Abstract
Key points
Incomplete development of the neural circuits that control breathing contributes to respiratory disorders in pre‐term infants. Manifestations include respiratory instability, prolonged apnoeas and poor ventilatory responses to stimuli.
Based on evidence suggesting that omega‐3 polyunsaturated fatty acids (n‐3 PUFA) improves brain development, we determined whether n‐3 PUFA supplementation (via the maternal diet) improves respiratory function in 10–11‐day‐old rat pups.
n‐3 PUFA treatment prolonged apnoea duration but augmented the relative pulmonary surface area and the ventilatory response to hypoxia. During hypoxia, the drop in body temperature measured in treated pups was 1 °C less than in controls. n‐3 PUFA treatment also reduced microglia cell density in the brainstem.
Although heterogeneous, the results obtained in rat pups constitute a proof of concept that n‐3 PUFA supplementation can have positive effects on neonatal respiration. This includes a more sustained hypoxic ventilatory response and a decreased respiratory inhibition during laryngeal chemoreflex.
Abstract
Most pre‐term infants present respiratory instabilities and apnoeas as a result of incomplete development of the neural circuits that control breathing. Because omega‐3 polyunsaturated fatty acids (n‐3 PUFA) benefit brain development, we hypothesized that n‐3 PUFA supplementation (via the maternal diet) improves respiratory function in rat pups. Pups received n‐3 PUFA supplementation from an enriched diet (13 g kg−1 of n‐3 PUFA) administered to the mother from birth until the experiments were performed (postnatal days 10–11). Controls received a standard diet (0.3 g kg−1 of n‐3 PUFA). Breathing was measured in intact pups at rest and during hypoxia (FiO2 = 0.12; 20 min) using whole body plethysmography. The duration of apnoeas induced by stimulating the laryngeal chemoreflex (LCR) was measured under anaesthesia. Lung morphology was compared between groups. Maternal n‐3 PUFA supplementation effectively raised n‐3 PUFA levels above control levels both in the blood and brainstem of pups. In intact, resting pups, n‐3 PUFA increased the frequency and duration of apnoeas, especially in females. During hypoxia, n‐3 PUFA supplemented pups hyperventilated 23% more than controls; their anapyrexic response was 1 °C less than controls. In anaesthetized pups, n‐3 PUFA shortened the duration of LCR‐induced apnoeas by 32%. The relative pulmonary surface area of n‐3 PUFA supplemented pups was 12% higher than controls. Although n‐3 PUFA supplementation augments apnoeas, there is no clear evidence of deleterious consequences on these pups. Based on the improved lung architecture and responses to respiratory challenges, this neonatal treatment appears to be beneficial to the offspring. However, further experiments are necessary to establish its overall safety.
Keywords: control of breathing, development, plasticity, prematurity
Key points
Incomplete development of the neural circuits that control breathing contributes to respiratory disorders in pre‐term infants. Manifestations include respiratory instability, prolonged apnoeas and poor ventilatory responses to stimuli.
Based on evidence suggesting that omega‐3 polyunsaturated fatty acids (n‐3 PUFA) improves brain development, we determined whether n‐3 PUFA supplementation (via the maternal diet) improves respiratory function in 10–11‐day‐old rat pups.
n‐3 PUFA treatment prolonged apnoea duration but augmented the relative pulmonary surface area and the ventilatory response to hypoxia. During hypoxia, the drop in body temperature measured in treated pups was 1 °C less than in controls. n‐3 PUFA treatment also reduced microglia cell density in the brainstem.
Although heterogeneous, the results obtained in rat pups constitute a proof of concept that n‐3 PUFA supplementation can have positive effects on neonatal respiration. This includes a more sustained hypoxic ventilatory response and a decreased respiratory inhibition during laryngeal chemoreflex.
Abbreviations
- cNTS
caudal region of the nucleus of solitary tractus
- DHA
docosahexaenoic acid
- EMG
electromyogram
- EPA
eicosapentaenoic acid
- Lm
mean linear intercept
- HVR
hypoxic ventilatory response
- LCR
laryngeal chemoreflex
- n‐3 PUFA
omega‐3 polyunsaturated fatty acids
- PFA
paraformaldehyde
- SpO2
O2 saturation
- TBS
Tris‐buffered saline
Introduction
Preterm birth is commonly associated with frequent apnoeas as a result of immaturity of the neural network that generates and regulates breathing. The recurrence of O2 desaturations and bradycardias that follow apnoeas is a major concern for clinicians because these events can have deleterious consequences on neurodevelopmental outcomes (Southall et al. 1982; Di Fiore et al. 2013). In the short term, exposing newborn rats to repeated hypoxaemias augments the O2 sensitivity of peripheral chemoreceptors (Pawar et al. 2008); the excessive hyperventilatory response resulting from this effect further promotes respiratory instabilities and apnoeas. Brainstem immaturity also precludes the co‐ordination of breathing with swallowing. As a result, the presence of liquids near the larynx triggers a powerful respiratory inhibition that aims to prevent the aspiration of foreign substances in the airways (Praud, 2010). This reflexive response (termed the laryngeal chemoreflex; LCR) results in prolonged apnoeas with significant O2 desaturations and bradycardias that can be life threatening.
The administration of ventilatory stimulants such as caffeine has been the mainstay of pharmacological treatment for apnoea of prematurity (Abu‐Shaweesh & Martin, 2008). Caffeine treatment is generally effective and long‐term effects may be positive (Finer et al. 2006; Montandon et al. 2006; Schmidt et al. 2007); however, it is estimated that 50% of infants do not respond to this treatment (Bairam et al. 1987; Poets et al. 1994) and there is no evidence that caffeine facilitates respiratory control development.
Over recent decades, a growing number of studies have documented the health benefits of polyunsaturated fatty acids of the omega‐3 family (n‐3 PUFA) (Siriwardhana et al. 2012; Vanden Heuvel, 2012; Zhang et al. 2014; McNamara et al. 2015; Schipper et al. 2016). α‐linolenic acid, the main n‐3 PUFA, is an essential nutrient, whereas docosahexaenoic acid (DHA; a key n‐3 PUFA metabolite) is an important constituent of cell membranes; DHA is thus highly concentrated in several tissues, including the brain, muscles and adipose tissue (Kuipers et al. 2012). Because it is necessary to support rapidly growing tissues, DHA is insufficient during the neonatal period, especially in preterm infants (Zhang et al. 2014). Consequently, DHA deficiency during early life has been linked with neurological disorders such as attention deficit/hyperactivity disorder, mood and psychotic disorders (McNamara et al. 2015). These observations indicate that DHA supplementation probably benefits growth and development in preterm infants.
With regard to the respiratory system, DHA benefits lung development of preterms by attenuating inflammation, thereby reducing the risk of bronchopulmonary dysplasia (Zhang et al. 2014; McNamara et al. 2015). However, the information concerning the brainstem respiratory network remains indirect. It is known that DHA may facilitate myelin sheath formation and is present in growth cone membranes and synapses where it promotes synapse formation and the stability of dendritic spines (McNamara et al. 2015). Microglia, which are mainly known as ‘scavengers of the CNS’, actively contribute to these developmental processes by eliminating excessive/inactive synapses (Paolicelli et al. 2011). DHA can stimulate phagocytosis by microglia (Hjorth et al. 2013), suggesting that these cells are important effectors of these beneficial effects of n‐3 PUFA on brain development. Taken together, these data led us to propose that n‐3 PUFA supplementation (via the maternal diet) improves respiratory function in rat pups. Rats are a highly relevant model for addressing this issue because the CNS of the rat is immature at birth (Mortola, 2001) with an overall degree of functionality (including brainstem respiratory network) comparable to a preterm infant (born at ∼28 weeks) (Clancy et al. 2001). Our main hypothesis was tested with diverse and complementary approaches: whole body plethysmography was used to measure breathing in intact, unanaesthetized pups. Measurements were performed at rest to assess the occurrence of apnoeas; this was followed by exposure to moderate hypoxia (FiO2 = 0.12 for 20 min) to evaluate the ventilatory and metabolic responses. In a distinct series of experiments, the LCR was measured in anaesthetized pups to evaluate the impact of n‐3 PUFA supplementation on reflexive cardiorespiratory responses without the confounding effects of metabolic depression. The effect of n‐3 PUFA treatment on lung morphology was assessed also. Finally, because microglia ‘shape’ synaptic connectivity in the developing brain and n‐3 PUFA can influence the phagocytic activity of these cells, immunohistochemistry was used to determine whether n‐3 PUFA supplementation affects the number and morphology of microglia present within the caudal region of the nucleus of solitary tractus (cNTS), the primary projection site for chemoreceptors associated with the hypoxic ventilatory response and LCR (Jordan, 2001). Because newborn males are more prone to develop respiratory control disorders than females (Mage & Donner, 2006), the sex of pups was considered aiming to evaluate potential sex‐based differences in the responses.
Methods
Animals and ethical approval
Experiments were performed on 136 Sprague–Dawley rat pups of both sexes aged between 10 and 11 days old; all animals used in the present study were born and raised in our animal care facilities. This age group was chosen because respiratory control has developed but is still immature (Mayer et al. 2014). Furthermore, preliminary experiments showed that this is the youngest age at which the laryngeal chemoreflex (LCR; series II) can be reliably studied under anaesthesia. The study consists of four distinct series of experiments; the details concerning the number of animals in each group appear in brackets when appropriate, as well as in Table 2.
Table 2.
Comparison of basal (normoxic) temperature, ventilatory and metabolic values obtained during plethysmographic recordings between pups (P10–11) receiving n‐3 PUFA supplementation (via maternal diet) and control pups (standard diet)
| Males | Females | ANOVA | |||||
|---|---|---|---|---|---|---|---|
| Parameter (baseline) | Control (n = 11) | n‐3 PUFA (n = 12) | Control (n = 13) | n‐3 PUFA (n = 10) | Treatment Effect P value | Sex effect P value | Factorial interaction P value |
| Minute ventilation () (ml BTPS min–1 per 100 g) | 228 ± 18 | 178 ± 16* | 249 ± 15 | 218 ± 18 | 0.02 | 0.07 | 0.56 |
| Breathing frequency (f R) (breaths min–1) | 194 ± 8 | 187 ± 6.2 | 207 ± 6 | 201 ± 6 | 0.33 | 0.05 | 0.98 |
| Tidal volume (V t) (ml BTPS per 100 g) | 1.1 ± 0.07 | 0.93 ± 0.06* | 1.2 ± 0.07 | 1.1 ± 0.07 | 0.01 | 0.20 | 0.45 |
| Body temperature (T b°) | 35.5 ± 0.1 | 35.7 ± 0.1 | 35.6 ± 0.09 | 35.7 ± 0.1 | 0.19 | 0.52 | 0.66 |
| Oxygen consumption () (ml STPD min–1 per 100 g) | 4.2 ± 0.6 | 4.4 ± 0.3 | 5.1 ± 0.4 | 3.7 ± 0.4 | 0.15 | 0.85 | 0.08 |
| Convective requirement ratio (/) | 62 ± 7 | 45 ± 4 | 51 ± 4 | 63 ± 6† | 0.69 | 0.47 | 0.01 |
*Significantly different from their respective control group at P ≤ 0.05.
†Significantly different from male n‐3 PUFA group at P ≤ 0.05.
All animals were maintained under standard laboratory conditions (12:12 h dark–light cycle, lights on 06.00 h; 21 °C,); the access to food and water was ad libitum during gestation and during the experimental treatment. Once the protocols were completed, animals were killed using an anaesthetic overdose in accordance with procedures approved by the Université Laval Animal Care Committee for this species and age group. The Université Laval Animal Care Committee approved all of the experimental procedures and protocols, which were performed in accordance with the guidelines of the Canadian Council on Animal Care. This study conforms with guidelines of The Journal of Physiology (Grundy, 2015).
Mating and n‐3 PUFA supplementation protocol
Male and female genitors were purchased (Charles River Canada, St Constant, QC, Canada). Nulliparous females were mated and delivered 10–15 pups. Two days after delivery, litters were culled to 12 pups with an approximately equal number of males and females when necessary. Gestating dams were randomly assigned to one of two diets: control or n‐3 PUFA supplemented (Harlan Teklad, Indianapolis, USA) (for diet composition, see Table 1). We choose this n‐3 PUFA supplementation dose because it was similar to those given for human study supplementation (Escamilla‐Nuñez et al. 2014). Furthermore, the enriched diet was designed to obtain an omega‐3/omega‐6 ratio near 1 (Table 1), which is commonly recommended (Simopoulos, 1999). The diet was given to the dams from birth until the day of experiments (P10–11). Although n‐3 PUFA supplementation during gestation can be beneficial to fetal development, we opted to begin supplementation after birth; pre‐term births often being unpredictable, this protocol is better aligned with clinical reality. Note that, for each experimental group, rat pups originated from at least two different litters to ensure that treatment‐related differences were not the result of a litter‐specific effect.
Table 1.
Composition of control and n‐3 PUFA enriched diets
| Concentration (g kg–1) | ||
|---|---|---|
| Compound | Control | n‐3 PUFA |
| Omega‐3 | 0.3 | 13 |
| Omega‐6 | 49 | 16 |
| Casein | 200 | 200 |
| l‐Cystine | 3 | 3 |
| Corn starch | 379.186 | 379.186 |
| Maltodextrin | 132 | 132 |
| Sucrose | 100 | 100 |
| Safflower oil linoleic | 45 | 45 |
| Corn oil | 25 | 25 |
| Cellulose | 50 | 50 |
| Mineral mix, AIN‐93G‐MX | 48 | 48 |
| Ferric citrate | 0.3 | 0.3 |
| Vitamin mix, AIN‐93‐VX | 15 | 15 |
| Choline bitartrate | 2.5 | 2.5 |
| TBHQ, anti‐oxidant | 0.014 | 0.014 |
Fatty acid analysis by gas chromatography
The effectiveness of n‐3 PUFA supplementation protocol was determined by comparing the fatty acid composition of the blood and brainstem homogenates between both experimental groups. Terminal blood samples were collected from anaesthetized pups by cardiac puncture with a heparinized syringe in a microcuvette 500K3 EDTA tube at the end of ventilatory measurements (Series I). The brainstem was also harvested, placed on dry ice and stored at −80 °C until the analysis by gas chromatography analysis service of our research centre was performed in accordance with standard protocols (Counil et al. 2009). Briefly, membranes of lysed erythrocytes were isolated by centrifugation (21 000 g for 15 min) and washed twice with 0.9% NaCl solution. Cell membranes were re‐suspended in 200 μl of the NaCl solution. Brainstems were homogenized using a glass potter using ∼25 mg of tissue. Lipids from these various samples were extracted along with a C‐17 phosphatidylcholine (Avanti Polar Lipids, Alabaster, AL, USA) as an internal standard in a chloroform:methanol:saline solution 0.9% (2:1:1, by volume) in accordance with a modified Folch method (Shaikh & Downar, 1981). Fatty acids were methylated following a transesterification reaction using a mix of methanol:benzene (4:1) and acetyl chloride at 95 °C for 1.5 h. Methylated fatty acids were finally analysed by gas chromatography coupled with a flame ionization detector.
Series I – Effects of n‐3 PUFA supplementation on respiratory physiology and related parameters
Recording of ventilation and indicators of metabolism in intact rat pups
These experiments were performed on intact, unrestrained and unanaesthetized rat pups (23 males and 23 females) using whole body plethysmography (Gulemetova & Kinkead, 2011). Briefly, the system consists of a 400 ml Plexiglas experimental chamber in which pressure differences between the experimental and reference chambers are measured with a differential pressure transducer (SenSym; Honeywell, Morris Plains, NJ, USA) with a fast response time (500 ms). After amplification, the flow signal was integrated by data analysis software (IOX; Emka Technologies, Falls Church, VA, USA) to obtain a tidal volume value. The system was calibrated by injecting a known volume of air (1 ml) into the chamber with a glass syringe at a rate corresponding to the air flow range typically generated by the rat. Fresh air (baseline) or a hypoxic gas mixture (12% O2) was delivered into the experimental chamber at a constant rate (280–300 ml min−1) with a bias flow regulator (PLY1020; Buxco Electronics, Sharon, CT, USA). Barometric pressure, chamber temperature (T C°), humidity and the rectal temperature of the animals (T b°) were also measured at the beginning and end of each experiment (Table 2) to correct the tidal volume values according to standard equations (Drorbaugh & Fenn, 1955). Calculation of oxygen consumption () was obtained as described previously (Mortola & Dotta, 1992). Chamber temperature was regulated at 30 °C using a servo controlled heating lamp (model TCAT‐2AC; Physitemp, Clifton, NJ, USA) to maintain rat pups within their thermoneutral zone (Schaeffer, 1998; Mortola, 2001; Malik & Fewell, 2003).
Protocols and data analysis
Normoxia
Each pup was first placed into the plethysmography chamber for ∼10 min prior to measuring rectal temperature with a small stainless steel thermocouple probe of appropriate size. The pup was then returned to chamber where it was allowed to acclimatize for ∼30 min. Baseline ventilatory and measurements were made under normoxic conditions for 15 min when the animal was quiet and the breathing signal was stable. Breathing frequency (f R), tidal volume (V t) and minute ventilation () were recorded. Baseline values of each ventilatory variable were obtained by averaging the last 10 min that preceded hypoxia.
The apnoea index was calculated under baseline conditions as we have done previously (Montandon et al. 2006). Briefly, based on established criteria (Mendelson et al. 1988), the apnoea index was determined by counting the number of apnoeas over 10 min of baseline recording. These results were then expressed as the number of apnoeic events per 10 min period. An apnoea was defined as an interruption of airflow for at least two breathing cycles. This criteria was based on the duration of an ‘average breathing cycle’ specific to each pup and was not based on population data; the data reported in Fig. 3 provide more detail regarding the mean duration of apnoeas in each group. The apnoeic index is the sum of two types of apnoeic pauses: spontaneous and post‐sigh apnoeas. A spontaneous apnoea was characterized by an interruption of flow that was not preceded by any significant change in tidal volume, whereas a post‐sigh apnoea was preceded by a breath with an amplitude twice the resting tidal volume.
Figure 3. n‐3 PUFA supplementation augments apnoea frequency in pups.
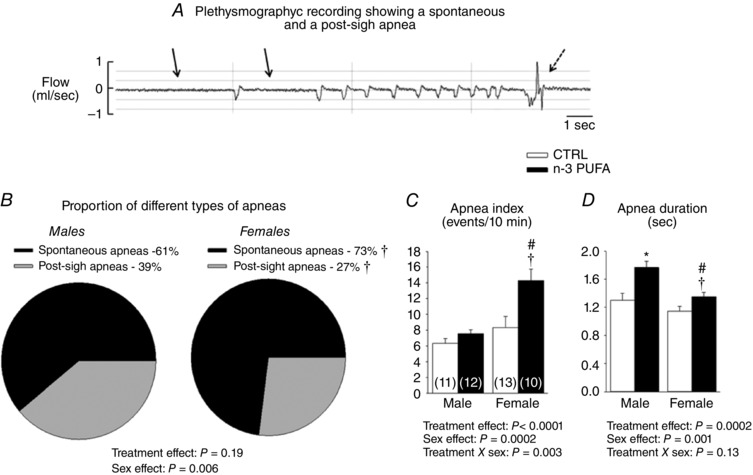
A, plethysmographic recording illustrating one example of a spontaneous (solid arrow) and a post‐sigh apnoea (dashed arrow) in rat pups during baseline conditions. B, proportion of apnoeas in male and female rat pups. C, apnoea index representing the number of total apnoeic events (spontaneous and post‐sigh) per unit of time. D, mean of apnoeas duration (spontaneous and postsigh). Histograms in (C) and (D) represent responses observed in male and female pups and were compared between animals that received n ‐3 PUFA supplementation (black bar) with controls (CTRL) (white bar). Numbers in brackets in (C) indicate the number of pups per group. Data are expressed as the mean ± SEM. Post hoc pairwise comparisons were performed only when warranted by ANOVA. Significantly different from male CTRL: * P ≤ 0.05. Significantly different from female CTRL: # P ≤ 0.05. Significantly different from male n ‐3 PUFA: † P ≤ 0.05.
Hypoxic ventilatory response (HVR)
Following baseline measurements (normoxia), pups were exposed to moderate hypoxia (12% O2) for 20 min. This level of hypoxia was reached within 2 min. The use of a pre‐mixed gas cylinder ensured consistency of the hypoxia protocol between experiments. The rapid increase in breathing frequency observed at the onset of hypoxia reflects the sensitivity of the carotid body to O2 (Powell et al. 1998). Accordingly, we evaluated the time course of the frequency response to hypoxia; the peak change in breathing frequency measured between the 3rd and the 4th minute of hypoxia was expressed as a percentage change from baseline and used as an index of O2 chemoreflex sensitivity, which is referred to as the ‘gain index’. The ‘steady state’ (or late phase) of the HVR was obtained by averaging the last 5 min of hypoxia. This value was expressed as percentage change from baseline also.
Series II – Effects of n‐3 PUFA supplementation on laryngeal chemoreflex stimulation in anaesthetized rat pups
To evaluate respiratory reflexes without the confounding effect of hypoxia‐induced metabolic depression, we measured the LCR, a set of cardiorespiratory responses induced by the presence of liquid in the upper airways based on a protocol developed previously (Xia et al. 2008). Briefly, each pup was anaesthetized with urethane (1 mg kg−1) and chloralose (20 mg kg−1) by i.p. injection. Once a surgical level of anaesthesia was reached (~20 min), the animal was placed in the supine position and a small thermistor probe was inserted into the rectum to record body temperature, which was controlled at 35 °C with a homeothermic blanket (Harvard Appratus; St‐Laurent, QC, Canada). O2 saturation (SpO2) and heart rate were measured via pulse oximetry (Mouse Ox, Starr Life Sciences; Oakmont, PA, USA) by placing a sensor onto the thigh of the pup. Hooked silver wires (diameter of 0.25 mm; Precision Instrument Inc., Sarasota, FL, USA) were introduced into the intercostal muscles in the lowest part of the rib cage to record an electromyogram (EMG) as an index of respiratory activity. A grounding wire was inserted s.c. into the skin over the abdomen. The EMG signal was amplified, filtered (model 1800; A‐M Systems, Carlsborg, WA, USA) and recorded with a data acquisition system (Windaq, DataQ Instrument, Akron, OH, USA). A midline skin incision was then made in the neck and the cervical trachea was freed from adjacent tissues. The recurrent and superior laryngeal nerves were identified and avoided. The trachea was cut partially with a transverse incision so that the lumen was visible. A polyethylene tubing (PE 50; Clay Adams, Parsippany, NJ, USA) was inserted slowly into the rostral part of the trachea and pushed gently towards the larynx; the tip of the catheter was positioned at the level of the cricoid cartilage. The pup's head was then tilted backward slightly to facilitate breathing via the opened trachea and ensure that the water injected near the larynx would drain via the nose and mouth and not be aspired into the lungs.
Experiments began once respiratory activity was stable for at least 10 min. Breathing frequency, heart rate and SpO2 were first measured under resting conditions. The LCR was activated by injecting water near the larynx. Three injections (10 μl each) were made using a 10 μl Hamilton syringe, starting at the beginning of inspiration. A 5 min recovery period was allowed between injections. We defined the duration of each apnoea as the period of apnoeas/respiratory disruption from the beginning of the stimulus until the onset of at least five regular breaths. O2 desaturation and bradycardias induced by laryngeal stimulation were defined as the minimum of values observed over the 5 min period that followed water application. The results were considered only when pups recovered prior to the next injection. Although 15% and 20% of pups died during the protocol, the mortality rate did not differ significantly between n‐3 PUFA supplemented pups and controls.
Series III: Impact of n‐3 PUFA supplementation on lung architecture
In a distinct series of experiments, the lung architecture was compared between control pups (five males and six females) and animals supplemented with n‐3 PUFA (eight males and six females).
Dissection of the lung
Lungs were harvested in accordance with a standard protocol (Jochmans‐Lemoine et al. 2015). Briefly, pups were deeply anaesthetized by an i.p. injection (0.1 ml per 100 g of body mass) of ketamine (87.5 mg ml−1) and xylazine (12.5 mg ml−1). The animal was perfused was with ice‐cold PBS (pH 7.2) through the left ventricle at a constant pressure of 24 cm H2O. A catheter was then fixed in the trachea and the lungs were inflated with 4% paraformaldehyde (PFA) for 30 min at a constant pressure of 24 cm H2O and then dissected. The total volume of the inflated lungs was measured by liquid displacement, the lungs were then kept in 4% PFA at room temperature for 24 h, the right and left lungs were separated and embedded in paraffin using the Tissue‐Tek VIP (Miles Scientific, Newark, DE, USA) and stored.
Lung histology and morphology
Paraffin‐embedded lungs were cut (5 μm) and then deparaffinized, coloured with Harris haematoxylin solution and mounted on glass slides. The images were captured using an optical microscope at a magnification of 100×. We randomly selected three non‐overlapping images from each slide using three slides per animal. The mean linear intercept (L m) was measured; from these values, we then calculated the relative alveolar surface area as S (m2 cm–3) = 4 V/L m, where V is the volume of one image. The product of the relative alveolar surface area × lung volume provided an estimate of the total alveolar surface area (Jochmans‐Lemoine et al. 2015). As ANOVA revealed no sex differences, data from males and females were pooled.
Series IV: n‐3 PUFA supplementation and brainstem microglia
In a distinct series of experiments, the number and morphology of microglia within the brainstem was compared between controls and n‐3 PUFA supplemented pups using Iba‐1 immunohistochemistry. Following deep anaesthesia with a solution of ketamine (80 mg kg−1)/xylazine (10 mg kg−1), pups were transcardially perfused with saline (0.9% NaCl) followed by 4% PFA. Brains were harvested and post‐fixed overnight in 4% PFA, and cryoprotected in 35% sucrose until they sank. Brains were frontally sectioned on a cryostat (20 μm). Slices were rinsed with Tris‐buffered saline (TBS) and blocked for 2 h with 1% BSA + 0.02% Triton X‐100; endogenous peroxidase activity was removed by incubation 30 min in 0.1% sodium borohydride in TBS. Slices were rinsed and incubated for 1 h at room temperature with antibody anti‐Iba‐1 (dilution 1:1000; Wako Chemicals, Tokyo, Japan) in 1% BSA + 0.02% Triton X‐100 in TBS and placed overnight at 4°C. Slices were rinsed and incubated with biotinylated anti‐rabbit secondary antibody (dilution 1:200; Vector Laboratories, Inc., Burlingame, CA, USA) in TBS + 1% BSA + 0.02% Triton X‐100, followed by 90 min in ABC complex (Vector Laboratories). A 5 min incubation with Fast DAB tablet (Sigma, St Louis, MO, USA) was performed. Slices were rinsed with TBS, dehydrated and mounted on glass slides. The cNTS was identified (bregma –14.30 mm) and a standardized shape of 22 μm2 was placed over area of interest. The number of Iba‐1 positive cells within that shape was counted on the right and left sides using Image J (NIH, Bethesda, MD, USA); only cells with soma completely within the pre‐defined area were counted. Because ANOVA did not reveal any lateralization, counts from the right and left sides were averaged. For each animal, data were obtained from four sections and the values were averaged.
In mature animals, the morphological index is commonly used as an indicator of functional state of microglia; however, it can also reflect the degree of maturity of these cells (Kaur & Ling, 1991; Wu et al. 1994; Harry & Kraft, 2012). The morphological index is calculated by dividing the soma's area by the area of the primary arborization; both were calculated with Image J. Microglia with an elevated morphological index have an amoeboid shape, which is associated with an immature (migrating) or challenged state. Conversely, cells with a low index are highly ramified; this morphology reflects a mature cell in a surveying state (Harry & Kraft, 2012).
Statistical analysis
Data were analysed using Statview, version 5.0 (SAS Institute, Cary, NC, USA). ANOVA was used to assess the effects of treatment (control vs. n‐3 PUFA supplementation) and sex (male vs. female); inspiratory gas (normoxia vs. hypoxia) was considered in the analysis of non‐normalized variables. In Series I, the effects of treatment on the relationship between the change in T b ° vs. body weight and apnoea index vs. O2 chemosensitivity were investigated using ANCOVA. These results were confirmed by correlation analysis using Fisher's to z test to determine whether a correlation coefficient is statistically different from zero. In Series II, the effect of repeated stimulation (carry‐over effect) was assessed statistically for each variable using ANOVA with a repeated measures design. Because durations of apnoea, O2 desaturations and bradycardias were unaffected by repeated stimulation, the three values obtained were averaged for each animal. When the results of the ANOVA revealed a significant effect of a factor (or factorial interaction), the analysis was followed by a post hoc Fisher's test. ANOVA results are mainly reported in graphic form; results from post hoc tests are indicated by symbols. All data are presented as the mean ± SEM. P < 0.05 was considered statistically significanct.
Results
Effectiveness and validation of maternal n‐3 PUFA supplementation on rat pups
By comparison with the control diet, n‐3 PUFA supplementation augmented the n‐3 PUFA ratio by 9% in pup's red blood cells membranes and by 6.5% in the brainstem (Fig. 1 A and B, respectively). Within the brainstem, the relative DHA level was 2.7% higher in n‐3 PUFA treated pups vs. controls (Fig. 1 C). Conversely, the treatment did not alter relative eicosapentaenoic acid (EPA) levels within the brainstem (Fig. 1 D); the α‐linolenic acid level was too low to be detectable by gas chromatography. On average, n‐3 PUFA supplemented pups weighed 6.3% more than controls (Fig. 2); at this age (P10–11), sex‐based differences in body weights were not significant.
Figure 1. Validation of experimental model.
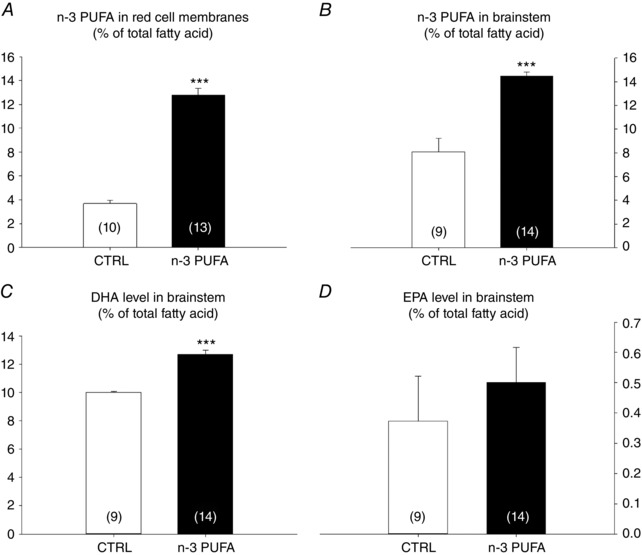
A, percentage of n‐3 PUFA (total fatty acids) present in red cell membranes. B, percentage of n‐3 PUFA (total fatty acids) present in brainstem homogenates. C and D, percentage of DHA (C) and EPA (D) in the brainstem. Data are expressed as the mean ± SEM. Post hoc pairwise comparisons were performed only when warranted by ANOVA. Significantly different from control: *** P ≤ 0.0001.
Figure 2. n‐3 PUFA supplementation increases body weight.
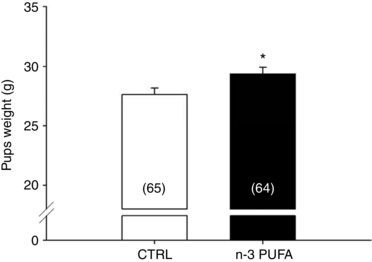
Comparison of body weight (g) in pups (10–11 days old) that received n‐3 PUFA supplementation (black bar) via maternal diet vs. pups raised by mothers receiving control diet (CTRL; white bar). Numbers in brackets indicate the number of pups. Note that data from male and females were pooled because there are no sex‐specific differences for these variables. Data are expressed as the mean ± SEM. Post hoc pairwise comparisons were performed only when warranted by ANOVA. Significantly different from CTRL: * P ≤ 0.05.
Series I – Effects of n‐3 PUFA supplementation on respiratory measurements in intact pups
Baseline respiratory data
Pups that received n‐3 PUFA supplementation showed a decrease in () compared to controls; this effect was most noticeable in males (Table 2). This relative hypopnoea was the result of a lower V T; f R did not differ significantly between groups. Measurements of indices of metabolic rate (T b and ) revealed no significant effect of treatment on these variables. Calculation of the convective requirement ratio (/) showed a sex‐specific hyperventilation that was significant in n‐3 PUFA supplemented females. Overall, f R was higher in females than males. Despite suggestive trends, minute ventilation did not differ between sexes.
n‐3 PUFA supplementation increases apnoea index in female pups
Analysis of the relative proportion of apnoeas (spontaneous vs. post‐sigh) (Fig. 3 A) showed that spontaneous apnoeas were generally more frequent in females than males (Fig. 3 B). n‐3 PUFA supplementation did not influence the type of apnoeas but augmented the total number of events significantly (Fig. 3 C). Total apnoeic events is the sum of spontaneous and post‐sigh apnoeas; both types were augmented by n‐3 PUFA supplementation. n‐3 PUFA supplementation augmented the frequency of apnoeas and this effect was more important in females in which the apnoea index was 42% greater than controls. Moreover, the apnoeas observed in n‐3 PUFA supplemented pups were generally longer than controls. Although apnoeas were slightly longer in males, the overall influence of n‐3 PUFA supplementation on apnoea duration was not sex‐specific (Fig. 3 D).
n‐3 PUFA supplementation delays the f R response to hypoxia in male pups
The f R increased rapidly at the onset of hypoxia (Fig. 4 A and B). In controls, a peak response was observed within 2 min, whereas n‐3 PUFA supplemented pups reached their plateau several min later. Overall, the tachypnoea observed in males was greater than females. Performing this analysis on absolute (non‐normalized) results yielded similar effects. The intensity of the f R response that occurs at the onset of hypoxia reflects the sensitivity (gain) of peripheral chemoreceptors and the O2 chemoreflex (Powell et al. 1998). Here, the gain index was calculated by averaging the frequency response over the 3rd and 4th minutes of hypoxia. Calculation of this index showed a decreased responsiveness at the onset of hypoxia in n‐3 PUFA supplemented pups (Fig. 4 C); this effect was not sex‐specific.
Figure 4. n‐3 PUFA supplementation delays the breathing frequency response at the onset of hypoxia.
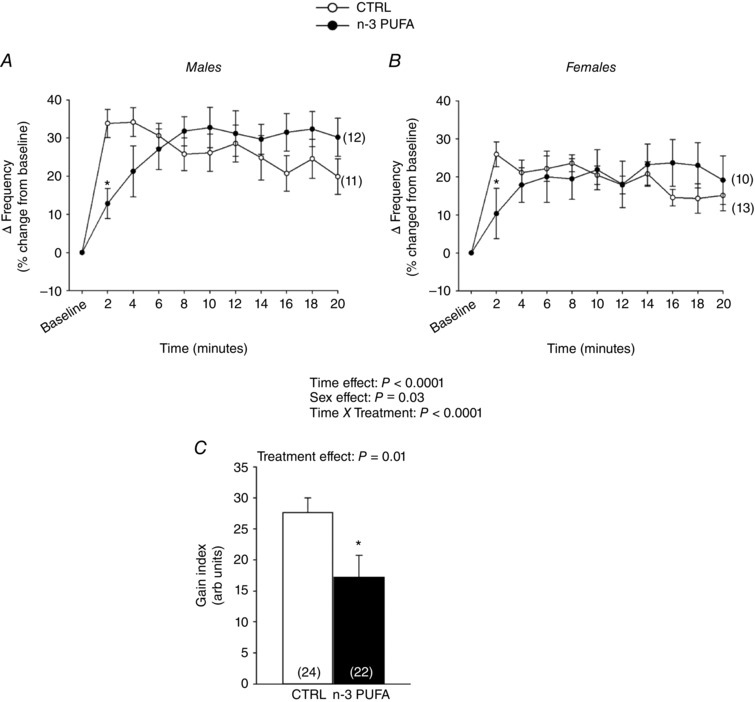
Time course of the breathing frequency response to hypoxia (12%). Data are expressed as percentage change from baseline (normoxia) and compared between groups and sexes: males (A) and females (B) that received n ‐3 PUFA supplementation (black circles) and control (CTRL) (white circles). C, gain index of the O2 chemoreflex calculated as the mean frequency increase observed over the 3rd and 4th minute of hypoxia; this value is reported in arbitrary units reflecting the percentage change from baseline. Numbers in brackets indicate the number of replicates in each group. Data are expressed as the mean ± SEM. Post hoc pairwise comparisons were performed only when warranted by ANOVA. Significantly different from CTRL: * P ≤ 0.05.
Effects of n‐3 PUFA supplementation on the relationship between O2 chemosensitivity and apnoeas
O2 chemoreflex gain is an important determinant of respiratory instability and apnoeas (Dempsey et al. 2010). Regardless of treatment, there was no significant relationship between O2 chemosensitivity and apnoea index in males (Fig. 5 A). However, there was a strong and inverse correlation between these variables in n‐3 PUFA supplemented females. This relationship was not observed in controls (Fig. 5 B).
Figure 5. Sex‐specific effects of n‐3 PUFA supplementation on the correlations between apnoea index and O2 chemosensitivity gain index.
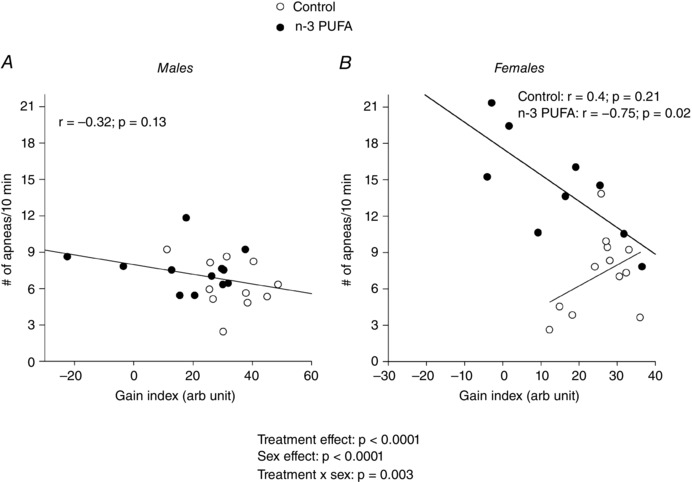
The gain index was obtained A) in males and B) females by calculating the mean increase in the breathing frequency between the 3rd and 4th minute of hypoxia. Data are expressed as absolute values (apnoeas number) and percentage change from baseline (breathing frequency). The treatment effects reported correspond to the ANOVA results.
Sex‐specific effects of n‐3 PUFA supplementation on ventilatory and metabolic responses to hypoxia
The response measured at the end of hypoxia was greater in n‐3 PUFA supplemented pups than controls (treatment effect: P = 0.007) (Fig. 6). In supplemented pups, V T depression was significantly less than controls (treatment effect: P = 0.004). Neither the and V T responses, nor the effects of n‐3 PUFA treatment were influenced by sex. Although the f R response was lower in females than males (sex effect: P = 0.03), it was not influenced by diet (treatment effect: P = 0.78). The depression measured at the end of hypoxia was the lowest in n‐3 PUFA supplemented females (treatment × sex: P = 0.02). Analysis of the convective requirement (/) at the end of hypoxia revealed no difference between groups (treatment effect: P = 0.99; data not shown).
Figure 6. Sex‐specific effects of n‐3 PUFA supplementation on minute ventilation, tidal volume, breathing frequency responses to hypoxia and O2 consumption in pups (P10–11).
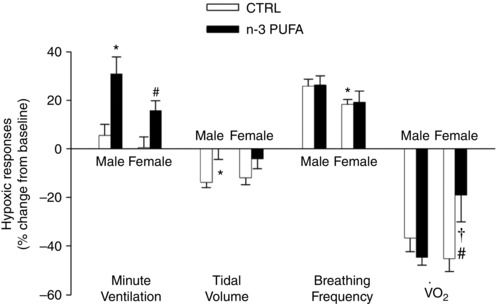
All variables were measured during normoxia (baseline) and following 20 min of exposure to moderate hypoxia (12% CO2). Histograms represent responses expressed as a percentage change from baseline and are compared between groups: pups that received n‐3 PUFA supplementation (black bar) and controls (white bar). Data are expressed as the mean ± SEM. The ANOVA results reported are for the entire data set. The number of replicates in each group is reported in Table 2. Post hoc pairwise comparisons were performed only when warranted by ANOVA. Significantly different from male CTRL group: * P ≤ 0.05. Significantly different from female CTRL group: # P ≤ 0.05. Significantly different from male n‐3 PUFA: † P ≤ 0.05.
n‐3 PUFA treatment attenuated the anapyrexic response to hypoxia (Fig. 7 A). Indeed, the T b° decrease in supplemented pups was, on average, 1 °C less than controls. Figure 7 B shows that, unlike controls, the importance of anapyrexia was independent of body weight in n‐3 PUFA supplemented pups.
Figure 7. Effects of n‐3 PUFA supplementation on anapyrexia response to hypoxia.
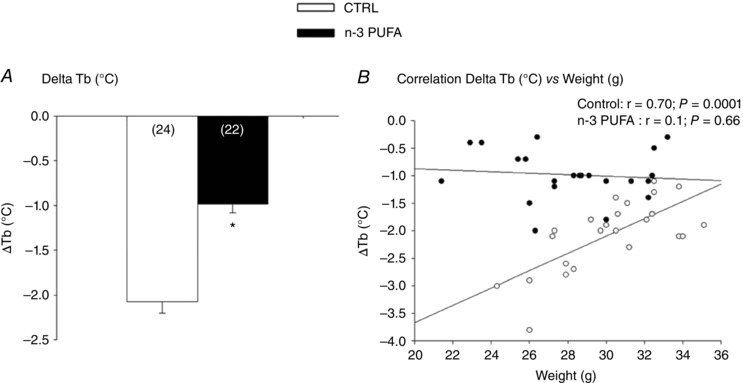
A, comparison of the decrease in T b° (body temperature) between n ‐3 PUFA supplemented and control (CTRL) pups. B, comparison of the relationship between ΔT b° and body weight in n ‐3 PUFA supplemented and control pups. Histograms represent responses expressed as the mean ± SEM; numbers in brackets indicate the number of pups per group. Males and females were analysed together because there was no sex‐specific difference for this variable. Significantly different from CTRL group: * P ≤ 0.05.
Series II – n‐3 PUFA treatment decreases apnoea duration following laryngeal chemoreflex (LCR) stimulation
Prior to LCR stimulation, resting breathing frequency of anaesthetized pups was greater in n‐3 PUFA supplemented pups than in controls (treatment effect: P = 0.05) and, overall, the frequency was higher in males than females (sex effect: P = 0.03) (Fig. 8). Basal SpO2 and heart rate were not influenced by n‐3 PUFA supplementation or sex (Fig. 9). Following LCR stimulation, the duration of the apnoeas measured in n‐3 PUFA supplemented pups was 32% shorter than in controls (Fig. 9 B). However, n‐3 PUFA supplementation had no effect on SpO2 and bradycardias and heart rate (Fig. 9 C and D, respectively).
Figure 8. n‐3 PUFA supplementation increases basal respiratory frequency in anaesthetized female pups.
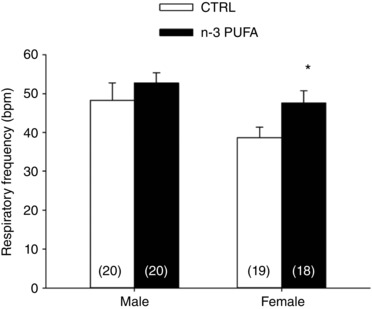
Respiratory frequency (beats per minute; bpm) during normoxia in pups that received n‐3 PUFA supplementation (black bars) or control (CTRL; white bars). Data are expressed as the mean ± SEM; numbers in brackets indicate the number of pups in each group. Post hoc pairwise comparisons were performed only when warranted by ANOVA. Significantly different from CTRL: * P ≤ 0.05.
Figure 9. n‐3 PUFA supplementation decreases apnoeas duration and has no effect on heart rate in anaesthetized pups during laryngeal chemoreflex (LCR) stimulation.
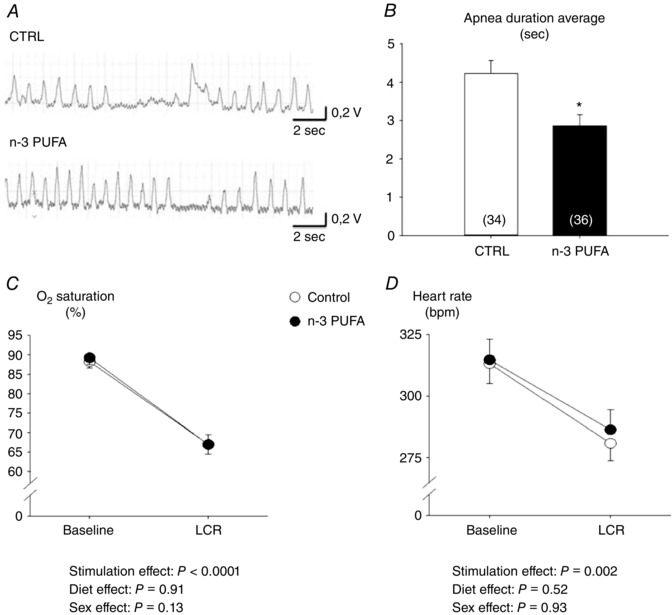
A, original recordings illustrating minute ventilation (red traces) and LCR stimulation in pups that received n ‐3 PUFA supplementation and controls (CTRL). B, mean of apnoeas duration elicited by LCR. C, mean of minimum saturation observed after LCR. D, mean of minimum heart rate observed after LCR. Histograms represent responses observed in pups that received n ‐3 PUFA supplementation (black bar) and CTRL (white bar). Males and females were analysed together because there were no sex‐specific differences for these variables. Data are expressed as the mean ± SEM. Post hoc pairwise comparisons were performed only when warranted by ANOVA. Significantly different from CTRL: * P ≤ 0.05.
Series III: n‐3 PUFA treatment augments the relative alveolar surface area in rat pups
Morphometric analysis of the photomicrographs of lungs from control (Fig. 10 A) and n‐3 PUFA treated pups (Fig. 10 B) showed that L m was lower in rats supplemented with n‐3 PUFA compared with controls and this effect was reflected in the relative alveolar surface area between controls and supplemented animals (Fig. 10 C and D, respectively); this effect was not sex‐specific. However, n‐3 PUFA supplementation had no effect on lung volume (Fig. 10 E) or the total alveolar surface area (data not shown).
Figure 10. n‐3 PUFA treatment improves relative alveolar surface area in pups (P10–11).
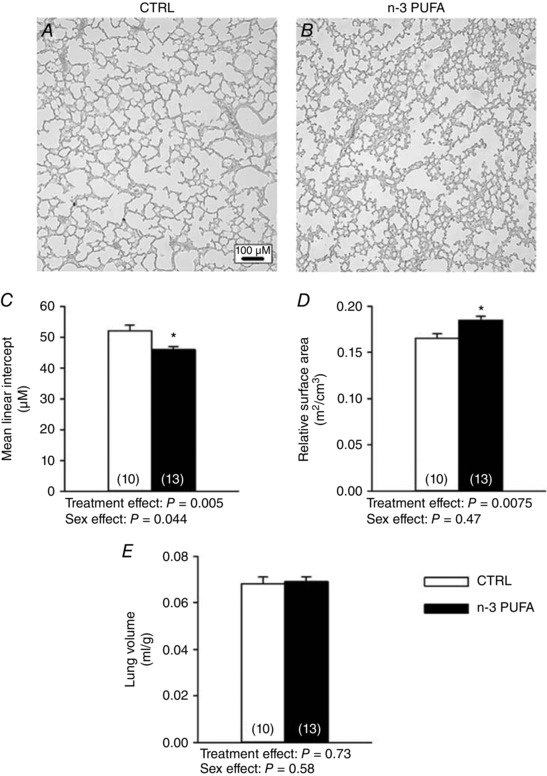
Typical images of the lung architecture obtained from control (CTRL) rat pup (A) and n‐3 PUFA supplemented pups (B). C, mean linear intercept (L m) (μm). D, relative alveolar surface area (m2 cm–3). E, lung volume (ml g–1). Because there was no sex‐specific effect, data from male and female pups were pooled; numbers in brackets indicate the number of replicates in each group. Significantly different from CTRL: * P ≤ 0.05.
Series IV: n‐3 PUFA supplementation affects brainstem microglia density and morphology
Quantification of Iba‐1 positive cells in the cNTS (Fig. 11 A) revealed that n‐3 PUFA treatment reduced microglial density by 60% in this region (Fig. 11 B–D). n‐3 PUFA supplementation augmented the morphological index and this effect was sex‐specific because significant differences were only noted in males (Fig. 11 D).
Figure 11. n‐3 PUFA treatment reduces the number of microglia and promotes an amoeboid phenotype in cNTS.
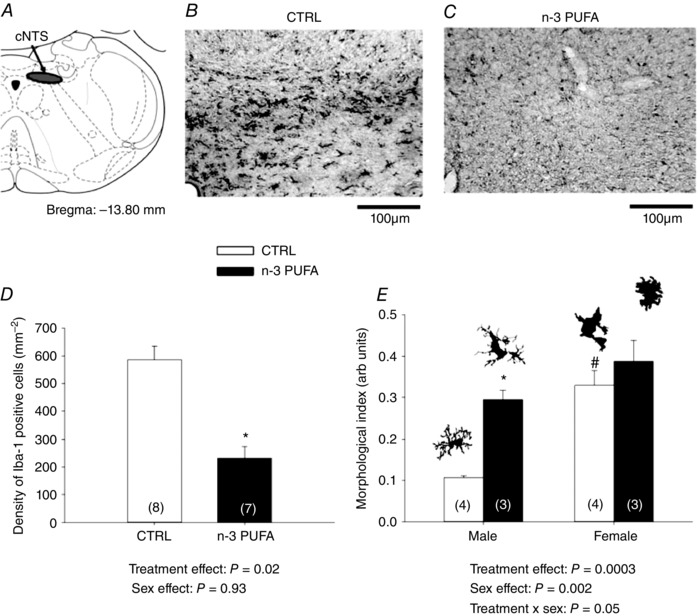
A, schematic representation of cNTS area (Paxinos & Watson, 1998). Photomicrographs illustrating Iba‐1 immunolabelling on tissue from control (CTRL) (B) and n‐3 PUFA supplemented pups (C). D, number of Iba‐1 positive cells in cNTS. E, morphological index of Iba‐1 positive cells in cNTS (illustration of microglia phenotype adapted with permission from Orłowski et al. 2003). Data are expressed as the mean ± SEM. Numbers in brackets indicate the number of replicates in each group. Post hoc pairwise comparisons were performed only when warranted by ANOVA. Significantly different from CTRL: * P ≤ 0.05. Significantly different from male CTRL: # P ≤ 0.05.
Discussion
Respiratory disorders related to neurological immaturity are a major cause of hospitalization and morbidity in preterm infants, yet therapeutic interventions aiming to alleviate this condition remain limited. Based on evidence indicating that n‐3 PUFA improves neurodevelopmental outcomes in this population (McNamara et al. 2015), we hypothesized that providing n‐3 PUFA supplementation to newborn via the maternal diet improves respiratory function in rat pups. At first, the increased frequency and duration of apnoeas observed in treated pups not only contradict our initial hypothesis, but also suggest that, at the studied dose, n‐3 PUFA supplementation worsens respiratory control. However, the subsequent demonstration that n‐3 PUFA supplementation improves physiological responses to respiratory challenges and augments relative alveolar surface area of the lungs brings us to re‐evaluate this preliminary interpretation.
Critique of methods
The growth of n‐3 PUFA‐related research has been associated with a diversity of experimental approaches used to manipulate fatty acid levels in developing newborn (both humans and rodents). Consequently, proper comparison of n‐3 PUFA levels achieved in the present study with those reporting beneficial effects is difficult. However, the n‐3 PUFA, DHA and EPA levels obtained in erythrocytes membrane compare favourably to studies of n‐3 PUFA supplementation in humans (Hurtado et al. 2015). Thus, final assessment of the potential therapeutic value of neonatal n‐3 PUFA supplementation is based on physiological outcomes. The impact of apnoeic events on development and health is mainly determined by the intensity of concomitant O2 desaturations and bradycardias (Southall et al. 1982; Poets, 2010). Accordingly, we attempted to evaluate the physiological consequences of apnoeas with pulse oximetry; however, we were unable to consistently maintain a probe on ‘resting’ pups for a sufficiently long period without sedation or anaesthesia to obtain reliable O2 saturation and/or heart rate data. This technical difficulty therefore prevents us from ruling out the possibility that the apnoeas observed in n‐3 PUFA pups were associated with significant O2 desaturations and bradycardias. We know, however, that exposing newborn to intermittent hypoxia for 10 consecutive days reduces the pups body weight by 13% (Rakusan et al. 2007) and augments the response of the carotid body to hypoxia ex vivo (Pawar et al. 2008). Here, n‐3 PUFA supplemented rats weighed 6% more than controls and measurements of the initial response to hypoxia provide no support for augmented O2 chemosensitivity. Based on these data, the lack of significant differences in basal respiratory/metabolic parameters, and the physiological responses measured at 10 days of age, there is no evidence indicating that the increased occurrence of apnoeas resulting from n‐3 PUFA supplementation had deleterious consequences on pups.
Origins of apnoeas in newborn pups
Respiratory instability and apnoeas observed in newborns (both rodents and infants) generally reflect the relative maturity of the respiratory network of the brainstem and the different types of apnoeas reveal the more specific mechanisms triggering them. Our initial quantification focused on spontaneous vs. post‐sigh apnoeas because it was not possible to measure apnoeas resulting from collapse of the upper airways or LCR stimulation without more invasive methods. n‐3 PUFA had no effect on the proportion of the two types of apnoeas that were observed but revealed a sexual dimorphism in the relative distribution of these events. This result further highlights sex‐based differences in respiratory regulation and may be linked to the effects of n‐3 PUFA supplementation on testosterone secretion (Phelan et al. 2011; Feng et al. 2015).
The majority of apnoeas occurred spontaneously, thereby indicating a cessation of the central respiratory command owing to insufficient respiratory drive. Several factors can be evoked to explain the origins of these apnoeas, including changes in sleep/wake state and stimulation originating from central (CO2) and peripheral chemoreceptors. Because the effects of n‐3 PUFA on sleep architecture and CO2 chemosensitivity were not measured, their potential roles remain unknown. Nevertheless, the lower tachypnoeic response measured at the onset of hypoxia in n‐3 PUFA supplemented pups suggests that reduced peripheral O2 chemosensitivity contributes to the increased propensity for spontaneous apnoeas in this group. However, correlation analyses show that this explanation is valid only in n‐3 PUFA females in which the O2 chemosensitivity index was inversely proportional to the apnoea index. This result contrasts with control females in which an opposite trend was observed. Although not statistically significant, this relationship suggests that, in this group, the hypocapnia resulting from the hypoxia‐induced hyperpnoea is a factor. Conversely, the absence of any relationship between the apnoea index and O2 chemosensitivity in males points to other (more complex) determinants of respiratory drive such as the interactions between various groups of chemoreceptors. As a whole, these observations indicate that the impact of n‐3 PUFA on the mechanisms underlying spontaneous apnoeas is heterogeneous and is greatly influenced by sex.
By comparison, post‐sigh apnoeas contributed less to the apnoea index but were nonetheless increased in n‐3 PUFA pups. The augmented breaths (sighs) originate from the brainstem network generating respiratory rhythm (Chapuis et al. 2014); they appear during fetal life and their frequency decrease progressively following birth (Thach & Taeusch, 1976; Chapuis et al. 2014). The duration of the ensuing apnoea is determined by the core rhythmogenic network, the strong inspiratory inhibition from pulmonary stretch receptors and the changes in arterial blood gases resulting from the transient increase in gas exchange; each of those mechanisms may contribute to the augmentation of post‐sigh apnoeas in n‐3 supplemented pups. We know, however, that n‐3 PUFA supplementation improves lung development by attenuating the impact of perinatal insults such as oxidation and inflammation (Ali et al. 2015) but, to the best of our knowledge, the effect of n‐3 PUFA supplementation during normal development has not been addressed. The protocol used in the present study was sufficient to augment the relative surface area, thus indicating an improved structural lung development. Given the modest size of the increase observed and the fact that n‐3 PUFA had no effect on lung volume, the functional impact may be limited at rest; however, improved gas exchange is plausible during an augmented breath and thus contribute to longer post‐sigh apnoeas in treated pups.
The LCR is an ensemble of physiological responses that prevent aspiration of foreign substances into the lower airways and lungs (Thach, 2001). In mature mammals, the presence of liquids near the larynx triggers coughing or swallowing. However, brainstem networks of preterm infants and young mammals are not sufficiently mature to co‐ordinate breathing with other motor commands and, as a result, the presence of liquids on the laryngeal mucosa inhibits inspiration (Praud, 1999; Praud & Reix, 2005). Although effective at protecting the airways, apnoeas resulting from LCR stimulation can be sufficiently long to be life threatening, especially in newborns, because the duration of LCR‐induced apnoeas is inversely proportional to developmental stage (Thach, 2001). Moreover, because sensory afferents from the laryngeal mucosa projecting to the cNTS are then relayed to brainstem parasympathetic neurons regulating heart rate, LCR stimulation also triggers profound bradycardias that can aggravate the impact of LCR‐induced apnoeas in newborns. With that in mind, comparing the intensity of LCR‐related cardio‐respiratory responses between groups is an excellent opportunity to test our hypothesis further because these experiments are performed at the same time as controlling sleep–wake states and body temperature. Furthermore, baseline data showing that breathing frequency, SpO2 and heart rate did not differ between groups suggest that respiratory drive was similar prior to LCR activation. Although the shorter apnoeas observed in n‐3 PUFA supplemented pups are consistent with a more mature respiratory control network, the lack of effect on O2 desaturations and bradycardias suggests that n‐3 PUFA does not affect the cardiovascular system.
n‐3 PUFA supplementation leads to a more sustained hypoxic ventilatory response
Newborns generally conform to reduced O2 availability by lowering metabolic demand and, as they develop, mammals progressively aim to maintain O2 levels constant by augmenting O2 uptake (Mortola, 2001). The dynamics of the HVR reflect these ontogenic changes in strategy such that, in newborns, the brisk tachypnoea at the onset of hypoxia is followed by ventilatory depression to reach levels near or even below the baseline level because metabolic depression is an important part of the response. By contrast, more mature mammals can sustain a significant hyperventilation throughout the hypoxic event and show a more modest reduction in metabolic demand (Bissonnette, 2000). Peripheral chemoreceptor development is highly plastic and, given the numerous structural and functional effects of n‐3 PUFA on the CNS, several hypotheses can be evoked to explain the delayed frequency response observed at the onset of hypoxia in supplemented pups. For example, increases in pro‐inflammatory cytokines following neonatal lipopolysaccharide treatment also reduce O2 sensitivity (Master et al. 2016). Based on the anti‐inflammatory properties of n‐3 PUFA treatment and the lung architecture reported in supplemented pups, an increase in inflammatory cytokines is probably not a cause of the reduced O2 sensing. Because experimental manipulations of dietary n‐3 PUFA can alter monoamines in the brain (Chalon, 2006; Vancassel et al. 2008), similar changes within the carotid bodies may alter development of O2 sensing.
The attenuation of HVR was transient such that, at the end of hypoxia, the response measured in n‐3 PUFA supplemented pups was greater than controls. Because this was achieved by minimizing V T depression, this difference suggests that neural inspiratory drive and/or muscle performance were improved by n‐3 PUFA. Furthermore, the and anapyrexic responses indicate that, overall, hypometabolism is a less important strategy for facing hypoxia in supplemented rats. In these pups, the anapyrexic response was less than controls and independent of body weight, thereby indicating that n‐3 PUFA reduced heat loss by improving autonomic regulation and/or benefited from a better insulation (increased body fat). This effect of n‐3 PUFA is an important observation considering that neonatal hypothermia has been associated with higher mortality and morbidity rates in premature babies (Lyu et al. 2015). Given their close inter‐relationship, the sex‐based differences in the importance of n‐3 PUFA effects on and T b° responses are difficult to reconcile. However, analysis of co‐variance indicates that the slightly lower body weight of females is a factor, suggesting that the linear correction applied to this variable may not be adequate. Although allometric corrections do exist for adults (Mortola et al. 1994), their validity has not been evaluated in pups.
n‐3 PUFA supplementation decreases microglia density and alters cell morphology in cNTS
During fetal life, microglia migrate from the yolk sac into the CNS via the circulation (Perry & Gordon, 1991; Wu et al. 1992, 1994; Ginhoux et al. 2010). Within the hippocampus of mice, the microglia density increases progressively following birth and reaches a plateau near postnatal day 15 (Paolicelli et al. 2011). Microglial morphology undergoes substantial changes during this process. In newborns, precursor cells are characterized by a round cell body (amoeboid shape) with no identifiable processes (Harry & Kraft, 2012) and, by postnatal day 14, rat microglia begin to show clear evidence of adult‐like ramification (Orlowski et al. 2003). The reduced microglia density observed in n‐3 PUFA supplemented pups indicates that this treatment delayed microglial colonization within the cNTS. This observation is consistent with the recent demonstration that n‐3 PUFA treatment improves blood–brain barrier integrity in newborn (Zhang et al. 2016) and is supported by the results of a morphological index analysis showing that the small amounts of Iba‐1 positive cells reaching the cNTS were immature, especially in males. Microglia play an important role in sexual differentiation of the brain and are highly responsive to testosterone (Lenz et al. 2013) and the sex‐based difference in morphology is consistent with this property, further suggesting that the n‐3 PUFA protocol influenced sex hormone secretion in pups. However, based on the reduced microglia density and generally immature phenotype following n‐3 PUFA treatment, the contribution of these cells to the establishment of synaptic circuits via phagocytosis of excessive synapses during early life is probably reduced. This, in turn, may explain why n‐3 PUFA supplemented pups are more responsive to respiratory stimuli. However, as a result of the importance of microglial function in brain development, this observation raises questions regarding the long‐term consequences of this treatment.
Clinical relevance, perspectives, and conclusions
In preterm infants, respiratory disorders related to immaturity of the brainstem control network are a major concern for clinicians both in the short and long term (Abu‐Shaweesh & Martin, 2008). Based on the results reported in the present study, further consideration should be given to n‐3 PUFA supplementation as an alternative treatment of respiratory disturbances associated with prematurity because several desirable respiratory traits were observed in pups following treatment. The improved ability to sustain a more robust hyperventilation combined with an augmented relative lung surface area will probably ameliorate gas exchange during hypoxia. The shorter apnoeas following LCR stimulation are of interest because n‐3 PUFA treatment may reduce the likelihood of related complications such as apparent life threatening event or sudden infant death syndrome. Finally, given that poor thermoregulation is a predictor of negative outcome in preterm infants (Lyu et al. 2015), the improved thermoregulation following n‐3 PUFA treatment is clinically relevant. Despite multiple approaches used to evaluate how n‐3 PUFA supplementation affects the respiratory system, several questions remain unaddressed but point to very promising research avenues. Moreover, given the growing interest for sex‐based differences in health and disease, a more detailed neuroendocrine assessment would be very informative.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
This research was performed in Dr Kinkead's laboratory at the Centre de Recherche du CHU de Québec, Hôpital St‐François d'Assise. LTL, CB, TS, IM and RK conceived and/or designed the work. CB, LTL, OM, AJL, OPP, TS, IM, VJ and RK acquired, analysed or interpreted data. CB, LTL, AJL, TS, IM, VJ and RK drafted the work or revised it critically for important intellectual content. All authors approved the final version of this manuscript; agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; and qualify for authorship.
Funding
This research was supported by operating grants from the Canadian Institutes of Health Research to RK (MOP‐119337) and to IM and the MOBYDIck research group (MOP‐133731).
Acknowledgements
We thank Mélanie Pelletier for animal care.
References
- Abu‐Shaweesh JM & Martin RJ (2008). Neonatal apnea: what's new? Pediatr Pulmonol 43, 937–944. [DOI] [PubMed] [Google Scholar]
- Ali M, Heyob KM, Velten M, Tipple TE & Rogers LK (2015). DHA suppresses chronic apoptosis in the lung caused by perinatal inflammation. Am J Physiol Lung Cell Mol Physiol 309, L441–L448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bairam A, Boutroy MJ, Badonnel Y & Vert P (1987). Theophylline versus caffeine: comparative effects in treatment of idiopathic apnea in the preterm infant. J Pediatr 110, 636–639. [DOI] [PubMed] [Google Scholar]
- Bissonnette JM (2000). Mechanisms regulating hypoxic respiratory depression during fetal and postnatal life. Am J Physiol Regul Integr Comp Physiol 278, R1391–R1400. [DOI] [PubMed] [Google Scholar]
- Chalon S (2006). Omega‐3 fatty acids and monoamine neurotransmission. Prostaglandins Leukot Essent Fatty Acids 75, 259–269. [DOI] [PubMed] [Google Scholar]
- Chapuis C, Autran S, Fortin G, Simmers J & Thoby‐Brisson M (2014). Emergence of sigh rhythmogenesis in the embryonic mouse. J Physiol 592, 2169–2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Darlington RB & Finlay BL (2001). Translating developmental time across mammalian species. Neuroscience 105, 7–17. [DOI] [PubMed] [Google Scholar]
- Counil E, Julien P, Lamarche B, Chateau‐Degat ML, Ferland A & Dewailly E (2009). Association between trans‐fatty acids in erythrocytes and pro‐atherogenic lipid profiles among Canadian Inuit of Nunavik: possible influences of sex and age. Br J Nutr 102, 766–776. [DOI] [PubMed] [Google Scholar]
- Dempsey JA, Veasey SC, Morgan BJ & O'Donnell CP (2010). Pathophysiology of sleep apnea. Physiol Rev 90, 47–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fiore JM, Martin RJ & Gauda EB (2013). Apnea of prematurity – Perfect storm. Respir Physiol Neurobiol 189, 213–222. [DOI] [PubMed] [Google Scholar]
- Drorbaugh JE & Fenn WO (1955). A barometric method for measuring ventilation in newborn infants. Pediatrics 16, 81–87. [PubMed] [Google Scholar]
- Escamilla‐Nuñez MC, Barraza‐Villarreal A, Hernández‐Cadena L, Navarro‐Olivos E, Sly PD & Romieu I (2014). Omega‐3 fatty acid supplementation during pregnancy and respiratory symptoms in children. Chest 146, 373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Ding Y, Liu J, Tian Y, Yang Y, Guan S & Zhang C (2015). Effects of dietary omega‐3/omega‐6 fatty acid ratios on reproduction in the young breeder rooster. BMC Vet Res 11, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finer NN, Higgins R, Kattwinkel J & Martin RJ (2006). Summary proceedings from the apnea‐of‐prematurity group. Pediatrics 117, S47–51. [DOI] [PubMed] [Google Scholar]
- Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM & Merad M (2010). Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology . J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulemetova R & Kinkead R (2011). Neonatal stress increases respiratory instability in rat pups. Respir Physiol Neurobiol 176, 103–109. [DOI] [PubMed] [Google Scholar]
- Harry GJ & Kraft AD (2012). Microglia in the developing brain: a potential target with lifetime effects. Neurotoxicology 33, 191–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjorth E, Zhu M, Toro VC, Vedin I, Palmblad J, Cederholm T, Freund‐Levi Y, Faxen‐Irving G, Wahlund LO, Basun H, Eriksdotter M & Schultzberg M (2013). Omega‐3 fatty acids enhance phagocytosis of Alzheimer's disease‐related amyloid‐beta42 by human microglia and decrease inflammatory markers. J Alzheimers Dis 35, 697–713. [DOI] [PubMed] [Google Scholar]
- Hurtado JA, Iznaola C, Peña M, Ruíz J, Peña‐Quintana L, Kajarabille N, Rodriguez‐Santana Y, Sanjurjo P, Aldámiz‐Echevarría L, Ochoa J, Lara‐Villoslada F & Group oBotN (2015). Effects of maternal Ω‐3 supplementation on fatty acids and on visual and cognitive development. J Pediatr Gastroenterol Nutr 61, 472–480. [DOI] [PubMed] [Google Scholar]
- Jochmans‐Lemoine A, Villalpando G, Gonzales M, Valverde I, Soria R & Joseph V (2015). Divergent physiological responses in laboratory rats and mice raised at high altitude. J Exp Biol 218, 1035–1043. [DOI] [PubMed] [Google Scholar]
- Jordan D (2001). Central nervous pathways and control of the airways. Respir Physiol 125, 67–81. [DOI] [PubMed] [Google Scholar]
- Kaur C & Ling EA (1991). Study of the transformation of amoeboid microglial cells into microglia labelled with the isolectin Griffonia simplicifolia in postnatal rats. Acta Anat (Basel) 142, 118–125. [DOI] [PubMed] [Google Scholar]
- Kuipers RS, Luxwolda MF, Offringa PJ, Boersma ER, Dijck‐Brouwer DA & Muskiet FA (2012). Fetal intrauterine whole body linoleic, arachidonic and docosahexaenoic acid contents and accretion rates. Prostaglandins Leukot Essent Fatty Acids 86, 13–20. [DOI] [PubMed] [Google Scholar]
- Lenz KM, Nugent BM, Haliyur R & McCarthy MM (2013). Microglia are essential to masculinization of brain and behavior. J Neurosci 33, 2761–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu Y, Shah PS, Ye XY, Warre R, Piedboeuf B, Deshpandey A, Dunn M, Lee SK & Canadian Neonatal N (2015). Association between admission temperature and mortality and major morbidity in preterm infants born at fewer than 33 weeks' gestation. JAMA Pediatr 169, e150277. [DOI] [PubMed] [Google Scholar]
- Mage DT & Donner M (2006). Female resistance to hypoxia: does it explain the sex difference in mortality rates? J Womens Health (Larchmt) 15, 786–794. [DOI] [PubMed] [Google Scholar]
- Malik SS & Fewell JE (2003). Thermoregulation in rats during early postnatal maturation: importance of nitric oxide. Am J Physiol Regul Integr Comp Physiol 285, R1366–R1372. [DOI] [PubMed] [Google Scholar]
- Master ZR, Porzionato A, Kesavan K, Mason A, Chavez‐Valdez R, Shirahata M & Gauda EB (2016). Lipopolysaccharide exposure during the early postnatal period adversely affects the structure and function of the developing rat carotid body. J Appl Physiol (1985) 121, 816–827. [DOI] [PubMed] [Google Scholar]
- Mayer CA, Di Fiore JM, Martin RJ & Macfarlane PM (2014). Vulnerability of neonatal respiratory neural control to sustained hypoxia during a uniquely sensitive window of development. J Appl Physiol (1985) 116, 514–521. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Vannest JJ & Valentine CJ (2015). Role of perinatal long‐chain omega‐3 fatty acids in cortical circuit maturation: mechanisms and implications for psychopathology. World J Psychiatry 5, 15–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson WB, Martin JV, Perlis M, Giesen H, Wagner R & Rapoport SI (1988). Periodic cessation of respiratory effort during sleep in adult rats. Physiol Behav 43, 229–234. [DOI] [PubMed] [Google Scholar]
- Montandon G, Bairam A & Kinkead R (2006). Long‐term consequences of neonatal caffeine on ventilation, occurrence of apneas, and hypercapnic chemoreflex in male and female rats. Pediatr Res 59, 519–524. [DOI] [PubMed] [Google Scholar]
- Mortola JP (2001). Respiratory physiology of newborn mammals: a comparative perspective. The Johns Hopkins University Press, Baltimore, MD. [Google Scholar]
- Mortola JP & Dotta A (1992). Effects of hypoxia and ambient temperature on gaseous metabolism of newborn rats. Am J Physiol Regul Integr Comp Physiol 263, R267–R272. [DOI] [PubMed] [Google Scholar]
- Mortola JP, Matsuoka T, Saiki C & Naso L (1994). Metabolism and ventilation in hypoxic rats: effect of body mass. Respir Physiol 97, 225–234. [DOI] [PubMed] [Google Scholar]
- Orlowski D, Soltys Z & Janeczko K (2003). Morphological development of microglia in the postnatal rat brain. A quantitative study. Int J Dev Neurosci 21, 445–450. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D & Gross CT (2011). Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–1458. [DOI] [PubMed] [Google Scholar]
- Pawar A, Peng YJ, Jacono FJ & Prabhakar NR (2008). Comparative analysis of neonatal and adult rat carotid body responses to chronic intermittent hypoxia. J Appl Physiol (1985) 104, 1287–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G & Watson C (1998). The rat brain in stereotaxic coordinates. Academic Press, San Diego. [DOI] [PubMed] [Google Scholar]
- Perry VH & Gordon S (1991). Macrophages and the nervous system. Int Rev Cytol 125, 203–244. [DOI] [PubMed] [Google Scholar]
- Phelan N, O'Connor A, Kyaw Tun T, Correia N, Boran G, Roche HM & Gibney J (2011). Hormonal and metabolic effects of polyunsaturated fatty acids in young women with polycystic ovary syndrome: results from a cross‐sectional analysis and a randomized, placebo‐controlled, crossover trial. Am J Clin Nutr 93, 652–662. [DOI] [PubMed] [Google Scholar]
- Poets CF (2010). Apnea of prematurity: what can observational studies tell us about pathophysiology? Sleep Medicine 11, 701–707. [DOI] [PubMed] [Google Scholar]
- Poets CF, Samuels MP & Southall DP (1994). Epidemiology and pathophysiology of apnoea of prematurity. Biol Neonate 65, 211–219. [DOI] [PubMed] [Google Scholar]
- Powell FL, Milsom WK & Mitchell GS (1998). Time domains of the hypoxic ventilatory response. Respir Physiol 112, 123–134. [DOI] [PubMed] [Google Scholar]
- Praud J‐P (2010). Upper airway reflexes in response to gastric reflux. Paediatr Respir Rev 11, 208–212. [DOI] [PubMed] [Google Scholar]
- Praud J‐P & Reix P (2005). Upper airways and neonatal respiration. Respir Physiol Neurobiol 149, 131–141. [DOI] [PubMed] [Google Scholar]
- Praud JP (1999). Larynx and neonatal apneas. Pediatr Pulmonol Suppl 18, 190–193. [PubMed] [Google Scholar]
- Rakusan K, Chvojkova Z, Oliviero P, Ostadalova I, Kolar F, Chassagne C, Samuel JL & Ostadal B (2007). ANG II type 1 receptor antagonist irbesartan inhibits coronary angiogenesis stimulated by chronic intermittent hypoxia in neonatal rats. Am J Physiol Heart Circ Physiol 292, H1237–H1244. [DOI] [PubMed] [Google Scholar]
- Schaeffer PJ (1998). The development of the ventilatory response to cold in very young rats. Comp Biochem Physiol A Mol Integr Physiol 119, 407–414. [DOI] [PubMed] [Google Scholar]
- Schipper L, Oosting A, Scheurink AJ, van Dijk G & van der Beek EM (2016). Reducing dietary intake of linoleic acid of mouse dams during lactation increases offspring brain n‐3 LCPUFA content. Prostaglandins Leukot Essent Fatty Acids 110, 8–15. [DOI] [PubMed] [Google Scholar]
- Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, Solimano A, Tin W & Caffeine for Apnea of Prematurity Trial G (2007). Long‐term effects of caffeine therapy for apnea of prematurity. N Engl J Med 357, 1893–1902. [DOI] [PubMed] [Google Scholar]
- Shaikh NA & Downar E (1981). Time course of changes in porcine myocardial phospholipid levels during ischemia. A reassessment of the lysolipid hypothesis. Circ Res 49, 316–325. [DOI] [PubMed] [Google Scholar]
- Simopoulos AP (1999). Essential fatty acids in health and chronic disease. Am J Clin Nutr 70, 560s–569s. [DOI] [PubMed] [Google Scholar]
- Siriwardhana N, Kalupahana NS & Moustaid‐Moussa N (2012). Health benefits of n‐3 polyunsaturated fatty acids: eicosapentaenoic acid and docosahexaenoic acid. Adv Food Nutr Res 65, 211–222. [DOI] [PubMed] [Google Scholar]
- Southall DP, Richards JM, Rhoden KJ, Alexander JR, Shinebourne EA, Arrowsmith WA, Cree JE, Fleming PJ, Goncalves A & Orme RL (1982). Prolonged apnea and cardiac arrhythmias in infants discharged from neonatal intensive care units: failure to predict an increased risk for sudden infant death syndrome. Pediatrics 70, 844–851. [PubMed] [Google Scholar]
- Thach BT (2001). Maturation and transformation of reflexes that protect the laryngeal airway from liquid aspiration from fetal to adult life. Am J Med 111 (Suppl 8A), 69S–77S. [DOI] [PubMed] [Google Scholar]
- Thach BT & Taeusch HW, Jr (1976). Sighing in newborn human infants: role of inflation‐augmenting reflex. J Appl Physiol 41, 502–507. [DOI] [PubMed] [Google Scholar]
- Vancassel S, Leman S, Hanonick L, Denis S, Roger J, Nollet M, Bodard S, Kousignian I, Belzung C & Chalon S (2008). n‐3 Polyunsaturated fatty acid supplementation reverses stress‐induced modifications on brain monoamine levels in mice. J Lipid Res 49, 340–348. [DOI] [PubMed] [Google Scholar]
- Vanden Heuvel JP (2012). Nutrigenomics and nutrigenetics of omega3 polyunsaturated fatty acids. Prog Mol Biol Transl Sci 108, 75–112. [DOI] [PubMed] [Google Scholar]
- Wu CH, Wen CY, Shieh JY & Ling EA (1992). A quantitative and morphometric study of the transformation of amoeboid microglia into ramified microglia in the developing corpus callosum in rats. J Anat 181, 423–430. [PMC free article] [PubMed] [Google Scholar]
- Wu CH, Wen CY, Shieh JY & Ling EA (1994). Down‐regulation of membrane glycoprotein in amoeboid microglia transforming into ramified microglia in postnatal rat brain. J Neurocytol 23, 258–269. [DOI] [PubMed] [Google Scholar]
- Xia L, Leiter JC & Bartlett D, Jr (2008). Laryngeal apnea in rat pups: effects of age and body temperature. J Appl Physiol (1985) 104, 269–274. [DOI] [PubMed] [Google Scholar]
- Zhang P, Lavoie PM, Lacaze‐Masmonteil T, Rhainds M & Marc I (2014). Omega‐3 long‐chain polyunsaturated fatty acids for extremely preterm infants: a systematic review. Pediatrics 134, 120–134. [DOI] [PubMed] [Google Scholar]
- Zhang W, Zhang H, Mu H, Zhu W, Jiang X, Hu X, Shi Y, Leak RK, Dong Q, Chen J & Gao Y (2016). Omega‐3 polyunsaturated fatty acids mitigate blood‐brain barrier disruption after hypoxic‐ischemic brain injury. Neurobiol Dis 91, 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]


