that rural populations in the southwest, mountain west, and adjacent regions of the US experience exposures to environmental pollutants sufficient to result in elevated risks for numerous health outcomes despite living in areas with lower levels of many anthropogenic pollutants characteristic of urban areas. Sources of these pollutants may differ from those in urban areas, but exposures are still identifiable as are associated adverse health outcomes. Rural populations in this region can experience exposures from anthropogenic sources such as resource extraction, smoking, and wood smoke, as well as from naturally occurring contaminants such as arsenic in drinking water and particulate matter from windblown dust.
There are many inherent challenges to conducting environmental population studies in rural areas. Due to sparse population density, and low environmental exposure levels, it is a challenge to obtain sufficient sample size to achieve statistical power. Therefore, the environmental epidemiology studies represented in this review provide important insight into the burden environmental exposures and health effects experienced by the rural populations in this region, often by inclusion of numerous similar populations across multiple regions with similar exposures.
The body of literature identified and summarized in this review consistently reported adverse health outcomes associated with environmental exposure to particulate matter, arsenic, cadmium, and other agents for rural, minority populations. These findings were also supported by a large US-wide cancer prevention study analysis that reported that arsenic and other metals had significant contribution to health risks through particulate matter exposure pathways [27].
It is important to emphasize that there is still an existing research gap in understanding the mechanisms through which arsenic and its methylated products influence cardiovascular risk, mortality, and type II diabetes development resulting in consistently adverse, severe health effects. There remains opportunity to build on this work to further address the environmental and health disparities observed among these populations. Resource and land use patterns among rural and minority populations often differ from those in urban areas, and extrapolation to exposure may require different modeling methods.
Additional studies that more explicitly identify sources of contaminant exposure and provide multiple and consistent methods to identify exposures, including spatial analytical methodologies, are urgently needed. Large-scale land-use information that can integrate with community settings, outdoor recreational activities and potential toxicant patterns would aid future intervention efforts to reduce exposures. Additionally, more inclusion of rural and minority populations in larger health studies would further illustrate existing disparities among populations with the ultimate goal to find solutions decreasing such health inequities.
Supplementary Material
Acknowledgments
Funding:
This work was supported by National Institutes of Health grants 1P50ES026102, 1P42ES025589, and 1U54MD00481106, and Assistance Agreement No. 83615701 awarded by the U.S. Environmental Protection Agency to the University of New Mexico Health Sciences Center. This work has not been formally reviewed by EPA. The views expressed are solely those of the authors and do not necessarily reflect those of the Agency.
Footnotes
Human and Animal Rights. This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Melissa Gonzales, University of New Mexico Health Sciences Center, School of Medicine, Department of Internal Medicine, MSC10 5550, Albuquerque, NM, 87131.
Esther Erdei, University of New Mexico Health Sciences Center, College of Pharmacy, Department of Pharmaceutical Sciences, MSC09 5360 Albuquerque, NM, 87131.
Joseph Hoover, University of New Mexico Health Sciences Center, College of Pharmacy, Department of Pharmaceutical Sciences, MSC09 5360 Albuquerque, NM, 87131.
Jacob Nash, University of New Mexico Health Sciences Center, Health Sciences Library and Information Center, MSC09 5100, Albuquerque, NM, 87131..
References
- 1.Ennis SR, Ríos-Vargas M, Albert NG. The Hispanic Population: 2010: U.S. Census Bureau; 2011. [Google Scholar]
- 2.Norris T, Vines PL, Hoeffel EM. The American Indian and Alaska Native Population: 2010 U.S. Census Bureau; 2012. [Google Scholar]
- 3.United States Census Bureau: Resident Population Data (Text Version) https://www.census.gov/2010census/data/apportionment-dens-text.php. Accessed 2/22/2018.
- 4.Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Information & Libraries Journal 2009;26(2):91–108. doi: 10.1111/j.1471-1842.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- 5.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Systematic Reviews 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards M, Hall J, Gong G, O’Bryant SE. Arsenic exposure, AS3MT polymorphism, and neuropsychological functioning among rural dwelling adults and elders: a cross-sectional study. Environ Health 2014;13(1):15.**The combined effects of arsenic exposure and a genetic polymorphism related to metabolism were included in the analysis of congitive function among members of rural cohort with a large proportion of Hispanics.
- 7.Gong G, O’Bryant SE. Low-level arsenic exposure, AS3MT gene polymorphism and cardiovascular diseases in rural Texas counties. Environmental Research 2012;113:52–7. [DOI] [PubMed] [Google Scholar]
- 8.Gong G, Basom J, Mattevada S, Onger F. Association of hypothyroidism with low-level arsenic exposure in rural West Texas. Environmental research 2015;138:154–60.* The association of arsenic exposure and hypothyroidism is assessed in a rural cohort with a large proportion of Hispanics.
- 9.Rodopoulou S, Chalbot MC, Samoli E, DuBois DW, San Filippo BD, Kavouras IG. Air pollution and hospital emergency room and admissions for cardiovascular and respiratory diseases in Doña Ana County, New Mexico. Environmental Research 2014;129:39–46.* An extensive characterization of air pollution sources was developed the authors as a component of their exposure assessment. This study also included a large number of hospital visits and admissions across seasons in a catchment area located on the U.S.-Mexico border that included a large proportion of Hispanics.
- 10.Rajkumar S, Fretts AM, Howard BV, Yeh F, Clark ML. The relationship between environmental tobacco smoke exposure and cardiovascular disease and the potential modifying effect of diet in a prospective cohort among american indians: The strong heart study. International Journal of Environmental Research and Public Health 2017;14(5).** A robust anaysis of the modifying effects of diet on the associaiton between environmental tobacco smoke and cardiovascular disease among a large cohort of rural-dwelling Native Americans.
- 11.Moon KA, Guallar Dr E, Umans Dr JG, Devereux Dr RB, Best Dr LG, Francesconi Dr KA et al. Association between exposure to low to moderate arsenic levels and incident cardiovascular disease. Annals of Internal Medicine 2013;159(10):649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newman JD, Navas-Acien A, Kuo C-C, Guallar E, Howard BV, Fabsitz RR et al. Peripheral Arterial Disease and Its Association With Arsenic Exposure and Metabolism in the Strong Heart Study. Am J Epidemiol 2016;184(11):806–17.** The associaiton of arsenic exposure and metabolism are both examined in this study of peripheral artery disease in a large, rural cohort of Native Americans.
- 13.Mateen FJ, Grau-Perez M, Pollak JS, Moon KA, Howard BV, Umans JG et al. Chronic arsenic exposure and risk of carotid artery disease: The Strong Heart Study. Environmental research 2017;157:127–34.* The prospective cohort study design permitted the authors to assess the associaiton between chronic arsenic exposure and carotid artery disease among a large, rural Native American study population.
- 14.Kim NH, Mason CC, Nelson RG, Afton SE, Essader AS, Medlin JE et al. Arsenic exposure and incidence of type 2 diabetes in Southwestern American Indians. Am J Epidemiol 2013;177(9):962–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuo C-C, Howard BV, Umans JG, Gribble MO, Best LG, Francesconi KA et al. Arsenic exposure, arsenic metabolism, and incident diabetes in the strong heart study. Diabetes Care 2015;38(4):620–7.** The association of arsenic exposure and metabolism are both examined in this study of incident diabetes in a large, rural cohort of Native Americans.
- 16.Gribble MO, Howard BV, Umans JG, Shara NM, Francesconi KA, Goessler W et al. Arsenic exposure, diabetes prevalence, and diabetes control in the Strong Heart Study. Am J Epidemiol 2012;176(10):865–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gribble MO, Crainiceanu CM, Howard BV, Umans JG, Francesconi KA, Goessler W et al. Body composition and arsenic metabolism: a cross-sectional analysis in the Strong Heart Study. Environ Health 2013;12:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng LY, Umans JG, Tellez-Plaza M, Yeh F, Francesconi KA, Goessler W et al. Urine arsenic and prevalent albuminuria: Evidence from a population-based study. American Journal of Kidney Diseases 2013;61(3):385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng LY, Umans JG, Yeh F, Francesconi KA, Goessler W, Silbergeld EK et al. The association of urine arsenic with prevalent and incident chronic kidney disease: evidence from the Strong Heart Study. Epidemiology 2015;26(4):601–12.* The association of arsenic exposure is examined in this study of incident chronic kidney disease in a large, rural cohort of Native Americans.
- 20.García-Esquinas E, Pollán M, Umans JG, Francesconi KA, Goessler W, Guallar E et al. Arsenic exposure and cancer mortality in a US-based prospective cohort: the strong heart study. Cancer Epidemiol Biomarkers Prev 2013;22(11):1944–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tellez-Plaza M, Guallar E, Howard BV, Umans JG, Francesconi KA, Goessler W et al. Cadmium Exposure and Incident Cardiovascular Disease. Epidemiology 2013;24(3):421–9. doi: 10.1097/EDE.0b013e31828b0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tellez-Plaza M, Guallar E, Fabsitz RR, Howard BV, Umans JG, Francesconi KA et al. Cadmium exposure and incident peripheral arterial disease. Circ Cardiovasc Qual Outcomes 2013;6(6):626–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franceschini N, Fry RC, Balakrishnan P, Navas-Acien A, Oliver-Williams C, Howard AG et al. Cadmium body burden and increased blood pressure in middle-aged American Indians: The Strong Heart Study. Journal of Human Hypertension 2017;31(3):225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Esquinas E, Pollan M, Tellez-Plaza M, Francesconi KA, Goessler W, Guallar E et al. Cadmium exposure and cancer mortality in a prospective cohort: the strong heart study. Environ Health Perspect 2014;122(4):363–70. doi: 10.1289/ehp.1306587.** The prospective cohort study design permitted the authors to assess the associaiton between chronic cadmium exposure and cancer mortality among a large, rural Native American study population.
- 25.McKenzie LM, Guo R, Witter RZ, Savitz DA, Newman LS, Adgate JL. Birth outcomes and maternal residential proximity to natural gas development in rural Colorado. Environmental Health Perspectives 2014;122(4):412–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang K, Arauz RF, Chen T-H, Cooper SP. Heat effects among migrant and seasonal farmworkers: a case study in Colorado. Occupational and environmental medicine 2016;73(5):324–8. [DOI] [PubMed] [Google Scholar]
- 27.Thurston GD, Ito K, Lall R, Burnett RT, Turner MC, Krewski D et al. NPACT Study 4. Mortality and Long-Term Exposure to PM2.5 and Its Components in the American Cancer Society’s Cancer Prevention Study II Cohort. National Particle Component Toxicity (NPACT) Initiative: Integrated Epidemiologic and Toxicologic Studies of the Health Effects of Particulate Matter Components Boston, MA: Health Effects Institute; 2013. Report No.: 177. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


