Abstract
Certain professionals have more exposure to animals and therefore an increased risk of zoonoses. Professional hunting dog caretakers work with upwards of 50 dogs and are exposed to zoonoses through exposure to multiple potentially infectious canine secretions or excretions, as well as to the ticks that dogs carry. Dog caretakers reported having found embedded ticks on their bodies 5.83 times more than environment-only controls. Zoonotic Lyme disease, first in the United States for morbidity due to a vector-borne infection, has dramatically expanded its geographic range over the last two decades. This finding emphasizes the increased risk of tick-borne diseases, including Lyme disease, based on dog exposure and in areas of disease emergence.
Keywords: : ticks, zoonotic, risk factors, Lyme disease, seroprevalence, occupational health
Introduction
Zoonotic diseases—diseases transmitted from animal to humans—account for >75% of emerging human pathogens, with domestic animals playing a significant role in the transmission (Blancou et al. 2005). Although interacting with pet species bears a risk of contracting a zoonotic infection, multiple sources describe the benefits of interaction with companion animals, including decreased blood pressure and better quality of life (Raina et al. 1999, Robinson and Pugh 2002, Parslow et al. 2005, Thorpe et al. 2006, Knight and Edwards 2008, Beetz et al. 2012, Orritt 2014, Day 2016). This interaction does not come without risk, as dogs are viewed as public health targets to prevent the spread of diseases such as rabies and leishmaniasis (Day 2016). Dogs are the predominant host of several tick species and are hosts for both nymph and adult Ixodes ticks (Hamer et al. 2009).
Lyme disease, a zoonotic disease caused by the bacterial spirochete Borrelia burgdorferi, transmitted via Ixodes scapularis, is the most widely reported zoonotic tick-borne disease in the United States (Wormser et al. 2006). More than 25,000 confirmed and probable cases of Lyme disease are reported in the United States each year, resulting in an incidence of about 10.54 cases per 100,000 individuals (Kuehn 2013, Mead 2015, Adams et al. 2016). The Centers for Disease Control and Prevention (CDC) estimates that there are as many as 300,000 cases each year, including cases from expanded geographic areas of the southern Mid-Atlantic and Midwestern regions including Iowa (Kuehn 2013, Mead 2015, Adams et al. 2016). Due to its zoonotic nature, Lyme disease is also of veterinary importance, seen most frequently in dogs. There was an 11% canine B. burgdorferi seroprevalence in 2009 (Bowman et al. 2009). Risk of Lyme disease in both dogs and people requires I. scapularis exposure. It has previously been demonstrated that pet owners have a slight, but statistically significant, increased exposure to ticks (Jones et al. 2018). It is unknown how an occupation with exposure to >50 dogs on a daily basis would change this risk.
The risk of zoonotic disease spread increases as interaction between mammalian hosts and people increases. Certain occupations or hobbies have dramatically more exposure to animals and therefore increased risk (Blancou et al. 2005). Reports have shown that bird watchers and deer hunters have a significantly higher risk of tick-borne diseases, including Lyme disease and Rocky Mountain spotted fever, mainly due to the time they spend in tick habitats (Bouchard et al. 2013, Kilpatrick et al. 2014). Fox hunters have similar exposure to tick habitat as other hunters, but also carry the potential risk of increased tick infestation and subsequent tick-borne diseases due to their exposure to >50 dogs daily.
How routine occupational exposure to many (>50) dogs, known hosts of B. burgdorferi that present with clinical Lyme disease, may increase exposure to ticks and/or zoonotic tick-borne diseases is unknown. It was hypothesized that individuals who work with hunting dogs would have higher exposures to ticks than people who are frequently in tick habitats without dogs. Subsequently, it was hypothesize that increased exposure to ticks would make hound caretakers more likely to be seropositive for B. burgdorferi. This cross-sectional study assessed the risk of tick exposure and Lyme disease within a population of hunting dog caretakers compared to individuals with similar environmental exposures but limited dog exposure.
Materials and Methods
Research participants
Ninety-four research participants were asked to take a risk assessment survey to establish tick and Lyme disease exposure based on a human subjects Institution Review Board–approved protocol from the University of Iowa. Research participants who voluntarily participated were included in the study if they were aged >18 years and spoke English. Hunting dog–exposed individuals were recruited for the study at dog shows held in VA and KS, representing multiple large hunting dog kennels predominantly from the Mid-Atlantic and Midwest regions. Individuals not exposed to dogs were recruited at meetings for the Iowa Audubon Society, with individuals who frequently travel to Lyme endemic areas of WI and MN to observe waterfowl and meetings of the regional Parks and Recreation Departments.
Survey tool for exposure to tick-borne infectious diseases
The survey included demographic questions (age, sex, and geographical location) and questions about spending time performing activities related to tick exposure. As a part of the survey, participants specifically answered questions about time spent outdoors, time spent walking or working in high grass or brush, and an open-ended question about recent medical diagnoses. In responding to this open-ended question, multiple respondents commented about previous or current personal Lyme disease signs, symptoms, and diagnoses by a medical professional with or without treatment. Research participants provided a blood spot after survey completion. Without exception, any self-reported individual's Lyme disease diagnosis occurred prior to this study's survey and blood sampling. Therefore, concordance of these two variables is low.
Definition of experimental groups
Exposure to hunting dogs was defined as working with a hunting dog kennel of ≥50 dogs. Unexposed, tick-environmental control individuals were bird watchers or individuals from county park departments, individuals without contact with a large number of dogs but similar tick environmental exposures.
Blood sampling
A blood spot was obtained using a ReliOn® or Unistik Travel Lancet. Blood was collected and dried on Whatman® 903 protein saver cards and stored in a locked box at room temperature. Serum was eluted from dried blood spots by sterile extraction of two circles from each Whatman 903 protein saver card, using a sterile hole puncher, and placing the circles in one well within a 96-well round-bottom plate. Elution buffer (125 μL), containing phosphate-buffered saline, 0.1% Tween-20, and 5% nonfat dry milk, was added, and samples were incubated for 16 h at 4°C after which serum was removed.
B. burgdorferi enzyme-linked immunosorbent assay
Isolated serum was tested using an Immunetics® C6 B. burgdoferi (Lyme) enzyme-linked immunosorbent assay (ELISA), which detects human immunoglobulin G (IgG)/immunoglobulin M (IgM).
Data management
Study data were collected and managed using Research Electronic Data Capture, an electronic data capture tool, hosted by the University of Iowa.
Statistical methods
To measure the association between exposure to multiple hunting dogs and the level of tick exposure, odds ratios (OR) with corresponding 95% confidence intervals (CI) were calculated. Pearson's chi-square or Fisher's exact test were used to test for significance where appropriate. In addition, demographic variables including age and sex were assessed for statistical differences between dog-exposed and non-exposed groups using chi-square analyses. Kappa coefficients were used to determine the correlation between self-reported Lyme disease and ELISA seropositivity. Statistical analyzes were performed using SAS v9.4 (SAS Institute, Cary, NC), and GraphPad Prism v6 (GraphPad Software, Inc., La Jolla, CA). Statistical significance was defined as p-values of ≤0.05.
Results
Group demographics
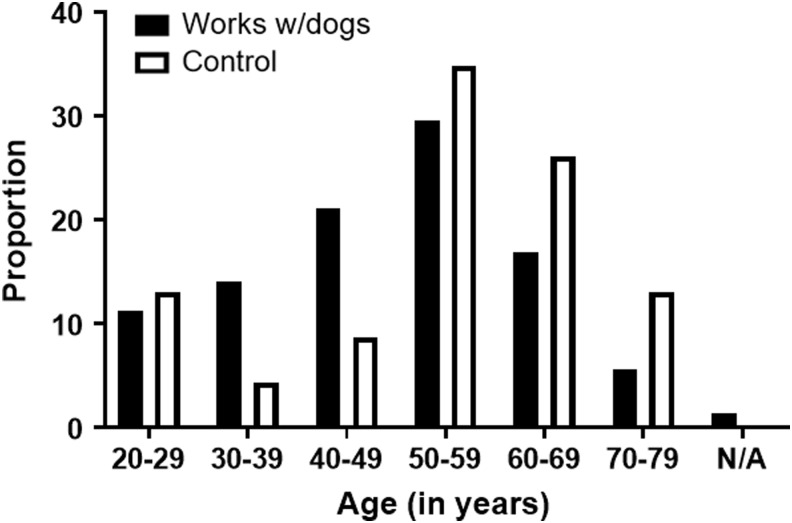
Ninety-four research participants completed a survey to assess animal and environmental exposures. Of those 94 research participants, >50% of unexposed and dog-exposed individuals were >50 years of age (52.1% of exposed, 73.9% of unexposed; Fig. 1). The majority of research participants in both groups were male (73.9% of unexposed and 57.8% of exposed). There was no statistically significant difference in age (chi-square, p-value = 0.48) or sex (chi-square, p-value = 0.17) between the two groups.
FIG. 1.
Age distribution of research participants separated by exposure dog-exposure status. Older ages predominate in the overall cohort. “Works with dogs” group represents those who work with hunting hounds. “No dogs” group was comprised of bird watchers or those who worked for the parks and recreation department with limited pet dog or no dog exposure (chi-square, p-value >0.49).
Geographic distribution of the dog-exposed cohort was distributed across the country, with a majority from the Midwest (25.4%) and Mid-Atlantic (19.7%) regions where Lyme disease is endemic or emerging (Table 1). The unexposed group was predominantly from emergent tick-borne disease areas of the Midwest. Further analysis was performed on a subset (38 dog exposed and 23 unexposed individuals) of the research participants who also provided a blood sample for serological testing for Lyme exposure.
Table 1.
Geographic Location and Sex Distribution of Research Participants
| Variable | Dog-exposed group | Environmental control group |
|---|---|---|
| Sex (male), % | 57.8 | 73.9 |
| Age, % | ||
| 20–30 | 11.3 | 13.0 |
| 30–40 | 14.1 | 4.4 |
| 40–50 | 21.1 | 8.7 |
| 50–60 | 29.6 | 34.8 |
| 60–70 | 16.9 | 26.1 |
| 70–80 | 5.6 | 13.0 |
| N/A | 1.4 | 0 |
| Location, % | ||
| Northeast | 2.8 | 0 |
| Mid-Atlantic | 19.7 | 0 |
| Southeast | 12.7 | 0 |
| Midwest | 25.4 | 100 |
| West | 1.4 | 0 |
| N/A | 38.0 | 0 |
Dog-exposed group: hunting dog caretakers; environmental control group: Audubon society members/State Parks employees.
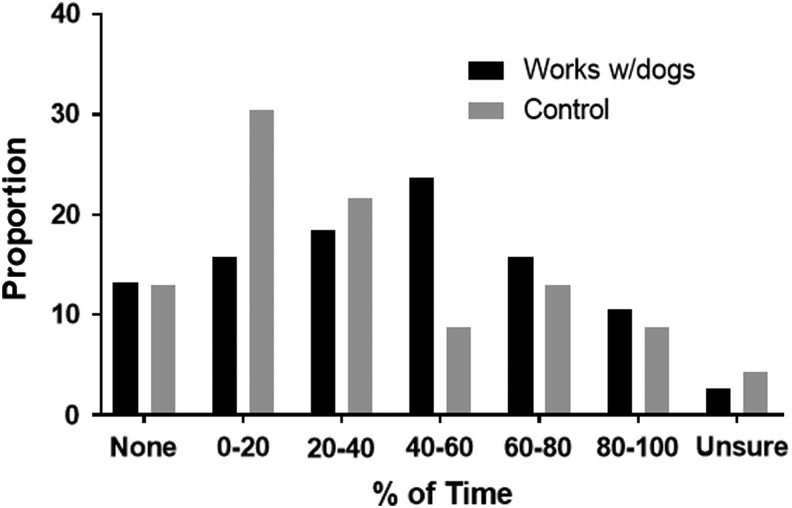
The majority of dog-exposed participants who provided a blood sample were enrolled at a Midwestern hound show. There were no statistically significant difference in age, sex, or geographic location between the dog-exposed and unexposed group participants who provided blood samples. Furthermore, both groups were found to have equal exposure to the outdoors and specifically had no statistically significant differences in time spent in brush or high grass (chi-square, p-value = 0.16; Fig. 2).
FIG. 2.
Distribution of percent of time spent in high brush or grass. Exposed and unexposed individuals spent a similar amount of time walking through brush or high grass. “Works with dogs” are those who are hunting hound caretakers; “no dogs” are those individuals who are bird watchers and parks and recreation employees (chi-squared, p-value >0.16).
Tick exposure significantly increased in dog-exposed group
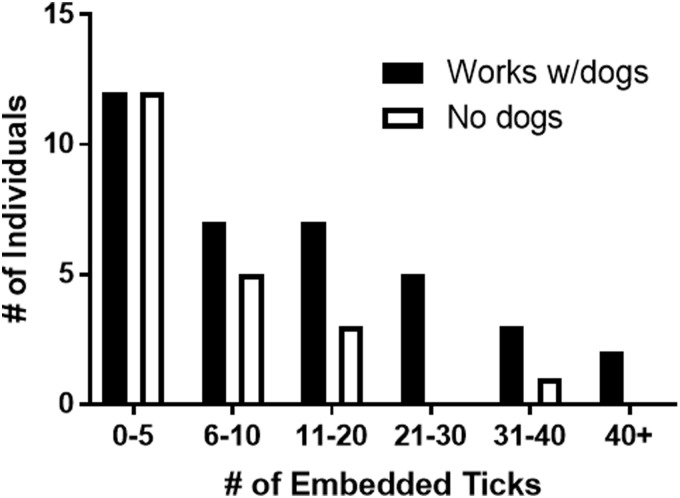
Due to contact with a large number of dogs who hunt through high grass and brush daily, it was hypothesized that individuals who worked with these hunting dogs would have higher exposures to ticks than people who were frequently in these environments but without dogs. Tick exposure was self-reported based on whether an individual had found a tick embedded in their skin within the past 3 years and if so how many (Fig. 3).
FIG. 3.
Tick exposure levels for dog-exposed and unexposed individuals. People who worked with many dogs were exposed to many ticks. “Works with dogs” are individuals who are hunting hound caretakers; “no dogs” are bird watchers and parks and recreation employees. Number of ticks determined as self-reported number of embedded ticks found in the past 3 years.
A total of 80.9% of individuals in the unexposed group reported either no tick exposures or exposure to between 0 and 10 ticks over the past 3 years. Individuals with dog exposure reported significantly higher exposure levels to ticks: 51.0% of individuals who reported having found an embedded tick on their body had found either 11–30 or >30 embedded ticks in the past 3 years. For regression analyses, low exposure was defined as <20 ticks embedded in the past 3 years, and high exposure was defined as >20. The odds of having a high exposure to ticks was 7.69 × greater among individuals who worked with hunting dogs compared to individuals in the environmental control group (OR = 7.69, 95% CI: 1.04–87.29, p-value = 0.0411; Table 2).
Table 2.
Tick Exposure Levels
| Group | High tick exposure (>20 ticks)a | Low tick exposure (≤20 ticks) |
|---|---|---|
| Dog-exposed (n = 38) | 10 | 26 |
| Control (n = 23) | 1 | 20 |
Individuals were asked whether they had found a tick embedded in their skin in the past 3 years and if so how many. For both groups, two individuals were not included in the analysis, as they answered “unsure” to the number of ticks they had found.
OR = 7.69, 95% CI: 1.04–87.29 (Fisher's exact test).
OR, odds ratio; CI, confidence interval.
Lyme seroprevalence and self-reported disease higher than U.S. average
It was hypothesized that individuals working with hounds could have higher Lyme disease seroprevalence because of higher tick exposure when compared to environmental control individuals. Perhaps unsurprisingly, given their equivalent high environmental exposure, there was high Borrelia seropositivity and self-reporting of Lyme disease diagnoses in both groups. A total of 8.5% of individuals who were exposed to dogs and 8.7% of individuals not exposed to dogs self-reported Lyme disease, whereas 23.7% of dog-exposed individuals and 17.4% of unexposed individuals were seropositive for B. burgdorferi. Of interest, this included individuals working for county parks in emergent areas of Southwestern Iowa where Ixodes spp. has not yet been reported (Oliver et al. 2017).
There was no statistically significant increase in B. burgdorferi seropositivity or self-reported Lyme disease in dog-exposed individuals compared to environmentally exposed controls (OR = 1.70, 95% CI: 0.51–5.44, p-value = 0.18; Table 3). The kappa coefficient, assessing the correlation between self-reported Lyme disease and seropositivity to B. burgdorferi in the dog-exposed group was −0.05 (95% CI: −0.143 to 0.042), suggesting a poor correlation between self-reporting and seropositivity. In the unexposed group, the kappa coefficient was 0.330 (95% CI: −0.249 to 0.909), suggesting that there was a fair but not good correlation between self-reporting and seropositivity in this group.
Table 3.
Assessment of Lyme Disease Prevalence in Cohort
| Lyme disease status | Dog exposed | Unexposed |
|---|---|---|
| Self-reported, % (n/total) | 8.5 (6/71) | 8.7 (2/23) |
| Seropositive, % (n/total) | 23.7 (9/38) | 17.4 (4/23) |
Self-reported Lyme disease was determined through survey completed by research participants. Individuals had the option to identify whether they had ever been diagnosed with a medical condition and if so what condition. Serological positivity for Lyme disease was determined by ELISA from serum obtained via blood spot. Thirty-eight individuals in the dog-exposed group and 23 individuals in the control group were tested via C6 ELISA for seropositivity to Borrelia burgdorferi. A total of 71 individuals in the dog-exposed group and 23 individuals completed the survey indicating recent diagnoses.
ELISA, enzyme-linked immunosorbent assay.
As self-reporting having been diagnosed with Lyme disease is a measure of clinical disease and serological testing a measure of exposure to a specific Borrelia antigen and creation of specific IgG/IgM antibodies, the kappa coefficients are as expected. The odds of seropositive and self-reported medical diagnosis of Lyme disease were 2.23 × greater in the study population due to environmental exposure, regardless of dog exposure, compared to Pennsylvania, the state in the United States with the highest rate of rate of probable and confirmed cases per 100,000 people in 2016 (OR = 2.23, 95% CI: 1.37–3.62, p-value = 0.0029; CDC 2018).
Conclusions
Previously, little was understood regarding occupational risks of repeated exposure to hunting dogs and potential increased transmission of zoonotic diseases. This study provides an important evaluation of how dog exposure significantly increased tick exposure in these individuals who work with hunting dogs. Individuals evaluated in this study take care of and train ≥50 hunting dogs for the main part of their working lives. Any ticks that come in contact with the dogs while the dogs are “walking out,” the daily exercise with hunt staff, or hunting with people on foot or on horseback, through high brush and grass, will also come in contact with the individuals handling the dogs when they are feeding, training, and providing other husbandry to these animals. Therefore, it is reasonable that individuals working with dogs would have an increased risk of tick exposure.
Individuals working with hunting hounds had a disproportionately increased exposure to Lyme disease, similar to other populations known to be at high risk of Lyme disease due to environmental exposures, such as bird watchers and individuals working for local departments of parks and recreation compared to the general public. The data from this study indicated an increase in seroprevalence or self-reported medical diagnosis of Lyme disease compared to the number of cases reported previously to the Iowa Department of Public Health across emergent Southwestern areas of Iowa in an at-risk population.
While recall bias regarding self-reported Lyme disease was inherent to this study due to the baseline understanding and concern regarding tick-borne diseases in both groups, this effect was likely non-differential, thus underlining the increase in seroprevalence or self-reported medical diagnosis of disease in Southwestern Iowa. Due to the small sample size within this study, these results warrant additional exploration into the seroprevalence of Lyme disease within Southwestern Iowa.
Dogs are three-life-stage (larva, nymph, and adult) hosts of Rhipicephalus sanguineus, the brown dog tick, or Dermacentor variabilis, the American dog tick, and only hosts for adult Ixodes spp., the vectors for B. burgdorferi (Rynkiewicz and Clay 2014, Burroughs et al. 2016). Perhaps this higher likelihood of dogs harboring dog-based ticks compared to Ixodes spp. is why seropositivity to B. burgdorferi was similar in dog caretakers and environmental control groups, despite higher, but perhaps not more Ixodes, tick exposure in dog caretakers. Dog-based tick exposure may instead increase the risk of Rickettsia rickettsii or Francisella tularensis infection, both transmitted by D. variabilis (Ammerman et al. 2004, Mani et al. 2016). These diseases would be logical targets for future study within these cohorts.
The findings of this study were limited by a small sample size, concerns of recall bias, likelihood that members of both the control and dog-exposed groups had additional tick exposure due to travel and less endemic geographical locations of study participants. Despite this, high rates of B. burgdorferi seropositivity in both groups suggest the need for better education of all participating groups regarding occupational risks of tick-borne diseases. Furthermore, future studies are warranted as habitat alterations and other factors increase the territory of Ixodes spp. and other ticks and their associated diseases.
Acknowledgment
This work was funded by a new faculty award from University of Iowa College of Public Health to CAP.
Author Disclosure Statement
No competing financial interests exist.
References
- Adams DA, Thomas KR, Jajosky RA, Foster L, et al. Summary of notifiable infectious diseases and conditions—United States, 2014. MMWR Morb Mortal Wkly Rep 2016; 63:1–152 [DOI] [PubMed] [Google Scholar]
- Ammerman NC, Swanson KI, Anderson JM, Schwartz TR, et al. Spotted-fever group Rickettsia in Dermacentor variabilis, Maryland. Emerg Infect Dis 2004; 10:1478–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beetz A, Uvnas-Moberg K, Julius H, Kotrschal K. Psychosocial and psychophysiological effects of human–animal interactions: The possible role of oxytocin. Front Psychol 2012; 3:234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blancou J, Chomel BB, Belotto A, Meslin FX. Emerging or re-emerging bacterial zoonoses: Factors of emergence, surveillance and control. Vet Res 2005; 36:507–522 [DOI] [PubMed] [Google Scholar]
- Bouchard C, Leighton PA, Beauchamp G, Nguon S, et al. Harvested white-tailed deer as sentinel hosts for early establishing Ixodes scapularis populations and risk from vector-borne zoonoses in southeastern Canada. J Med Entomol 2013; 50:384–393 [DOI] [PubMed] [Google Scholar]
- Bowman D, Little SE, Lorentzen L, Shields J, et al. Prevalence and geographic distribution of Dirofilaria immitis, Borrelia burgdorferi, Ehrlichia canis, and Anaplasma phagocytophilum in dogs in the United States: Results of a national clinic-based serologic survey. Vet Parasitol 2009; 160:138–148 [DOI] [PubMed] [Google Scholar]
- Burroughs JE, Thomasson JA, Marsella R, Greiner EC, et al. Ticks associated with domestic dogs and cats in Florida, USA. Exp Appl Acarol 2016; 69:87–95 [DOI] [PubMed] [Google Scholar]
- CDC. Reported cases of Lyme disease by state or locality, 2006–2016. 2018. https://www.cdc.gov/lyme/stats/tables.html Accessed July9, 2018
- Day MJ. The CALLISTO project: A summary. J Comp Pathol 2016; 155:S1–S7 [DOI] [PubMed] [Google Scholar]
- Hamer SA, Tsao JI, Walker ED, Mansfield LS, et al. Use of tick surveys and serosurveys to evaluate pet dogs as a sentinel species for emerging Lyme disease. Am J Vet Res 2009; 70:49–56 [DOI] [PubMed] [Google Scholar]
- Jones EH, Hinckley AF, Hook SA, Meek JI, et al. Pet ownership increases human risk of encountering ticks. Zoonoses Public Health 2018; 65:74–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick HJ, LaBonte AM, Stafford KC. The relationship between deer density, tick abundance, and human cases of Lyme disease in a residential community. J Med Entomol 2014; 51:777–784 [DOI] [PubMed] [Google Scholar]
- Knight S, Edwards V. In the company of wolves: The physical, social, and psychological benefits of dog ownership. J Aging Health 2008; 20:437–455 [DOI] [PubMed] [Google Scholar]
- Kuehn BM. CDC estimates 300,000 US cases of Lyme disease annually. JAMA 2013; 310:1110 [DOI] [PubMed] [Google Scholar]
- Mani RJ, Morton RJ, Clinkenbeard KD. Ecology of tularemia in Central US endemic region. Curr Trop Med Rep 2016; 3:75–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead PS. Epidemiology of Lyme disease. Infect Dis Clin North Am 2015; 29:187–210 [DOI] [PubMed] [Google Scholar]
- Oliver JD, Bennett SW, Beati L, Bartholomay LC. Range expansion and increasing Borrelia burgdorferi infection of the tick Ixodes scapularis (Acari: Ixodidae) in Iowa, 1990–2013. J Med Entomol 2017; 54:1727–1734 [DOI] [PubMed] [Google Scholar]
- Orritt R. Dog ownership has unknown risks but known health benefits: We need evidence based policy. BMJ 2014; 349:g4081 [DOI] [PubMed] [Google Scholar]
- Parslow RA, Jorm AF, Christensen H, Rodgers B, et al. Pet ownership and health in older adults: Findings from a survey of 2,551 community-based Australians aged 60–64. Gerontology 2005; 51:40–47 [DOI] [PubMed] [Google Scholar]
- Raina P, Waltner-Toews D, Bonnett B, Woodward C, et al. Influence of companion animals on the physical and psychological health of older people: An analysis of a one-year longitudinal study. J Am Geriatr Soc 1999; 47:323–329 [DOI] [PubMed] [Google Scholar]
- Robinson RA, Pugh RN. Dogs, zoonoses and immunosuppression. J R Soc Promot Health 2002; 122:95–98 [DOI] [PubMed] [Google Scholar]
- Rynkiewicz EC, Clay K. Tick community composition in Midwestern US habitats in relation to sampling method and environmental conditions. Exp Appl Acarol 2014; 64:109–119 [DOI] [PubMed] [Google Scholar]
- Thorpe RJ, Jr, Kreisle RA, Glickman LT, Simonsick EM, et al. Physical activity and pet ownership in year 3 of the health ABC study. J Aging Phys Act 2006; 14:154–168 [DOI] [PubMed] [Google Scholar]
- Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: Clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43:1089–1134 [DOI] [PubMed] [Google Scholar]