Abstract
The innate immune responses triggering production of type I interferons and inflammatory cytokines constitute a nonspecific innate resistance that eliminates invading pathogens including viruses. The activation of innate immune signaling through pattern recognition receptors (PRRs) is by sensing pathogen-associated molecular patterns derived from viruses. According to their distribution within cells, PRRs are classified into three types of receptors: membrane, cytoplasmic, and nuclear. Kaposi’s sarcoma-associated herpesvirus (KSHV), a large DNA virus, replicates in the nucleus. Its genome is protected by capsid proteins during transport in the cytosol. Multiple PRRs are involved in KSHV recognition. To successfully establish latent infection, KSHV has evolved to manipulate different aspects of the host antiviral innate immune responses. This review presents recent advances in our understanding about the activation of the innate immune signaling in response to infection of KSHV. It also reviews the evasion strategies used by KSHV to subvert host innate immune detection for establishing a persistent infection.
Keywords: KSHV, PRRs, Innate immune response, Evasion strategies
1. Introduction
Kaposi’s sarcoma-associated herpesvirus (KSHV), a γ2 herpesvirus, is etiologically associated with Kaposi’s sarcoma (KS) and two other rare lymphoproliferative disorders: a variant of multicentric Castleman disease (MCD) and primary effusion lymphoma (PEL) [1–4]. Like all other herpesviruses, KSHV displays two distinct life modes, latent and lytic replication. Following primary infection, both lytic and latent genes are expressed. After abortive lytic replication, latent cycle is founded. Latency is the default state that maintains KSHV genome as a multicopy, circular episomal DNA with only a small portion of latent genes expressed [5, 6]. Viruses undergo lytic replication under certain circumstances such as hypoxia or immune suppression, leading to a complete panel of viral genes expression and production of infectious progeny virus [7–10].
The innate immune response is a relatively nonspecific response that is the host first line to defense against invading pathogens before they establish full infection in cells. Induction of inflammatory cytokines and type I interferon (IFN) response is critical for host innate defense mechanisms. Host cells employ an array of constitutively expressed, germline-encoded pattern-recognition receptors (PRRs) to recognize pathogen-derived and conserved pathogen associated molecular patterns (PAMPs). Recognition subsequently evokes PRR-adaptor-transcriptional factor signaling cascades to induce inflammatory cytokines and type I IFN production [11, 12]. Six types of PRRs have been identified including toll-like receptors (TLRs), retinoic acid-inducible gene-I (RIG-I)-like receptors (RLRs), absent in melanoma 2 (AIM2)-like receptors (ALRs), nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs), cytosolic DNA-sensing receptors and nuclear DNA sensors [13].
Data suggest that most host innate immune systems can be activated by KSHV infection. However, KSHV has evolved a variety of sophisticated countermeasures, encoding numerous genes and microRNAs that interfere with the host innate immune response. These countermeasures are critical for establishing persistent infection [13]. This brief review covers the up-to-data advances in understanding the cellular sensing of KSHV infection and KSHV strategies for escaping host innate immune signaling pathways.
1. Kaposi’s sarcoma-associated herpesvirus
KSHV (also called human herpesvirus type 8) is a member of the human γ-herpesvirus family, which was discovered in 1994 from KS tissue by Chang and Moore using representational difference analysis [1]. By 1996, the Chang and Moore groups had sequenced and cloned the entire viral genome [14].
1.1. Virion structure
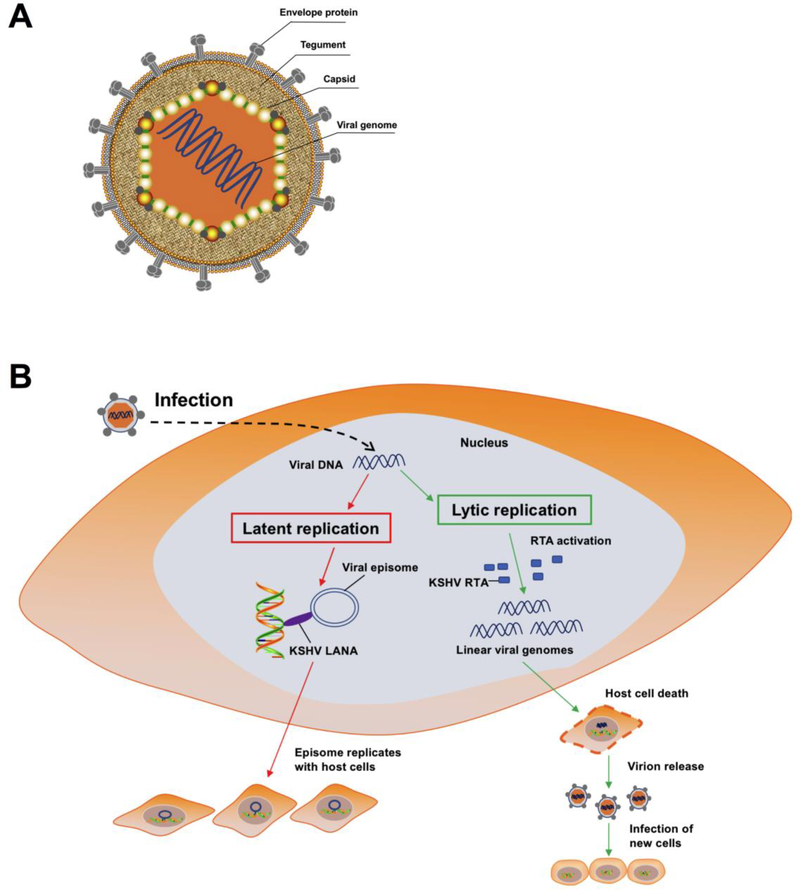
Similar to the other members of the rhadinovirus genus of herpesviruses, KSHV virions exhibit an icosahedral capsid protecting the KSHV genome. The capsid is surrounded by lipid-containing envelope. A highly organized and dense proteinaceous layer localizes between the capsid and the envelope, is called the tegument layer (Fig. 1a).
Figure 1.
(a) Schematic presentation of KSHV structure. KSHV virions exhibit an icosahedral nucleocapsid surrounded by a lipid bilayer envelope, and a tegument layer between the capsid and envelope. (b) Schematic representation of KSHV life cycle in infected cells. KSHV life cycle contains two phases of infection: a short lytic replication and a persistent latent replication. During latency, LANA protein tethers KSHV episome to host cell chromosome. KSHV episome replicates along with host cell dividing during KSHV latent infection. Upon exogenous stimuli, e.g. cellular stress, valproate, butyrate etc., KSHV can be induced to switch from latency to lytic replication. During this phase, the RTA promoter is activated and most viral genes are expressed. During lytic replication, the KSHV genome replicates in a rolling cycle mechanism. Linear genomes are packaged into capsids, and then host cell are destroyed, leading to release of new virions to infect new host cells.
The KSHV envelope is studded with glycoproteins that have key functions in mediating viral entry and viral recognition by host cell surface receptors. Seven viral glycoproteins have been identified, including gB (ORF8), K8.1, gH (ORF22), gM (ORF39), gL (ORF47), gN (ORF53), and ORF68 [15–20].
The tegument layer is a morphologically amorphous structure that remains largely undefined. Based on the specific biochemical criteria of tegument components and mass spectrometry analysis of mature KSHV virions, several tegument proteins were identified: ORF11, ORF21, ORF33, ORF38, ORF45, ORF50, ORF52, ORF55, ORF63, ORF64 and ORF75 [5, 20, 21]; more would be characterized in the future. Tegument proteins have key roles at various stages of the KSHV life cycle. They are involving virion tegumentation, assembly, trafficking, replication and egress processes, and mediate host innate immunity to facilitate the persistent infection [22].
The KSHV capsid is a trangulation number of 16 icosahedral lattice with 12 pentons, 150 hexons, and 320 triplexes. Five capsid proteins have been identified [23]: ORF25 encoded major capsid protein that forms hexons and pentons; ORF26 encoded triplex dimer protein TRI-2; ORF62 encoded triplex monomer protein TRI-1; ORF65 encoded small capsid protein; and the ORF17.5 encoded scaffolding [24]. Within this capsid is a large DNA genome consisting of a unique coding region of about 140kb flanked by multiple copies of GC-rich terminal-repeats [14].
1.2. Two phases of the KSHV cycle
Like other herpesviruses, KSHV cycle displays two stages of infection, including transient lytic replication and persistent latent infection. Latency is the default state of KSHV infection, in which only a few viral genes are expressed to maintain the viral genome, without infectious virions production [6] (Fig. 1b). The latent genes are ORF73 encoded latency-associated nuclear antigen (LANA); ORF71 encoded the viral FLICE inhibitory protein (v-FLIP); ORF72 encoded v-cyclin; Kaposin encoded by K12; and 12 pre-microRNAs [25]. LANA, a major KSHV latent protein, is the most extensively investigated latent gene and is responsible for maintenance of latent cycle by keeping the viral genome as a circular episome [26] and inhibiting lytic gene expression [27, 28].
Different exogenous stimuli induce viruses to switch from latency to lytic reactivation. During KSHV lytic reactivation, a full panel of viral gene are expressed. The viral genome is replicated using the rolling cycle mechanism, and infectious progeny virion are assembled and released from host cells [29] (Fig. 1b). Upon lytic reactivation, transcription of three classes of lytic genes, the immediate early (IE), early (E), and late (L) genes, is activated [30]. IE genes are K3, K4, K4.1, K4.2, K8α, K8.2, ORF29b, ORF45, ORF48, ORF50, and ORF70, etc; E genes are K1, K2/vIL6, K5, K8, K8.1A, K9, K12, ORF6, ORF21, ORF37, ORF57, ORF59, PAN RNA, Viral interferon regulatory factor (vIRF) 1, ORF65 and ORF74, etc; and L genes encode structural proteins of KSHV, such as a small viral capsid protein and membrane glycoproteins involved in the process of virion particles’ assembly and maturation [31–33]. KSHV ORF50 encoded the replication and transcriptional activator (RTA) protein is a key lytic switch regulator. RTA is the exclusive viral protein that is necessary and sufficient to drive the lytic replication cycle [34, 35].
1.3. KSHV related diseases and treatment
KSHV is etiologically linked to the development of KS, a common AIDS-associated malignant tumor, as well as two other rare lymphoproliferative diseases, MCD and PEL.
KS is a tumor most likely with endothelial origin that arises in skin, gastrointestinal tract, oral cavity, lymph node and organs [36]. KS is one of most prevalent tumor in subequatorial African countries and can be classified into four subtypes including classic KS, iatrogenic KS, endemic KS, and AIDS-KS [37]. Aging is a key risk factor for classic KS, which typically affects older men of Mediterranean, Eastern European, South American origins and Northwest of China [38–40]. Endemic KS is described in geographically-restricted regions of sub-Saharan Africa and predominantly in young men, which tended to be more aggressive than classic KS and can be developed into fatal [41]. Iatrogenic KS is identified in patients receiving immunosuppressive therapy, such as organ transplant recipients [42]. With the advent of AIDS pandemic, increasing the incidence of KS among young homosexual men males [43]. This variant is more aggressive and has been called AIDS-KS. KS is the most common tumor significant cause of morbidity and mortality in HIV-infected individuals. Patients with AIDS-related KS present multifocal skin lesions and frequent organ morbidity that is more commonly life-threatening [44]. Survival of patients with KS has remained poor due to absence of effective drugs or specific treatment, highly active antiretroviral treatment and combination therapy are effective on AIDs-related KS [45, 46].
PEL is a KSHV-positive B lymphoma with body cavity effusion, which frequently occurs in HIV-infected individuals [3]. Poor survival of PEL in the conventional chemotherapy [47]. Another B-cell lymphoproliferative associated with KSHV is MCD, which presents the symptom of multiple enlarged lymph nodes [48]. KSHV-related MCD mainly occurs in HIV-positive individuals, which can be treated with chemotherapy and steroids with poor prognosis [49].
Vaccinating with KSHV-encoded proteins may produce antibodies to neutralize KSHV virus in animal experiments [50]. As KSHV infection is common in African countries and remains a major cause of morbidity and mortality in HIV-infected patients, moreover, there is no definite cure therapies, developing new approaches for treatment KSHV is needed.
2. Recognition by membrane receptors
TLRs are transmembrane receptors that are localized in the plasma membrane and endosomal membrane. TLRs have an extracellular motif, a transmembrane region, and a conserved cytoplasmic toll/interleukin-1 receptor (TIR) domain. TLR1, 2, 4, 5, 6, 10 and 11 are on the plasma membrane, while TLR3, 7, 8, 9, 12 and 13 localize in endosomal membranes. TLRs recognize PAMPs derived from viruses, including CpG DNA, single-stranded RNA (ssRNA), double-stranded RNA and proteins from viruses. TLRs recognize ligands, leading to recruitment of TIR-containing adaptors such as TRIF or MyD88, which interact with the TIR domain of TLRs. This interaction allows the recruitment and activation of downstream kinases including IRAKs (1, 2 and 4) and TRAFs (3 and 6). Kinase activation results in additional downstream kinases activation and the release of NF-κB or phosphorylation of IRF3/7, which allows IRF3/7 or NF-κB to translocate to the nucleus. Translocation leads to proinflammatory and type I IFN responses [51, 52]. Both plasma membrane and endosomal TLRs are involved in KSHV sensing, and KSHV also modulates TLR signaling to establish long-term infection (Fig. 2).
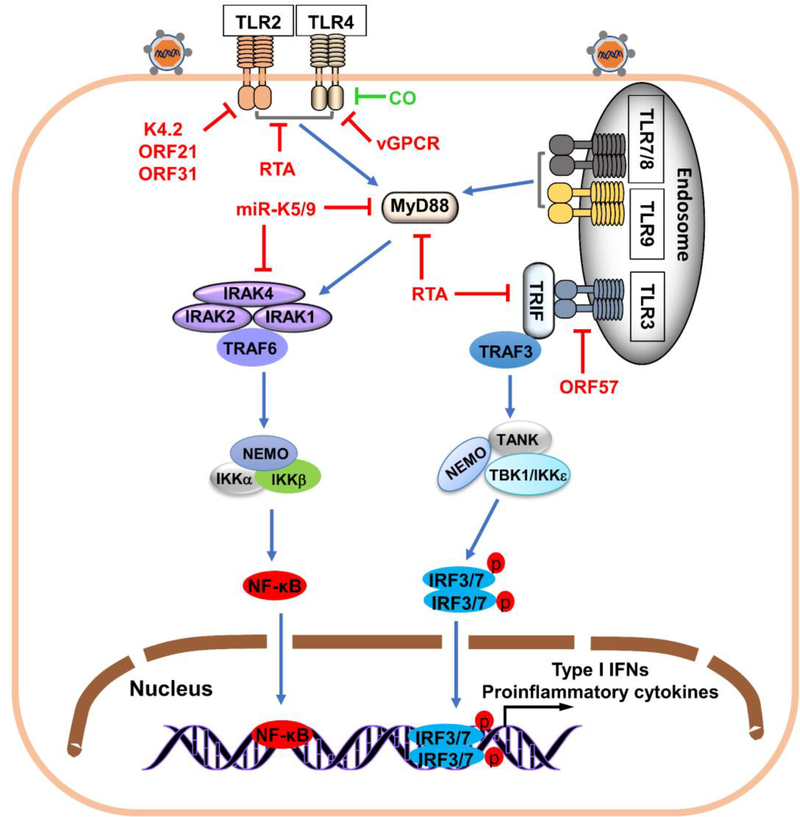
Figure 2.
Activation and evasion of membrane PRR-mediated antiviral response by KSHV. Following KSHV infection of cells, specific TLRs are activated, initiating recruitment of adaptor proteins MyD88 or TRIF. MyD88 recruits IRAK4, IRAK1, IRAK2 and TRAF6, triggering activation of IKK complex of NEMO and IKKαβ that mediate nuclear translocation of NF-κB, essential for IFN-β production. Recruitment of TRIF to TLRs activates recruitment of TRAF3 and subsequent activation of virus-mediated kinases (TBK1 and IKKε), triggering IRF3/7 phosphorylation, which homodimerizes, translocates to the nucleus, and triggers type I IFN induction. KSHV-encoded proteins (red) inhibit immune responses initiated by TLRs. K4.2, ORF21 and ORF31 inhibit TLR2-dependent signaling activation. RTA encoded by ORF50 reduces TLR2 and TLR4 and disrupts plasma membrane localization of TLR2 and TLR4. TLR4 is targeted by vGPCR, central in TLR4 downregulation. E3 ubiquitin ligase KSHV RTA promotes proteasomal degradation of MyD88 and TRIF. KSHV miR-K9 and miR-K5 inhibit TLR signaling by targeting MyD88 and IRAK1. ORF57 inhibits TLR3-mediate signaling by blocking TLR3 phosphorylation. KSHV uses CO (green) to inhibit TLR4 signaling.
2.1. TLR2 and TLR4
TLR2 and TLR4 are expressed by a wide range of cell types including myeloid dendritic cells (DCs), neutrophils, monocytes/macrophages, T and B lymphocytes, epithelial cells, and synovial fibroblast-like cells [53]. TLR2 and TLR4 can sense PAMPs from microbial infections. Bacterial lipopolysaccharide is a well-known ligand for TLR4. Viral proteins such as retroviral envelopes [54], respiratory syncytial virus F protein [55], and vesicular stomatitis virus glycoprotein G [56] are also TLR4 agonists. TLR2 recognizes different ligands, for instance lipoproteins, peptidoglycans, lipotechoic acids, zymosan, mannan, and glycosylphosphatidylinositol-anchored mucins-like glycoproteins from Trypanosoma cruzi [57]. Both TLR2 and TLR4 are involved in γ-herpesvirus detection. TLR2 detects murine γherpesvirus-68 (MHV-68) and Epstein–Barr virus and TLR4 to detect KSHV. Macrophages from TLR4 knockout mice lead to higher KSHV gene expression, and TLR4 silencing with specific siRNA in human lymphatic endothelial cells also results in higher KSHV gene expression. Infection with KSHV inhibits the activation of TLR2- and TLR4- mediated signaling [58, 59]. These results indicate that TLR2 and TLR4 are crucial for detecting KSHV invasion. KSHV uses ORF31, ORF21, K4.2, and RTA to suppress the activation of TLR2-dependent signal pathway. RTA, the master switch molecule of KSHV that can initiate virus lytic replication from latent state, functions as an E3 ligase and disrupts plasma membrane localization of TLR2 and TLR4 and strongly reduces the expression level of TLR2 and TLR4 [59]. However, the further mechanism of RTA-induced degradation of TLR2 and TLR4 is poorly understood. Because RTA is localized in the nucleus and the two TLRs are on the cell surface, RTA likely acts through an indirect mechanism via unidentified cellular protein(s) that mediate TLR degradation. KSHV vIRF1 inhibits IRF-mediated transcription that in turn represses TLR4 transcription [60–62]; KSHV vGPCR promotes phosphorylation of ERK, which also contributes to TLR4 suppression [58]. Some studies show that carbon monoxide (CO) blocks activation of TLR4 signaling by inhibiting the interaction between TLR4 and its downstream adaptor proteins [63, 64]. Interestingly, the heme oxygenase-1 (HO-1) is a host protein that is co-opted by KSHV, resulting in increased levels of intracellular CO that inhibit TLR4 signaling to promote KSHV infection [65].
2.2. TLR3
TLR3 is highly expressed in innate immune cells and is crucial for virus-mediated innate immune responses. TLR3 senses viral double-stranded RNA, triggering signal transduction by recruiting TRIF, which leads to the induction of type I IFN and other cytokines [66]. However, TLR3 antiviral function is controversial. TLR3-deficient mice are susceptible to encephalomyocarditis virus [67], mouse cytomegalovirus [68], coxsackievirus B3/B4 [69, 70], and poliovirus [71], but normally more resistant than wild type to influenza virus [72], West Nile virus [73], and herpes simplex virus 1 [74]. KSHV de novo infection of human monocytes upregulates expression of TLR3 that strongly induces TLR3-specific chemokines and cytokines including IFN-β1, CCL2, CXCL10, and IRF1 [75]. However, at later times post infection, TLR3 levels are downregulated due to suppression of TLR3-mediated activation of type I IFN transcription reporters by KSHV vIRFs. In response to TLR3 activation, only vIRF1 leads to decreased phosphorylation and nuclear translocation of IRF3. The result means that each vIRFs has a unique mechanism for prevent TLR3-mediated signaling [76, 77]. A study reported that ORF57, an early lytic RNA-binding protein, blocks poly I:C-induced TLR3 phosphorylation that is required for recruiting downstream adaptor proteins and then activating further signaling cascades; however, the detailed mechanism remains unclear [78].
2.3. TLR7/8 and TLR9
TLR7 is predominantly expressed in plasmacytoid DCs (pDCs), and recognizes ssRNA derived from RNA viruses, such as influenza virus, vesicular stomatitis virus, and human immunodeficiency virus [53]. Human TLR8 is primarily expressed in macrophages and myeloid DCs, and also senses viral ssRNA [66]. Expression of TLR9 is highly elevated in pDCs, B cells, and macrophages, and can be activated by unmethylated CpG motifs of dsDNA and dsDNA viruses such as mouse cytomegalovirus [79] and herpes simplex viruses 1 and 2 [80, 81], mediating type I IFN production. TLR7 and TLR9 are the two TLRs known to be expressed in pDCs so far. They are vital for activating the type I IFN pathway against invading viruses such as Epstein–Barr virus [82]. A study showed that KSHV infects and activates human pDCs, resulting in activating TLR9 signaling pathway and leading to increased IFN-α production and secretion [83]. This finding indicates that TLR9 recognizes KSHV during primary infection. In contrast to activation of TLR3 by KSHV primary infection of monocytes and TLR9 by infection of pDCs, activation of TLR7/8 leads to KSHV reactivation from latency. Further study revealed that sspoly-U-induced TLR7/8 response activates the KSHV RTA promoter and promotes lytic gene replication and expression. After secondary viral infects the KSHV-infected B lymphocyte cells, TLR7/TLR8 signaling can be activated, which induces reactivation of KSHV to ensure new infectious particle production and transmission, allowing propagation of the secondary virus [84].
2.4. TRIF and MyD88
TLRs-mediated innate immune signaling is classified into the MyD88-dependent and MyD88-indpendent pathways. The MyD88-dependent pathway is activated by all TLRs except TLR3, while the MyD88-indpendent pathway is mediated by TRIF and triggered by TLR3 and TLR4. Upon activation, ubiquitin ligase TRAF6 and downstream kinases IRAK1/4 are recruited to MyD88. This causes the TAK1 activation and the activation of inhibitor of nuclear factor-κB (IκB) kinase complex, resulting in induction of NF-κB-mediated transcription of inflammatory cytokines [66]. TRIF associates with TRAF3, activating TBK1 and IKKε complex that containing NEMO and TANK, leading to the activation and nuclear import of IRF3/IRF7 and the subsequent production of type I IFNs [51, 85]. Since MyD88 and TRIF are crucial for TLR signaling pathways, it is reasonable that KSHV employs various strategies to block the function of MyD88 and TRIF. RTA possesses ubiquitin E3 ligase activity and degrades a number of host proteins through the proteasome pathway. Meyer et al. reported that the TLR-TRIF pathway activation facilitates the expression of RTA. RIP1 may be involved in TRIF-mediated increasing expression of RTA protein, with KSHV RTA in turn degrading TRIF via the proteasomal pathway to block activation of TLR signaling pathways [86, 87]. RTA protein directly interacts with MyD88 and downregulates MyD88 expression via the proteasomal pathway to impair TLR signaling [88]. KSHV-encoded miR-K5 and miR-K9 inhibit TLRs signal pathway by targeting MyD88 and IRAK1 [89].
3. Recognition by cytoplasmic receptors
3.1. RLRs
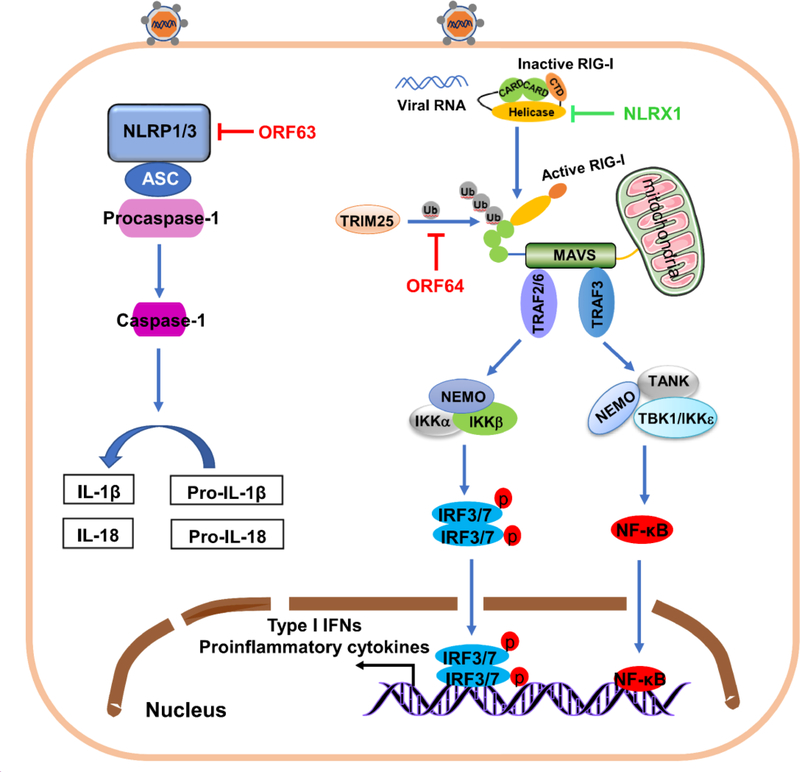
The RLR family has three members including retinoic acid-inducible gene-I (RIG-I), melanoma differentiation gene 5 (MDA5), and laboratory of genetics and physiology 2 (LGP2) [90]. RLRs are the main family of cytosolic RNA sensors and are critical for RNA virus recognition and antiviral innate immune responses [91, 92]. RIG-I has two N-terminal caspase activation and recruitment domains (CARDs), an RNA helicase region and a C-terminal motif. Under steady state conditions, RIG-I CARDs bind to the helicase domain that is under autoinhibition. Upon binding the specific molecular features of viral RNA such as 5′-triphosphate the conformation of RIG-I changes, mediated by ATPase/helicase activity, resulting in the exposure of CARDs. This step triggers robust K63 ubiquitination of RIG-I by TRIM25 and oligomerization of RIG-I. Next, RIG-I interacts with the adaptor protein MAVS through its CARDs, which results in MAVS oligomerization. Downstream, TRAF3/TBK1/IKKε and TRAF6/IKK further activate IRF3/7 driving IFN expression, and NF-κB, stimulating proinflammatory cytokine expression (Fig. 3). Growing evidence suggests that RIG-I pathway also takes part in recognizing DNA viruses including herpesviruses [93, 94]. A study showed that in cells depleted for RIG-I, cause an increased viral genome-wide transcription during primary KSHV infection and leads to increased production of infectious virions during the reactivation. This result suggests that RIG-I senses KSHV during various stages of its cycle [95]. Many short and long noncoding RNAs are transcribed by KSHV genome [96, 97], and some transcripts might be recognized by RIG-I. However, PAN RNA, the most abundant non-coding RNA produced in KSHV infected cells, is unable to induce RIG-I activation [98]. So far, the underlying mechanism by which RIG-I recognizes KSHV following infection is not fully elucidated and is worthy of future studies. Interestingly, a study suggests that KSHV encodes protein(s) that bind directly or indirectly to NLRX1, which is a negative regulator of RIG-I to disrupt type I IFN activation [99, 100]. In addition, KSHV ORF64 with deubiquitinase activity, downregulates RIG-I-mediated type I IFN production by inhibit RIG-I ubiquitination that mediated by TRIM25, leading to inhibit activation of type I IFN signal pathway [98]. The interaction between KSHV and RIG-I pathway is in Figure 3.
Figure 3.
Activation and evasion of cytoplasmic PRR-mediated antiviral response by KSHV. Following KSHV infection, RIG-I exposes its CARD domain for TRIM25 binding, which conjugates K63-polyubiquitin chains to RIG-I and activates RIG-I. Activation of RIG-I causes binding to adaptor protein MAVS on mitochondria, activating IRF3/7 and NF-κB and inducing production of type I IFN and pro-inflammatory cytokines. Viral deubiquitinase KSHV ORF64 (red) targets and inhibits TRIM25-mediated RIG-I ubiquitination and blocks IFN signaling. KSHV uses cell protein NLRX1 (green) to block RIG-I-mediated signaling. On activation, NLRP1/3 binds downstream adaptor ASC and substrate procaspase-1, forming the inflammasome. Caspase-1is activated, processing pro-IL-1 and pro-IL-18 into active IL-1 and IL-18 and regulating type I IFN and inflammatory pathways. KSHV encodes ORF63 protein (red), a viral homolog of human NLRP1 without CARD and PYD effector domains of its cellular counterpart, suppressing NLRP1- and NLRP3-mediated signaling by interacting with NLRP1 and NLRP3.
3.2. NLRs
NLRs have a common domain organization with a central NOD domain, an N-terminal effector region and a C-terminal leucine-rich repeat motif. Based on their N-terminal motif, NLRs are divided into three subfamilies: CARD-containing NODs, PYD-containing NALPs, or BIR-containing NAIPs. leucine-rich repeat are ligand-sensing motifs. These motifs recognize PAMPs to allow NLRs to recruit downstream adaptor protein ASC forming a complex that in turn recruits and activates caspase-1, then it ultimately causes processing and maturation of proinflammatory cytokines IL-1 and IL-18 (Fig. 3), which contributes to host inflammatory responses [101–103]. NLRP1 signaling inhibits KSHV lytic reactivation from latency and generation of progeny virus. KSHV might encode a viral homolog of human NLRP1, ORF63, that heteroligomerizes with NLRP1, NLRP3 and NOD2, but without CARD and PYD domains. Oligomerization impairs NLRP1 and NLRP3 oligomerization, ultimately blocking NLRP1 and NLRP3-dependent innate immune responses [104]. NLRX1, a member of the NLRs, localizes to the mitochondria and associates with MAVS to disrupt RIG-I-MAVS interaction [99]. NLRX1 also sequesters STING from interaction with TBK1 [105]. Therefore, NLRX1 is a negative regulator of innate immune signaling. Zhe et al. reported that NLRX1 expression benefits KSHV lytic replication (Fig. 3) [100]. How KSHV use NLRX1 protein to modulate its reactivation remains unclear because no upregulation or downregulation of NLRX1 is observed at either the transcript or protein level during KSHV reactivation.
3.3. cGAS
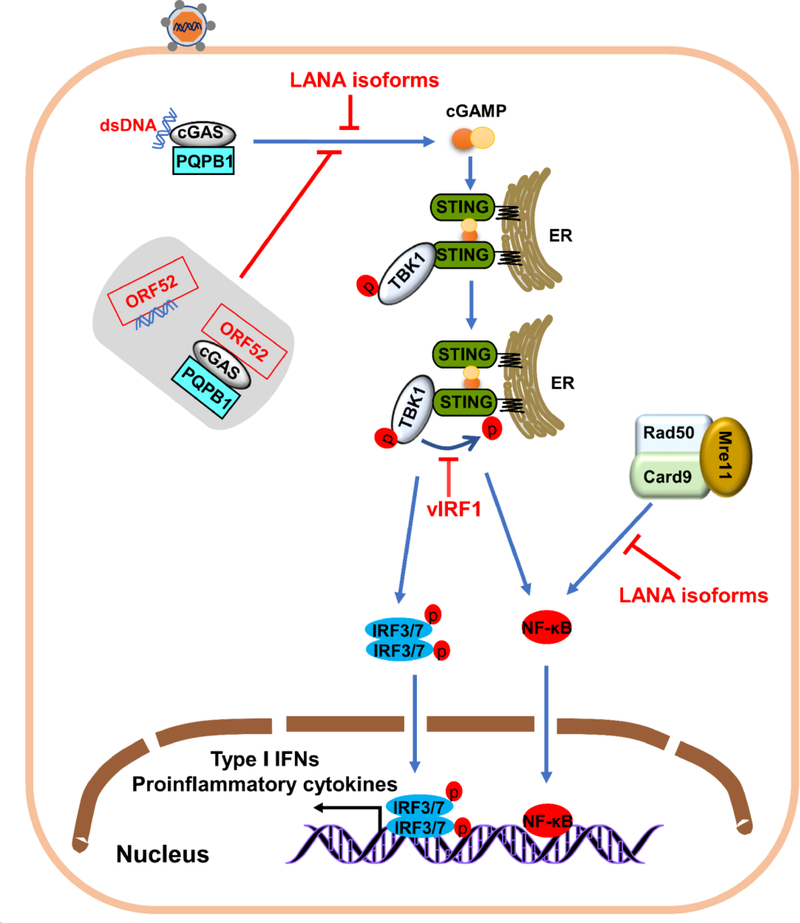
Detection of pathogenic DNA is crucial for activating innate immune responses for eliminating DNA virus. Many DNA sensors have been found in the cytoplasm [106–110]. Among these sensors, cGAMP synthase (cGAS) has a principal function in the herpesviruses-induced innate immune response [111]. After cGAS and its DNA-binding partner PQBP1 recognizing viral DNA, the synthesis of cyclic GMP-AMP (cGAMP) from intracellular ATP and GTP serves as the second messenger which interacts with STING. Active STING recruits and activates TBK1. TBK1 phosphorylates STING, which is critical for activation of IRF3 and NF-κB. Ultimately, activated IRF3 and NF-κB translocate into the nucleus to initiate antiviral responses [112–114] (Fig. 4). CGAS also associates with Beclin-1, which induces the autophagy of infected cells to eliminate virus infection [115]. CGAS−/− mice fails to induce a type I IFN response to HSV, MHV-68, and vaccinia virus [116, 117], and cGAS−/− mice are highly susceptible to lethal infections. In addition, the cGAS-STING signaling pathway is activated upon human and mouse cytomegalovirus infection, and cGAS−/− mice and STING−/− mice exhibit low levels of type I IFN and high viral titers in tissues [118]. The cGAS-STING axis also has antiviral effects against KSHV. To successfully establish persistent infection, KSHV has evolved to target the key progresses of cGAS-mediated antiviral innate immune signaling. Although LANA is primary localized in the nucleus, studies found that full-length LANA and isoforms of LANA can are in the cytoplasm during lytic replication [119, 120], and some cytoplasmic isoforms of LANA target cGAS to inhibit cGAS-mediated antiviral innate immune responses [121]. Ma et al. found that KSHV vIRF1 targets STING, an important DNA sensing adaptor, blocking the association between STING and TBK1, and TBK1-mediated phosphorylation of STING, which are essential for the response to viral infection [122]. KSHV ORF52, a tegument protein, is a late lytic gene that directly blocks cGAS enzymatic activity by associating with both DNA and cGAS [123], and inhibits binding of cGAS and PQBP1 to HexlM1. This inhibition prevents IRF3 phosphorylation, which is pivotal in the innate immune defense against viral infection [124].
Figure 4.
Activation and evasion of cytoplasmic DNA sensors-mediated antiviral response by KSHV. DNA binding to cGAS and DNA-binding partner PQBP1 activates enzymatic activity. CGAS catalyzes production of cGAMP, a second messenger that activates STING, a dimer that recruits TBK1 to promote its phosphorylation. Phosphorylated STING recruits IRF3 and triggers IRF3 phosphorylation by TBK1. Activation of IRF3 causes NF-κB to enter the nucleus and trigger innate immune responses. KSHV-encoded proteins (red) inhibit cGAS and STING-dependent responses. Tegument protein ORF52 binds DNA and cGAS and directly inhibits cGAS-dependent DNA sensing. Cytoplasmic LANA isoforms inhibit cGAS-STING-dependent induction of interferon by directly interacting with cGAS. KSHV protein. VIRF1 disrupts STING-TBK1 interaction and prevents STING phosphorylation and activation by interacting with STING. Cytoplasmic Rad50, Mre11 and CARD9 sense cytoplasmic DNA and activate NF-κB-mediated innate antiviral responses. Cytoplasmic KSHV LANA isoforms (red) target Rad50 and Mre11 to interfere with activation of the NF-κB cascade.
3.4. MRN complex members
Mre11-Rad50-NBS1 (MRN) complex (Fig. 4), a sensor of dsDNA breaks that is responsible for detecting DNA damage and activating DNA repair cascades [125, 126]. Studies suggest that MRN complex also senses cytoplasmic DNA and activates the NF-κB pathway to control of KSHV replication [127–129]. Giuseppe et al. showed that cytosolic LANA isoforms also recruit members of the MRN repair complex, inhibiting the NF-κB signaling activation that induced by cytosolic DNA and increasing lytic replication in KSHV-infected cells [130].
4. Recognition by nuclear receptors
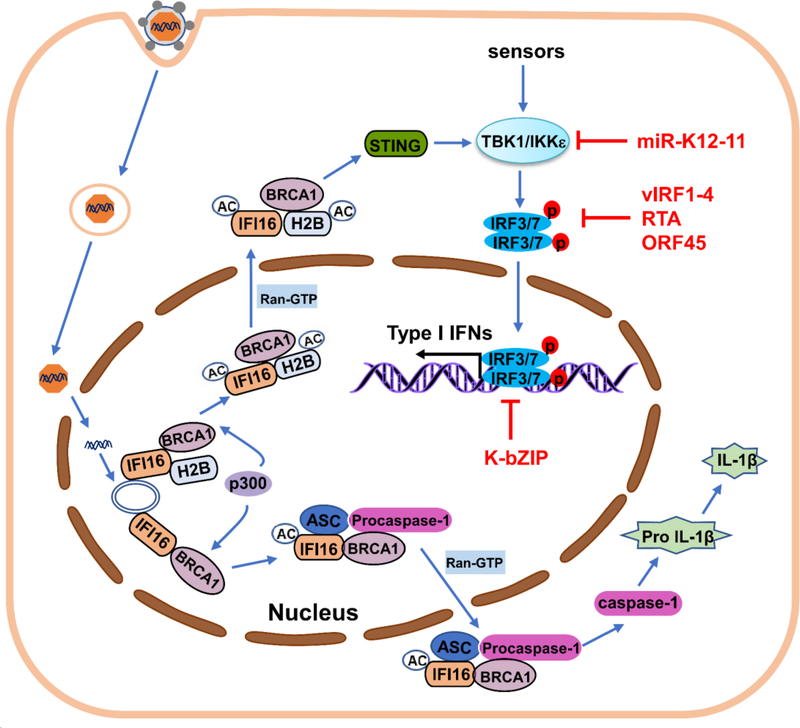
Due to the similarities between viral and human DNA, the intracellular surveillance which recognizes viral DNA is supposed to occur solely in cytoplasmic and endosomal compartments without containing host DNA molecules [131]. But, in many cases of nucleus- replicating DNA viruses, capsid is released only by nuclear import that shelters viral genomes during cytoplasmic transport progress. Hence, cytosolic DNA sensors have only a little chance to contact viral DNA molecules. Studies have demonstrated the existence of nuclear DNA sensors that sense viral DNA to trigger host antiviral response [132–135]. Interferon γ-inducible protein 16 (IFI16), a member of the ALR family, is a key DNA sensor for detecting nuclear pathogenic DNA and is pivotal in type I IFN production and inflammasome activation [132, 136]. KSHV enters cells in a cell type-dependent manner, trafficking into the endosome and releasing capsid near the nuclear pore to deliver viral DNA into the nucleus. Soon after KSHV DNA entry into the nucleus, IFI16, in complex with BRCA1 or BRCA1-H2B, recognizes KSHV genomes. This recognition leads to BRCA1-mediated p300 recruitment, interaction with IFI16, and acetylation of IFI16 and H2B by p300. Cytoplasmic translocation of acetylated IFI16-H2B-BRCA1 complex by RanGTP results in association with STING from a macromolecular complex, leading into phosphorylation of IRF3 and activation of the type I IFN pathway. Independent of H2B-IFI16-BRCA1, IFI16-BRCA1 recognizes KSHV genome and then leads to formation of BRCA1-acetylated IFI16-ASC-procaspase-1 inflammasome complex which promotes procaspase-1 activation in the nucleus, interaction with RanGTPase, translocation of cytoplasm and induction of IL-1β, which results in inflammation responses [137, 138] (Fig. 5). Roy et al. found that KSHV uses IFI16 acts as a transcriptional repressor to bind the lytic gene promoter, maintaining latency and repressing lytic transcripts [139]. KSHV may have evolved to usurp the mechanism of the IFI16 inflammasome pathway and IFI16 functions as a transcription repressor to drive KSHV-associate inflammatory cytokine syndromes. KSHV late protein(s) may be involved in the ubiquitination and proteasomal degradation of IFI16. However, since IFI16 is important for sensing DNA viral infection, more in-depth analysis is needed to be identified which KSHV protein(s)-mediated proteasomal degradation of IFI16 and other possible mechanisms that regulate IFI16-mediated signaling by KSHV.
Figure 5.
Activation of nuclear DNA sensor-mediated antiviral response by KSHV with regulation of downstream kinases of PRR-mediated signal pathways. After KSHV genome entry into the nucleus, IFI16 associates with BRCA1-H2B or BRCA1 and binds the viral genome, leading to BRCA1-mediated p300 recruitment, interaction with IFI16, and acetylation (Ac) of IFI16 and H2B by p300. Acetylated IFI16-H2B-BRCA1 complex transports to the cytoplasm and associates with STING, leading into TBK1 and IRF3/7 phosphorylation, IRF3/7 nuclear translocation and type I IFN production. Acetylated IFI16-BRCA1 interacts with ASC and procaspase-1 in the nucleus. BRCA1-IFI16-ASC-procaspase-1 complex is transported to the cytoplasm via Ran-GTP, activating caspase-1. IL-1β generation results in inflammasome-IL-1β responses. Signaling pathways activated by the different classes of PRRs converge at the level of the IKK complex and related kinases IKKε and TBK1 to activate IRF3/7, inducing IFN production. KSHV encodes proteins (red) that block downstream signaling cascades of PRRs. KSHV miR-K12–11 inhibits IKKε-mediated IRF1/7 phosphorylation by inhibiting IKKε expression. KSHV harbors four viral IRFs (vIRF1–4) with different mechanisms to block IRF3/7-mediated IFN transcriptional activity. KSHV RTA promotes proteasome-dependent degradation of IRF3/7 by directly targeting IRF7 or associating with cellular E3 ligase. KSHV ORF45 interacts with ORF7 and competitively inhibits IRF7 phosphorylation by IKKε and TBK1. KSHV encodes K-bZIP protein that binds directly to the IFN-β promoter and prevents IRF3 from binding to the IFN-β promoter, inhibiting type I IFN production.
The interferon induction inducible protein X (IFIX), a member of the PYHIN family, is a nuclear DNA sensor that binds HSV-1 DNA upon infection and promotes antiviral cytokine expression. HSV-1 has evolved a viral host suppression mechanism to inhibit IFIX functions via host proteasome-dependent degradation [135]. Given that IFIX binds DNA substrates in a sequence-independent manner, IFIX may have a similar function in KSHV infection although further work is needed. In addition, cGAS is reported to distribute to both the cytoplasm and nucleus of human keratinocytes and fibroblasts cells, and interacts with IFI16 to promotes IFI16 stability. However, cGAS is not recruited to the incoming viral genomes [140]. These findings suggest that cGAS may perform auxiliary functions that enhance IFI16 stability to prolong or enable signal potentiation. Whether cGAS also recognizes viral DNA genome in host nucleus to initiate nucleus-originating immune signaling remains to be investigated.
5. Molecules downstream of PRRs
Most PRRs activate IRF3 and IRF7 for the transcription of type I interferon. IRF3 and IRF7 serve as transcription factors and undergo phosphorylation, dimerization and translocation into the nucleus upon virus infection, then directly bind to IFN promoters to stimulate type I IFN expression [141]. Thus, viruses have evolved various immune escape countermeasures to inhibit IRF-mediated type I IFN activation (Fig. 5). KSHV harbors four viral IRFs (vIRF1–4) with significant homology to host IRFs. Studies suggest that each of the vIRFs subverts the IFN-mediated antiviral response via a different mechanism. vIRF1 directly targets p300 to interfere with CBP/p300-IRF3 complex formation and inhibits the activity of p300 histone acetyltransferase, leading to block IRF3-mediated the activation of IFN signaling [142]. vIRF2 also inhibits expression of the IFN-stimulated genes by IRF1 and IRF3 [143]. VIRF3 inhibits IRF7 DNA binding activity by targeting the DNA-binding domain of IRF7 [144]. Moreover, vIRF4 efficiently blocks IRF7 dimerization by interacting with IRF7, ultimately suppressing IRF7-mediated IFN transcriptional activity [145]. RTA acts as a ubiquitin E3 ligase that interacts with IRF7 for proteasome-mediated degradation [146] and cooperates with cellular ubiquitin E3 ligases, such as RAUL, to facilitate proteasome-dependent degradation of IRF3 and IRF7 [147]. KSHV tegument protein ORF45 targets IRF7 but without interfere with the association between IRF7 and IKKε/TBK1. ORF45 competes with IRF7 for phosphorylation by TBK1/IKKε, blocking the activation and nuclear translocation of IRF7, leading to inhibition of the transactivation of IFN signaling [148–150]. K-bZIP is a viral transcription factor that blocks IRF3 occupancy of the IFN-β promoter by directly binding the region of the IFN-β promoter and impairing IFN-β production [151]. Liang et al. reported a KSHV miRNA, miR-K12–11, that attenuates IFN activation by inhibiting IKKε-mediated phosphorylation of IRF3/IRF7 to suppress antiviral innate immunity [152].
As reviewed here, PRRs signaling can be turned on by sensing KSHV infection, triggering antiviral innate immune responses. However, KSHV has also evolved elegant countermeasures to escape the innate immune system for persistent viral infection and pathogenesis.
6. Conclusions
Studies on recognition of invading virus by host innate immune systems have come a long way. A number of well-characterized viral sensors in the membrane and cytoplasm have been identified and have the ability to recognize KSHV infection. However, these sensors have limited opportunities to contact the KSHV genome because KSHV deposits and replicates the DNA genome exclusively within host nuclei. Therefore, nuclear viral DNA sensors may be critical for the response against invading DNA viruses. IFI16 might not be the only DNA sensor in the nucleus to detect the KSHV DNA genome. The existence of other novel DNA sensors in nucleus remains to be studied. If more sensors exist, how they regulate DNA virus infection and how host proteins modulate these sensors and mediate innate immune signaling remains to be studied. Also to be studied is how DNA sensors are regulated by viral proteins. Finally, further insights and developments in new nuclear DNA sensors will contribute to devising new antiviral strategies against KSHV infection for treatment of KSHV-associated diseases. IFI16 and IFIX recognize viral DNA independent of sequence. However, host genes and DNA viral genes co-exist in the nucleus during viral infection. Hence, differentiating between self and foreign genes, which may be related to modification of viral genomes by cellular proteins, is important. The mechanism by which nuclear DNA sensors distinguish between self-DNA and nonself-DNA remains to be investigated. Future studies are critical for delineating nuclear viral DNA sensors and will help us understand the processes of antiviral signaling events and provide potential therapeutic targets for drug interventions.
In summary, nearly all known PRRs are involved in recognizing KSHV infection, and then activate host antiviral innate immune responses. However, KSHV has achieved a balance in host by evolving various strategies to evade host innate immune response and maintain long-term infection. During these processes, KSHV encodes viral proteins or utilizes host genes to interfere with the activation of cellular antiviral immune signaling. Therefore, the understanding of how KSHV activates host antiviral immunity and how KSHV escapes from host innate immune surveillance will lead to develop novel therapeutic targets and vaccine targets for the treatment and prevention of KSHV-associated diseases in the future.
Acknowledgments
Due to the page limitation, we regret we omitted some important references. This work was supported by the National Key R&D Program of China (2016YFA0502100), the Natural Science Foundation for Distinguished Young Scholars (81425017), and the National Institutes of Health (7R01AI116442) to K.L..
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Chang Y, Cesarman E, Pessin MS et al. (1994) Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 266:1865–1869 [DOI] [PubMed] [Google Scholar]
- [2].Soulier J, Grollet L, Oksenhendler E et al. (1995) Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood 86:1276–1280 [PubMed] [Google Scholar]
- [3].Nador RG, Cesarman E, Chadburn A et al. (1996) Primary effusion lymphoma: a distinct clinicopathologic entity associated with the Kaposi’s sarcoma-associated herpes virus. Blood 88:645–656 [PubMed] [Google Scholar]
- [4].Neipel F, Fleckenstein B (1999) The role of HHV-8 in Kaposi’s sarcoma. Semin Cancer Biol 9:151–164 [DOI] [PubMed] [Google Scholar]
- [5].Bechtel JT, Winant RC, Ganem D (2005) Host and viral proteins in the virion of Kaposi’s sarcoma-associated herpesvirus. J Virol 79:4952–4964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cai Q, Verma SC, Lu J et al. (2010) Molecular biology of Kaposi’s sarcoma-associated herpesvirus and related oncogenesis. Advances in virus research 78:87–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Grundhoff A, Ganem D (2004) Inefficient establishment of KSHV latency suggests an additional role for continued lytic replication in Kaposi sarcoma pathogenesis. J Clin Invest 113:124–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Krishnan HH, Naranatt PP, Smith MS et al. (2004) Concurrent expression of latent and a limited number of lytic genes with immune modulation and antiapoptotic function by Kaposi’s sarcoma-associated herpesvirus early during infection of primary endothelial and fibroblast cells and subsequent decline of lytic gene expression. J Virol 78:3601–3620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wen KW, Damania B (2010) Kaposi sarcoma-associated herpesvirus (KSHV): molecular biology and oncogenesis. Cancer Lett 289:140–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mariggio G, Koch S, Schulz TF (2017) Kaposi sarcoma herpesvirus pathogenesis. Philos Trans R Soc Lond B Biol Sci 372: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124:783–801 [DOI] [PubMed] [Google Scholar]
- [12].Kawai T, Akira S (2007) Antiviral signaling through pattern recognition receptors. J Biochem 141:137–145 [DOI] [PubMed] [Google Scholar]
- [13].Lee HR, Choi UY, Hwang SW et al. (2016) Viral Inhibition of PRR-Mediated Innate Immune Response: Learning from KSHV Evasion Strategies. Mol Cells 39:777–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Russo JJ, Bohenzky RA, Chien MC et al. (1996) Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc Natl Acad Sci U S A 93:14862–14867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Baghian A, Luftig M, Black JB et al. (2000) Glycoprotein B of human herpesvirus 8 is a component of the virion in a cleaved form composed of amino- and carboxyl-terminal fragments. Virology 269:18–25 [DOI] [PubMed] [Google Scholar]
- [16].Akula SM, Pramod NP, Wang FZ et al. (2001) Human herpesvirus 8 envelope-associated glycoprotein B interacts with heparan sulfate-like moieties. Virology 284:235–249 [DOI] [PubMed] [Google Scholar]
- [17].Wang FZ, Akula SM, Pramod NP et al. (2001) Human herpesvirus 8 envelope glycoprotein K8.1A interaction with the target cells involves heparan sulfate. J Virol 75:7517–7527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Naranatt PP, Akula SM, Chandran B (2002) Characterization of gamma2-human herpesvirus-8 glycoproteins gH and gL. Archives of virology 147:1349–1370 [DOI] [PubMed] [Google Scholar]
- [19].Koyano S, Mar EC, Stamey FR et al. (2003) Glycoproteins M and N of human herpesvirus 8 form a complex and inhibit cell fusion. J Gen Virol 84:1485–1491 [DOI] [PubMed] [Google Scholar]
- [20].Zhu FX, Chong JM, Wu L et al. (2005) Virion proteins of Kaposi’s sarcoma-associated herpesvirus. J Virol 79:800–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lan K, Kuppers DA, Verma SC et al. (2005) Induction of Kaposi’s sarcoma-associated herpesvirus latency-associated nuclear antigen by the lytic transactivator RTA: a novel mechanism for establishment of latency. J Virol 79:7453–7465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sathish N, Wang X, Yuan Y (2012) Tegument Proteins of Kaposi’s Sarcoma-Associated Herpesvirus and Related Gamma-Herpesviruses. Front Microbiol 3:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wu L, Lo P, Yu X et al. (2000) Three-dimensional structure of the human herpesvirus 8 capsid. J Virol 74:9646–9654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nealon K, Newcomb WW, Pray TR et al. (2001) Lytic replication of Kaposi’s sarcoma-associated herpesvirus results in the formation of multiple capsid species: isolation and molecular characterization of A, B, and C capsids from a gammaherpesvirus. J Virol 75:2866–2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Uppal T, Jha HC, Verma SC et al. (2015) Chromatinization of the KSHV Genome During the KSHV Life Cycle. Cancers 7:112–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ballestas ME, Chatis PA, Kaye KM (1999) Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science 284:641–644 [DOI] [PubMed] [Google Scholar]
- [27].Lu F, Day L, Gao SJ et al. (2006) Acetylation of the latency-associated nuclear antigen regulates repression of Kaposi’s sarcoma-associated herpesvirus lytic transcription. J Virol 80:5273–5282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Shamay M, Krithivas A, Zhang J et al. (2006) Recruitment of the de novo DNA methyltransferase Dnmt3a by Kaposi’s sarcoma-associated herpesvirus LANA. Proc Natl Acad Sci U S A 103:14554–14559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Aneja KK, Yuan Y (2017) Reactivation and Lytic Replication of Kaposi’s Sarcoma-Associated Herpesvirus: An Update. Front Microbiol 8:613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jenner RG, Alba MM, Boshoff C et al. (2001) Kaposi’s sarcoma-associated herpesvirus latent and lytic gene expression as revealed by DNA arrays. J Virol 75:891–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wang SE, Wu FY, Yu Y et al. (2003) CCAAT/enhancer-binding protein-alpha is induced during the early stages of Kaposi’s sarcoma-associated herpesvirus (KSHV) lytic cycle reactivation and together with the KSHV replication and transcription activator (RTA) cooperatively stimulates the viral RTA, MTA, and PAN promoters. J Virol 77:9590–9612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Deng H, Young A, Sun R (2000) Auto-activation of the rta gene of human herpesvirus-8/Kaposi’s sarcoma-associated herpesvirus. J Gen Virol 81:3043–3048 [DOI] [PubMed] [Google Scholar]
- [33].Song MJ, Deng H, Sun R (2003) Comparative study of regulation of RTA-responsive genes in Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8. J Virol 77:9451–9462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lukac DM, Kirshner JR, Ganem D (1999) Transcriptional activation by the product of open reading frame 50 of Kaposi’s sarcoma-associated herpesvirus is required for lytic viral reactivation in B cells. J Virol 73:9348–9361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sun R, Lin SF, Staskus K et al. (1999) Kinetics of Kaposi’s sarcoma-associated herpesvirus gene expression. J Virol 73:2232–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Laurent C, Meggetto F, Brousset P (2008) Human herpesvirus 8 infections in patients with immunodeficiencies. Human pathology 39:983–993 [DOI] [PubMed] [Google Scholar]
- [37].Hengge UR, Ruzicka T, Tyring SK et al. (2002) Update on Kaposi’s sarcoma and other HHV8 associated diseases. Part 2: pathogenesis, Castleman’s disease, and pleural effusion lymphoma. The Lancet Infectious diseases 2:344–352 [DOI] [PubMed] [Google Scholar]
- [38].Ruocco E, Ruocco V, Tornesello ML et al. (2013) Kaposi’s sarcoma: etiology and pathogenesis, inducing factors, causal associations, and treatments: facts and controversies. Clinics in dermatology 31:413–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Regnier-Rosencher E, Guillot B, Dupin N (2013) Treatments for classic Kaposi sarcoma: a systematic review of the literature. Journal of the American Academy of Dermatology 68:313–331 [DOI] [PubMed] [Google Scholar]
- [40].Zheng J, Yang Y, Cui M et al. (2017) Prevalence of Kaposi’s sarcoma-associated herpesvirus in Uygur and Han populations from the Urumqi and Kashgar regions of Xinjiang, China. Virologica Sinica 32:396–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Dollard SC, Butler LM, Jones AM et al. (2010) Substantial regional differences in human herpesvirus 8 seroprevalence in sub-Saharan Africa: insights on the origin of the “Kaposi’s sarcoma belt”. International journal of cancer 127:2395–2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Seckin D, Demirag A, Hizel N et al. (2000) Absence of Epstein-Barr virus DNA sequences in iatrogenic Kaposi’s sarcomas of renal transplant recipients. Transplantation proceedings 32:554–555 [DOI] [PubMed] [Google Scholar]
- [43].Centers for Disease C (1981) Kaposi’s sarcoma and Pneumocystis pneumonia among homosexual men--New York City and California. MMWR Morbidity and mortality weekly report 30:305–308 [PubMed] [Google Scholar]
- [44].Krown SE, Metroka C, Wernz JC (1989) Kaposi’s sarcoma in the acquired immune deficiency syndrome: a proposal for uniform evaluation, response, and staging criteria. AIDS Clinical Trials Group Oncology Committee . Journal of clinical oncology : official journal of the American Society of Clinical Oncology 7:1201–1207 [DOI] [PubMed] [Google Scholar]
- [45].Dittmer DP, Richards KL, Damania B (2012) Treatment of Kaposi sarcoma-associated herpesvirus-associated cancers. Front Microbiol 3:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yanik EL, Napravnik S, Cole SR et al. (2013) Incidence and timing of cancer in HIV-infected individuals following initiation of combination antiretroviral therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 57:756–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Boulanger E, Daniel MT, Agbalika F et al. (2003) Combined chemotherapy including high-dose methotrexate in KSHV/HHV8-associated primary effusion lymphoma. American journal of hematology 73:143–148 [DOI] [PubMed] [Google Scholar]
- [48].Saeed-Abdul-Rahman I, Al-Amri AM (2012) Castleman disease. The Korean journal of hematology 47:163–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Carbone A, De Paoli P, Gloghini A et al. (2015) KSHV-associated multicentric Castleman disease: A tangle of different entities requiring multitarget treatment strategies. International journal of cancer 137:251–261 [DOI] [PubMed] [Google Scholar]
- [50].Sakamoto K, Asanuma H, Nakamura T et al. (2010) Immune response to intranasal and intraperitoneal immunization with Kaposi’s sarcoma-associated herpesvirus in mice. Vaccine 28:3325–3332 [DOI] [PubMed] [Google Scholar]
- [51].Kumar H, Kawai T, Akira S (2009) Toll-like receptors and innate immunity. Biochem Biophys Res Commun 388:621–625 [DOI] [PubMed] [Google Scholar]
- [52].Chen L, DiPietro LA (2017) Toll-Like Receptor Function in Acute Wounds. Adv Wound Care (New Rochelle) 6:344–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Blasius AL, Beutler B (2010) Intracellular toll-like receptors. Immunity 32:305–315 [DOI] [PubMed] [Google Scholar]
- [54].Rassa JC, Meyers JL, Zhang Y et al. (2002) Murine retroviruses activate B cells via interaction with toll-like receptor 4. Proceedings of the National Academy of Sciences of the United States of America 99:2281–2286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Haynes LM, Moore DD, Kurt-Jones EA et al. (2001) Involvement of toll-like receptor 4 in innate immunity to respiratory syncytial virus. Journal of virology 75:10730–10737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Georgel P, Jiang Z, Kunz S et al. (2007) Vesicular stomatitis virus glycoprotein G activates a specific antiviral Toll-like receptor 4-dependent pathway. Virology 362:304–313 [DOI] [PubMed] [Google Scholar]
- [57].Kawai T, Akira S (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nature immunology 11:373–384 [DOI] [PubMed] [Google Scholar]
- [58].Lagos D, Vart RJ, Gratrix F et al. (2008) Toll-like receptor 4 mediates innate immunity to Kaposi sarcoma herpesvirus. Cell Host Microbe 4:470–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Bussey KA, Reimer E, Todt H et al. (2014) The gammaherpesviruses Kaposi’s sarcoma-associated herpesvirus and murine gammaherpesvirus 68 modulate the Toll-like receptor-induced proinflammatory cytokine response. J Virol 88:9245–9259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Coscoy L (2007) Immune evasion by Kaposi’s sarcoma-associated herpesvirus. Nat Rev Immunol 7:391–401 [DOI] [PubMed] [Google Scholar]
- [61].Moore PS, Chang Y (2003) Kaposi’s sarcoma-associated herpesvirus immunoevasion and tumorigenesis: two sides of the same coin? Annu Rev Microbiol 57:609–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Rehli M, Poltorak A, Schwarzfischer L et al. (2000) PU.1 and interferon consensus sequence-binding protein regulate the myeloid expression of the human Toll-like receptor 4 gene. J Biol Chem 275:9773–9781 [DOI] [PubMed] [Google Scholar]
- [63].Nakahira K, Kim HP, Geng XH et al. (2006) Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. The Journal of experimental medicine 203:2377–2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Wang XM, Kim HP, Nakahira K et al. (2009) The heme oxygenase-1/carbon monoxide pathway suppresses TLR4 signaling by regulating the interaction of TLR4 with caveolin-1. J Immunol 182:3809–3818 [DOI] [PubMed] [Google Scholar]
- [65].Botto S, Gustin JK, Moses AV (2017) The Heme Metabolite Carbon Monoxide Facilitates KSHV Infection by Inhibiting TLR4 Signaling in Endothelial Cells. Front Microbiol 8:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lester SN, Li K (2014) Toll-like receptors in antiviral innate immunity. Journal of molecular biology 426:1246–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Hardarson HS, Baker JS, Yang Z et al. (2007) Toll-like receptor 3 is an essential component of the innate stress response in virus-induced cardiac injury. American journal of physiology Heart and circulatory physiology 292:H251–258 [DOI] [PubMed] [Google Scholar]
- [68].Tabeta K, Georgel P, Janssen E et al. (2004) Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proceedings of the National Academy of Sciences of the United States of America 101:3516–3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Negishi H, Osawa T, Ogami K et al. (2008) A critical link between Toll-like receptor 3 and type II interferon signaling pathways in antiviral innate immunity. Proceedings of the National Academy of Sciences of the United States of America 105:20446–20451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Richer MJ, Lavallee DJ, Shanina I et al. (2009) Toll-like receptor 3 signaling on macrophages is required for survival following coxsackievirus B4 infection. PloS one 4:e4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Abe Y, Fujii K, Nagata N et al. (2012) The toll-like receptor 3-mediated antiviral response is important for protection against poliovirus infection in poliovirus receptor transgenic mice. Journal of virology 86:185–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Le Goffic R, Balloy V, Lagranderie M et al. (2006) Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS pathogens 2:e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Wang T, Town T, Alexopoulou L et al. (2004) Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nature medicine 10:1366–1373 [DOI] [PubMed] [Google Scholar]
- [74].Zhang SY, Jouanguy E, Ugolini S et al. (2007) TLR3 deficiency in patients with herpes simplex encephalitis. Science 317:1522–1527 [DOI] [PubMed] [Google Scholar]
- [75].West J, Damania B (2008) Upregulation of the TLR3 pathway by Kaposi’s sarcoma-associated herpesvirus during primary infection. J Virol 82:5440–5449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Jacobs SR, Gregory SM, West JA et al. (2013) The viral interferon regulatory factors of kaposi’s sarcoma-associated herpesvirus differ in their inhibition of interferon activation mediated by toll-like receptor 3. J Virol 87:798–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Jacobs SR, Stopford CM, West JA et al. (2015) Kaposi’s Sarcoma-Associated Herpesvirus Viral Interferon Regulatory Factor 1 Interacts with a Member of the Interferon-Stimulated Gene 15 Pathway. J Virol 89:11572–11583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Sharma NR, Majerciak V, Kruhlak MJ et al. (2017) KSHV inhibits stress granule formation by viral ORF57 blocking PKR activation. PLoS Pathog 13:e1006677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Krug A, French AR, Barchet W et al. (2004) TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity 21:107–119 [DOI] [PubMed] [Google Scholar]
- [80].Lund J, Sato A, Akira S et al. (2003) Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. The Journal of experimental medicine 198:513–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Krug A, Luker GD, Barchet W et al. (2004) Herpes simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor 9. Blood 103:1433–1437 [DOI] [PubMed] [Google Scholar]
- [82].Quan TE, Roman RM, Rudenga BJ et al. (2010) Epstein-Barr virus promotes interferon-alpha production by plasmacytoid dendritic cells. Arthritis and rheumatism 62:1693–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].West JA, Gregory SM, Sivaraman V et al. (2011) Activation of plasmacytoid dendritic cells by Kaposi’s sarcoma-associated herpesvirus. J Virol 85:895–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Gregory SM, West JA, Dillon PJ et al. (2009) Toll-like receptor signaling controls reactivation of KSHV from latency. Proc Natl Acad Sci U S A 106:11725–11730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Yamamoto M, Sato S, Hemmi H et al. (2003) Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301:640–643 [DOI] [PubMed] [Google Scholar]
- [86].Ahmad H, Gubbels R, Ehlers E et al. (2011) Kaposi sarcoma-associated herpesvirus degrades cellular Toll-interleukin-1 receptor domain-containing adaptor-inducing beta-interferon (TRIF). J Biol Chem 286:7865–7872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Meyer F, Ehlers E, Steadman A et al. (2013) TLR-TRIF pathway enhances the expression of KSHV replication and transcription activator. J Biol Chem 288:20435–20442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Zhao Q, Liang D, Sun R et al. (2015) Kaposi’s sarcoma-associated herpesvirus-encoded replication and transcription activator impairs innate immunity via ubiquitin-mediated degradation of myeloid differentiation factor 88. J Virol 89:415–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Abend JR, Ramalingam D, Kieffer-Kwon P et al. (2012) Kaposi’s sarcoma-associated herpesvirus microRNAs target IRAK1 and MYD88, two components of the toll-like receptor/interleukin-1R signaling cascade, to reduce inflammatory-cytokine expression. J Virol 86:11663–11674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Yoneyama M, Fujita T (2008) Structural mechanism of RNA recognition by the RIG-I-like receptors. Immunity 29:178–181 [DOI] [PubMed] [Google Scholar]
- [91].Loo YM, Gale M Jr. (2011) Immune signaling by RIG-I-like receptors. Immunity 34:680–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Martin-Vicente M, Medrano LM, Resino S et al. (2017) TRIM25 in the Regulation of the Antiviral Innate Immunity. Frontiers in immunology 8:1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Cheng G, Zhong J, Chung J et al. (2007) Double-stranded DNA and double-stranded RNA induce a common antiviral signaling pathway in human cells. Proc Natl Acad Sci U S A 104:9035–9040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Chiu YH, Macmillan JB, Chen ZJ (2009) RNA polymerase III detects cytosolic DNA and induces type I interferons through the RIG-I pathway. Cell 138:576–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].West JA, Wicks M, Gregory SM et al. (2014) An important role for mitochondrial antiviral signaling protein in the Kaposi’s sarcoma-associated herpesvirus life cycle. J Virol 88:5778–5787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Chandriani S, Xu Y, Ganem D (2010) The lytic transcriptome of Kaposi’s sarcoma-associated herpesvirus reveals extensive transcription of noncoding regions, including regions antisense to important genes. J Virol 84:7934–7942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Dresang LR, Teuton JR, Feng H et al. (2011) Coupled transcriptome and proteome analysis of human lymphotropic tumor viruses: insights on the detection and discovery of viral genes. BMC genomics 12:625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Inn KS, Lee SH, Rathbun JY et al. (2011) Inhibition of RIG-I-mediated signaling by Kaposi’s sarcoma-associated herpesvirus-encoded deubiquitinase ORF64. J Virol 85:10899–10904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Moore CB, Bergstralh DT, Duncan JA et al. (2008) NLRX1 is a regulator of mitochondrial antiviral immunity. Nature 451:573–577 [DOI] [PubMed] [Google Scholar]
- [100].Ma Z, Hopcraft SE, Yang F et al. (2017) NLRX1 negatively modulates type I IFN to facilitate KSHV reactivation from latency. PLoS Pathog 13:e1006350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Kanneganti TD, Lamkanfi M, Nunez G (2007) Intracellular NOD-like receptors in host defense and disease. Immunity 27:549–559 [DOI] [PubMed] [Google Scholar]
- [102].Wen H, Miao EA, Ting JP (2013) Mechanisms of NOD-like receptor-associated inflammasome activation. Immunity 39:432–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Coutermarsh-Ott S, Eden K, Allen IC (2016) Beyond the inflammasome: regulatory NOD-like receptor modulation of the host immune response following virus exposure. J Gen Virol 97:825–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Gregory SM, Davis BK, West JA et al. (2011) Discovery of a viral NLR homolog that inhibits the inflammasome. Science 331:330–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Guo H, Konig R, Deng M et al. (2016) NLRX1 Sequesters STING to Negatively Regulate the Interferon Response, Thereby Facilitating the Replication of HIV-1 and DNA Viruses. Cell Host Microbe 19:515–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Dempsey A, Bowie AG (2015) Innate immune recognition of DNA: A recent history. Virology 479–480:146–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Man SM, Karki R, Kanneganti TD (2016) AIM2 inflammasome in infection, cancer, and autoimmunity: Role in DNA sensing, inflammation, and innate immunity. Eur J Immunol 46:269–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Zhang X, Brann TW, Zhou M et al. (2011) Cutting edge: Ku70 is a novel cytosolic DNA sensor that induces type III rather than type I IFN. J Immunol 186:4541–4545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Kim T, Pazhoor S, Bao M et al. (2010) Aspartate-glutamate-alanine-histidine box motif (DEAH)/RNA helicase A helicases sense microbial DNA in human plasmacytoid dendritic cells. Proc Natl Acad Sci U S A 107:15181–15186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Sun L, Wu J, Du F et al. (2013) Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339:786–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Xia P, Wang S, Gao P et al. (2016) DNA sensor cGAS-mediated immune recognition. Protein Cell 7:777–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Yoh SM, Schneider M, Seifried J et al. (2015) PQBP1 Is a Proximal Sensor of the cGAS-Dependent Innate Response to HIV-1. Cell 161:1293–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Ablasser A, Schmid-Burgk JL, Hemmerling I et al. (2013) Cell intrinsic immunity spreads to bystander cells via the intercellular transfer of cGAMP. Nature 503:530–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Burdette DL, Vance RE (2013) STING and the innate immune response to nucleic acids in the cytosol. Nat Immunol 14:19–26 [DOI] [PubMed] [Google Scholar]
- [115].Liang Q, Seo GJ, Choi YJ et al. (2014) Crosstalk between the cGAS DNA sensor and Beclin-1 autophagy protein shapes innate antimicrobial immune responses. Cell Host Microbe 15:228–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Li XD, Wu J, Gao D et al. (2013) Pivotal roles of cGAS-cGAMP signaling in antiviral defense and immune adjuvant effects. Science 341:1390–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Schoggins JW, MacDuff DA, Imanaka N et al. (2014) Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature 505:691–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Lio CW, McDonald B, Takahashi M et al. (2016) cGAS-STING Signaling Regulates Initial Innate Control of Cytomegalovirus Infection. Journal of virology 90:7789–7797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Davis DA, Naiman NE, Wang V et al. (2015) Identification of Caspase Cleavage Sites in KSHV Latency-Associated Nuclear Antigen and Their Effects on Caspase-Related Host Defense Responses. PLoS Pathog 11:e1005064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Garrigues HJ, Howard K, Barcy S et al. (2017) Full-length isoforms of KSHV LANA accumulate in the cytoplasm of cells undergoing the lytic cycle of replication. J Virol [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Zhang G, Chan B, Samarina N et al. (2016) Cytoplasmic isoforms of Kaposi sarcoma herpesvirus LANA recruit and antagonize the innate immune DNA sensor cGAS. Proc Natl Acad Sci U S A 113:E1034–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Ma Z, Jacobs SR, West JA et al. (2015) Modulation of the cGAS-STING DNA sensing pathway by gammaherpesviruses. Proc Natl Acad Sci U S A 112:E4306–4315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Wu JJ, Li W, Shao Y et al. (2015) Inhibition of cGAS DNA Sensing by a Herpesvirus Virion Protein. Cell Host Microbe 18:333–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Morchikh M, Cribier A, Raffel R et al. (2017) HEXIM1 and NEAT1 Long Non-coding RNA Form a Multi-subunit Complex that Regulates DNA-Mediated Innate Immune Response. Mol Cell 67:387–399 e385 [DOI] [PubMed] [Google Scholar]
- [125].Lamarche BJ, Orazio NI, Weitzman MD (2010) The MRN complex in double-strand break repair and telomere maintenance. FEBS Lett 584:3682–3695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Rupnik A, Lowndes NF, Grenon M (2010) MRN and the race to the break. Chromosoma 119:115–135 [DOI] [PubMed] [Google Scholar]
- [127].Roth S, Rottach A, Lotz-Havla AS et al. (2014) Rad50-CARD9 interactions link cytosolic DNA sensing to IL-1beta production. Nat Immunol 15:538–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Trigg BJ, Ferguson BJ (2015) Functions of DNA damage machinery in the innate immune response to DNA virus infection. Curr Opin Virol 15:56–62 [DOI] [PubMed] [Google Scholar]
- [129].Kondo T, Kobayashi J, Saitoh T et al. (2013) DNA damage sensor MRE11 recognizes cytosolic double-stranded DNA and induces type I interferon by regulating STING trafficking. Proc Natl Acad Sci U S A 110:2969–2974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Mariggio G, Koch S, Zhang G et al. (2017) Kaposi Sarcoma Herpesvirus (KSHV) Latency-Associated Nuclear Antigen (LANA) recruits components of the MRN (Mre11-Rad50-NBS1) repair complex to modulate an innate immune signaling pathway and viral latency. PLoS Pathog 13:e1006335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Diner BA, Lum KK, Cristea IM (2015) The emerging role of nuclear viral DNA sensors. J Biol Chem 290:26412–26421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Kerur N, Veettil MV, Sharma-Walia N et al. (2011) IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi Sarcoma-associated herpesvirus infection. Cell Host Microbe 9:363–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Orzalli MH, DeLuca NA, Knipe DM (2012) Nuclear IFI16 induction of IRF-3 signaling during herpesviral infection and degradation of IFI16 by the viral ICP0 protein. Proc Natl Acad Sci U S A 109:E3008–3017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Diner BA, Li T, Greco TM et al. (2015) The functional interactome of PYHIN immune regulators reveals IFIX is a sensor of viral DNA. Mol Syst Biol 11:787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Crow MS, Cristea IM (2017) Human Antiviral Protein IFIX Suppresses Viral Gene Expression during Herpes Simplex Virus 1 (HSV-1) Infection and Is Counteracted by Virus-induced Proteasomal Degradation. Mol Cell Proteomics 16:S200–S214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Unterholzner L, Keating SE, Baran M et al. (2010) IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol 11:997–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Ansari MA, Dutta S, Veettil MV et al. (2015) Herpesvirus Genome Recognition Induced Acetylation of Nuclear IFI16 Is Essential for Its Cytoplasmic Translocation, Inflammasome and IFN-beta Responses. PLoS Pathog 11:e1005019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Iqbal J, Ansari MA, Kumar B et al. (2016) Histone H2B-IFI16 Recognition of Nuclear Herpesviral Genome Induces Cytoplasmic Interferon-beta Responses. PLoS Pathog 12:e1005967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Roy A, Dutta D, Iqbal J et al. (2016) Nuclear Innate Immune DNA Sensor IFI16 Is Degraded during Lytic Reactivation of Kaposi’s Sarcoma-Associated Herpesvirus (KSHV): Role of IFI16 in Maintenance of KSHV Latency. J Virol 90:8822–8841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Orzalli MH, Broekema NM, Diner BA et al. (2015) cGAS-mediated stabilization of IFI16 promotes innate signaling during herpes simplex virus infection. Proc Natl Acad Sci U S A 112:E1773–1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Honda K, Takaoka A, Taniguchi T (2006) Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity 25:349–360 [DOI] [PubMed] [Google Scholar]
- [142].Lin R, Genin P, Mamane Y et al. (2001) HHV-8 encoded vIRF-1 represses the interferon antiviral response by blocking IRF-3 recruitment of the CBP/p300 coactivators. Oncogene 20:800–811 [DOI] [PubMed] [Google Scholar]
- [143].Fuld S, Cunningham C, Klucher K et al. (2006) Inhibition of interferon signaling by the Kaposi’s sarcoma-associated herpesvirus full-length viral interferon regulatory factor 2 protein. J Virol 80:3092–3097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Joo CH, Shin YC, Gack M et al. (2007) Inhibition of interferon regulatory factor 7 (IRF7)-mediated interferon signal transduction by the Kaposi’s sarcoma-associated herpesvirus viral IRF homolog vIRF3. J Virol 81:8282–8292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Hwang SW, Kim D, Jung JU et al. (2017) KSHV-encoded viral interferon regulatory factor 4 (vIRF4) interacts with IRF7 and inhibits interferon alpha production. Biochem Biophys Res Commun 486:700–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Yu Y, Wang SE, Hayward GS (2005) The KSHV immediate-early transcription factor RTA encodes ubiquitin E3 ligase activity that targets IRF7 for proteosome-mediated degradation. Immunity 22:59–70 [DOI] [PubMed] [Google Scholar]
- [147].Yu Y, Hayward GS (2010) The ubiquitin E3 ligase RAUL negatively regulates type i interferon through ubiquitination of the transcription factors IRF7 and IRF3. Immunity 33:863–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Zhu FX, Sathish N, Yuan Y (2010) Antagonism of host antiviral responses by Kaposi’s sarcoma-associated herpesvirus tegument protein ORF45. PLoS One 5:e10573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Sathish N, Zhu FX, Golub EE et al. (2011) Mechanisms of autoinhibition of IRF-7 and a probable model for inactivation of IRF-7 by Kaposi’s sarcoma-associated herpesvirus protein ORF45. J Biol Chem 286:746–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Liang Q, Fu B, Wu F et al. (2012) ORF45 of Kaposi’s sarcoma-associated herpesvirus inhibits phosphorylation of interferon regulatory factor 7 by IKKepsilon and TBK1 as an alternative substrate. J Virol 86:10162–10172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Lefort S, Soucy-Faulkner A, Grandvaux N et al. (2007) Binding of Kaposi’s sarcoma-associated herpesvirus K-bZIP to interferon-responsive factor 3 elements modulates antiviral gene expression. J Virol 81:10950–10960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Liang D, Gao Y, Lin X et al. (2011) A human herpesvirus miRNA attenuates interferon signaling and contributes to maintenance of viral latency by targeting IKKepsilon. Cell Res 21:793–806 [DOI] [PMC free article] [PubMed] [Google Scholar]