Abstract
Mounting evidence suggests that site-appropriate loading of implanted extracellular matrix (ECM) bioscaffolds and the surrounding microenvironment is an important tissue remodeling determinant, although the role at the cellular level in ECM-mediated skeletal muscle remodeling remains unknown. This study evaluates crosstalk between progenitor cells and macrophages during mechanical loading in ECM-mediated skeletal muscle repair. Myoblasts were exposed to solubilized ECM bioscaffolds and were mechanically loaded at 10% strain, 1 Hz for 5 h. Conditioned media was collected and applied to bone marrow-derived macrophages followed by immunolabeling for proinflammatory M1-like markers and proremodeling M2-like markers. Macrophages were subjected to the same loading protocol and their secreted products were collected for myoblast migration, proliferation, and differentiation analysis. A mouse hind limb unloading volumetric muscle loss model was used to evaluate the effect of loading upon the skeletal muscle microenvironment after ECM implantation. Animals were sacrificed at 14 or 180 days. Isometric torque production was tested and tissue sections were immunolabeled for macrophage phenotype and muscle fiber content. Results show that loading augments the ability of myoblasts to promote an M2-like macrophage phenotype following exposure to ECM bioscaffolds. Mechanically loaded macrophages promote myoblast chemotaxis and differentiation. Lack of weight bearing impaired muscle remodeling as indicated by Masson's Trichrome stain. Isometric torque was significantly increased following ECM implantation when compared to controls, a response not present in the hind limb-unloaded group. This work provides an important mechanistic insight of the effects of rehabilitation upon ECM-mediated remodeling and could have broader implications in clinical practice, advocating multidisciplinary approaches to regenerative medicine, emphasizing rehabilitation.
Keywords: : macrophage, extracellular matrix, volumetric muscle loss, mechanical loading
Introduction
Severe skeletal muscle injury, such as volumetric muscle loss (VML), as a result of trauma, tumor ablation, or prolonged denervation, is a challenging problem in civilian and military medicine with significant clinical and economic consequences.1–3 Treatment strategies include muscle grafts, orthotic devices, and/or physical rehabilitation and typically leave patients with a bleak prognosis of persistent strength and functional deficits.4–6 To address this unmet clinical need, a wide variety of strategies in the field of tissue engineering/regenerative medicine have been investigated as alternative therapeutic approaches to VML.
The use of acellular biologic scaffolds composed of mammalian extracellular matrix (ECM) as an inductive myogenic template has been investigated for more than two decades in multiple animal models, a variety of tissues, and types of injury, and with diverse bioscaffold source tissues. Outcomes have ranged from successful to unsatisfactory.7–16 The findings from these preclinical studies served as the basis for the clinical use of ECM bioscaffolds for VML treatment in a pilot, 13-patient cohort study, with promising results, including partial restoration of muscle strength, function, and range of motion.17–19 Although the preliminary clinical outcome of ECM bioscaffold-based treatment for VML is encouraging, it is unlikely that the use of ECM bioscaffolds alone will yield complete restoration of skeletal muscle function.
Evidence suggests that mechanical loading is not only beneficial but also necessary for musculoskeletal development, strength and endurance gains, fatigue resistance, and even acute regeneration.20–25 However, the role of mechanical loading in the context of ECM bioscaffold-mediated repair following VML is not fully understood.
Innate immunity is an essential component of the host response to injury and also plays a regulatory role in tissue development and homeostasis. Moreover, macrophage presence and phenotype have been shown to be a critical regulator of skeletal muscle regeneration and predictive of downstream ECM-mediated tissue repair following injury. Since mechanical loading has been shown to have myogenic benefits, it is plausible that incorporation of early postinjury mechanical loading may foster a proregenerative crosstalk between macrophages and myogenic progenitor cells following ECM implantation for VML repair. The objective of this study was to investigate the role of mechanical stimulation upon the macrophage and myoblast response in ECM bioscaffold-mediated VML repair, in well-accepted in vitro and in vivo models.
Materials and Methods
Overview of study design
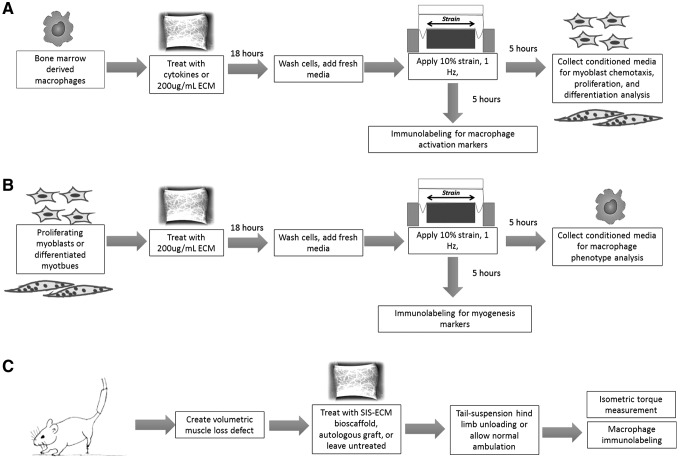
The role of mechanical stimulation upon cell processes known to be important in ECM bioscaffold-mediated skeletal muscle remodeling was evaluated in a two-part study (Fig. 1). In vitro characterization of the macrophage and myoblast response to concurrent exposure to ECM degradation products and mechanical stimulation was conducted (FlexCell International, Burlington, NC). Conditioned media (containing secreted factors) from myoblasts and macrophages following exposure to ECM degradation products and/or mechanical strain were collected. The corresponding cell type was then exposed to these conditioned media to evaluate the effect on well-established functional outcome metrics (Fig. 1A, B). In a parallel study, the effect of mechanical stimulation on ECM bioscaffold-mediated skeletal muscle reconstruction was evaluated using tail suspension-induced hind limb unloading in an established in vivo model of VML (Fig. 6).
FIG. 1.
Overview of experimental design. The goal of this study was to determine the ability of mechanical stimulation in the presence of ECM degradation products to promote skeletal muscle remodeling. The following questions were posed: (A) how does mechanical loading affect macrophage phentoype in the presence of an ECM bioscaffold and how is the macrophage secretome altered in the context of promoting myoblast chemotaxis, proliferation, and differentiation? (B) How does mechanical loading affect myoblast differentiation in the presence of an ECM bioscaffold and how does the myoblast/myotube secretome change in the context of promoting a change in activation in macrophages? (C) What is the effect of absence of mechanical loading upon the ECM-mediated skeletal muscle repair microenvironment? ECM, extracellular matrix.
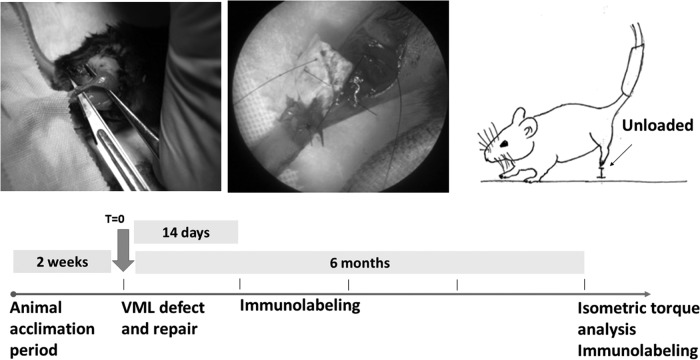
FIG. 6.
Overview of animal model of VML and hind limb unloading. C57bl/6 mice were subjected to tail-suspension to achieve hind limb unloading for 2 weeks before surgery. Surgical excision of the distal gastrocnemius and proximal Achilles was replaced with an SIS-ECM bioscaffold or an autologous graft, or left untreated. Animals were either allowed to walk normally or subjected to hind limb unloading and sacrificed according to the indicated timeline. VML, volumetric muscle loss.
Preparation of ECM bioscaffolds
Small intestinal submucosa extracellular matrix (SIS-ECM) was prepared as previously described.26–28 Briefly, porcine small intestine was obtained (Animal Biotech Industries, Danboro, PA) and the stratum compactum, muscularis mucosa, and tunica submucosa were isolated from the jejunum of animals weighing ∼240–260 lbs. Peracetic acid, deionized H2O, and phosphate buffer saline washes were used to decellularize the tissue. SIS-ECM sheets were lyophilized to form sheets or milled to form a powder. The powder was then solubilized with 1.0% pepsin as previously described for the preparation of an ECM hydrogel to yield a 10 mg/mL solution of ECM degradation products.29
Isolation and culture of murine bone marrow-derived macrophages
Murine bone marrow-derived macrophages were isolated and cultured as previously described.30 Briefly, bone marrow was isolated from the femurs and tibias of female C57BL/6 mice and was subsequently cultured in complete growth media, including Dulbecco's modified Eagles medium (DMEM; Gibco, Grand Island, NY), 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA), 10% L929 supernatant, 0.1% beta-mercaptoethanol (Gibco), 100 U/mL penicillin, 100 μg/mL streptomycin, 10 mM nonessential amino acids (Gibco), and 10 mM HEPES buffer, for 7 days with complete media changes every 48 h until mature bone marrow-derived macrophages were obtained, as confirmed by F4/80 immunolabeling. Macrophages were cultured on Uniflex culture plates (FlexCell International) for mechanical loading.
Myoblast culture
C2C12 myoblasts (ATCC) were cultured in accordance with ATCC guidelines. The cells were grown in DMEM/high glucose (4500 mg/L), 10% FBS, and 1% penicillin–streptomycin. Cells were grown at 37°C, 5% CO2, and were assayed at ∼80% confluence.
ECM treatment and mechanical loading of myoblasts and macrophages
Macrophages and myoblasts were treated with solubilized SIS-ECM at 200 μg/mL in media containing 10% FBS and 1% penicillin/streptomycin in DMEM/high glucose for 18 h or treated with canonical activation controls of interferon gamma (IFNγ) and lipopolysaccharide (LPS) to derive a proinflammatory macrophage phenotype, or IL-4 to derive a proremodeling phenotype. Cells designated for mechanical loading were plated at 1 × 105 cells per well on 35 mm collagen I-coated 6-well Uniflex culture plates (FlexCell International) and were subjected to 10% uniaxial strain at a cyclic rate of 1 Hz using the F-4000 FlexCell machine after being cultured to ∼70% confluence. Cells were kept in normal growth media with or without SIS-ECM to analyze the effect of mechanical loading upon cell phenotype, or were mechanically loaded in serum-free, ECM-free media for secreted product collection for crosstalk analyses.
Immunolabeling analyses
Macrophages and myoblasts were fixed following mechanical strain with 2% paraformaldehyde for 20 min and then washed with phosphate-buffered saline (PBS). Cells were then immunolabeled for macrophage activation markers iNOS and Fizz1 or myogenic markers MyoD, desmin, and sarcomeric myosin. Before immunolabeling, cells were incubated for 1 h in a blocking solution consisting of 0.1% Triton-X 100, 0.1% Tween-20, 2% bovine serum albumin, and 4% normal goat serum. Cells were then incubated with the following primary antibodies diluted in the blocking solution for 16 h at 4°C: (1) monoclonal anti-F4/80 (abcam, Cambridge, MA) as a pan macrophage marker at 1:200, (2)polyclonal anti-iNOS (abcam) as an Mpro-inflammatory macrophage marker at 1:100, (3) polyclonal anti-Fizz1 (Peprotech, Rocky Hil, NJ) as an Mpro-remodeling macrophage marker at 1:200, (4) monoclonal anti-MyoD (ThermoFisher, Pittsburgh, PA) at 1:500 as an early myogenic marker, (5) monoclonal anti-desmin (abcam), and (6) anti-sarcomeric myosin heavy chain (Developmental Studies Hybridoma Bank, University of Iowa) at 1:500. Cells were then washed with PBS and incubated in Alexa Fluor 488 goat anti-rat, goat anti-rabbit, or goat anti-mouse diluted to 1:200 in the blocking solution for 1 h at room temperature and nuclei were counterstained with 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI).
Myoblast chemotaxis
The effect of macrophage-conditioned media upon C2C12 skeletal muscle myoblast chemotaxis was examined using a modified Boyden Chamber cell migration assay as previously described.31 C2C12 myoblasts were cultured in starvation media (DMEM, 0.5% FBS, and 1% penicillin/streptomycin) for 18 h before use. Cells were then trypsinized, resuspended in growth factor-free DMEM, and transferred to a 15 mL conical tube for 1-h incubation at 37°C, 5% CO2. Polycarbonate chemotaxis membranes with 8 μm pores were coated with 0.05 mg/mL collagen type I. Macrophage-conditioned media (MIFNy+LPS, MIL-4, MECM, and their mechanically loaded counterparts) or positive (DMEM with 20% FBS) or negative (growth factor-free DMEM) controls were added to the lower wells of Neuro Probe 48-well microchemotaxis chamber. Collagen-coated membranes were placed over the chemoattractants and 2 × 105 cells were added to each of the upper wells of the chamber. Cells were allowed to migrate across the chamber for 3 h at 37°C, 5% CO2. Following the migration period, nonmigrating cells were scraped from the upper side of the membrane using a rubber scraper. Migrated cells that attached to the bottom of the membrane were fixed with 95% methanol and stained with DAPI before imaging. Membranes were imaged using a Zeiss Axiovert microscope and the number of migrated cells was quantified using a CellProfiler pipeline.
Myoblast mitogenesis
C2C12 myoblasts were seeded in normal growth media at 1 × 103 cells per well in a 96-well plate. Media were switched to starvation media (DMEM, 0.5% FBS, 1% penicillin/streptomycin) for 18 h. Following the starvation period, cells were treated with one of the macrophage-secreted product samples or positive (DMEM with 20% FBS) or negative (growth factor-free DMEM) control media for 18 h. Treatments were supplemented with 10 μM 5-bromo-2′deoxyuridine (BrdU) for the final 4 h. Cells were fixed with 95% methanol for 10 min and washed with PBS. Cells were then treated with 2 N HCl for 30 min at 37°C. Following HCl treatment, cells were blocked using the previously described blocking solution for 1 h at room temperature. Following the blocking period, cells were incubated in G3G4 (Anti-BrdU) antibody (Developmental Studies Hybridoma Bank, University of Iowa) at a dilution of 1:1000 for 16 h at 4°C. After primary antibody incubation, cells were washed thrice with PBS and incubated in Alexa Fluor donkey anti-mouse 488 secondary at a dilution of 1:300 for 1 h at room temperature before being subjected to DAPI nuclear stain. BrdU incorporation was imaged using a Zeiss Axiovert microscope and quantified using an ImageJ macro.
Myoblast myogenesis
High-serum media (10% FBS) and low-serum media (1% FBS, 1% horse serum) were used as negative and positive controls for C2C12 myotube formation as described previously.32 These media will be referred to as proliferation and differentiation media, respectively. Myogenic differentiation potential following exposure to macrophage-secreted products was determined by examining myotube formation. C2C12 myoblasts were cultured in proliferation media until they reached ∼70% confluence. Media were then changed to treatment media consisting of a 1:1 solution of macrophage supernatants and 20% FBS DMEM to yield a final serum concentration of 10% FBS, or controls of proliferation or differentiation media. Following 4–5 days or when differentiation media controls showed myotube formation, cells were fixed for immunolabeling with 2% paraformaldehyde. Fixed cells were blocked according to the previously described protocol for 1 h at room temperature and incubated with anti-sarcomeric myosin heavy chain as described previously. Images of five 20X fields were taken for each well using a Zeiss Axiovert microscope.
Surgical procedure and hind limb unloading
All animal studies were conducted in accordance with the University of Pittsburgh Institutional Animal Care and Use Committee (IACUC) guidelines. Seventy-two C57bl/6 mice (Jackson Laboratories, Bar Harbor ME) were randomly divided into six equal groups. All mice were subjected to tail suspension to achieve hind limb unloading with full access to the cage floor for two weeks to acclimate before the surgical procedure. A mouse model of VML was used to evaluate the in vivo remodeling response to SIS-ECM in the presence or absence of mechanical load. A 5 mm segment including the distal third of the gastrocnemius and proximal half of the Achilles tendon was surgically excised (Fig. 6), or left uninjured as a healthy control. In the VML groups, the segmental defect was repaired with an SIS-ECM sheet or autologous test sample, or was allowed to heal normally without intervention (i.e., no treatment). The device or autologous graft was placed over the proximal and distal stumps and fixed in place with interrupted 7–0 Prolene sutures. The skin closure was subcuticular with absorbable sutures. Animals were then allocated into the tail-suspended hind limb-unloaded group or the normal ambulation group. Animals were checked twice daily to assure slippage from the tail-suspension apparatus had not occurred, and were sacrificed at 14 or 180 days postsurgery.
Tissue harvest and immunolabeling
Animals were sacrificed at their predetermined time point and the gastrocnemius and Achilles tendon unit was excised from the surrounding tissue, fixed in neutral-buffered formalin, and paraffin embedded. Sections were deparaffinized and rehydrated using a graded ethanol series and subjected to heat-mediated antigen retrieval with a citrate buffer (pH = 8). Following antigen retrieval, tissue sections were incubated in the blocking solution consisting of 0.1% Triton X-100, 0.1% Tween-20, 2% bovine serum albumin, and 4% goat serum to prevent nonspecific antibody binding. After the blocking step, tissue sections were incubated with the following primary antibodies diluted in the blocking solution for 16 h at 4°C: (1) anti-iNOS (abcam) and (2) anti-Fizz1 (Peprotech). After incubation in primary antibody, tissue sections were washed with PBS and incubated for 1 h at room temperature with fluorescent secondary antibody diluted in the blocking solution: (1) Alexa Fluor 488 goat anti rabbit at 1:200. Sections were washed again and counterstained with DAPI and cover-slipped for imaging using a Nuance multispectral microscope with appropriate fluorescent filter sets.
Isometric torque measurement
Functional analysis was performed by measuring isometric torque production of the gastrocnemius 180 days postsurgery using a protocol previously described.33 This method allows the determination of contractile properties of the isolated gastrocnemius, while maintaining normal muscle orientation, innervation, and vascular supply. Animals were anesthetized while the hind limb was stabilized by platform supports with the foot in the flexed position. Needle electrodes were inserted into the belly of the muscle injury site ∼2–3 μm beneath the skin. Muscles were stimulated at nine different frequencies (1–200 Hz) with a 2-min rest period between each frequency. Twitch contraction and tetanic contraction were analyzed using a Dynamic Muscle Analysis program (Aurora Scientific, Inc., Canada) and data were normalized to the animals' weights.
Statistical analysis
All statistical analysis was conducted using SPSS Statistical Analysis Software (IBM, Chicago, IL). Data were normally distributed and tested for homogeneity of variance. Data were compared between groups using a one-way independent ANOVA and Tukey's post hoc analysis with an alpha value of 0.05. Error bars represent standard deviation. A two-way ANOVA and Fisher's least significant difference (LSD) post-hoc analysis were used to compare isometric torque between treatment groups at each frequency with an alpha value of 0.05. Error bars represent standard deviation.
Results
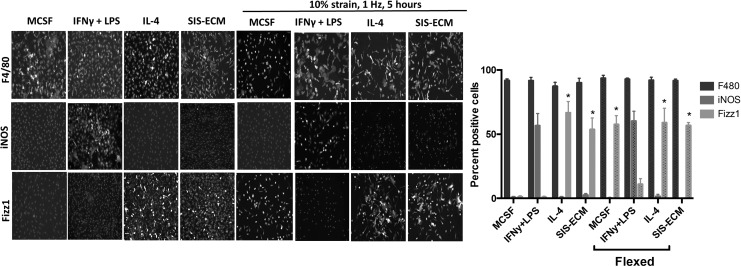
Cyclic mechanical strain promotes a Fizz1 ± macrophage phenotype
After 18 h, degradation products derived from SIS-ECM promote a predominant iNOS−/Fizz1+ macrophage phenotype. When exposed to mechanical load, naive macrophages begin to express the M2-like marker Fizz1 similar to IL-4 and ECM-treated resting macrophages (Fig. 2).
FIG. 2.
Cyclic mechanical strain of bone marrow-derived macrophages. Macrophages were subjected to 10% mechanical strain for 5 h using the FlexCell system. Five-hour mechanical strain resulted in an F480+/iNOS−/Fizz1+ macrophage phenotype, suggesting that mechanical strain alone can promote macrophage activation toward a proremodeling phenotype. (*p < 0.05 when compared to the MCSF-unflexed group, n = 5, error bars represent standard deviation). MCSF, macrophage colony stimulating factor.
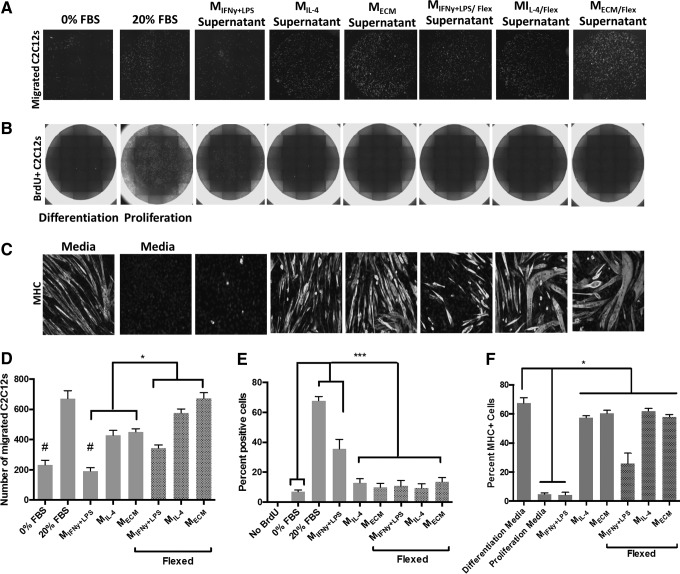
Cyclic mechanical strain promotes a proremodeling macrophage phenotype that induces myoblast chemotaxis
As previously shown, naive macrophages treated with SIS-ECM behave similar to macrophages treated with IL-4.27,28 Specifically, both promote a significant increase in myoblast chemotaxis and myotube formation (Fig. 3A, D, C, and F). In contrast, macrophages treated with IFNy+LPS increase myoblast mitogenesis. Interestingly, when mechanically strained, all macrophage types (IFNy+LPS treated, IL-4 treated, or SIS-ECM treated) promote a significant increase in myoblast chemotaxis when compared to their resting, unstrained counterparts (Fig. 3D).
FIG. 3.
The effect of ECM and mechanical stimulation upon the macrophage secretome. Macrophages were treated with activating cytokines IFNy+ LPS or IL-4, or 200 μg/mL of ECM degradation products for 18 h. After 18 h, cells were washed and media were replaced with serum-free, ECM-free media. Macrophages were strained for 5 h and their conditioned media were collected for C2C12 experiments. (A, D) IL-4-treated and ECM-treated macrophages promote increased C2C12 migration. Mechanically loaded macrophages significantly increase C2C12 migration compared to the unloaded control macrophages and the low-serum negative control. (B, E) IFNy+LPS-treated macrophages promote increased C2C12 mitogenesis. Mechanically loaded macrophages significantly increase C2C12 mitogenesis compared to the unloaded and proliferation media positive control. (C, F) IL-4-stimulated and M-ECM-stimulated macrophages significantly increase C2C12 myogenesis. Mechanically loaded macrophages significantly increase C2C12 myogenesis compared to the unloaded and proliferation media negative control. (#p < 0.05 when compared to the 20% FBS control, *p < 0.05 compared to the indicated groups, ***p < 0.01 when compared to the 20% FBS and IFNy+LPS groups, n = 5, error bars represent standard error of the mean). FBS, fetal bovine serum.
Cyclic mechanical strain promotes a proremodeling macrophage phenotype that reduces myoblast proliferation and promotes myoblast differentiation
IFNy+LPS-stimulated macrophages promote a significant increase in the number of BrdU-positive proliferating myoblasts (Fig. 3B, E). However, if IFNy+LPS-stimulated macrophages are first subjected to mechanical strain, this response is significantly diminished and is similar to IL-4-treated or SIS-ECM-treated macrophages (Fig. 3B, E). Mechanical strain applied to IFNy+LPS-stimulated macrophages also increased the ability of their collective secretome to promote C2C12 myotube formation (Fig. 3C, F).
Cyclic mechanical strain combined with SIS-ECM treatment promotes myoblast differentiation
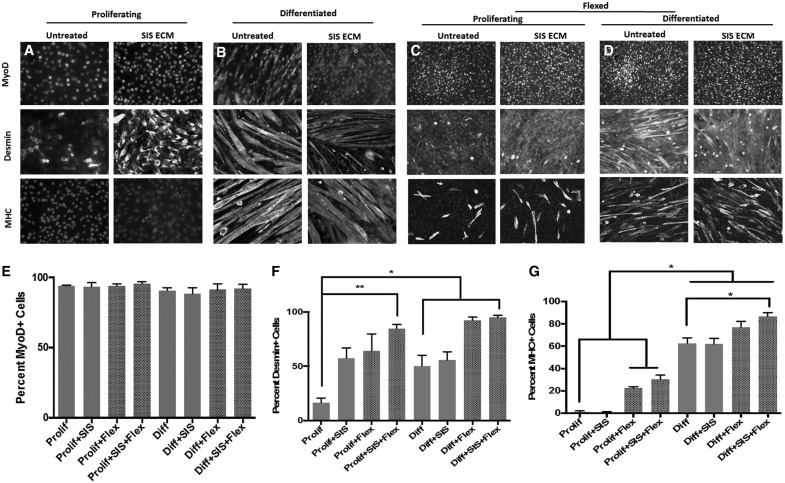
Treatment of proliferating myoblasts with SIS-ECM coupled with mechanical strain resulted in a significant increase in desmin expression (Fig. 4F). Consistent with previous findings, the application of mechanical strain alone resulted in sarcomeric myosin heavy chain positive myotube formation in the proliferating group, suggesting the ability of mechanical strain to promote myoblast differentiation as shown in Figure 4G.34 This response was augmented by the addition of SIS-ECM treatment. Furthermore, when subjected to both SIS-ECM treatment and mechanical strain, the number of differentiated myotubes expressing the terminal differentiation marker, sarcomeric myosin heavy chain, significantly increased compared to the resting, untreated myotubes (Fig. 4F).
FIG. 4.
Cyclic mechanical strain of C2C12 myoblasts. (A, C) Myoblasts were cultured and either kept in their proliferation media or (B, D) allowed to differentiate to form myotubes. (C, D) Cells were treated with solubilized ECM bioscaffolds for 18 h or were left in media and were then mechanically strained for 5 h, or (A, B) were left unstrained. (E) All treatments were associated with MyoD expression. (F) SIS-ECM treatment increased expression of desmin, with an additional increase after myoblasts were mechanically strained. (G) Mechanical strain increased myotube formation shown by myosin heavy chain (MHC) expression. (*p < 0.05, **p < 0.01, n = 5, error bars represent standard error of the mean). SIS-ECM, small intestinal submucosa extracellular matrix.
Cyclic mechanical strain promotes myotube-mediated macrophage activation toward a proremodeling phenotype
It is well established that the secretome of stem cells can promote a proremodeling macrophage phenotype.35 The secretome of proliferating C2C12 myoblasts (i.e., those upstream of the terminal differentiation pathway) can shift macrophages toward an iNOS−/Fizz1+ phenotype regardless of whether the myoblasts are treated with SIS-ECM or mechanically strained (Fig. 5). However, when allowed to exit the cell cycle and fuse to form myotubes following serum withdrawal, the secretome of C2C12 myotubes does not alter macrophage phenotype toward an iNOS−/Fizz1+ phenotype. In contrast, when first treated with SIS-ECM, the C2C12 myotube secretome is altered and promotes the iNOS−/Fizz1+ macrophage phenotype (Fig. 5). A similar response is seen when myotubes are mechanically strained. Exposure to both SIS-ECM and mechanical strain augments this response; specifically, there is a significant increase in the number of Fizz1+ macrophages after exposure to this secretome (Fig. 5F).
FIG. 5.
The effect of ECM and mechanical stimulation upon the myoblast secretome. C2C12 cells were cultured in proliferation media or allowed to form myotubes in differentiation media culture. Myoblasts or myotubes were treated with 200 μg/mL of ECM degradation products for 18 h, after which the media were replaced with serum-free, ECM-free media and the cells were subjected to mechanical strain. Conditioned media were collected and added to bone marrow-derived macrophage for 18 h and the cells were fixed for immunolabeling. (A) Exposure times were normalized to cytokine stimulated controls. (B, D) The secretome of proliferating myoblasts promotes an iNOS−/Fizz1+ macrophage phenotype, (C) however, differentiated myotubes do not promote the same effect. Treating myotubes with ECM degradation products, however, alters their secretome allowing them to promote a Fizz1+ macrophage phenotype. (E) This response is augmented when ECM-treated myotubes are subjected to mechanical strain. (F) Percentage of iNOS- and Fizz1-positive macrophages were quantified using CellProfiler. (#p < 0.05 for iNOS expression when compared to the untreated control, *p < 0.05 for Fizz1 expression when compared to the untreated control, n = 4, error bars represent standard error of the mean).
Lack of mechanical stimulation alters the macrophage activation response in ECM-mediated skeletal muscle remodeling
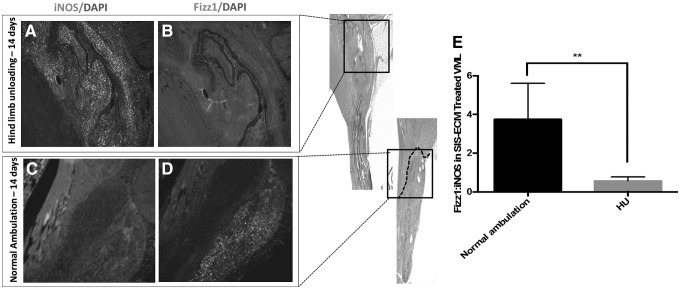
After 14 days, hind limb unloading results in a significant decrease in the Fizz1+:iNOS+ macrophage infiltrate within the defect site (Fig. 7).
FIG. 7.
Marophage and myoblast response to hind limb unloading following ECM-mediated VML remodeling. (A) Hind limb unloading results in an increased infiltration of iNOS+ macrophages at 14 days following implantation and (B) decreased Fizz1+ macrophage infiltration in contrast to the (C, D) normal ambulation control. (E) Quantification of the Fizz1+:iNOS+ macrophage ratio across all animals showed a significant decrease following 14 days of hind limb unloading (**p < 0.01, error bars represent standard error of the mean).
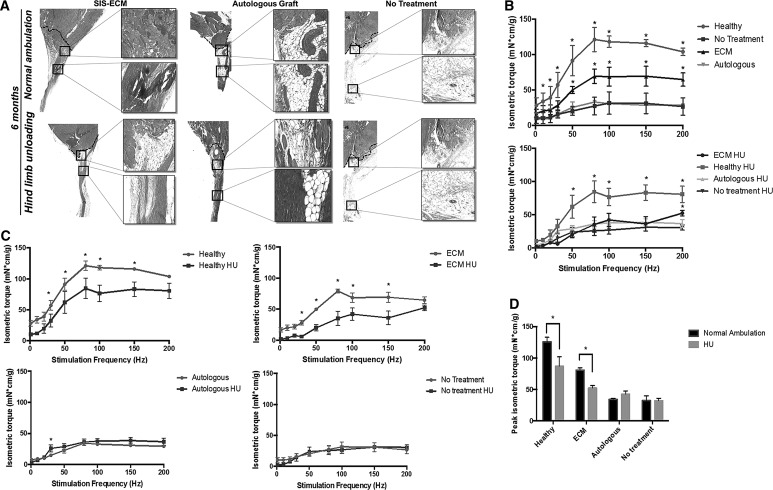
Lack of mechanical stimulation hinders ECM bioscaffold-mediated constructive and functional remodeling in a mouse model of musculotendinous injury
SIS-ECM treatment promotes site-appropriate tissue deposition following VML (i.e., gastrocnemius-Achilles musculotendinous unit) injury in the mouse hind limb (Fig. 8), whereas fatty tissue deposition and disorganized connective tissue occur in the autologous graft or untreated controls (Fig. 8). When prevented from bearing weight on their hind limbs, the SIS-ECM treatment results in more adipose tissue deposition and less muscle formation within the defect site when compared to the normal ambulation SIS-ECM-treated group (Fig. 8). Isometric torque production in the SIS-ECM-treated group is significantly increased compared to the autologous graft-treated and graft-untreated control groups following after 6 months when animals were allowed to bear weight normally (Fig. 8). This functional gain is significantly diminished in the absence of mechanical loading upon the hind limbs (Fig. 8). Peak isometric torque is reduced in both the SIS-ECM-treated groups and the healthy control animals in the absence of hind limb weight-bearing (Fig. 8). In general, there is a positive correlation between histologically evident new skeletal muscle tissue formation and a regain of isometric torque following injury.
FIG. 8.
The effect of mechanical stimulation upon constructive remodeling following ECM implantation in a mouse model of VML. (A) Masson's trichrome staining shows site-appropriate tissue deposition following ECM bioscaffold implantation in mouse VML after 6 months in contrast to fatty tissue deposition and disorganized connective tissue formation in the autologous graft and untreated groups. Hind limb unloading for 6 months decreases the deposition of site-appropriate tissue. (B) ECM treatment results in a significant improvement in gastrocnemius isometric torque production when compared to the untreated and autologous control groups. Hind limb unloading diminishes this increase in force production. (C) Hind limb unloading significantly decreases isometric torque production in the ECM-treated animals and the healthy controls. (D) Peak isometric torque production is significantly decreased following hind limb unloading in the healthy control and ECM groups (HU, hindlimb unloading, *p < 0.05, n = 4, error bars represent standard error).
Discussion
The results of this study show that mechanical stimulation fosters a proremodeling macrophage and myoblast crosstalk with both direct and indirect promotion of an M2-like macrophage phenotype and increased myoblast chemotaxis and differentiation after exposure to the macrophage secretome. Lack of mechanical stimulation mitigates ECM-mediated skeletal muscle remodeling and reduces restoration of function. This less robust functional response was associated with a reduced M2:M1 macrophage ratio at early time points. The M2:M1 ratio has been shown to be a critical regulator of downstream skeletal muscle remodeling.36
The mechanisms by which ECM bioscaffolds promote restoration of innervated, vascularized, skeletal muscle tissue with associated increased strength and improvement in functional performance in VML patients have not been totally elucidated, but it is well established that ECM bioscaffolds modify the default wound healing response by promoting an early transition in macrophage phenotype and mobilization of progenitor cells.19,28 The successful clinical use of ECM bioscaffolds for VML has been coupled with aggressive, early (i.e., immediate) physical rehabilitation. This regimen of postoperative physical therapy has been considered an important contributor to downstream functional remodeling when ECM bioscaffolds are used.17,19,37 This study supports this concept and provides evidence of the complex signaling and, to at least some extent, dependency between mechanical loading, the innate immune system, and stem/progenitor cell development and differentiation. The incorporation of structured mechanical loading (i.e. physical therapy), supported by data such as that provided in this study, could narrow the gap between some of the disparate outcomes seen with studies of ECM-mediated skeletal muscle remodeling.14,38,39
While the benefits of muscle loading and exercise have long been recognized, the impact upon the innate immune response and benefits of early mechanical loading (i.e., within 24–48 h after injury and ECM placement) in the presence of a biomaterial intervention have not been appreciated. Macrophages are key regulators of many complex physiologic processes not only in wound healing but also in tissue homeostasis and development, and these cells appear to have particular importance in regulating skeletal muscle regeneration. The importance of macrophages has also been investigated in the broader context of ECM bioscaffold-mediated tissue repair.27,30,36,40–46 The results of this study confirm earlier findings that ECM-based signaling molecules influence macrophage behavior both directly27,30,41 and through myogenic progenitor signaling mechanisms.30 These findings also show that mechanical stimulation can accentuate this response. While sensitivity of myoblasts to cyclic loading has been extensively studied, the importance of the early macrophage response to mechanical cues has not been generally appreciated. Previous work has shown that cell elasticity and cyclic strain can regulate macrophage phenotype and drive biomaterial design.47,48 Although in vitro cyclic loading of macrophages was shown to push macrophages toward a proremodeling phenotype, this study also shows a similar in vivo macrophage response. These findings show that macrophage behavior can be influenced by not only changing surface topography of a bioscaffold substrate but also by providing external cues in the form of mechanical loading, following severe skeletal muscle injury. Seminal studies investigating the response of macrophages to substrates of varying elasticities and rigidities have shown that changes in the cytokine secretion profile and gene/surface marker expression patterns of macrophages occur through mechanotransduction pathways, including actin polymerization, activation of stretch-sensitive ion channels, and/or activation or denaturation of G-proteins.21,48,49 Through these and other mechanisms, a link between mechanics and biologic processes can be established, allowing for another controllable factor to drive the design of biomaterials, and the proscription of rational physical therapy/rehabilitation regimens to facilitate functional skeletal muscle restoration.
Although attempts to directly relate in vitro strain regimens to the complexities of the in vivo response to injury are generally overreaching, studies have shown that altering strain regimens can promote both beneficial and detrimental cell responses.47,50,51 This study did not investigate a range of straining protocols, but it is noteworthy that mechanical loading in general, and in combination with cues from ECM bioscaffold degradation products, can foster a constructive crosstalk between myoblasts and macrophages. The results of this crosstalk include immunomodulation, myoblast chemotaxis and differentiation, site-appropriate tissue deposition, and increased isometric torque production. Future work would logically be aimed at investigating strain-related changes to macrophages, skeletal muscle progenitor cells, myofiber alignment, angiogenesis, and innervation among other important components of functional skeletal muscle regeneration. Such strain-related variables would include a range of strain magnitude, duration, and rate in both in vitro constructs and rehabilitation medicine.
There are several limitations to this study. Macrophage phenotype cannot be characterized by analysis of a single marker as this inevitably ignores other important aspects such as the metabolic state, gene expression pattern, the secretome, and functional capacity.52,53 This limitation being recognized, iNOS and Fizz1 are frequently utilized as proinflammatory and immunomodulatory macrophage markers, respectively, and they have been shown to be regulated by mechanical loading and play important roles in ECM remodeling, following acute skeletal muscle injury.54–56 Changes in arginine metabolism by iNOS have been widely investigated in skeletal muscle and can affect muscle health,54,55 and therefore iNOS and Fizz1 were chosen as macrophage markers in this study.
Another limitation of this study is the use of only one cell type for analysis of the skeletal muscle response to injury. Skeletal muscle regeneration in vivo involves the coordination of many cell types, not only myoblasts but also satellite cells,57 perivascular stem cells,58,59 and other myogenic progenitor cells.58–60 The effect of mechanical stimulation has been investigated particularly in satellite cell involvement,23,61,62 but likely will affect most if not all of the cell types involved in skeletal muscle regeneration. Finally, although the hind limb unloading model sufficiently reduced weight-bearing in the remodeling gastrocnemius/Achilles, it did not allow for complete immobilization. It is noteworthy, although, that the lack of weight-bearing alone was clearly detrimental to the remodeling process after ECM intervention. Future work should evaluate the effects of complete immobilization, and conversely, overloading of the gastrocnemius upon remodeling outcomes to better understand the mechanisms through which mechanical stimulation contributes to skeletal muscle repair.
Conclusion
The results of this work substantiate the relevance and importance of incorporation of mechanical cues in conjunction with acellular biologic scaffold therapies to support skeletal muscle remodeling following VML. Appropriate mechanical loading may narrow the gap between ECM bioscaffold-mediated constructive remodeling and complete skeletal muscle regeneration, and has important implications when utilizing biologic scaffold therapies for VML in clinical practice.
Acknowledgments
The authors gratefully acknowledge the work of Lori Walton for histology and Xi Yin and Bing Wang for force production analysis. J.D. was supported by the National Science Foundation Graduate Research Fellowship. C.L.D. received support from the National Institute of Biomedical Imaging and Bioengineering (Award No. IR03EB018889-01A1) and the DoD-VA Extremity Trauma & Amputation Center of Excellence (Public Law 110–417, National Defense Authorization Act 2009, Section 723).
Disclosure Statement
The views expressed in this article are those of authors and do not necessarily reflect the official policy of the Departments of the Army, Navy, Defense, nor the United States Government. No competing financial interests exist.
References
- 1.Grasman J.M., Zayas M.J., Page R.L., and Pins G.D. Biomimetic scaffolds for regeneration of volumetric muscle loss in skeletal muscle injuries. Acta Biomater 25, 2, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grogan B.F., Hsu J.R.; Skeletal Trauma Research Consortium. Volumetric muscle loss. J Am Acad Orthop Surg 19 Suppl 1, S35, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Owens B.D., Kragh J.F., Jr., Macaitis J., Svoboda S.J., and Wenke J.C. Characterization of extremity wounds in Operation Iraqi Freedom and Operation Enduring Freedom. J Orthop Trauma 21, 254, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Lin C.H., Lin Y.T., Yeh J.T., and Chen C.T. Free functioning muscle transfer for lower extremity posttraumatic composite structure and functional defect. Plast Reconstr Surg 119, 2118, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Moneim M.S., and Omer G.E. Latissimus dorsi muscle transfer for restoration of elbow flexion after brachial plexus disruption. J Hand Surg Am 11, 135, 1986 [DOI] [PubMed] [Google Scholar]
- 6.Tu Y.K., Yen C.Y., Ma C.H., Yu S.W., Chou Y.C., Lee M.S., and Ueng S.W. Soft-tissue injury management and flap reconstruction for mangled lower extremities. Injury 39 Suppl 4, 75, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Chen X.K., and Walters T.J. Muscle-derived decellularised extracellular matrix improves functional recovery in a rat latissimus dorsi muscle defect model. J Plast Reconstr Aesthet Surg 66, 1750, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Merritt E.K., Hammers D.W., Tierney M., Suggs L.J., Walters T.J., and Farrar R.P. Functional assessment of skeletal muscle regeneration utilizing homologous extracellular matrix as scaffolding. Tissue Eng Part A 16, 1395, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Sicari B.M., Agrawal V., Siu B.F., Medberry C.J., Dearth C.L., Turner N.J., and Badylak S.F. A murine model of volumetric muscle loss and a regenerative medicine approach for tissue replacement. Tissue Eng Part A 18, 1941, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valentin J.E., Turner N.J., Gilbert T.W., and Badylak S.F. Functional skeletal muscle formation with a biologic scaffold. Biomaterials 31, 7475, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner N.J., Yates A.J., Jr., Weber D.J., Qureshi I.R., Stolz D.B., Gilbert T.W., and Badylak S.F. Xenogeneic extracellular matrix as an inductive scaffold for regeneration of a functioning musculotendinous junction. Tissue Eng Part A 16, 3309, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Piccoli M., Urbani L., Alvarez-Fallas M.E., Franzin C., Dedja A., Bertin E., Zuccolotto G., Rosato A., Pavan P., Elvassore N., De Coppi, P., and Pozzobon M. Improvement of diaphragmatic performance through orthotopic application of decellularized extracellular matrix patch. Biomaterials 74, 245–55, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Turner N.J., Badylak J.S., Weber D.J., and Badylak S.F. Biologic scaffold remodeling in a dog model of complex musculoskeletal injury. J Surg Res 176, 490, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Aurora A., Roe J.L., Corona B.T., and Walters T.J. An acellular biologic scaffold does not regenerate appreciable de novo muscle tissue in rat models of volumetric muscle loss injury. Biomaterials 67, 393, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Wolf M.T., Dearth C.L., Sonnenberg S.B., Loboa E.G., and Badylak S.F. Naturally derived and synthetic scaffolds for skeletal muscle reconstruction. Adv Drug Deliv Rev 84, 208, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daly K.A., Wolf M., Johnson S.A., and Badylak S.F. A rabbit model of peripheral compartment syndrome with associated rhabdomyolysis and a regenerative medicine approach for treatment. Tissue Eng Part C Methods 17, 631, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Sicari B.M., Rubin J.P., Dearth C.L., Wolf M.T., Ambrosio F., Boninger M., Turner N.J., Weber D.J., Simpson T.W., Wyse A., Brown E.H., Dziki J.L., Fisher L.E., Brown S., and Badylak S.F. An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Sci Transl Med 6, 234ra58, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mase V.J., Jr., Hsu J.R., Wolf S.E., Wenke J.C., Baer D.G., Owens J., Badylak S.F., and Walters T.J. Clinical application of an acellular biologic scaffold for surgical repair of a large, traumatic quadriceps femoris muscle defect. Orthopedics 33, 511, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Dziki J.B., Badylak S., Yabroudi M., Sicari B., Ambrosio A., Stearns K., Turner N., Wyse A., Boninger M., Brown E., Rubin J. An acellular biologic scaffold treatment for volumetric muscle loss: results of a thirteen patient cohort study. Nat Regen Med 1, 1, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cezar C.A., Roche E.T., Vandenburgh H.H., Duda G.N., Walsh C.J., and Mooney D.J. Biologic-free mechanically induced muscle regeneration. Proc Natl Acad Sci U S A 113, 1534, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gieni R.S., and Hendzel M.J. Mechanotransduction from the ECM to the genome: are the pieces now in place? J Cell Biochem 104, 1964, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Tidball J.G., Lavergne E., Lau K.S., Spencer M.J., Stull J.T., and Wehling M. Mechanical loading regulates NOS expression and activity in developing and adult skeletal muscle. Am J Physiol 275(1 Pt 1), C260, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Kohno S., Yamashita Y., Abe T., Hirasaka K., Oarada M., Ohno A., Teshima-Kondo S., Higashibata A., Choi I., Mills E.M., Okumura Y., Terao J., and Nikawa T. Unloading stress disturbs muscle regeneration through perturbed recruitment and function of macrophages. J Appl Physiol (1985) 112, 1773, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ambrosio F., Ferrari R.J., Distefano G., Plassmeyer J.M., Carvell G.E., Deasy B.M., Boninger M.L., Fitzgerald G.K., and Huard J. The synergistic effect of treadmill running on stem-cell transplantation to heal injured skeletal muscle. Tissue Eng Part A 16, 839, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ambrosio F., Kadi F., Lexell J., Fitzgerald G.K., Boninger M.L., and Huard J. The effect of muscle loading on skeletal muscle regenerative potential: an update of current research findings relating to aging and neuromuscular pathology. Am J Phys Med Rehabil 88, 145, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Badylak S.F., Lantz G.C., Coffey A., and Geddes L.A. Small intestinal submucosa as a large diameter vascular graft in the dog. J Surg Res 47, 74, 1989 [DOI] [PubMed] [Google Scholar]
- 27.Dziki J.L., Wang D.S., Pineda C., Sicari B.M., Rausch T., and Badylak S.F. Solubilized extracellular matrix bioscaffolds derived from diverse source tissues differentially influence macrophage phenotype. J Biomed Mater Res A 105, 138–147, 2017 [DOI] [PubMed] [Google Scholar]
- 28.Dziki J.L., Sicari B.M., Wolf M.T., Cramer M.C., and Badylak S.F. Immunomodulation and mobilization of progenitor cells by extracellular matrix bioscaffolds for volumetric muscle loss treatment. Tissue Eng Part A 22, 1129, 2016 [DOI] [PubMed] [Google Scholar]
- 29.Freytes D.O., Martin J., Velankar S.S., Lee A.S., and Badylak S.F. Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials 29, 1630, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Sicari B.M., Dziki J.L., Siu B.F., Medberry C.J., Dearth C.L., and Badylak S.F. The promotion of a constructive macrophage phenotype by solubilized extracellular matrix. Biomaterials 35, 8605, 2014 [DOI] [PubMed] [Google Scholar]
- 31.Sicari B.M., Zhang L., Londono R., and Badylak S.F. An assay to quantify chemotactic properties of degradation products from extracellular matrix, Methods Mol Biol 1202, 103, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yaffe D., and Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 270, 725, 1977 [DOI] [PubMed] [Google Scholar]
- 33.Distefano G., Ferrari R.J., Weiss C., Deasy B.M., Boninger M.L., Fitzgerald G.K., Huard J., and Ambrosio F. Neuromuscular electrical stimulation as a method to maximize the beneficial effects of muscle stem cells transplanted into dystrophic skeletal muscle. PLoS One 8, e54922, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang Y.J., Chen Y.J., Huang C.W., Fan S.C., Huang B.M., Chang W.T., Tsai Y.S., Su F.C., and Wu C.C. Cyclic stretch facilitates myogenesis in C2C12 myoblasts and rescues thiazolidinedione-inhibited myotube formation. Front Bioeng Biotechnol 4, 27, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.English K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol Cell Biol 91, 19, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Brown B.N., Londono R., Tottey S., Zhang L., Kukla K.A., Wolf M.T., Daly K.A., Reing J.E., and Badylak S.F. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater 8, 978, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ambrosio F., Wolf S.L., Delitto A., Fitzgerald G.K., Badylak S.F., Boninger M.L., and Russell A.J. The emerging relationship between regenerative medicine and physical therapeutics. Phys Ther 90, 1807, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corona B.T., and Greising S.M. Challenges to acellular biological scaffold mediated skeletal muscle tissue regeneration. Biomaterials 104, 238, 2016 [DOI] [PubMed] [Google Scholar]
- 39.Badylak S.F., Dziki J.L., Sicari B.M., Ambrosio F., and Boninger M.L. Mechanisms by which acellular biologic scaffolds promote functional skeletal muscle restoration. Biomaterials 103, 128, 2016 [DOI] [PubMed] [Google Scholar]
- 40.Brown B.N., Sicari B.M., and Badylak S.F. Rethinking regenerative medicine: a macrophage-centered approach. Front Immunol 5, 510, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slivka P.F., Dearth C.L., Keane T.J., Meng F.W., Medberry C.J., Riggio R.T., Reing J.E., and Badylak S.F. Fractionation of an ECM hydrogel into structural and soluble components reveals distinctive roles in regulating macrophage behavior. Biomater Sci 2, 1521, 2014 [DOI] [PubMed] [Google Scholar]
- 42.Wolf M.T., Dearth C.L., Ranallo C.A., LoPresti S.T., Carey L.E., Daly K.A., Brown B.N., and Badylak S.F. Macrophage polarization in response to ECM coated polypropylene mesh. Biomaterials 35, 6838, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown B.N., Ratner B.D., Goodman S.B., Amar S. and Badylak S.F. Macrophage polarization: an opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials 33, 3792, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valentin J.E., Stewart-Akers A.M., Gilbert T.W., and Badylak S.F. Macrophage participation in the degradation and remodeling of extracellular matrix scaffolds. Tissue Eng Part A 15, 1687, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown B.N., Valentin J.E., Stewart-Akers A.M., McCabe G.P., and Badylak S.F. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials 30, 1482, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Badylak S.F., Valentin J.E., Ravindra A.K., McCabe G.P., and Stewart-Akers A.M. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A 14, 1835, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Ballotta V., Driessen-Mol A., Bouten C.V., and Baaijens F.P. Strain-dependent modulation of macrophage polarization within scaffolds. Biomaterials 35, 4919, 2014 [DOI] [PubMed] [Google Scholar]
- 48.Patel N.R., Bole M., Chen C., Hardin C.C., Kho A.T., Mih J., Deng L., Butler J., Tschumperlin D., Fredberg J.J., Krishnan R., and Koziel H. Cell elasticity determines macrophage function. PLoS One 7, e41024, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Throm Quinlan A.M., Sierad L.N., Capulli A.K., Firstenberg L.E., and Billiar K.L. Combining dynamic stretch and tunable stiffness to probe cell mechanobiology in vitro. PLoS One 6, e23272, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cortes P., Riser B.L., Yee J., and Narins R.G. Mechanical strain of glomerular mesangial cells in the pathogenesis of glomerulosclerosis: clinical implications, Nephrol Dial Transplant 14, 1351, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Bouten C.V., Dankers P.Y., Driessen-Mol A., Pedron S., Brizard A.M., and Baaijens F.P. Substrates for cardiovascular tissue engineering. Adv Drug Deliv Rev 63, 221, 2011 [DOI] [PubMed] [Google Scholar]
- 52.Murray P.J., Allen J.E., Biswas S.K., Fisher E.A., Gilroy D.W., Goerdt S. Gordon S., Hamilton J.A., Ivashkiv L.B., Lawrence T., Locati M., Mantovani A., Martinez F.O., Mege J.L., Mosser D.M., Natoli G., Saeij J.P., Schultze J.L., Shirey K.A., Sica A., Suttles J., Udalova I., van Ginderachter J.A., Vogel S.N., and Wynn T.A. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mosser D.M., and Edwards J.P. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8, 958, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Villalta S.A., Nguyen H.X., Deng B., Gotoh T., and Tidball J.G. Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Hum Mol Genet 18, 482, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tidball J.G. Interactions between muscle and the immune system during modified musculoskeletal loading. Clin Orthop Relat Res 403 Suppl, S100, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Vassilakopoulos T., Govindaraju K., Parthenis D., Eidelman D.H., Watanabe Y., and Hussain S.N. Nitric oxide production in the ventilatory muscles in response to acute resistive loading. Am J Physiol Lung Cell Mol Physiol 292, L1013, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Motohashi N., and Asakura A. Muscle satellite cell heterogeneity and self-renewal. Front Cell Dev Biol 2, 1, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tedesco F.S., Dellavalle A., Diaz-Manera J., Messina G., and Cossu G. Repairing skeletal muscle: regenerative potential of skeletal muscle stem cells. J Clin Invest 120, 11, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dellavalle A., Maroli G., Covarello D., Azzoni E., Innocenzi A., Perani L., Antonini S., Sambasivan R., Brunelli S., Tajbakhsh S., and Cossu G. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun 2, 499, 2011 [DOI] [PubMed] [Google Scholar]
- 60.Buckingham M. Myogenic progenitor cells and skeletal myogenesis in vertebrates. Curr Opin Genet Dev 16, 525, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Ciciliot S., and Schiaffino S. Regeneration of mammalian skeletal muscle. Basic mechanisms and clinical implications. Curr Pharm Des 16, 906, 2010 [DOI] [PubMed] [Google Scholar]
- 62.Wang X.D., Kawano F., Matsuoka Y., Fukunaga K., Terada M., Sudoh M., Ishihara A., and Ohira Y. Mechanical load-dependent regulation of satellite cell and fiber size in rat soleus muscle. Am J Physiol Cell Physiol 290, C981, 2006 [DOI] [PubMed] [Google Scholar]