Abstract
The emerging field of regenerative engineering offers a great challenge and an even greater opportunity for materials scientists and engineers. How can we develop materials that are highly porous to permit cellular infiltration, yet possess sufficient mechanical integrity to mimic native tissues? How can we retain and deliver bioactive molecules to drive cell organization, proliferation, and differentiation in a predictable manner? In the following perspective, we highlight recent studies that have demonstrated the vital importance of each of these questions, as well as many others pertaining to scaffold development. We posit hybrid materials synthesized by molecular decoration and molecular imprinting as intelligent biomaterials for regenerative engineering applications. These materials have potential to present cell adhesion molecules and soluble growth factors with fine-tuned spatial and temporal control, in response to both cell-driven and external triggers. Future studies in this area will address a pertinent clinical need, expand the existing repertoire of medical materials, and improve the field’s understanding of how cells and materials respond to one another.
Keywords: Regenerative engineering, Drug delivery, Bioconjugation, Molecular imprinting, Intelligent biomaterials
Lay Summary
Regenerative engineering seeks to combine our growing understandings of materials, stem cells, and developmental boilogy to generate therapeutic and curative treatments for a range of diseases. In this perspective, we discuss the utility and limitations of existing materials employed for regenerative engineering applications. These materials balance the dynamic need to provide mechanical strength, present therapeutic biomolecules, permit cell entry, and degrade over time. Then, we present recent developments in the field of materials science, which have generated hybrids of natural and synthetic origin. These blended, conjugated, and/or functionalized materials engage in intelligent and responsive interactions with the biological host. Specific interaction-response examples are discussed for the regeneration of nerve, bone, and cardiac muscle. In the future, intelligent materials for regenerative engineering will respond dynamically to signals produced by a patient’s cells or administered in a clinical intervention to facilitate tissue growth, healing, and recovery.
Introduction
Regenerative engineering calls for a “convergence” of advanced materials, stem cell science, and developmental biology to reconstruct complex tissues and organs and address the source of a diverse array of diseases [1]. For scientists and engineers, regenerative engineering offers unique applications for existing materials, generates new issues with dynamic cellbiomaterial environments, and challenges our understanding of natural tissue development, modification, and repair.
The challenge of regenerative engineering, for materials scientists in particular, lies in its inherent complexity. It is insufficient to design a new hydrogel biomaterial, for example, to yield a unique molecular architecture or a new physical property. Instead, a specific and rational purpose must guide material design; this material must interact with cells in a productive and therapeutic manner. Cells must adhere, spread, proliferate, and even differentiate, and they will rely on soluble or matrix-bound factors to guide these processes. Will this biomaterial be natural or synthetic in origin? How will cells respond to the environment provided by the material scaffold? Conversely, how will the material respond to cellular cues? How will the material overcome transport limitations in the delivery of growth factors, removal of waste products, and maintenance of normoxia?
Addressing these questions will involve the development of new and creative hybrid materials composed of natural and synthetic components. This development must be guided by an understanding of the established fundamentals of all disciplines, including materials, stem cell science, and developmental biology, as well as a willingness to explore new and complex assemblies. Finally, robust in vitro and in vivo assessments will be required to evaluate complex phenomena, including (but not limited to) cell adhesion and infiltration, protein retention and delivery, cell-driven scaffold modification, and scaffold-driven cell behavior.
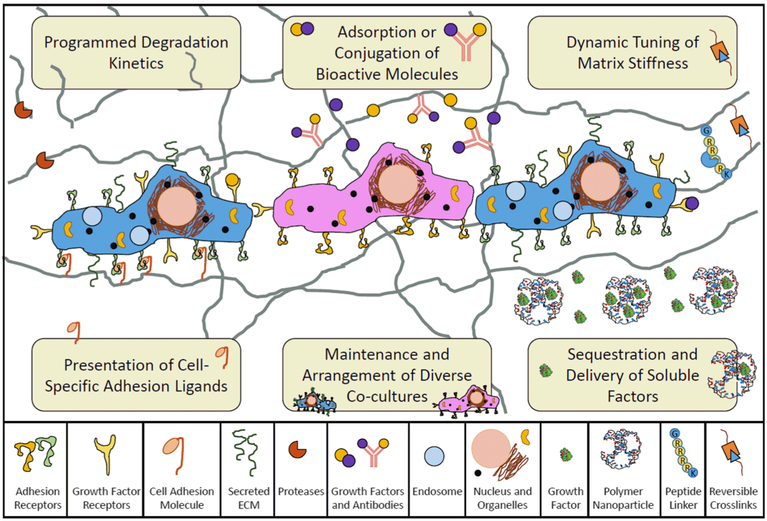
In the following perspective, we posit functionally decorated and molecularly imprinted polymers as intelligent biomaterial scaffolds. These materials have the potential to address various limitations of existing systems, including the presentation of cell adhesion molecules, maintenance of diverse cocultures, sequestration and delivery of soluble factors, dynamic tuning of matrix stiffness, and controlled degradation (Fig. 1). We explore the motivation and materials-based approaches for achieving each of these scaffold hallmarks. In particular, we highlight various advancements in molecularly decorated polymers, as well as polymers imprinted with cells or proteins, which address current challenges in regenerative engineering.
Fig. 1.
Intelligent biomaterial scaffolds must present growth factors and other molecules in a rational spatiotemporal manner, possess an appropriate modulus, and degrade with predictable and controlled kinetics in order to facilitate the specific cell functions of diverse co-cultures for regenerative engineering
Applications of Responsive and Recognitive Polymers in Regenerative Engineering
Critical Evaluation of Existing Scaffolds
Current tissue engineering strategies for regenerative engineering applications belong to three main categories. These include (i) injection of cells directly into the tissue of interest; (ii) implantation of a cell containing three-dimensional (3D) tissue structure; and (iii) implantation of a scaffold-based system that presents bioactive molecules to promote cell migration, proliferation, and differentiation [2]. The developed scaffolds must be biocompatible and biodegradable, offer similar mechanical properties to that of the tissue of interest, and provide a porous architecture to promote cell penetration and the diffusion of nutrients [3]. In addition, these scaffolds must mimic the host microenvironment where cells interact with and respond to the biochemical and biophysical cues obtained from the surrounding environment [4]. Thus, the material properties of these engineered constructs are critical in determining subsequent cell functions [4]. Challenges of existing regenerative engineering scaffolds include implementing finetuned material porosity while maintaining specific mechanical properties, retaining and delivering specific growth factors with ideal release profiles, and controlling cell arrangement within the tissue-engineered construct.
Development of Complex 3D Assemblies with Fine-Tuned Chemistry, Porosity, and Mechanical Properties
Scaffold porosity plays a vital role in directing tissue formation and function. A material must have sufficient porosity to allow cell penetration and the diffusion of oxygen and nutrients, which is particularly important in the absence of a vascular network. The degree of porosity also affects the mechanical properties of a material, with an increase in porosity resulting in a decrease in stiffness [5]. Furthermore, pore size and interconnectivity throughout the scaffold greatly influence cell growth and extracellular matrix secretion.
Hydrogels have emerged as leading tissue engineering constructs for regenerative engineering applications due to their biocompatibility and similarity to the native extracellular matrix [5]. However, control of specific 3D hydrogel properties, such as porosity, remains a challenge. Previous studies have employed several fabrication techniques to obtain interconnecting, highly porous, 3D scaffolds. These conventional fabrication techniques utilized to control the overall porosity of hydrogels include solvent casting/particle leaching, gas foaming, electrospinning, and freeze drying [6]. However, these methods do not allow the precise control of internal hydrogel architecture or the formation of complex structures [5]. In addition, these existing methods often produce scaffolds with poor mechanical properties and frequently require the use of toxic solvents [6]. Recently, advanced micropatterning and micromolding techniques have been developed to provide control over the hydrogel’s microarchitecture and pore features [5, 6]. Overall, control of complex features within hydrogels is necessary to guide the development and integration of these tissue engineered constructs with the native environment. We will highlight some of the bioconjugation chemistries that are particularly amenable to biomaterial patterning, as well as strategies for the dynamic tuning of scaffold stiffness, in the following sections.
Growth Factor Retention, Sequestration, and Delivery
The growth factor signaling system is vital for instructing specific cell functions. Cell-cell signaling in response to various growth factors regulates critical processes including cell migration, proliferation, and differentiation. The critical role of growth factors in maintaining and controlling many basic cell functions elucidates their use in scaffolds to promote and facilitate tissue regeneration. Many growth factors induce therapeutic outcomes, such as bone regeneration or neovascularization [2]. Methods of incorporating bioactive factors into polymer matrices include chemical immobilization and physical encapsulation. In the preparation of polymer-growth factor systems, it may be necessary to modify the polymer matrix chemically or to physically encapsulate growth factors in order to enhance the therapeutic efficiency [2]. The use of polymer matrices as growth factor carriers enables specific regulation of growth factor release rates. Through these mechanisms, scientists and engineers can optimize the presentation and local concentration of growth factors to promote tissue regeneration outcomes.
Researchers typically employ one of a variety of techniques to present growth factors within hydrogels. Two common strategies are physical adsorption and covalent immobilization. Adsorption of growth factors to polymeric matrices is primarily a result of secondary or intermediate interactions through intermediate proteins or molecules [2]. Release kinetics of these adsorbed growth factors are dependent on the strength of association between the bioactive factor and polymer, as well as environmental conditions (i.e., temperature, pH). Alternatively, covalent conjugation of growth factors to polymer carriers has the ability to achieve prolonged release. Polymerization or post-synthesis modification reactions can be utilized to incorporate these bioactive molecules covalently. A variety of fabrication techniques, on the other hand, such as solvent casting/particulate leaching, freeze drying, phase separation, phase emulsion, gas foaming, and in situ polymerization accomplish physical encapsulation of growth factors, where diffusion controls the kinetics of release. Alternate release profiles (i.e., polymeric structures capable of burst and sustained release) can be achieved by combining fabrication methods [2].
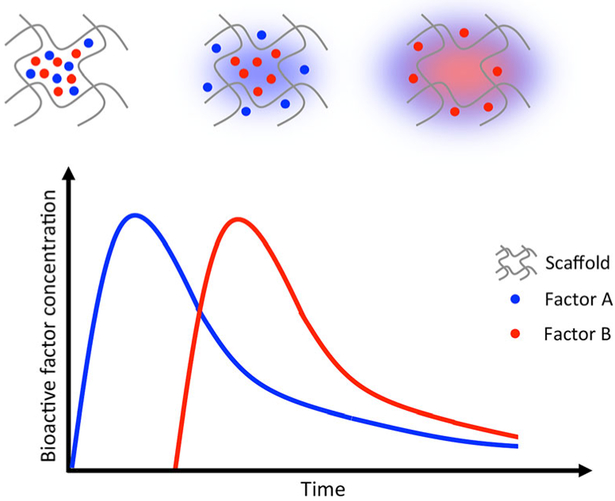
Growth factor incorporation into polymer matrices has been utilized as a strategy for delivering single components, simultaneously delivering multiple growth factors or sequentially delivering multiple growth factors (Fig. 2). Systems engineered for the delivery of single or multiple growth factors have been used in bone, cardiac, and nerve regeneration applications. For example, Kaigler et al. utilized an injectable alginate system for the delivery of vascular endothelial growth factor (VEGF) to promote bone regeneration in rats using guided bone regeneration procedures. Topical application of the VEGF hydrogel on critical-sized rat cranial defects resulted in an increased blood vessel density and greater bone regeneration as compared to controls [7]. Moreover, a dual-growth factor delivery system for osteochondral tissue repair in rabbits was reported by Lu et al. [8]. Oligo(poly(ethylene glycol) fumarate) hydrogels embedded with gelatin microparticles containing insulin-like growth factor-1 (IGF-1) and bone morphogenetic protein-2 (BMP-2) were implanted in a medial femoral condyle osteochondral defect. Twelve weeks after implantation, the dual delivery of IGF-1 and BMP-2 resulted in superior subchondral bone repair and greater bone growth at the defect margin, as compared to a similar system that delivered IGF-1 alone [8]. Furthermore, Awada et al. reported the sequential delivery of angiogenic growth factors to improve cardiac function following myocardial infarction. Platelet-derived growth factor (PDGF) in a heparin-based coacervate and VEGF were embedded in a fibrin hydrogel and implanted in a rat myocardial infarction model [9]. Implantation of the sequential growth factor-releasing gels resulted in improved cardiac function by increasing the ventricular wall thickness, reducing fibrosis, and maintaining cardiac viability in the infarcted myocardium.
Fig. 2.
Scaffolds for regenerative engineering applications can be loaded with multiple bioactive factors and tailored to deliver these factors sequentially with fine-tuned spatiotemporal control. Adapted from Lee et al. [2]
Material Patterning and Alignment for the Retention and Arrangement of Cells
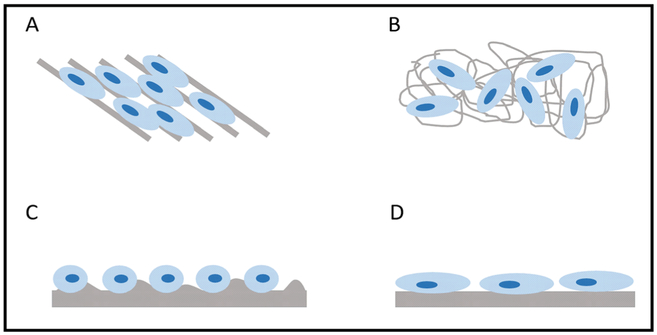
Chemical and physical surface characteristics of biomaterials influence the behavior of cells. The topography of these materials can be controlled through patterning and alignment (Fig. 3). Micro- and nanopatterns on biomaterial surfaces are fabricated using soft lithography, photolithography, plasma lithography, or thermal lithography [10]. In addition, material alignment can be controlled using soft lithography, as well as electrospinning techniques. Previous studies have reported that microstructured surfaces affect the cytoskeleton and cell morphology, while nanostructured surfaces influence cell functions such as alignment and proliferation [10, 11]. In a study by Gaharwar et al., poly(glycerol sebacate) (PGS) and poly(ε-caprolactone) (PCL) were electrospun to create anisotropic materials with randomly oriented and highly aligned fibers. Endothelial cells cultured on the aligned PGS-PCL fibers exhibited greater proliferation and alignment compared to those cultured on the randomly oriented materials. In addition, alignment of the fibers enabled the endothelial cells to form organized constructs, an important aspect for vascularized tissue structures [12].
Fig. 3.
Influence of material alignment and topography on cell arrangement and morphology. a Materials with aligned structures can result in the alignment of cells, whereas b randomly oriented materials may result in the random arrangement of cells. c Rough surfaces may lead to round cell morphologies, compared to d flat surfaces which can increase cell spreading
Furthermore, surface topography has a large effect on cell morphology. Kumar et al. investigated the effects of surface roughness on human bone marrow stromal cell (hBMSC) functions. Freeform-fabricated scaffolds of PCL were solvent-etched to create surface roughness. In the absence of osteogenic supplements, hBMSCs cultured on etched scaffolds were round in morphology and spread over a small area, while cells cultured on un-etched scaffolds had large, spread morphologies. Additionally, etched scaffolds induced osteogenic differentiation of hBMSCs, while un-etched scaffolds did not impact hBMSC differentiation [13].
Aiding the Regeneration of Complex Tissues with Multiple Cell Types
Co-cultures can mimic native tissues that contain diverse cell types for regenerative engineering applications. Researchers can utilize these constructs as a tool to enhance our understanding of cell-cell and cell-material interactions when multiple cell types are present. However, simultaneous utilization of multiple cell lines presents many challenges with significant limitations. The contributions of different cell types to measured responses are difficult to delineate, and similarly cell-cell, as opposed to cell-material interactions, are challenging to distinguish. Thus, proper experimental design strategies are needed in order to determine the appropriate contributions. Although co-culture experimentation can be complex, significant advancements in tissue engineering have been reported utilizing these systems [14]. Specifically, co-culture systems have been employed to enhance our understanding of cross talk and stimuli that are exchanged between cells.
In cardiac tissue engineering, much effort has been focused on using cardiomyocytes for the development of a cardiac patch to replace damaged myocardial tissue. However, two major shortcomings have emerged, namely maintaining long-term viability of cardiomyocytes in mono-culture and achieving synchronized contractions. In order to overcome these limitations, researchers have investigated the use of cocultures of cardiomyocytes and fibroblasts. Hussain et al. fabricated an electrospun-3D chitosan scaffold, on which neonatal rat cardiomyocytes and murine 3T3-J2 fibroblasts were co-cultured. Cardiomyocyte-fibroblast co-cultures presented polarized cardiomyocyte morphology, while mono-cultures lost cardiomyocyte polarity. In addition, co-cultures showed evidence of synchronized contractions that involved large cellular networks [15]. Co-cultures have also been utilized to promote bone, cartilage, skin, and neural regeneration [14]. For example, osteoblasts have been co-cultured with endothelial cells and endothelial progenitor cells to promote the formation of capillaries and improve vascularization for the purpose of bone regeneration [16, 17]. In the case of neural tissue regeneration, Schwann cells have been co-cultured with adiposederived stem cells and neurons to promote cell differentiation and neurite growth [18, 19]. In combination with co-cultures, biomaterials and growth factors construct complex 3D tissue mimics. With these recent and cutting-edge findings, the area of regenerative engineering is a topic of ongoing research.
Molecularly Functional or Decorated Polymers
A pervasive strategy for the fabrication of intelligent biomaterials is to select a base material which possesses an ideal macroscopic property (i.e., mechanical stability, biocompatibility, degradability, pH responsiveness, temperature responsiveness, or otherwise). Then, researchers chemically functionalize the material with moieties, which respond to environmental cues, interact with matrix-present or secreted biomolecules, or provide adhesion molecules for various cell lineages [20, 21]. Our vast repertoire of natural and synthetic materials, coupled with advanced engineering of biomolecules (DNA aptamers, peptides, recombinant proteins, and more), enables the employment of this strategy to regenerate countless tissues.
Natural-Synthetic Hybrid Biomaterials
A major debate in the field of regenerative engineering lies in the employment of natural or synthetic materials for the scaffold bulk [22]. Natural materials are isolated from a biological source and are typically biocompatible and biodegradable within the host. On the other hand, synthetic materials offer tunable mechanical properties, porosity, and a wide range of chemical moieties that have advantageous properties for sequestering and delivering bioactive molecules. There are certainly advantages and limitations of both natural and synthetic materials, and it is not the focus of this perspective to contribute to that debate. Rather, we herein will discuss the usage of modified natural or functionalized synthetic materials to perform desired biological functions.
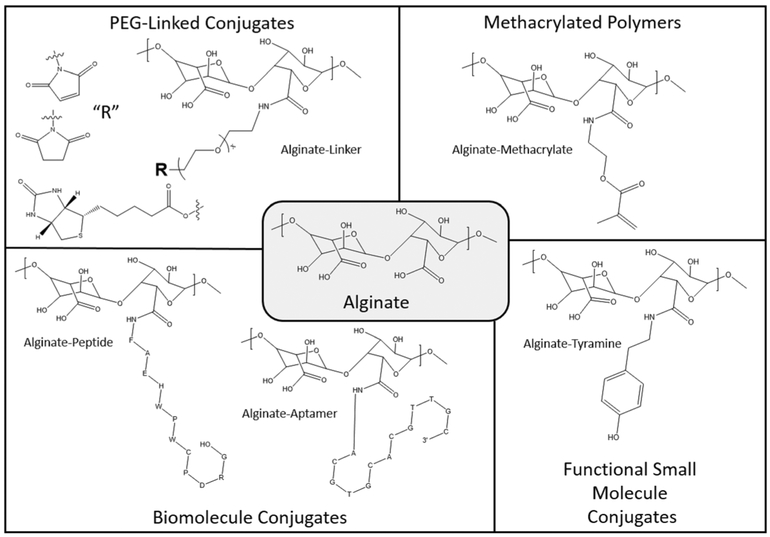
By example, alginate is a natural biopolymer that is utilized frequently for regenerative engineering applications [23]. With a carbodiimide-mediated coupling reaction, carboxylic acids that are naturally present in alginate can be functionalized with a variety of moieties through a stable amide bond [24, 25]. These moieties can be natural or synthetic in origin and perform a variety of functions within the ultimate scaffold. For example, methacrylate functionalization contributes a polymerizable double bond, which can participate in chemical cross-linking via ultraviolet or chemical initiation [26]. This chemical cross-linking contributes to enhanced hydrogel stability and elevated mechanical integrity, as compared to alginate gels cross-linked with calcium alone. Alternate functional groups, such as aromatic moieties, increase the hydrophobicity of the scaffold, lead to physical cross-linking, alter the adsorption of serum proteins, and enhance the capacity to load and release small molecules [27].
Chemical coupling can also covalently link bioactive molecules to the hydrogel backbone. For example, a DNA aptamer or peptide can be conjugated through a 5’ or Nterminal amine, respectively, with the pendant carboxylic acids on alginate through the same carbodiimide-mediated coupling reaction [28, 29]. These biomolecules engage with both polymer chains and the physiological environment to produce an engineered function. DNA aptamers or peptides can interact with a complementary sequence or protein receptor, respectively, to induce reversible, physical cross-linking [30]. These cross-links are dynamically tunable through the presentation of competing proteins, peptides, or nucleic acids in solution. More commonly, conjugated biomolecules serve as cell adhesion molecules or function to enhance the adsorption, retention, or release of growth factors [31]. Frequently, in order to optimize the affinity of biomolecules or conjugate a whole active protein, a poly(ethylene glycol) (PEG) spacer is employed [32]. In the case of alginate, a bifunctional PEG with an amine and a “terminal moiety” of optimal length links to alginate’s pendant carboxylic acid groups through an amide bond. The terminal moiety such as an N-hydroxysuccinimide (NHS) ester for amine conjugation, a maleimide for thiol conjugation, or biotin for forming an avidin-biotin complex is selected appropriately so that coupling with a given ligand will be obtained at high yield. A summary of common functionalizations, using alginate as a base material, is presented in Fig. 4. Methacrylic acid- or acrylic acid-containing polymers can substitute for alginate if an application requires a synthetic system amenable to the same functionalization chemistry.
Fig. 4.
Carbodiimide-mediated coupling reactions can functionalize the carboxylic acid moieties of alginate, at ambient conditions, with aminecontaining natural or synthetic ligands
Biomaterial Engineering to Promote Specific Interactions with Proteins or Cells
Moving toward the synthesis of functionalized polymers, which interact with cells and tissues for regenerative purposes, the specific identities of conjugated ligands are imperative. While protein-based biomaterial gels can naturally contain cell adhesion peptides, polysaccharide materials (i.e., chitosan, hyaluronic acid, alginate) and synthetic biomaterials do not [33]. The most popular approach to promote cell attachment and spreading on these biomaterials is to conjugate the tripeptide arginyl-glycyl-aspartic acid (RGD) [34], although other approaches which blend non-adhesive biomaterials with extracellular matrix proteins (i.e., collagen, laminin, entactin, heparin sulfate) are also effective [35]. When fabricating these peptide-material conjugates or polymer-protein blends, previously described methods (carbodiimide-mediated coupling, physical adsorption) are effective. In some biomaterial cases, however, the appropriate carboxylic acid or amine moieties are not present for covalent ligand coupling. Alternatively, a specific application may require adhesion ligands in a specified geometry or area, necessitating a coupling chemistry more amenable to photolithography. In these instances, peptides with a terminal cysteine will participate in a thiol-ene addition reaction upon exposure to specific wavelengths of visible light [36].
Molecularly Functional Polymers in the Future of Regenerative Engineering
The major advantage of molecularly functional polymers for regenerative engineering appli-cations is our extensive repertoire of biomaterials, linkers, and ligands that synergistically provide a diverse array of spatiotemporal properties. We have the ability to pattern biomaterials with cell adhesion ligands, conjugate or adsorb bioactive proteins, specify initial porosity, and modulate stiffness. In the future, it will become even more important to develop biomaterials that dynamically present hidden ligands, release encapsulated drug payloads, alter matrix mechanics, and degrade, each in response to cellular and/or external triggers.
As an example of such a dynamic system, researchers have developed liposome and gold nanorod-containing biomaterials, which soften or stiffen in response to tuned near infrared light stimuli [37]. An array of linker molecules are available for biomaterial design, such that an environmental stimulus (i.e., pH, presence of reducing agent, proteolytic enzymes) [38] or external trigger (light) [39] detaches polymeric coatings and reveals new polymer surfaces. These systems have been employed for targeted drug delivery and tissue engineering applications and could be readily translated to generate next-generation scaffolds for regenerative engineering. These dynamic materials will become critically important as the field continues to progress toward the regeneration of complex tissues, where a single scaffold must facilitate the growth and differentiation of multiple cell types over time.
Molecularly Imprinted Polymers
Researchers originally fabricated molecularly imprinted polymers (MIPs) for applications in affinity chromatography, where sorbents imprinted with a desired synthetic product separated and isolated specific template products with enhanced specificity. In the formation of MIPs, functional monomers, which possess moieties that interact favorably with a template molecule, selfassemble and polymerize in the presence of a molecular template. Following purification, which extracts entrapped template molecules and removes unreacted impurities, the final MIP material possesses void cavities, which have affinity for the original template. Originally, MIPs were composed of synthetic monomers with a high degree of cross-linking, in an effort to create specific nanocavities for small molecule enantiomers [40]. However, as MIPs have emerged in the biomedical domain, MIPs fabricated from synthetic materials with low degrees of cross-linking, as well as natural materials, have shown promise for the recognition, retention, and even delivery of macromolecules [41]. Our research group has been especially active in this area, and we believe that MIP biomaterials could address numerous challenges in regenerative engineering.
MIPs for Biomedical Applications
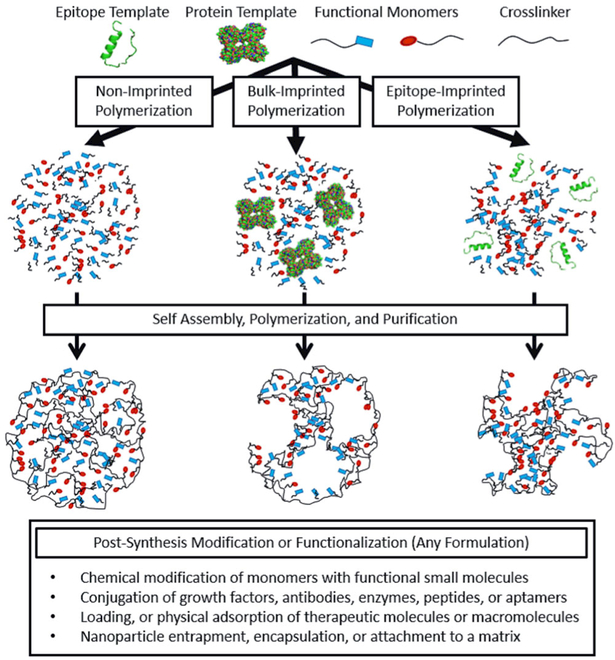
MIPs for biomedical applications, including biosensing, drug delivery, and regenerative medicine, have been an active area of research for several decades. The field originated with a number of papers by our group and others, which looked at molecular recognition using star polymers [42, 43], molecular imprinting of glucose [44], moiety imprinting [41], and protein imprinting [45]. In the years that have followed, MIPs in the form of nanoparticles, microparticles, and films have been fabricated to address diverse medical problems ranging from the recognition of hyperglycemia in a diabetic host, to the sustained ocular delivery of macromolecule drugs [46]. Molecular imprinting for biomedical applications has also resulted in an increased diversity of imprinting templates, which now include small molecules, proteins, peptide epitopes, and cells (Fig. 5).
Fig. 5.
Proteins, peptide epitopes, or cells (not shown) can serve as templates for molecularly imprinted polymerizations. Following polymerization and purification, chemical modification of pendant functional moieties, as described previously, can generate complex material structures
Moreover, the development of MIPs for applications in regenerative engineering is an area of future study [47]. Some recent studies, mostly designed for biosensing applications, suggest that rationally designed MIP materials are capable of recognizing template cells [48, 49]. A few studies even suggest that molecular imprinting of specific cell lineages can guide the differentiation of pluripotent cells [50, 51]. Further studies are necessary to determine the specific mechanisms through which the topography, or any remaining byproducts of template cells, can guide cell differentiation.
Cell-Imprinted Biomaterials
In cell imprinting, polymeric materials are synthesized on the surface of a cell-coated substrate, such as a glass slide. As the majority of published studies on cell imprinting have been conducted for biosensing applications, or toward the development of microbial fuel cells, the cell-imprinted polymer materials were synthesized directly, or cast post-synthesis, on the surface of a relevant substrate, such as a platinum-poly(ethylene terephthalate) electrode [52] or quartz-crystal microbalance sensor [53]. The polymerized MIP materials employed in these studies were composed of a diverse array of functionalities, including poly(ethylene oxide), poly(urethane), and poly(acrylamide).
Several key findings have emerged from studies of cell imprinting. Ren et al. demonstrated that cells fixed with paraformaldehyde and glutaraldehyde (4 and 2% by volume, respectively) were a viable substrate for cell imprinting. This finding is particularly important, as polymerization conditions frequently involve high concentrations of cytotoxic monomers, organic solvents, UV irradiation or heat, each which would jeopardize the viability of live cultures [54]. In a separate study, DePorter et al. imprinted HEK293, HeLa, and MRC-9 cell lines in poly(acrylamide) hydrogels [55]. While cells failed to adhere to non-imprinted poly(acrylamide), MIP poly(acrylamide) served as a suitable surface for the adhesion and proliferation of each cell lineage. The authors posited, herein, that the cells’ topographical features, which were imparted by the imprinting protocol, promoted cell-selective adhesion to the MIP poly(acrylamide). Additional data presented in the manuscript also suggests that cellular fragments (such as peptides, proteins, lipids, and nucleic acids) remained in some of the MIP formulations, which could also contribute to subsequent cell adhesion [55].
Cell-imprinting studies have affirmed the importance of surface chemistry and topography in the generation of scaffolds for regenerative engineering. The inclusion of a cellular template during polymerization should not alter the chemistry or mechanical properties of the hydrogel bulk, but rather modify the surface with remaining cellular fragments and generate cell-specific topographical features. Modification of a surface through cell imprinting, to promote specific cellular interactions without compromising the stiffness or porosity of the bulk material, could certainly prove useful in the future of regenerative engineering.
Recent Advancements in Protein Imprinting for Therapeutic Applications
Recent studies within macromolecular imprinting have established new ways in which these materials could sequester or deliver therapeutic proteins within a scaffold. By example, White et al. developed a silicone-based MIP contact lens, which exhibited the sustained release of 120 kDa hydroxypropyl methylcellulose (HPMC). MIP formulations eluted HPMC at a sustained dose of approximately 60 μg/day for 53 days, as opposed to non-imprinted formulations, which sustained release for only 5–11 days. This study demonstrated that, by enhancing the material’s affinity for a macromolecular template, molecular imprinting can lead to controlled or sustained delivery of macromolecular therapeutics [56].
Additionally, Culver et al. recently conducted a study, which looked at the adsorption of numerous proteins, which differed in molecular weight and isoelectric point, to lysozymeimprinted poly(acrylamide-co-(dimethylamino)ethyl methacrylate-co-methacrylic acid) microgels [57]. Molecular imprinting increased the adsorption capacity of the microgels for all tested proteins in non-competitive conditions. Similar increases in adsorption capacity, relative to non-imprinted controls, were observed for all high isoelectric point templates. Most interestingly, both imprinted and non-imprinted polymer formulations were selective for lysozyme in competitive solutions. The study concluded that polymer formulation had the greatest impact on protein selectivity and that molecular imprinting increases the adsorption capacity of a cross-linked polymer network for both template and non-template proteins [57]. Building upon this work, we imprinted three high isoelectric point proteins (trypsin, lysozyme, and cytochrome c) within poly(acrylamide-co-(diethyamino)ethyl methacrylate-co-methacrylic acid) microgels. Similar to the results of the study by Culver et al. polymer formulation, relative to protein isoelectric point, dictated the polymers’ protein selectivity, and molecular imprinting increased the adsorption capacity for all high isoelectric point templates in both non-competitive and competitive conditions. Through an analysis of material composition, we recognized that all MIP formulations had entrapped template remaining following polymerization and purification. The observed inability to extract imprinting templates completely is consistent with most of the literature. This suggested that the remaining template could be precisely what is enhancing the materials’ affinity for subsequent proteins. Additionally, it affirmed the feasibility of imprinting with a low-cost template that shares structural similarity with an expensive therapeutic protein as a cost-efficient strategy for enhancing the protein-material affinity [58].
MIPs in the Future of Regenerative Engineering
Molecularly decorated polymers have given researchers the tools to develop materials with spatiotemporal control over biomaterial stiffness, degradation, and surface chemistry. Simultaneously, advancements in molecular imprinting offer a reminder of the importance of material topography and the importance of considering accessibility and affordability during biomaterial design. Advancements in both areas offer researchers a repertoire of techniques to alter a biomaterial’s affinity for target molecules, proteins, or cells.
We believe that MIPs will find utility in regenerative engineering through two major avenues. First, MIP micro- and nanomaterials can be incorporated as a component of an existing scaffold. This MIP would be engineered to have affinity for a small molecule or protein, which modulates some aspect of the regenerative process, such as cell adhesion, migration, proliferation, or differentiation. A MIP particle, patterned in specific scaffold locations and loaded with this bioactive molecule of interest, could serve as a depot and sustain local drug delivery to surrounding cells for extended time. Conversely, a void MIP particle could act as a reservoir and capture cell-secreted molecules, increasing their local re-tention time to modulate cell behavior. This area of investiga-tion is mostly an extension of recent efforts and applications in biomolecule imprinting. Second, MIP materials could directly target cells through the recognition of surface-presented biomarkers or receptors. This avenue is more of an extension of whole-cell imprinting, with an added temporal component, as the distribution of various membrane-bound receptors changes over time.
MIPs in regenerative engineering are not separate from molecularly decorated polymers, but rather the two go hand in hand. We outline some of the synthetic strategies, which could become pertinent to generate MIP conjugate hybrid materials, in Fig. 5. Molecular imprinting and ligand conjugation (aptamer, peptide, small molecule, or otherwise) could prove extensively useful to further enhance polymer-biomolecule affinity. Furthermore, future studies using MIPs for regenerative purposes will need to take motivation and inspiration from decorated functional polymers to generate MIP coatings that degrade in response to cellular and external triggers. Conversely, protein-MIPs themselves present an additional functionalization tool. For example, MIP nanomaterials could engage in reversible cross-linking with polymer chains that present conjugated template molecules, offering another strategy for modulating matrix mechanics and degradation.
Conclusions and Remarks
Regenerative engineering offers great promise as a means of developing curative options for conditions that lack sufficient therapeutic regimens for today’s patients. These conditions range from spinal cord injuries necessitating neuroregeneration [59, 60], to myocardial infarctions requiring prompt cardiac tissue repair [61, 62]. In this perspective, we discussed some of the major materials-based strategies and considerations when developing scaffolds for regenerative engineering applications. Existing techniques and strategies have generated promising biomaterials and exciting results toward the regeneration of many tissue types. These studies, however, have also made evident the complex considerations in scaffold development, which merit further investigation, and range from mechanical properties, porosity, and presentation of bioactive factors, to the dynamic tuning of stiffness, biodegradation, and the orientation of co-cultured cells.
Molecularly decorated and molecularly imprinted hydrogels have the potential to address shortcomings of existing scaffolds for regenerative engineering applications. Numerous facile bioconjugation techniques are available to decorate the bulk of new natural or synthetic scaffolds with small molecules, peptides, aptamers, and proteins. These conjugated molecules can interact with each other for the purpose of gel mechanical integrity, with adsorbed molecules to determine release profiles, or with cells to promote adhesion and motility. Molecular imprinting with protein or cellular templates can also alter the composition and topography of either scaffolds or nanomaterials that are entrapped within. These molecular decoration and molecular imprinting strategies, while typically studied separately, should be employed synergistically to address future challenges in regenerative engineering.
In the introduction to this perspective, we raised numerous questions that biomaterials scientists within the field of regenerative engineering must answer. It is our hope that this perspective sheds light on the complexity of these seemingly simple inquiries.
Acknowledgements
The authors would like to acknowledge financial support from the UT-Portugal Collaborative Research program (CoLAB). JRC and MEW are supported by NSF Graduate Research Fellowships.
References
- 1.Laurencin CT, Khan Y. Regenerative engineering. Sci Transl Med 2012;4:160. [DOI] [PubMed] [Google Scholar]
- 2.Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface. 2011;8(55):153–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Brien FJ. Biomaterials & scaffolds for tissue engineering. Mater Today. 2011;14(3):88–95. [Google Scholar]
- 4.Loh QL, Choong C. Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng Part BRev 2013;19(6):485–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Annabi N, Nichol JW, Zhong X, Ji C, Koshy S, Khademhosseini A, et al. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng Part B Rev 2010;16(4):371–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bajaj P, Schweller RM, Khademhosseini A, West JL, Bashir R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu Rev Biomed Eng 2014;16:247–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaigler D, Silva EA, Mooney DJ. Guided bone regeneration (GBR) utilizing injectable vascular endothelial growth factor (VEGF) delivery gel. J Periodontol 2013;84(2):230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu S, Lam J, Trachtenberg JE, Lee EJ, Seyednejad H, van den Beucken JJJP, et al. Dual growth factor delivery from bilayered, biodegradable hydrogel composites for spatially-guided osteochondral tissue repair. Biomaterials. 2014;35(31):8829–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Awada HK, Johnson NR, Wang Y. Sequential delivery of angiogenic growth factors improves revascularization and heart function after myocardial infarction. J Control Release Off J Control Release Soc 2015;207:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeon H, Simon CG, Kim G. A mini-review: cell response to microscale, nanoscale, and hierarchical patterning of surface structure. J Biomed Mater Res B Appl Biomater 2014;102(7):1580–94. [DOI] [PubMed] [Google Scholar]
- 11.Wang P-Y, Thissen H, Kingshott P. Modulation of human multipotent and pluripotent stem cells using surface nanotopographies and surface-immobilised bioactive signals: a review. Acta Biomater 2016;45:31–59. [DOI] [PubMed] [Google Scholar]
- 12.Gaharwar AK, Nikkhah M, Sant S, Khademhosseini A. Anisotropic poly (glycerol sebacate)-poly (ε-caprolactone) electrospun fibers promote endothelial cell guidance. Biofabrication. 2014;7(1):015001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar G, Waters MS, Farooque TM, Young MF, Simon CG. Freeform fabricated scaffolds with roughened struts that enhance both stem cell proliferation and differentiation by controlling cell shape. Biomaterials. 2012;33(16):4022–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Battiston KG, Cheung JWC, Jain D, Santerre JP. Biomaterials in coculture systems: towards optimizing tissue integration and cell signaling within scaffolds. Biomaterials. 2014;35(15):4465–76. [DOI] [PubMed] [Google Scholar]
- 15.Hussain A, Collins G, Yip D, Cho CH. Functional 3-D cardiac co-culture model using bioactive chitosan nanofiber scaffolds. Biotechnol Bioeng 2013;110(2):637–47. [DOI] [PubMed] [Google Scholar]
- 16.Ghanaati S, Fuchs S, Webber MJ, Orth C, Barbeck M, Gomes ME, et al. Rapid vascularization of starchpoly(caprolactone) in vivo by outgrowth endothelial cells in coculture with primary osteoblasts. J Tissue Eng Regen Med 2011;5(6):e136–43. [DOI] [PubMed] [Google Scholar]
- 17.Unger RE, Dohle E, Kirkpatrick CJ. Improving vascularization of engineered bone through the generation of proangiogenic effects in coculture systems. Adv Drug Deliv Rev 2015;94:116–25. [DOI] [PubMed] [Google Scholar]
- 18.Suri S, Schmidt CE. Cell-laden hydrogel constructs of hyaluronic acid, collagen, and laminin for neural tissue engineering. Tissue Eng Part A. 2010;16(5): 1703–16. [DOI] [PubMed] [Google Scholar]
- 19.Tomita K, Madura T, Sakai Y, Yano K, Terenghi G, Hosokawa K. Glial differentiation of human adipose-derived stem cells: implications for cell-based transplantation therapy. Neuroscience. 2013;236:55–65. [DOI] [PubMed] [Google Scholar]
- 20.Cheng R, Meng F, Deng C, Klok H-A, Zhong Z. Dual and multistimuli responsive polymeric nanoparticles for programmed sitespecific drug delivery. Biomaterials. 2013;34(14):3647–57. [DOI] [PubMed] [Google Scholar]
- 21.Steichen SD, Caldorera-Moore M, Peppas NA. A review of current nanoparticle and targeting moieties for the delivery of cancer therapeutics. Eur J Pharm Sci 2013;48(3):416–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sionkowska A Current research on the blends of natural and synthetic polymers as new biomaterials: review. Prog Polym Sci 2011;36(9):1254–76. [Google Scholar]
- 23.Sun J, Tan H. Alginate-based biomaterials for regenerative medicine applications. Materials. 2013;6(4):1285–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakajima N, Ikada Y. Mechanism of amide formation by carbodiimide for bioconjugation in aqueous media. Bioconjug Chem 1995;6(1):123–30. [DOI] [PubMed] [Google Scholar]
- 25.Sehgal D, Vijay IK. A method for the high efficiency of watersoluble carbodiimide-mediated amidation. Anal Biochem 1994;218(1):87–91. [DOI] [PubMed] [Google Scholar]
- 26.Rouillard AD, Berglund CM, Lee JY, Polacheck WJ, Tsui Y, Bonassar LJ, et al. Methods for photocrosslinking alginate hydrogel scaffolds with high cell viability. Tissue Eng Part C Methods. 2010;17(2):173–9. [DOI] [PubMed] [Google Scholar]
- 27.Sakai S, Hirose K, Taguchi K, Ogushi Y, Kawakami K. An injectable, in situ enzymatically gellable, gelatin derivative for drug delivery and tissue engineering. Biomaterials. 2009;30(20):3371–7. [DOI] [PubMed] [Google Scholar]
- 28.Soontornworajit B, Zhou J, Shaw MT, Fan T-H, Wang Y. Hydrogel functionalization with DNA aptamers for sustained PDGF-BB release. Chem Commun 2010;46(11):1857–9. [DOI] [PubMed] [Google Scholar]
- 29.Santiago LY, Nowak RW, Rubin JP, Marra KG. Peptide-surface modification of poly (caprolactone) with laminin-derived sequences for adipose-derived stem cell applications. Biomaterials. 2006;27(15):2962–9. [DOI] [PubMed] [Google Scholar]
- 30.Yang H, Liu H, Kang H, Tan W. Engineering target-responsive hydrogels based on aptamer- target interactions. J Am Chem Soc 2008;130(20):6320–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol 2005;23(1):47–55. [DOI] [PubMed] [Google Scholar]
- 32.Stefanick JF, Ashley JD, Kiziltepe T, Bilgicer B. A systematic analysis of peptide linker length and liposomal polyethylene glycol coating on cellular uptake of peptidetargeted liposomes. ACS Nano 2013;7(4):2935–47. [DOI] [PubMed] [Google Scholar]
- 33.Shelke NB, James R, Laurencin CT, Kumbar SG. Polysaccharide biomaterials for drug delivery and regenerative engineering. Polym Adv Technol 2014;25(5):448–60. [Google Scholar]
- 34.Bellis SL. Advantages of RGD peptides for directing cell associa-tion with biomaterials. Biomaterials. 2011;32(18):4205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Badylak SF. Regenerative medicine and developmental biology: the role of the extracellular matrix. Anat Rec B New Anat 2005;287(1):36–41. [DOI] [PubMed] [Google Scholar]
- 36.DeForest CA, Polizzotti BD, Anseth KS. Sequential click reactions for synthesizing and patterning three-dimensional cell microenvironments. Nat Mater 2009;8(8):659–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stowers RS, Allen SC, Suggs LJ. Dynamic phototuning of 3D hydrogel stiffness. Proc Natl Acad Sci 2015;112(7): 1953–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang X-Z, Du J-Z, Dou S, Mao C-Q, Long H-Y, Wang J. Sheddable ternary nanoparticles for tumor acidity-targeted siRNA delivery. ACS Nano 2011;6(1):771–81. [DOI] [PubMed] [Google Scholar]
- 39.DeForest CA, Anseth KS. Cytocompatible clickbased hydrogels with dynamically tunable properties through orthogonal photoconjugation and photocleavage reactions. Nat Chem 2011. ;3(12):925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andersson LI, Mosbach K. Enantiomeric resolution on molecularly imprinted polymers prepared with only non-covalent and non-ionic interactions. J Chromatogr A. 1990;516(2):313–22. [DOI] [PubMed] [Google Scholar]
- 41.Byrne ME, Hilt JZ, Peppas NA. Recognitive biomimetic networks with moiety imprinting for intelligent drug delivery. J Biomed Mater Res A. 2008;84(1):137–47. [DOI] [PubMed] [Google Scholar]
- 42.Keys KB, Andreopoulos FM, Peppas NA. Poly (ethylene glycol) star polymer hydrogels. Macromolecules. 1998;31(23):8149–56. [Google Scholar]
- 43.Oral E, Peppas NA. Responsive and recognitive hydrogels using star polymers. J Biomed Mater Res A. 2004;68(3):439–47. [DOI] [PubMed] [Google Scholar]
- 44.Oral E, Peppas NA. Hydrophilic molecularly imprinted poly (hydroxyethyl-methacrylate) polymers. J Biomed Mater Res A. 2006;78(1):205–10. [DOI] [PubMed] [Google Scholar]
- 45.Shi H, Tsai W-B, Garrison MD, Ferrari S, Ratner BD. Templateimprinted nanostructured surfaces for protein recognition. Nature. 1999;398(6728):593–7. [DOI] [PubMed] [Google Scholar]
- 46.Alvarez-Lorenzo C, Concheiro A. Molecularly imprinted polymers for drug delivery. J Chromatogr B. 2004;804(1):231–45. [DOI] [PubMed] [Google Scholar]
- 47.Neves MI, Wechsler ME, Gomes ME, Reis RL, Granja PL, Peppas NA. Molecularly imprinted intelligent scaffolds for tissue engineering applications. Tissue Eng Part B Rev 2017;23(1):27–43. [DOI] [PubMed] [Google Scholar]
- 48.Kunath S, Panagiotopoulou M, Maximilien J, Marchyk N, Sänger J, Haupt K. Cell and tissue imaging with molecularly imprinted polymers as plastic antibody mimics. Adv Healthc Mater 2015;4(9):1322–6. [DOI] [PubMed] [Google Scholar]
- 49.Ren K, Zare RN. Chemical recognition in cellimprinted polymers. ACS Nano 2012;6(5):4314–8. [DOI] [PubMed] [Google Scholar]
- 50.Bonakdar S, Mahmoudi M, Montazeri L, Taghipoor M, Bertsch A, Shokrgozar MA, et al. Cell imprinted substrates modulate the differentiation, redifferentiation, and transdifferentiation. ACS Appl Mater Interfaces. 2016;8(22):13777–84. [DOI] [PubMed] [Google Scholar]
- 51.Mahmoudi M, Bonakdar S, Shokrgozar MA, Aghaverdi H, Hartmann R, Pick A, et al. Cell-imprinted substrates direct the fate of stem cells. ACS Nano 2013;7(10):8379–84. [DOI] [PubMed] [Google Scholar]
- 52.Lee M-H, Thomas JL, Chen W-J, Li M-H, Shih C-P, Lin H-Y. Fabrication of bacteria-imprinted polymer coated electrodes for microbial fuel cells. ACS Sustain Chem Eng 2015;3(6):1190–6. [Google Scholar]
- 53.Yilmaz E, Majidi D, Ozgur E, Denizli A. Whole cell imprinting based Escherichia coli sensors: a study for SPR and QCM. Sens Actuators B Chem 2015;209:714–21. [Google Scholar]
- 54.Ren K, Banaei N, Zare RN. Sorting inactivated cells using cell-imprinted polymer thin films. ACS Nano 2013;7(7):6031–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.DePorter SM, Lui I, McNaughton BR. Programmed cell adhesion and growth on cellimprinted polyacrylamide hydrogels. Soft Matter 2012;8(40):10403–8. [Google Scholar]
- 56.White CJ, McBride MK, Pate KM, Tieppo A, Byrne ME. Extended release of high molecular weight hydroxypropyl methylcellulose from molecularly imprinted, extended wear silicone hydrogel contact lenses. Biomaterials. 2011;32(24):5698–705. [DOI] [PubMed] [Google Scholar]
- 57.Culver HR, Steichen SD, Peppas NA. A closer look at the impact of molecular imprinting on adsorption capacity and selectivity for protein templates. Biomacromolecules. 2016;17(12):4045–53. [DOI] [PubMed] [Google Scholar]
- 58.Clegg JR, Zhong JX, Irani AS, Gu J, Spencer DS, Peppas NA. Characterization of protein interactions with molecularly imprinted hydrogels that possess engineered affinity for high isoelectric point biomarkers. J Biomed Mater Res Part A. 2017:00A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsintou M, Dalamagkas K, Seifalian AM. Advances in regenerative therapies for spinal cord injury: a biomaterials approach. Neural Regen Res 2015;10(5):726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Daly W, Yao L, Zeugolis D, Windebank A, Pandit A. A biomaterials approach to peripheral nerve regeneration: bridging the peripheral nerve gap and enhancing functional recovery. J R Soc Interface. 2012;9(67):202–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burdick JA, Mauck RL, Gorman JH, Gorman RC. Acellular biomaterials: an evolving alternative to cell-based therapies. Sci Transl Med 2013;5(176):176ps4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garbern JC, Lee RT. Cardiac stem cell therapy and the promise of heart regeneration. Cell Stem Cell. 2013;12(6):689–98. [DOI] [PMC free article] [PubMed] [Google Scholar]