Abstract
As the incidence of cardiovascular disease continues to climb worldwide, there is a corresponding increase in demand for surgical interventions involving vascular grafts. The current gold standard for vascular grafts is autologous vessels, an option often excluded due to disease circumstances. As a result, many patients must resort to prosthetic options. While widely available, prosthetic grafts have been demonstrated to have inferior patency rates compared with autologous grafts due to inflammation and thrombosis. In an attempt to overcome these limitations, many different materials for constructing vascular grafts, from modified synthetic nondegradable polymers to biodegradable polymers, have been explored, many of which have entered the translational stage of research. This article reviews these materials in the context of large animal models, providing an outlook on the preclinical potential of novel biomaterials as well as the future direction of vascular graft research.
Keywords: : vascular grafts, tissue-engineered vascular grafts, large animal models, biomaterials
Introduction
The global burden of cardiovascular disease is on the rise, currently affecting one in three American adults and projected to affect one in two by 2030.1 Many of these diseases require surgical intervention with grafts to correct perfusion insufficiencies and anatomic defects, including coronary and peripheral artery disease, end-stage renal disease (ESRD), and congenital heart defects. Approximately 400,000 bypasses, 446,000 AV fistulas, and over 6000 repairs for congenital heart defects occur annually, highlighting the exceeding demand for limited autologous graft options.1
Existing prosthetic options are limited to expanded polytetrafluoroethylene (ePTFE), polyethylene terephthalate (Dacron®), and polyurethane.2 While prosthetic grafts are satisfactory in large-diameter applications, they fail to demonstrate long-term patency comparable to that of autologous grafts in small-diameter (<6 mm) settings due to their susceptibility to inflammation, thrombosis, intimal hyperplasia, and consequent compliance mismatch with the host vessels.3–9
The ultimate solution to combat the inflammation and thrombosis in existing prosthetic grafts is to create vascular prostheses that closely mimic the physiological responses and mechanical properties of autologous vessels without eliciting an immunogenic response. Structural components of blood vessels such as collagen and elastin promote compliance matching to host vessels, while the endothelial lining provides resistance against inflammation and thrombosis.10 Accordingly, the focus in vascular engineering has been on the facilitation of graft remodeling and reconstitution of native vessel wall anatomy.11 Other important criteria to consider in the search for biomaterials for vascular grafts are ease of surgical anastomoses, resistance to infection, and off-the-shelf availability.10
Biomaterials, currently being investigated for use in vascular grafts, span a broad range of chemical and mechanical properties, but can be broadly categorized as nondegradable and biodegradable. Nondegradable biomaterials are composed of synthetic polymers of ePTFE and Dacron while biodegradable materials are composed of natural and synthetic polymers as well as decellularized tissue scaffolds. An important conceptual subcategory of vascular grafts that transcends the boundaries of biodegradability is tissue-engineered vascular grafts (TEVGs). Fundamentally, TEVGs require cells and a tubular scaffold. However, the attractiveness of TEVGs for clinical application lies in its projected ability to grow, remodel, and repair in vivo, offering the ideal solution to the thrombogenic conundrum.12
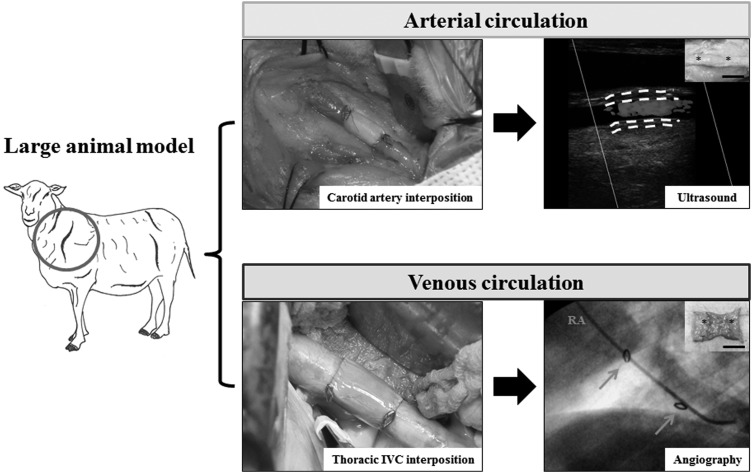
Before human application, the clinical potential of vascular grafts must be assessed in vivo in animal models (Fig. 1). Consideration for the selection of animal models include availability, cost, immunogenicity, implantability, reproducibility, and most importantly, similarity to normal human physiology.13
FIG. 1.
Vascular graft testing in large animal models. Grafts are implanted into large animal models in either the arterial or venous circulation at the location indicated by the circle. Representative procedures at the time of implantation and follow-up at 6 months' postimplantation are shown. White dotted lines indicate the luminal and adventitial surfaces of the graft, yellow arrows indicate clip markings, and asterisks demarcate the suture line. IVC, inferior vena cava; RA, right atrium. Scale bar represents 1.0 cm.
While small animal models such as mice and rabbits are widely available and relatively inexpensive to maintain, they lack similarity to human physiology with regard to vascular anatomy and hemodynamics and thus are only useful for dissecting the mechanical and molecular mechanisms of graft.14 In contrast, large animal models, including dogs, pigs, and sheep, are limited in availability and costly to maintain, but are generally better able to mimic human physiology, although some variability is present between species.
For example, ovine models show the most similarity to humans in terms of the inflammatory response and thrombogenicity, while canine models are hyperthrombogenic, a property exploited for more rigorous vascular graft testing, but do not demonstrate spontaneous graft endothelialization.14 On the contrary, porcine models exhibit endothelialization of the graft but consequentially are prone to intimal hyperplasia.14,15
The ideal animal model to simulate the normal human microenvironment would combine the thrombogenicity of the ovine model and the spontaneous endothelialization of the porcine model. In contrast, a combination of the hyperthrombogenicity of the canine model and the exaggerated endothelial healing response of the porcine model may create a more accurate depiction of diseased conditions. While it is apparent that there is no perfect animal model for human physiology and pathophysiology, testing grafts in large animals is essential for evaluation of long-term patency, graft remodeling, and ultimately, clinical translation.
Modified Synthetic Nondegradable Polymers: ePTFE and Dacron
Synthetic nondegradable polymers have been widely applied as vascular grafts due to the ease with which their mechanical properties can be modified. Two of the most common polymers used are ePTFE and Dacron (polyester). ePTFE is frequently used in femoropopliteal bypasses in the absence of available autologous vessels, whereas Dacron is more often used for large caliber aortic replacements. In canine carotid and femoral artery interposition models, Dacron grafts had increased early-platelet deposition compared to autologous vessel implants and exhibited 54% patency at 1 month.16 Unlike the other biomaterials discussed in this review, ePTFE and Dacron vascular grafts have already been in clinical application for over 50 years with extensive literature documenting clinical outcomes.17
As a replacement for large caliber (>8 mm) and medium caliber (6–8 mm) arteries, the thrombogenicity of prosthetic grafts can be largely offset by anticoagulation therapy, demonstrating 5-year patency rates rivaling that of autologous grafts at 90%.8,9 However, even with rigorous anticoagulation and antiplatelet therapy, the 5-year primary patency rates for small-diameter prosthetic grafts in patients remain markedly inferior to that of autologous options in lower extremity bypasses: 44–62% for ePTFE and Dacron compared with 71–75% for autologous bypasses.3 The poor patency rates of these polymers have prompted the development of new methods that primarily focus on promoting endothelialization. Two general methods of enhancing endothelialization have been tested in large animal models: cell seeding and surface modifications (Table 1).
Table 1.
Preclinical Large Animal Studies Using Synthetic Nonbiodegradable Polymer-Derived Vascular Grafts
| Authors and year | Animal species | n | Graft type | Inner diameter of graft (mm) | Implantation model | Follow-up time (days) | Patency rate (%) |
|---|---|---|---|---|---|---|---|
| Clagett et al. (1984)19 | Dog | 16 | EC-seeded Dacron | 10 | Thoracoabdominal aorta bypass | 84 | N/A |
| Bhattacharya et al. (2000)18 | Dog | 10 | BMC-seeded ePTFE and PET composite | 8 | Descending thoracic aorta interposition | 28 | 100 |
| Fields et al. (2002)20 | Dog | 10 | EC-seeded ePTFE | 4 | Femoral artery interposition | 42 | 100 |
| Li et al. (2005)24 | Sheep | 6 | P15-treated ePTFE | 8 | Femoral artery/vein and carotid artery/jugular vein arteriovenous shunt | 28 | 100 |
| Rotmans et al. (2005)23 | Pig | 11 | CD34-coated ePTFE | 5 | Carotid artery/internal jugular vein arteriovenous shunt | 28 | 73 |
BMC, bone marrow cell; EC, endothelial cell; ePTFE, expanded polytetrafluoroethylene; PET, polyethylene terephthalate; N/A, not available.
TEVGs produced by electrostatic seeding of ePTFE and polyester grafts with host-derived endothelial cells (ECs) or EC precursors (CD34+ bone marrow cells) demonstrated reduced thrombosis, attributed to the production of prostacyclin by seeded EC, and increased surface endothelialization with increased microvessels in the neointima and graft wall in canine femoral artery and thoracoabdominal aorta interposition models.18–20 The observed success of EC-seeded prosthetic grafts in canine models have also been corroborated by clinical trials, which showed that seeding of ePTFE femoropopliteal bypasses with EC increased the 1-year patency rate by 1.5 times and the 5-year patency rate by 3.5 times compared with unseeded controls.21,22
Surface modification of prosthetic grafts essentially applies the same principle as cell seeding, except the ECs that are autoseeded after implantation. In one study using a porcine arteriovenous graft model where ePTFE grafts were coated with human anti-CD34 monoclonal antibody, 85% of the graft surface was covered with ECs after 28 days compared with the 32% covered in uncoated grafts.23 Similar results were also observed in a study using P15 (a Type-I collagen cell adhesion domain peptide)-treated ePTFE vascular grafts in ovine arteriovenous graft models.24
In terms of improving the patency rates of synthetic nondegradable prosthetic grafts such as ePTFE and Dacron, both EC-seeding and surface modification approaches have demonstrated success with regard to enhancing surface endothelialization and improving graft patency in large animal studies. However, these studies were much less effective in promoting the adhesion and proliferation of smooth muscle cells, an important characteristic of arterial walls, suggesting the possibility of targeting cell types in addition to ECs. Yet, because these prosthetic grafts are nondegradable, it may be less crucial to fully reconstitute the arterial anatomy than it is to simply extend the patency rate to a level comparable to that of autologous grafts.
According to the clinical trials published by Meinhart et al., the 5-year patency of EC-seeded ePTFE grafts was 73.8%, a figure that matched the reported 5-year patency of autologous saphenous vein grafts (72%).3,21 While more translational studies moving from large animal models to humans are needed to establish the relative efficacy of cell seeding and surface modification of nondegradable prosthetic grafts, current methods have shown considerable promise in leveling out the playing field between autologous grafts and prosthetic grafts.
Biodegradable Scaffolds
Decellularized tissue scaffolds
The rationale behind using decellularized vessels are reduced antigenicity and preservation of native three-dimensional tissue architecture, which theoretically would facilitate host cell migration through the provision of physiological adhesion molecules.25 Decellularized small intestinal submucosa (dSIS) has additionally been shown to have antibacterial properties.26 Since the approach of using dSIS to construct vascular grafts emerged in the late 1980s, many preclinical implantation experiments have been conducted in large animal models (Table 2).
Table 2.
Preclinical Large Animal Studies Using Decellularized Tissue Scaffold-Derived Vascular Grafts
| Authors and year | Animal species | n | Graft type | Inner diameter of graft (mm) | Implantation model | Follow-up time (days) | Patency rate (%) |
|---|---|---|---|---|---|---|---|
| Badylak et al. (1989)27 | Dog | 12 | Autogenous dSIS | 10 | Infrarenal aorta interposition | 364 | 75 |
| Lantz et al. (1990)28 | Dog | 18 | Autogenous dSIS | 4.3 | Carotid and femoral artery interposition | 336 | 75 |
| Hiles et al. (1995)29 | Dog | 8 | Xenogenic porcine dSIS | 10 | Infrarenal aorta interposition | 60 | 100 |
| Sandusky et al. (1995)30 | Dog | 8 | Xenogenic porcine dSIS | 3.5–5.0 | Carotid artery interposition | 180 | 87.5 |
| Robotin-Johnson et al. (1998)34 | Pig | 11 | Autogenous dSIS | 36.8 | SVC interposition | 90 | 100 |
| Nemcova et al. (2001)31 | Dog | 5 | Xenogenic porcine dSIS | 4 | Femoral artery interposition | 63 | 89 |
| Roeder et al. (2001)32 | Dog | 7 | dSIS | 5–8 | Carotid artery interposition | 63 | 100 |
| Kaushal et al. (2001)41 | Sheep | 11 | EPC-seeded xenogenic porcine decellularized iliac vessels | 4 | Carotid artery interposition | 130 | 100 |
| Conklin et al. (2002)36 | Dog | 2 | Heparin-treated xenogenic porcine decellularized carotid artery | <5 | Carotid artery bypass | 67 | 100 |
| Tamura et al. (2003)39 | Dog | 1 | Heparin-treated xenogenic porcine decellularized carotid artery | — | Abdominal aorta interposition | 126 | N/A |
| Cho et al. (2005)77 | Dog | 12 | BMC-seeded canine decellularized carotid artery | 3 | Carotid artery interposition | 56 | 100 |
| Ketchedjian et al. (2005)37 | Sheep | 16 | Decellularized sheep pulmonary trunk | — | Pulmonary artery or aortic artery patches | 140 | N/A |
| Cho et al. (2006)40 | Dog | 6 | BMC-seeded canine decellularized abdominal aorta | 7 | Abdominal aorta interposition | 56 | 100 |
| Hinds et al. (2006)33 | Pig | 6 | Elastin-strengthened xenogenic porcine dSIS | 4.3 | Carotid artery interposition | 0.25 | 33 |
| Tillman et al. (2012)38 | Sheep | 11 | EC-seeded xenogenic porcine decellularized carotid artery | 5 | Common carotid artery/external jugular vein arteriovenous shunt | 180 | 0 |
| Syedain et al. (2014)42 | Sheep | 9 | Decellularized graft derived from fibrin gel and ovine dermal fibroblasts | 4 | Carotid artery interpostion | 168 | 100 |
| Koobatian et al. (2016)35 | Sheep | 12 | Heparin and VEGF-treated xenogenic porcine dSIS | 5 | Carotid artery interposition | 90 | 92 |
dSIS, decellularized small intestinal submucosa; EPC, endothelial progenitor cell; SVC, superior vena cava; VEGF, vascular endothelial growth factor.
Badylak et al. was the first to evaluate dSIS in large animal models, specifically as large-diameter interposition grafts in canine aortas.27 These first grafts had a higher elastic modulus and lower tensile strength compared to endogenous vessels, and while they did not exhibit endothelialization, the relatively high patency offered a potential solution to the plaguing problem of thrombosis. Development of small-diameter dSIS saw progressive success in recapitulating the properties of native vessels.
Initially, small-diameter dSIS in canine models exhibited no intimal formation and had low short-term graft patency due to graft occlusion, dilation, and rupture.28 Subsequent canine studies implemented grafts with increased tensile strength, although compliance was still a fraction that of a native vessel; dilation and rupture were rare.29 The grafts experienced complete endothelialization with smooth muscle cells in the neointima and patency was increased to 87.5–100% at 9–12 weeks postimplantation.30,31 Mechanical remodeling of the dSIS grafts was further demonstrated by Roeder et al., who compared the mechanical properties of the grafts preimplantation and postexplantation and observed increased compliance over time, suggesting that the grafts were remodeling to approach the mechanical parameters of autologous vessels.32
Further breakthroughs in dSIS vascular grafts involved surface modifications in porcine models. Elastin is a natural polymer with low tensile strength, limiting its use as an arterial conduit. However, the tensile strength of elastin is boosted 10-fold upon strengthening with dSIS and the enhanced graft showed increased patency in carotid interposition models compared with ePTFE grafts.33 Another surface modification that has been explored is heparin. Heparin-bound dSIS implanted into SVC of piglets was 100% patent at 90 days, although two out of nine grafts did show early signs of stricture and aneurysmal change.34 Importantly, the implanted grafts grew with the animals, increasing in circumference and length with the native vessel without jeopardizing mechanical strength or interfering with the remodeling process.
The approach of treating dSIS with heparin was applied further by Koobatian et al., who coated dSIS with heparin-bound vascular endothelial growth factor to prevent thrombosis and promote endothelialization.35 Within 3 months, complete endothelialization had occurred with the endothelium aligned in the direction of flow with smooth muscle cells organized circumferentially in the medial layer; the smooth muscle cells also exhibited vascular contractility in response to vasoconstriction agonists.
Small-diameter decellularized vascular grafts have also been derived from the carotid artery, abdominal aorta, pulmonary trunk, and iliac vessels, although literature on preclinical evaluation in large animal models is much less robust than for dSIS.10,14 In contrast to dSIS, decellularized carotid arteries exhibit compliance similar to that of native vessels.33
In terms of large animal studies, decellularized carotid artery grafts have mainly been investigated in canine models and relied on cell seeding (TEVGs) and surface modification to overcome the challenges of prosthetic grafts. TEVGs constructed from decellularized carotid arteries seeded with bone marrow cells increased patency to 8 weeks from the 2 weeks for unseeded grafts.37 However, when EC-seeded TEVGs were implanted in sheep, all grafts developed venous anastomotic stenosis by 4 months as commonly seen in clinical arteriovenous graft failure.38 Heparinization of decellularized carotid arteries seems to have found considerable success as testing in canine models revealed full patency at four and half months postimplantation.39
Taking a different approach from direct surface modification, Cho et al. administered granulocyte colony-stimulating factor subcutaneously postimplantation and saw enhanced endothelialization with reduced intimal hyperplasia.40 Similar successes were observed for decellularized pulmonary trunks implanted as patches into the pulmonary aorta of sheep.37 In addition to exhibiting full patency at 130 days postimplantation, endothelial progenitor cell-seeded decellularized iliac vessels were also shown to exhibit contractile activity and nitric oxide-mediated vasodilation akin to endogenous vessels.41
A remarkably different approach has been undertaken, in which decellularized grafts are synthesized in vitro using dermal fibroblasts and a fibrin gel mold.42 Grafts were implanted into ovine femoral arteries and found to be 100% patent at 24 weeks with extensive recellularization and with compliance and mechanical properties comparable to that of native arteries. Importantly, this approach produced grafts that have off-the-shelf availability, as the grafts were synthesized 5 weeks before implantation and could be stored stably until implantation.
The use of decellularized biological scaffolds has been widely explored in vascular graft development due to its inherent physiologic properties, conferring the ability to adapt to and grow with the host, overcoming a major pitfall of synthetic nondegradable polymer grafts. In addition, vessels used for subsequent decellularization and implantation can be harvested autologously, reducing the reactive inflammatory response, effectively decreasing the risk of thrombosis and increasing patency.
While the use of autologous vessels to combat thrombosis is a sound conceptual idea, the practicality of this method falls short when considering that goal of vascular graft research is to find alternatives to overcome the supply limitation of autologous vessels in cases of systemic vascular disease. Therefore, the focus has shifted away from using autologous implantation toward xenogenic implantation, using surface modifications and cell-seeding (TEVGs). While preclinical experiments using decellularized scaffolds have demonstrated the ability to withstand arterial flow as well as the potential to reconstitute native vessel anatomy, they must be able to overcome the limited off-the-shelf availability of using biological tissues before becoming a possibility in clinical practice.
Natural polymers
Fibrin is a natural protein that has been explored as a biomaterial for vascular graft, particularly TEVG, synthesis because it promotes cell adhesion, has high biocompatibility, and can be easily isolated from host blood.43 However, its lack of stability often limits its application.44 Two general approaches have emerged in response to this limitation, enabling the application of fibrin in translational vascular graft research: fibrin gels and fibrin compaction.
ECs, smooth muscle cells, and bone marrow-derived smooth muscle progenitor cells embedded in fibrin gel tubes show physiological contractility in response to vasoreactive agonists and displayed complete endothelialization with circumferentially aligned smooth muscle cells in the media layer in as little as 5 weeks postimplantation into sheep.45,46 The second approach of fibrin compaction, utilized in the sheep model, while demonstrating recapitulation of native artery wall anatomy, saw patency rates that fell noticeably short of autologous vessels.43
Hyaluronan-based scaffolds (HYAFF-11) were developed to target the insufficiency of elastin biosynthesis in small-diameter vascular grafts in many large animal studies. While graft patency was only 70% at 5 months due to intimal hyperplasia and thrombosis, the patent grafts showed complete degradation and replacement of the scaffold with a neoartery with laminar circumferential arrangement of smooth muscle and elastin fibers.47
Few studies have evaluated the preclinical potential of natural polymer-derived vascular grafts in large animals, with a large spread of synthetic method as well as patency results (Table 3). The deterrence in the use of natural polymer-derived vascular grafts is largely attributed to the difficulty in balancing graft stability and graft degradation. While the ease of degradation facilitates the replacement of the implanted graft with neoartery, the simultaneous graft instability jeopardizes the integrity of the graft during the initial remodeling process. However, natural polymers have shown substantial promise in smoothing out the transition between graft degradation and host cell recruitment, leading to a remodeled vessel bearing remarkable resemblance to native arteries.
Table 3.
Preclinical Large Animal Studies Using Natural Polymer-Derived Vascular Grafts
| Authors and year | Animal species | n | Graft type | Inner diameter of graft (mm) | Implantation model | Follow-up time (days) | Patency rate (%) |
|---|---|---|---|---|---|---|---|
| Swartz et al. (2005)45 | Sheep | 4 | SMC-embedded fibrin gel | 4 | Jugular vein interposition | 105 | 100 |
| Liu et al. (2007)46 | Sheep | 3 | BM-SMPC-embedded fibrin gel | 4 | Jugular vein interposition | 35 | 100 |
| Zavan et al. (2008)47 | Pig | 10 | Hyaluronan-based scaffold (HYAFF-11) | 4 | Carotid artery interposition | 150 | 70 |
| Aper et al. (2016)43 | Sheep | 6 | EC and SMC-seeded compacted fibrin | 5.3–6.0 | Carotid artery interposition | 180 | 50 |
BM-SMPC, bone marrow-derived smooth muscle progenitor cell; SMC, smooth muscle cell.
Biodegradable synthetic polymers
Scaffolds made from biodegradable polymers have been widely explored as an approach to recapitulate autologous vessel anatomy and function in large animal trials (Table 4). Theoretically, as the scaffolds degrade, the seeded cells and the host's cells will endothelialize the graft and secrete extracellular matrix (ECM), allowing for the seamless transition from a synthetic to endogenous vessel. Widely used materials include polyglycolic acid (PGA), polylactic acid (PLA), and poly ɛ-caprolactone (PCL). Polymers or combinations of polymers are used to customize mechanical properties and degradation speed.48
Table 4.
Preclinical Large Animal Studies Using Biodegradable Synthetic Polymer-Derived Vascular Grafts
| Authors and year | Animal species | n | Graft type | Inner diameter of graft (mm) | Implantation model | Follow-up time (days) | Patency rate (%) |
|---|---|---|---|---|---|---|---|
| Shinoka et al. (1998)49 | Sheep | 7 | PGA/PLGA | 15 | Pulmonary artery interposition | 168 | 100 |
| Niklason et al. (1999)51 | Pig | 4 | PGA | <6 | Right saphenous artery interposition | 24 | 100 |
| Shum-Tim et al. (1999)66 | Sheep | 11 | PGA/PHA | 7 | Abdominal aorta interposition | 150 | 100 |
| Watanabe et al. (2001)60 | Dog | 4 | Femoral vein cell-seeded PGA/PLCL | 10 | IVC interposition | 180 | 100 |
| Izhar et al. (2001)75 | Dog | 8 | PELA | 6 | Carotid artery interposition | 90 | 100 |
| Matsumura et al. (2003)57 | Dog | 16 | BMC-seeded PLCL | 8 | IVC interposition | 56 | 100 |
| Iwai et al. (2004)56 | Dog | 20 | PLGA with collagen microsponge | 15 | Pulmonary artery patch | 180 | N/A |
| Hibino et al. (2005)59 | Dog | 13 | Venous cell or bone marrow-seeded or unseeded PGA/PLCL | 8 | IVC interposition | 28 | 100 for seeded |
| 40% for unseeded | |||||||
| Hoerstrup et al. (2006)67 | Sheep | 14 | PGA/PHA | 18 | Pulmonary artery interposition | 700 | 100 |
| Matsumura et al. (2006)63 | Dog | 34 | BMC-seeded PGA/PLCL | 8 | IVC interposition | 180 | N/A |
| Roh et al. (2007)61 | Sheep | 8 | BMVC-seeded PGA/PLCL | 13 | IVC interposition | 30 | 100 |
| Brennan et al. (2008)50 | Sheep | 8 | PGA/PLCL | 13 | IVC interposition | 180 | 100 |
| Torikai et al. (2008)54 | Pig | 15 | PGA/PLA | 10 | Thoracic aorta interposition | 360 | 100 |
| Yokota et al. (2008)55 | Dog | 16 | PGA/PLA | 4 | Carotid artery and coronary artery bypass | 360 | 100 |
| Zhang et al. (2008)64 | Dog | 16 | BMSC-seeded PGA/PLCL | 6 | Abdominal aorta interpostion | 90 | 100 |
| Dahl et al. (2011)52 | Dog | 8 | PGA | 3–4 | Carotid artery and coronary artery bypass | 360 | 83 |
| Cummings et al. (2012)69 | Sheep | 14 | Myofibroblast and EC-seeded PGA/PHA | — | Pulmonary artery interposition | 560 | 100 |
| Kelm et al. (2012)68 | Sheep | 14 | PGA/PHA | 18 | Pulmonary artery interposition | 1680 | N/A |
| Matsumura et al. (2013)62 | Dog | 7 | PGA/PLCL | 8 | Pulmonary artery interposition | 360 | 100 |
| Wang et al. (2013)65 | Dog | 20 | Heparin-treated PLA/PLCL and polyurethane-collagen type I composite | <6 | Femoral artery interposition | 168 | 88.9 |
| Lu et al. (2013)76 | Dog | — | Heparin-treated PCL | 2.5 | |||
| Mrowczynski et al. (2014)53 | Pig | 11 | PCL | 4 | Carotid artery interposition | 30 | 78 |
| Zhou et al. (2014)73 | Dog | 6 | EC-seeded PCL/CS | 3 | Carotid artery interposition | 90 | 83 |
| Fukunishi et al. (2016)58 | Sheep | 6 | PCL/CS | 5 | Carotid artery interposition | 180 | 67 |
| Fukunishi et al. (2016)58 | Sheep | 6 | PGA/PLCL | 12 | IVC interposition | 180 | 100 |
BMSC, bone marrow stromal cell; BMVC, bone marrow-derived vascular cells; CS, chitosan; IVC, inferior vena cava; PCL, poly ɛ-caprolactone; PELA, poly(ethylene glycol); PGA, polyglycolic acid; PHA, polyhydroxyalkanoate; PLA, polylactic acid; PLCL, poly (L-lactide-co-ɛ-caprolactone); PLGA, poly(lactic-co-glycolic acid).
The feasibility of using biopolymers was first demonstrated by Shinoka et al., who implanted a TEVG, consisting of a PGA tubular scaffold seeded with autologous ECs and myofibroblasts, as an interposition graft into the pulmonary artery of lambs.49 The PGA scaffolds were absent upon histological examination by week 11 and the remodeled vascular grafts showed endothelialization as well as ECM production. Similar results were observed in PGA scaffolds implanted in the venous microenvironment, specifically as inferior vena cava (IVC) interposition grafts in lambs.50 These PGA grafts remained patent for up to 24 days in porcine saphenous artery grafts and up to 1 year in canine coronary bypass models, showing short- and long-term patency.51,52
PCL nanofiber grafts have not been as extensively tested in large animal models as PGA grafts; one short-term study revealed that PCL grafts had improved patency and endothelialization compared with ePTFE controls and also showed significant macrophage infiltration and thrombosis formation.53
PGA has a short degradation period, which confers an advantage in terms of remodeling, but is also a pitfall as the scaffold may degrade before the remodeled graft has attained adequate mechanical strength. Therefore, PGA has been explored as copolymers with PLA and PCL, which have longer degradation periods. While PGA and PLA have similar tensile strength and elasticity, PCL is notably different in that it has much less tensile strength but increased elasticity.48
A few large animal studies have looked at the translational potential of PGA-PLA, or poly (lactic-co-glycolic acid) (PLGA), copolymer grafts, which consists of a PGA core surrounded by woven PLA for mechanical reinforcement. The PGA core was completely degraded by 2 months postimplantation while the PLA sheath remained unabsorbed for the duration of the study.54,55
Torikai et al. implanted relatively large-diameter grafts (10 mm) into the descending thoracic aorta of pigs and observed complete endothelialization with smooth muscle cells in the subendothelium, which had good collagen content but poor elastin deposition.54 In contrast, a patch graft in canine pulmonary arteries revealed elastin content comparable to native vessels at 6 months postimplant.56 These remodeled vessels also demonstrated physiologic vasomotor responsiveness to norepinephrine, showing functional recapitulation on top of gross anatomic mimicry.
Small-diameter (4 mm) PGA-PLA grafts have been tested as well as carotid bypass grafts in canine models.55 As the previous study, endothelialization occurred with subendothelial smooth ECs and upon mechanical evaluation, it was found that the tensile strength and elastic modulus were comparable to that of native carotids. Importantly, despite the small diameter and high-flow vascular environment, these grafts remained patent at 12 months postimplantation with no signs of thrombosis or aneurysmal transformation.
Another combination, PLA-PCL copolymer, commonly referred to as poly (L-lactide-co-ɛ-caprolactone) (PLCL), has been evaluated in a TEVG study utilizing a canine IVC interposition model.57 This study demonstrated that bone marrow cells seeded onto the biodegradable scaffold expressed endothelial lineage markers at 8 weeks after implantation but did not explicitly evaluate the mechanical or histological properties of the scaffold itself.
All three common biopolymers (PGA, PLA, and PCL) have been integrated into vascular grafts and have undergone extensive preclinical testing in large animal models; PLCL can either be interwoven or layered with PGA. Compared with PGA-PLA grafts, PGA-PLGL grafts have a longer degradation period of 6 months.58,59
The success of large-diameter (≥6 mm) PGA-PLCL grafts have been very reproducible in both ovine and canine models, consistently showing endothelialization with ECM and smooth muscle deposition similar to that of native vessels and the absence of dilation, thrombosis, or calcification.58,60–62 However, one canine study reported poor vascular smooth muscle layer development at 12 months, although hydroxyproline and elastin content of the remodeled graft was similar to that of a native vessel while another study reported nonuniform distribution of subendothelial smooth muscle cells, leading to lesions within the graft.60,62
Furthermore, in a canine IVC interposition study, TEVGs constructed from PGA/PLCL seeded with bone marrow-derived vascular cells have demonstrated biochemical mimicry of native vessels, specifically the IVC, by releasing nitric oxide in a dose-dependent manner in response to acetylcholine stimulation.63 The same study also showed that the inflammatory response attenuates as the biopolymer scaffold is replaced by autologous components, with CD4+ T cell infiltration inversely correlated to percentage degradation of the scaffold, providing a proof-of-concept for biodegradable vascular grafts in general. Small-diameter PGA-PLCL grafts have also experienced considerable preclinical success in overcoming thrombogenesis, the main challenge of small-diameter prosthetic vessels.64,65
A novel approach that has been explored is binding heparin to scaffolds such that the scaffolds continuously release heparin after implantation to discourage thrombosis and subsequent graft stenosis. Using this approach, one group reported an extension of the patency period by 24 weeks in a canine femoral artery replacement model.65
One emerging copolymer is PGA and polyhydroxyalkanoate (PHA); PHA has higher tensile strength than PGA and provides mechanical strength to the graft as the PGA layer is being remodeled.66
PGA-PHA grafts have only been tested in ovine models thus far, but has shown clinical potential as successes have been reproducible. In aortic, IVC, and pulmonary artery interposition graft systems, extensive remodeling of PGA-PHA grafts occurred, with proteoglycan percentage, DNA content, and endothelial surface proteins reminiscent of native vessels with no evidence of thrombosis, calcification, stenosis, or aneurysm.66–68 However, two groups did note that these grafts, while mechanically stronger due to higher collagen content, had less elastic properties than native vessels.67,68 An upregulation of matrix metalloproteinases, markers of ECM remodeling, have been correlated with collagen deposition in these grafts, providing evidence that the environment provided by PGA-PHA grafts are conducive to vascular remodeling.69
Recently, a novel hybrid polymer of synthetic PCL and chitosan (CS) has been investigated in several large animal models. CS is a natural protein with high biocompatibility, low toxicity, antibacterial properties, and rapid degradation.70,71 Unfortunately, its cationic nature has the potential to facilitate aggregation of anionic platelets.72
Very small-diameter grafts (3 mm) have been constructed and evaluated in canine and ovine models, both of which have demonstrated adequate endothelialization with ECM composition close to that of autologous vessels.73,74 Perhaps due to the combination of the small diameter and the thrombogenic tendency of CS, unseeded scaffolds had patency rates below 100% at 3 and 6 months after implantation. However, seeding of the graft with outgrowth endothelial cells produced TEVGs that could increase patency significantly, from 17% to 83% at 3 months postimplantation.73
Another possibility for small-diameter vascular grafts is Lycra (polyester-polyurethane copolymer) fibers bound by poly(ethylene glycol) (PELA)-PGA copolymer, which seals off initially the porous scaffold. In the canine common carotid interposition model, this graft exhibited limited neointima formation with a lack of organized ECM but interestingly showed increased compliance over time, eventually to a degree close to that of the canine aorta.75 In addition, the porosity and hydrophilicity of the Lycra fibers and PELA-PGA copolymer, respectively, were found to be positively correlated to endothelialization and vascularization of the graft. Small-diameter heparin-conjugated PCL/polyurethane-collagen type I composite grafts have also been briefly evaluated in the canine model, revealing patent grafts at 8 weeks after implantation without aneurismal dilatation or stenosis.76
Vascular grafts constructed from biodegradable synthetic polymers demonstrate the most versatility and potential of all the biomaterials discussed thus far. Unlike vascular grafts made from decellularized vessels and natural polymers that have a narrow window of adaptation with regard to mechanical and degradative properties, the distinct properties of individual biopolymers have enabled the emergence of customized hybrid and composite grafts that continues to close the functional gap between synthetic grafts and autologous vessels.
Currently, the methodology of vascular graft research using biodegradable synthetic polymers entails a substantial amount of trial-and-error with biomaterial combinations and subsequent fine-tuning of the proportions of each biomaterial. Furthermore, the emergence of novel biomaterials, synthetic protocols, cell-surface modifications, and cell-seeding technologies provides additional parameters that not only expands the scope and depth of research but also increases the chances of stumbling upon the perfect alternative to autologous grafts.
Outlook
Global burden of cardiovascular disease and ESRD continues to climb while treatment options remain limited to small diameter autologous vessels and suboptimal large-diameter prosthetic grafts. Novel biomaterials have emerged as a promising solution to meet this increasing demand but these grafts must first overcome many challenges in both design and evaluation before becoming a clinical possibility. These grafts must be able to withstand systemic arterial pressure, resist inflammation, and have the capacity to remodel, ideally acquiring native vessel anatomy. Importantly, the thrombogenic tendencies of current prosthetic grafts must be overcome, allowing for long-term patency of small-diameter vascular grafts.
To gauge the clinical potential of vascular graft candidates, they must be evaluated in large animal models, which simulates, although imperfectly, the human vascular environment. As mentioned in the introduction, there are many barriers to the routine use of large animal models for preclinical evaluation of vascular grafts; chief among these is the exorbitant cost of maintaining the animals and facilities. There are two major ways that this obstacle can be surmounted: high-throughput in vitro screening and increased graft implantations per animal.
Rigorous in vitro experimentation with graft candidates would increase selectivity for the grafts that make it to the preclinical testing stage, ensuring that each large animal subject is used prudently. Double-graft implantation in which the bilateral symmetry of the vascular system is exploited to implant both the candidate graft and control graft into the same animal would not only produce more methodically sound experiments but also maximize the utility of each animal.
It is important to note that the cost of preclinical testing in large animals may be minimized but will never become zero because large animal models bridge the chasm between basic science research and clinical trials. Ultimately, the cost of preclinical experimentation becomes trivial in comparison to the cost of running clinical trials with human subjects.
While many biomaterials and synthetic technologies are currently in the translational stage of development undergoing testing in large animals, there are no standard protocols for preclinical testing specific to the intended purpose of each vascular graft. Thus, clinical trials must rely on preclinical results from studies that are not directly comparable and often lack valid controls. Standardized preclinical protocols, ideally producing models that recapitulate severe pathophysiology, and grafts that are deliberately “over-engineered” to withstand chronic inflammatory and diseased microenvironments would improve predictability when translating to human clinical trials.
As this review has outlined, there are many materials, natural as well as synthetic and biodegradable as well as nonbiodegradable, that are currently available and being explored in the development of vascular grafts with long-term patency rates rivaling that of autologous grafts. Based on existing studies in large animal models, vascular grafts constructed from biodegradable synthetic polymers show the most clinical potential because of the unique mechanical and degradative properties of the polymers themselves. Superimposed onto the versatile combinations of individual polymers are the varying synthetic techniques that offer additional layers of fine-tuning. Furthermore, surface modifications and preimplantation cell seeding will be imperative to graft remodeling and maximizing long-term patency.
Compared to decellularized tissue scaffolds and natural polymers, the development of a clinically applicable vascular graft using biodegradable synthetic polymers may be a lengthier and more tedious process, simply due to the large number of manipulatable parameters. As evidenced by Tables 1–4, surface-modified vascular grafts and TEVGs composed from decellularized vessels and from biodegradable synthetic polymers exhibit high patency rate most consistently and thus may be the first ones to proceed to clinical trial. However, given the inherent compliance mismatch of the decellularized tissue with the host vessels, it is likely that most of the vascular grafts moving to clinical trials will be combinations of biodegradable synthetic polymers.
There have been promising results seen in large animal models as the challenges are addressed using a combination of cell sourcing, graft surface modifications, and the utilization of biodegradable materials that collectively facilitate graft remodeling. Large animal studies have provided evidence of graft remodeling and in many instances have been able to largely recapitulate native vessel anatomy via histological analysis. There is direct evidence that this new generation of grafts has improved patency compared with existing prosthetic options, as many studies have exploited ePTFE grafts as a control. This may be an important step in facilitating the movement from large animal studies to human clinical trials.
Nonetheless, with continual elucidation of biochemical pathways in vessel endothelialization and the discovery of novel polymers and synthetic techniques, the development of a small-diameter vascular graft with long-term patency, and good off-the-shelf availability may not be far off in the horizon.
Financial Support
There was no specific funding support for this study.
Disclosure Statement
No competing financial interests exist.
References
- 1.Mozaffarian D., Benjamin E.J., Go A.S., et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation 133, e38, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Kannan R.Y., Salacinski H.J., Butler P.E., Hamilton G., and Seifalian A.M. Current status of prosthetic bypass grafts: a review. J Biomed Mater Res Part B Appl Biomater 74, 570, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Ziegler K.R., Muto A., Eghbalieh S.D.D., and Dardik A. Basic data related to operative infrainguinal revascularization procedures: a twenty year update. Ann Vasc Surg 25, 413, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abbott W.M., Megerman J., Hasson J.E., L'Italien G., and Warnock D.F. Effect of compliance mismatch on vascular graft patency. J Vasc Surg 5, 376, 1987 [PubMed] [Google Scholar]
- 5.Conte M.S. The ideal small arterial substitute: a search for the Holy Grail? FASEB J 12, 43, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Whittemore A.D., Kent K.C., Donaldson M.C., Couch N.P., and Mannick J.A. What is the proper role of polytetrafluoroethylene grafts in infrainguinal reconstruction? J Vasc Surg 10, 299, 1989 [DOI] [PubMed] [Google Scholar]
- 7.Kapadia M.R., Popowich D.A., and Kibbe M.R. Modified prosthetic vascular conduits. Circulation 117, 1873, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Brewster D.C. Current controversies in the management of aortoiliac occlusive disease. J Vasc Surg 25, 365, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Ricotta J.J. Vascular conduits: an overview. In: Rutherford R.B., ed. Vascular Surgery, 6th ed. Philadelphia, PA: Elsevier-Saunders, 2005, pp. 688–695 [Google Scholar]
- 10.Ravi S., and Chaikof E.L. Biomaterials for vascular tissue engineering. Regen Med 5, 107, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Humphrey J.D. Mechanics of the arterial wall: review and directions. Crit Rev Biomed Eng 23, 1, 1995 [PubMed] [Google Scholar]
- 12.Pashneh-Tala S., MacNeil S., and Claeyssens F. The tissue-engineered vascular graft—past, present, and future. Tissue Eng Part B Rev 22, 68, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbott W.M., Callow A., Moore W., et al. Evaluation and performance standards for arterial prostheses. J Vasc Surg 17, 746, 1993 [DOI] [PubMed] [Google Scholar]
- 14.Swartz D.D., and Andreadis S.T. Animal models for vascular tissue-engineering. Curr Opin Biotechnol 24, 916, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstone R.N., McCormack M.C., Khan S.I., et al. Photochemical tissue passivation reduces vein graft intimal hyperplasia in a swine model of arteriovenous bypass grafting. J Am Heart Assoc 5, e003856, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen B.T., Mathias C.J., Sicard G.A., Welch M.J., and Clark R.E. Platelet deposition on vascular grafts. The accuracy of in vivo quantitation and the significance of in vivo platelet reactivity. Ann Surg 203, 318, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chlupac J., Filova E., and Bacakova L. Blood vessel replacement: 50 years of development and tissue engineering paradigms in vascular surgery. Physiol Res 58 Suppl 2, S119, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Bhattacharya V., McSweeney P.A., Shi Q., et al. Enhanced endothelialization and microvessel formation in polyester grafts seeded with CD34(+) bone marrow cells. Blood 95, 581, 2000 [PubMed] [Google Scholar]
- 19.Clagett G.P., Burkel W.E., Sharefkin J.B., et al. Platelet reactivity in vivo in dogs with arterial prostheses seeded with endothelial cells. Circulation 69, 632, 1984 [DOI] [PubMed] [Google Scholar]
- 20.Fields C., Cassano A., Makhoul R.G., et al. Evaluation of electrostatically endothelial cell seeded expanded polytetrafluoroethylene grafts in a canine femoral artery model. J Biomater Appl 17, 135, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Meinhart J., Deutsch M., and Zilla P. Eight years of clinical endothelial cell transplantation. Closing the gap between prosthetic grafts and vein grafts. ASAIO J 43, M515, 1997 [PubMed] [Google Scholar]
- 22.Zilla P., Deutsch M., Meinhart J., et al. Clinical in vitro endothelialization of femoropopliteal bypass grafts: an actuarial follow-up over three years. J Vasc Surg 19, 540, 1994 [DOI] [PubMed] [Google Scholar]
- 23.Rotmans J.I., Heyligers J.M., Verhagen H.J., et al. In vivo cell seeding with anti-CD34 antibodies successfully accelerates endothelialization but stimulates intimal hyperplasia in porcine arteriovenous expanded polytetrafluoroethylene grafts. Circulation 112, 12, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Li C., Hill A., and Imran M. In vitro and in vivo studies of ePTFE vascular grafts treated with P15 peptide. J Biomater Sci Polym Ed 16, 875, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Schenke-Layland K., Vasilevski O., Opitz F., et al. Impact of decellularization of xenogeneic tissue on extracellular matrix integrity for tissue engineering of heart valves. J Struct Biol 143, 201, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Shell D.H.t. Croce M.A., Cagiannos C., et al. Comparison of small-intestinal submucosa and expanded polytetrafluoroethylene as a vascular conduit in the presence of gram-positive contamination. Ann Surg 241, 995; discussion 1001, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Badylak S.F., Lantz G.C., Coffey A., and Geddes L.A. Small intestinal submucosa as a large diameter vascular graft in the dog. J Surg Res 47, 74, 1989 [DOI] [PubMed] [Google Scholar]
- 28.Lantz G.C., Badylak S.F., Coffey A.C., Geddes L.A., and Blevins W.E. Small intestinal submucosa as a small-diameter arterial graft in the dog. J Invest Surg 3, 217, 1990 [DOI] [PubMed] [Google Scholar]
- 29.Hiles M.C., Badylak S.F., Lantz G.C., et al. Mechanical properties of xenogeneic small-intestinal submucosa when used as an aortic graft in the dog. J Biomed Mater Res 29, 883, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Sandusky G.E., Lantz G.C., and Badylak S.F. Healing comparison of small intestine submucosa and ePTFE grafts in the canine carotid artery. J Surg Res 58, 415, 1995 [DOI] [PubMed] [Google Scholar]
- 31.Nemcova S., Noel A.A., Jost C.J., et al. Evaluation of a xenogeneic acellular collagen matrix as a small-diameter vascular graft in dogs—preliminary observations. J Invest Surg 14, 321, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Roeder R.A., Lantz G.C., and Geddes L.A. Mechanical remodeling of small-intestine submucosa small-diameter vascular grafts—a preliminary report. Biomed Instrum Technol 35, 110, 2001 [PubMed] [Google Scholar]
- 33.Hinds M.T., Rowe R.C., Ren Z., et al. Development of a reinforced porcine elastin composite vascular scaffold. J Biomed Mater Res A 77, 458, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Robotin-Johnson M.C., Swanson P.E., Johnson D.C., Schuessler R.B., and Cox J.L. An experimental model of small intestinal submucosa as a growing vascular graft. J Thorac Cardiovasc Surg 116, 805, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Koobatian M.T., Row S., Smith R.J., et al. Successful endothelialization and remodeling of a cell-free small-diameter arterial graft in a large animal model. Biomaterials 76, 344, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conklin B.S., Richter E.R., Kreutziger K.L., Zhong D.S., and Chen C. Development and evaluation of a novel decellularized vascular xenograft. Med Eng Phys 24, 173, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Ketchedjian A., Jones A.L., Krueger P., et al. Recellularization of decellularized allograft scaffolds in ovine great vessel reconstructions. Ann Thorac Surg 79, 888; discussion 896, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Tillman B.W., Yazdani S.K., Neff L.P., et al. Bioengineered vascular access maintains structural integrity in response to arteriovenous flow and repeated needle puncture. J Vasc Surg 56, 783, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Tamura N., Nakamura T., Terai H., et al. A new acellular vascular prosthesis as a scaffold for host tissue regeneration. Int J Artif Organs 26, 783, 2003 [PubMed] [Google Scholar]
- 40.Cho S.W., Lim J.E., Chu H.S., et al. Enhancement of in vivo endothelialization of tissue-engineered vascular grafts by granulocyte colony-stimulating factor. J Biomed Mater Res A 76, 252, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Kaushal S., Amiel G.E., Guleserian K.J., et al. Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med 7, 1035, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Syedain Z.H., Meier L.A., Lahti M.T., Johnson S.L., and Tranquillo R.T. Implantation of completely biological engineered grafts following decellularization into the sheep femoral artery. Tissue Eng Part A 20, 1726, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aper T., Wilhelmi M., Gebhardt C., et al. Novel method for the generation of tissue-engineered vascular grafts based on a highly compacted fibrin matrix. Acta Biomater 29, 21, 2016 [DOI] [PubMed] [Google Scholar]
- 44.Ahmed T.A., Dare E.V., and Hincke M. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng Part B Rev 14, 199, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Swartz D.D., Russell J.A., and Andreadis S.T. Engineering of fibrin-based functional and implantable small-diameter blood vessels. Am J Physiol Heart Circ Physiol 288, H1451, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Liu J.Y., Swartz D.D., Peng H.F., et al. Functional tissue-engineered blood vessels from bone marrow progenitor cells. Cardiovasc Res 75, 618, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Zavan B., Vindigni V., Lepidi S., et al. Neoarteries grown in vivo using a tissue-engineered hyaluronan-based scaffold. FASEB J 22, 2853, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Fukunishi T., Shoji T., and Shinoka T. Nanofiber composites in vascular tissue engineering. In: Rangasamy J., and Shantikumar N., eds. Nanofiber Composites for Biomedical Applications, 1st ed. Cambridge, United Kingdom: Woodhead Publishing, 2017, pp. 455–481 [Google Scholar]
- 49.Shinoka T., Shum-Tim D., Ma P.X., et al. Creation of viable pulmonary artery autografts through tissue engineering. J Thorac Cardiovasc Surg 115, 536; discussion 545, 1998 [DOI] [PubMed] [Google Scholar]
- 50.Brennan M.P., Dardik A., Hibino N., et al. Tissue-engineered vascular grafts demonstrate evidence of growth and development when implanted in a juvenile animal model. Ann Surg 248, 370, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Niklason L.E., Gao J., Abbott W.M., et al. Functional arteries grown in vitro. Science 284, 489, 1999 [DOI] [PubMed] [Google Scholar]
- 52.Dahl S.L., Kypson A.P., Lawson J.H., et al. Readily available tissue-engineered vascular grafts. Sci Transl Med 3, 68ra69, 2011 [DOI] [PubMed] [Google Scholar]
- 53.Mrowczynski W., Mugnai D., de Valence S., et al Porcine carotid artery replacement with biodegradable electrospun poly-e-caprolactone vascular prosthesis. J Vasc Surg 59, 210, 2014 [DOI] [PubMed] [Google Scholar]
- 54.Torikai K., Ichikawa H., Hirakawa K., et al. A self-renewing, tissue-engineered vascular graft for arterial reconstruction. J Thorac Cardiovasc Surg 136, 37; 45.e31, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Yokota T., Ichikawa H., Matsumiya G., et al. In situ tissue regeneration using a novel tissue-engineered, small-caliber vascular graft without cell seeding. J Thorac Cardiovasc Surg 136, 900, 2008 [DOI] [PubMed] [Google Scholar]
- 56.Iwai S., Sawa Y., Ichikawa H., et al. Biodegradable polymer with collagen microsponge serves as a new bioengineered cardiovascular prosthesis. J Thorac Cardiovasc Surg 128, 472, 2004 [DOI] [PubMed] [Google Scholar]
- 57.Matsumura G., Miyagawa-Tomita S., Shin'oka T., Ikada Y., and Kurosawa H. First evidence that bone marrow cells contribute to the construction of tissue-engineered vascular autografts in vivo. Circulation 108, 1729, 2003 [DOI] [PubMed] [Google Scholar]
- 58.Fukunishi T., Best C.A., Sugiura T., et al. Preclinical study of patient-specific cell-free nanofiber tissue-engineered vascular grafts using 3-dimensional printing in a sheep model. J Thorac Cardiovasc Surg 153, 924, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hibino N., Shin'oka T., Matsumura G., Ikada Y., and Kurosawa H. The tissue-engineered vascular graft using bone marrow without culture. J Thorac Cardiovasc Surg 129, 1064, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Watanabe M., Shin'oka T., Tohyama S., et al. Tissue-engineered vascular autograft: inferior vena cava replacement in a dog model. Tissue Eng 7, 429, 2001 [DOI] [PubMed] [Google Scholar]
- 61.Roh J.D., Brennan M.P., Lopez-Soler R.I., et al. Construction of an autologous tissue-engineered venous conduit from bone marrow-derived vascular cells: optimization of cell harvest and seeding techniques. J Pediatr Surg 42, 198, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Matsumura G., Isayama N., Matsuda S., et al. Long-term results of cell-free biodegradable scaffolds for in situ tissue engineering of pulmonary artery in a canine model. Biomaterials 34, 6422, 2013 [DOI] [PubMed] [Google Scholar]
- 63.Matsumura G., Ishihara Y., Miyagawa-Tomita S., et al. Evaluation of tissue-engineered vascular autografts. Tissue Eng 12, 3075, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Zhang L., Zhou J., Lu Q., Wei Y., and Hu S. A novel small-diameter vascular graft: in vivo behavior of biodegradable three-layered tubular scaffolds. Biotechnol Bioeng 99, 1007, 2008 [DOI] [PubMed] [Google Scholar]
- 65.Wang S., Mo X.M., Jiang B.J., et al. Fabrication of small-diameter vascular scaffolds by heparin-bonded P(LLA-CL) composite nanofibers to improve graft patency. Int J Nanomed 8, 2131, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shum-Tim D., Stock U., Hrkach J., et al. Tissue engineering of autologous aorta using a new biodegradable polymer. Ann Thorac Surg 68, 2298; discussion 2305, 1999 [DOI] [PubMed] [Google Scholar]
- 67.Hoerstrup S.P., Cummings Mrcs I., Lachat M., et al. Functional growth in tissue-engineered living, vascular grafts: follow-up at 100 weeks in a large animal model. Circulation 114, I159, 2006 [DOI] [PubMed] [Google Scholar]
- 68.Kelm J.M., Emmert M.Y., Zurcher A., et al. Functionality, growth and accelerated aging of tissue engineered living autologous vascular grafts. Biomaterials 33, 8277, 2012 [DOI] [PubMed] [Google Scholar]
- 69.Cummings I., George S., Kelm J., et al. Tissue-engineered vascular graft remodeling in a growing lamb model: expression of matrix metalloproteinases. Eur J Cardiothorac Surg 41, 167, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chupa J.M., Foster A.M., Sumner S.R., Madihally S.V., and Matthew H.W. Vascular cell responses to polysaccharide materials: in vitro and in vivo evaluations. Biomaterials 21, 2315, 2000 [DOI] [PubMed] [Google Scholar]
- 71.No H.K., Park N.Y., Lee S.H., and Meyers S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int J Food Microbiol 74, 65, 2002 [DOI] [PubMed] [Google Scholar]
- 72.Yao Y., Wang J., Cui Y., et al. Effect of sustained heparin release from PCL/chitosan hybrid small-diameter vascular grafts on anti-thrombogenic property and endothelialization. Acta Biomater 10, 2739, 2014 [DOI] [PubMed] [Google Scholar]
- 73.Zhou M., Qiao W., Liu Z., et al. Development and in vivo evaluation of small-diameter vascular grafts engineered by outgrowth endothelial cells and electrospun chitosan/poly(epsilon-caprolactone) nanofibrous scaffolds. Tissue Eng Part A 20, 79, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fukunishi T., Best C.A., Sugiura T., et al. Tissue-engineered small diameter arterial vascular grafts from cell-free nanofiber PCL/chitosan scaffolds in a sheep model. PLoS One 11, e0158555, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Izhar U., Schwalb H., Borman J.B., et al. Novel synthetic selectively degradable vascular prostheses: a preliminary implantation study. J Surg Res 95, 152, 2001 [DOI] [PubMed] [Google Scholar]
- 76.Lu G., Cui S.J., Geng X., et al. Design and preparation of polyurethane-collagen/heparin-conjugated polycaprolactone double-layer bionic small-diameter vascular graft and its preliminary animal tests. Chin Med J 126, 1310, 2013 [PubMed] [Google Scholar]
- 77.Cho S., Lim S., Kim I., et al. Small-diameter blood vessels engineered with bone marrow-derived cells. Annals of surgery 241, 506, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]