Abstract
The solution-favored conformations of the 13-residue disordered peptide, indolicidin (Ile1-Leu2-Pro3-Trp4-Lys5-Trp6-Pro7-Trp8-Trp9-Pro10-Trp11-Arg12-Arg13), are evaluated using electrospray ionization (ESI) coupled to ion mobility spectrometry-mass spectrometry (IMS-MS). The ESI-IMS-MS distributions for the dominant [M+4H]4+ ions indicate that three populations of structures coexist in a range of aqueous to non-aqueous solutions (water:dioxane, water:trifluoroethanol, and water:hexafluoroisopropanol). Conformer types and their relative abundances change in response to different solution environments suggesting that the gas phase conformers reflect on the solution populations present in different solvent environments. Collisional activation of isolated gas phase conformations with IMS-IMS-MS experiments provides additional insight about the relative stabilities of different structural types in the absence of solvent. Simulated annealing studies suggest that proline configuration may be important for the presence of multiple conformations.
Keywords: Disordered peptide, Ion mobility, Mass spectrometry, Cross section, Indolicidin, Antimicrobial structure, Conformation
Graphical Abstract

1. Introduction
The classification of peptide and protein structures as “disordered” or “unstructured” raises a number of questions regarding the dynamics and functions of such species. For example, over what timescales, and in what environments do such disorder prevail? It is known that a wide range of disordered proteins is present in cells. Computations suggest that ~10%–20% of full-length eukaryotic proteins and ~25%–40% of protein domains may be disordered [1]. Despite the lack of defined structures, such species display a variety of functions in signaling and regulatory pathways [2–4], contain regions prone to proteolysis and post-translational modifications [5], and are associated with different diseases [2,4,6].
The widespread existence of disordered proteins, their biological and pathological significance, as well as the limitations of current techniques in assessing their inherent conformational heterogeneity have created a need for alternative analytical methods to study disordered systems. In recent years, electrospray ionization (ESI) coupled to ion mobility spectrometry-mass spectrometry (IMS-MS) has emerged as a means to gain insight into the structures of biomolecules present in solution [7–11]. The evaporative cooling process of ESI can trap metastable conformers arising from solution [12,13], and these preserved solution-like conformations can be separated based on their size and charge with IMS-MS [7–12]. Several studies [14–21] have employed IMS-MS techniques to probe the conformational properties of disordered proteins such as α-synuclein [15], HMGA (high-mobility group A) protein [16], human salivary proline rich protein IB5 [17], apo-cytochrome c and apo-osteocalcin [18], DNA-binding domain of p53 [19,20], and Swi5-Srf1 protein complex [21]. While most of these studies are focused on studying disordered proteins, few efforts have been made to probe the conformational ensembles of disordered peptides [18].
In the present study, we use ESI-IMS-MS to study the conformational properties of a disordered peptide indolicidin (Ile1-Leu2-Pro3-Trp4-Lys5-Trp6-Pro7-Trp8-Trp9-Pro10-Trp11-Arg12-Arg13), a 13-residue antimicrobial peptide that demonstrates potent activity against Gram-positive and Gram-negative bacteria [22], fungi [23], HIV-I [24], and protozoa [25]. Residues 7–10 of indolicidin constitute a Pro-Trp-Trp-Pro (or PWWP) motif, commonly found in PWWP domain proteins named after this motif [26]. Both indolicidin [26,27] and PWWP domains of larger proteins [28,29] are involved in DNA binding. Previous circular dichroism studies on indolicidin have shown that the peptide is unstructured in aqueous and bulk organic solution, but adopts a poly-L-proline type II helix or a β-turn conformation in lipids [27,30]. Prior nuclear magnetic resonance (NMR) studies of indolicidin in water provide evidence of multiple conformations; however, no structure was determined due to the overlap of NMR signals and poor sensitivity [27]. In the presence of 50% trifluoroethanol (TFE) in water, two types of structures are deduced from the NMR studies [27]; each type has a rigid backbone involving 5th through 11th residues (Lys5-Trp6-Pro7-Trp8-Trp9-Pro10-Trp11), while C- and N-terminus are disordered. Within the rigid backbone, different modes of contact between two WPW (Trp6-Pro7-Trp8 and Trp9-Pro10-Trp11) motifs are important in distinguishing the two families of structures [27]. Similarly, NMR studies in dodecylphosphocholine (DPC) and sodium dodecyl sulfate (SDS) show well-defined region from 3rd to 11th residues (Pro3-Trp4-Lys5-Trp6-Pro7-Trp8-Trp9-Pro10-Trp11), and 5th to 11th residues (Lys5-Trp6-Pro7-Trp8-Trp9-Pro10-Trp11), respectively [31].
2. Experimental section
2.1. IMS-MS analysis
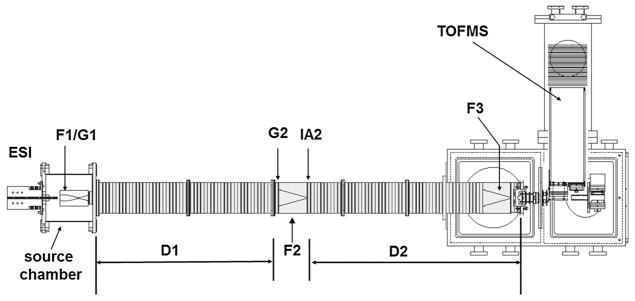
Details regarding the home-built IMS-MS instrument [32,33] (shown in Fig. 1) and the theory of IMS-MS techniques [34–38] have been reported elsewhere. A TriVersa NanoMate autosampler (Advion, Ithaca, NY) was used as a nanoelectrospray ionization source. The ESI source was operated under gentle conditions with no activation voltage or heat applied to the source. Ions were trapped in a Smith geometry ion funnel [39] and pulsed into the drift tube (~1.83 m in length) filled with ~3.0 Torr of helium buffer gas at 300 K. Under the influence of a uniform electric field, the ions undergo collisions with the buffer gas and are separated based on overall size-to-charge ratio [40]. As ions exit the drift tube, they were directed to time-of-flight (TOF) MS instrument for mass analysis. Because the mobility experiment is carried out on a millisecond time scale and the mass analysis requires only microseconds, IMS and MS information can be recorded in a nested fashion [41]. As a result, a two-dimensional representation (2D-plot) of the dataset was obtained, where mass spectra were acquired within individual drift time windows [41].
Fig. 1.
Schematic diagram of the instrument used to probe solution-related structures of indolicidin. Different parts of the instrument are indicated on the diagram, including three ion funnels (F1, F2, F3), a two-meter long drift tube (D1 and D2), and an ion activation region (IA2).
2.2. Calculation of collision cross section
Values of collision cross sections (Ω) are determined from the measured drift time (tD) by the following relation [34]:
where ze is the total charge of the ion, kB is the Boltzmann’s constant, MI and MB are the masses of the ion and buffer gas respectively, E is the electric field, L is the length of the drift tube, T is the temperature, P is the pressure, and N is the number density of buffer gas at standard temperature and pressure (STP). In the present experiment, the drift tube is operated at ~10 V cm−1, and radio frequencies varying from 390 kHz to 440 kHz are applied to the instrument funnels (F1, F2 and F3; Fig. 1).
2.3. Two-dimensional IMS–IMS experiments
The presence of an electrostatic gate G2 (Fig. 1) at the entrance of F2 (Fig. 1) allows for the selection of a narrow distribution of ions with a given mobility to conduct two-dimensional IMS–IMS experiments. Activation of the selected ions was performed at IA2 (Fig. 1) by increasing the voltage between the two lenses constituting the activation region (IA2, Fig. 1). Resulting distributions from activation were measured in the second part of the drift tube (D2, Fig. 1) and finally mass analyzed by the TOF MS. In the present work, ions pertaining to conformers B and C were selected (selection width 10 μs) and gradually activated to voltages ranging from 10 V to 100 V.
2.4. Molecular dynamics simulations
The experimental IMS–IMS activation process can be used to anneal selected structures, and complementary molecular dynamics simulations can be used to find suitable candidate structures that may correlate with the experimental data, providing insight into what conformers may be present for indolicidin. Simulated annealing studies of indolicidin in vacuum were performed with GROMACS 4.6.5 [42] software using the OPLS-AA/L all atom force field [43]. Atomic coordinates for eight candidate solution-phase structures, containing different stereoisomeric forms of the three Xaa-Pro peptide bonds, were generated from the NMR structure deposited in the Protein Data Bank (PDB code, 1G89) [27]. The cis-and trans-configuration of the Xaa-Pro peptide bond in the NMR structure was altered using PyMOL (DeLano Scientific, San Carlos, CA, USA) [44]. For each candidate solution-phase structures, four sites of protonation were assigned at Lys3, Arg12, Arg14, and the N-terminus. The LINCS algorithm [45] was used to constrain all peptide covalent bonds and no cut-offs were applied for periodic boundary conditions and non-bonding interactions. Each initial starting structure was then subjected to an energy minimization process to ensure that there was no steric clashes or inappropriate geometry in the system. Similar to previous approach [9], the resulting structure from the minimization process was heated up to 500 K for 5 ps (each time step = 1 fs), equilibrated at the same temperature for 10 ps, and finally cooled to 298 K for 5 ps. Simulations were then performed at 298 K for 10 ps and the trajectory for each run of simulation was sampled at 10 ps. This annealing cycle of heating and subsequent cooling was repeated 100 times (total run of 3 ns), where the resulting structure from each run became the input structure for the next round of the annealing process. As a result, 100 annealed structures were created from each initial structure, generating a total of 800 annealed structures. The theoretical collision cross sections values for the 10 low-energy structures from each candidate solution structure were calculated by the trajectory method (TM) in MOBCAL [35]. Single representative geometry with relatively low energy and calculated cross section within ± 2% of the measured cross section was then selected to be the proposed structure. For all the conformers, majority of the low-energy structures with matching cross section had same cis- and trans- configuration. Hydrogen bonds in the representative geometries for conformers A, B, and C were determined with Visual molecular dynamics (VMD) software [46]. The distance cut-off of 4.0 and the angle cut-off of 40 were used to search for the hydrogen bonds. Turn- and coil-like structures present in the geometries of conformer A, B, and C were identified using the default feature in VMD [46].
2.5. Sample preparation
Indolicidin extracted from bovine neutrophils (>95% purity) was purchased (GenScript USA Inc.; Piscataway, NJ) and used without further purification. Solution compositions ranged from 100:0 to 10:90 water:organic solvent (v:v). Dioxane, TFE, and hexafluoroisopropanol (HFIP) were used as organic solvents.
3. Results and discussion
3.1. Nested IMS-MS measurements of indolicidin in water
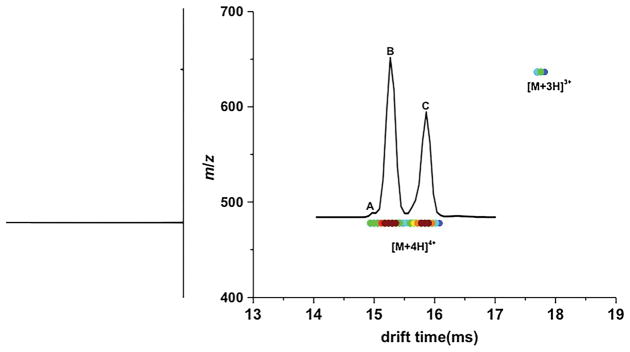
Fig. 2 shows the 2D-plot from the nested IMS-MS measurement of indolicidin when electrosprayed from pure water solution. In the mass spectrum, [M+4H]4+ is the dominant charge state (accounts for >95% of the total ion population) and thus is the focus of this study. The mobility distribution shows three peaks corresponding to families of co-existing conformers, i.e., A at 14.94 ms comprising ~3% of the distribution, followed by B at 15.24 ms comprising ~53% of the distribution and C at 15.84 ms comprising ~44% of the distribution. The observation of multiple conformations in water is in agreement with data from NMR studies [27].
Fig. 2.
Two-dimensional plot of indolicidin in water obtained by IMS-MS experiment. Ion intensities are plotted as a function of drift time and m/z. The corresponding mass spectrum, obtained by integrating all drift time bins for each m/z value, is shown on the left side of the plot. [M+4H]4+ and [M+3H]3+ ions of indolicidin are labeled. The mobility distribution for [M+4H]4+ indolicidin ions is overlaid with the plot. Two major conformers (B and C) and a minor conformer (A) are indicated.
3.2. Mobility distributions of indolicidin ions in different solutions
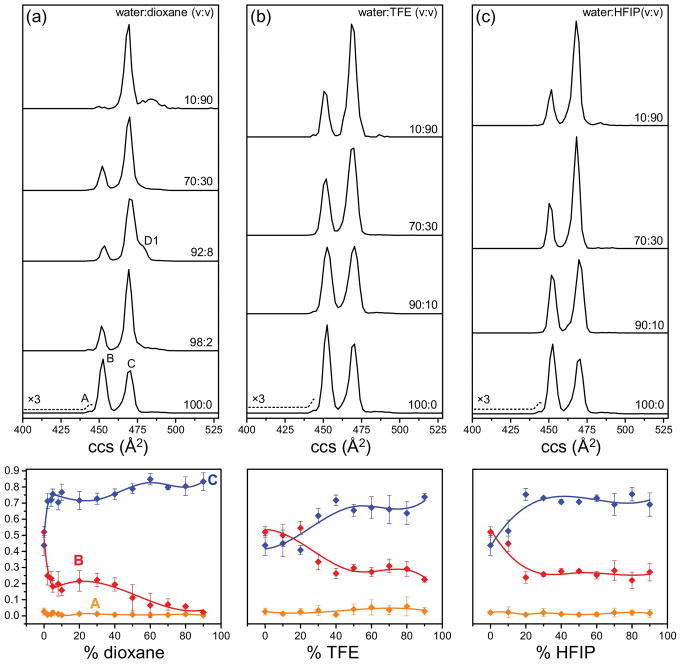
Fig. 3 shows the mobility distributions of indolicidin obtained from solutions with varying contents of dioxane, TFE, and HFIP. In water, the three observed peaks correspond to the collision cross sections of Ω = 441 Å2 (A), Ω = 450 Å2 (B), and Ω = 468 Å2 (C). As the amount of organic solvent increases in solution, the relative abundance of conformer C increases accompanied by a decrease in the relative abundance of conformer B. The presence of two major conformers (B and C) in 50:50 water:TFE solution is consistent with the observations from NMR studies [27]. New elongated features, not present in pure water solution, are observed in solution with increasing proportion of organic solvent in the solution. For instance, new features with Ω = 485 Å2 and Ω = 490 Å2 are present in the mobility distribution obtained from solutions with 90% dioxane, TFE, and HFIP. These peaks might arise from solvent complexes that dissociate after IMS separation, but before mass detection. Such solvent adduct peaks have also been also observed previously [47]. The low dielectric constant of the solution with high amount of organic solvent might promote the formation of these complexes. The role of non-polar solvent in promoting aggregation has been demonstrated before as well [48].
Fig. 3.
Solution-related structures of indolicidin. Mobility distributions for [M+4H]4+ ions of indolicidin from solutions of (a) water:dioxane, (b) water:TFE, and (c) water:HFIP. The proportion of organic solvent (v:v) increases from bottom to top as indicated. The bottom trace represents the mobility distribution from pure water solution. Normalized intensities of different conformer types as a function of solution composition are shown in bottom panel. Conformers A, B, and C are represented in orange, red, and blue, respectively.
Dioxane, TFE, and HFIP are commonly used solvents for structural studies in solution because of their role in increasing protein helicity [49–51]. The non-polar nature of dioxane is known to promote intramolecular hydrogen bonds in proteins, and is associated with an increase in their helical properties [49]. TFE also promotes intramolecular hydrogen bonding and stabilizes the secondary structure in peptides by displacing water molecules surrounding the peptides [50]. It is interesting to note that even a low percent of dioxane (2%) in solution induces a significant difference in the conformational distribution of indolicidin (relative abundances of conformer B and C are 25% and 72%, respectively). We also notice that solutions with 10–30% HFIP induce a greater change in the relative abundances of conformers B and C when compared to solutions with 10–30% TFE. For example, in solution with 10% TFE, conformer B and C are present in equal abundances; however, the relative abundance of conformer C (53%) is greater than conformer B (45%) in solution with 10% HFIP. This observation supports the idea that HFIP is better than TFE at stabilizing secondary structures in peptides due to the added fluorine atoms and bulkier isopropyl groups [50]. The influence of these organic solvents on the gas phase conformers of indolicidin suggest that different states in solution can generate dissimilar gas phase conformers upon dehydration, and can be distinguished with IMS-MS.
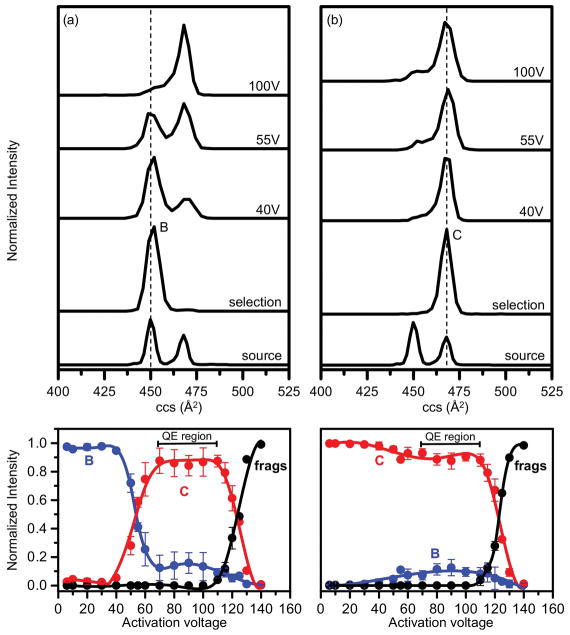
3.3. Relative stabilities of conformers in [M±4H]4± distribution of indolicidin
Fig. 4 shows the mobility profiles obtained when a narrow distribution of ions corresponding to conformer B (Ω = 450 Å2) or conformer C (Ω = 468 Å2) is selected at G2 (Fig. 1) and submitted to the annealing process associated with collisional activation. Conformer C is present in low abundance when conformer B is selected at G2 (Fig. 4a). However, no feature corresponding to conformer B is observed upon selection of conformer C (Fig. 4b). At low activation voltages (10–40 V), selection and activation of both conformers B and C produce no major change. The transition of conformer B to conformer C is observed when the activation voltage is increased from 40 V to 70 V (Fig. 4a). In the case of conformer B, conformers B and C are present in equal abundances at 55 V; however, conformer C dominates the distribution at 70 V. Beyond 70 V, no change is observed in the populations of conformers B and C. Conformer C, on the other hand, is relatively stable (Fig. 4b), and doesn’t undergo major structural changes. Conformer B is present in low abundance only at high activation voltages (40–70 V). Independent of the initial conformer selected, higher activation voltages produce a quasi-equilibrium distribution [8] and favor the formation of elongated conformer C. This observation is consistent with prior studies that show how all annealed ions, above a certain energy barrier between states, collapse to form the same quasi-equilibrium populations [8].
Fig. 4.
IMS-IMS-MS experiments on conformers B and C. Mobility distribution obtained from the selection and activation of (a) conformer B and (b) conformer C. The bottom trace in both panels represents the source distribution from pure water solution, prior to any selection and activation. The activation voltage for each mobility distribution is indicated. Normalized intensities of conformers B and C as well as fragment ions are plotted as a function of increasing activation voltages in the bottom panel. The intensities of conformer B, conformer C, and populations of fragments (frags) are represented by blue, red, and black, respectively. Quasi-equilibrium (QE) region before fragmentation is denoted with bracketed line. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
It is interesting to note unique behaviors displayed by conformers B and C upon activation. Conformer B favors the formation of conformer C at high activation voltages. It can, thus, be inferred that conformer B present at low activation voltages is a solution-like structure that retains some memory of elements relevant to its initial structure in solution. Conformer C remains as the stable conformation type in all-activating conditions, and most likely represents the stable extended structure present in solution. The inability of conformer C to form conformer B in vacuo further strengthens the argument that conformer B is a solution-related structure, which is not stable in vacuo but accessible only in the presence of solvent. Our solution studies also demonstrate how aqueous environment is important for the stability of conformer B since nonaqueous environment with higher content of organic solvent favored conformer C.
3.4. Insight into various structures of peptide using molecular dynamics simulations
Simulated annealing studies were performed to get a feeling for the types of structures present for indolicidin ions. The proposed low-energy structures for various conformers are shown in Fig. 5. The outcome of the simulated studies suggests that the cis–trans isomerization of proline residues may be important for the presence of multiple conformations. In the suggested geometries of conformers A (Ωcalculated = 435 Å2) and B (Ωcalculated = 445 Å2), the configuration of proline residues is cis-Pro3, trans-Pro7, and trans-Pro10. On the other hand, the proposed structure of conformer C (Ωcalculated = 473 Å2) has proline residues in trans-Pro3, cis-Pro7, and trans-Pro10 configurations. Since the proposed structures of conformers A and B are very similar, it is possible that they originate from the same structure in solution. However, we take great care to minimize any activation in the source during electrospray. So, we believe that these two conformers originate from unique solution structures. Previous studies have shown that the hydrogen-bonding interactions around the protonated N-terminus seems to play an important role in the stability of cis-Pro configuration in various peptides [9,52–54]. Consistent with these earlier findings, compact conformers A and B (with cis-Pro3) are stabilized by hydrogen-bonding interactions involving the protonated N-terminus. In conformer A, a distinct bend near the N-terminus is stabilized by two hydrogen bonds; one between the protonated N-terminus and carbonyl oxygen of Lys3 and other between the NH group of Trp8 and carbonyl oxygen of Leu2. On the other hand, the bend for conformer B is stabilized by a single hydrogen bond between the protonated N-terminus and Lys3. Meanwhile, no hydrogen-bonding interactions involving N-terminus is found in elongated conformer C.
Fig. 5.
Suggested low-energy structures with molecular modeling. Proposed gas phase structures for different conformers of indolicidin (labeled as conformer A, B, and C) obtained from simulated annealing studies in vacuum. The charged sites are located at Lys3, Arg12, Arg14, and the protonated N-terminus. Coil like structures are represented in yellow and turn like structures are represented in pink. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
4. Summary and conclusions
IMS-MS is used to monitor the solution-induced changes in the conformational populations of indolicidin. When electrosprayed from water, [M+4H]4+ charge state of indolicidin exists as an ensemble of two major conformers (B and C) and one minor conformer (A). When the content of organic solvents (dioxane, TFE, and HFIP) increases in solution, the elongated conformer C is more favored and becomes the dominant conformer type. The stabilities of conformers B and C in solvent-free environment are further probed with IMS-IMS-MS experiments. Both IMS-MS and IMS-IMS-MS studies suggest that compact conformer B is favored in aqueous solution but not in gas phase. In contrast, conformer C is favored in vacuo and in nonaqueous environment. Molecular modeling suggests that cis-trans isomerization of proline residues may influence the structures of indolicidin. Overall, we find evidence for at least four stable conformer types present for indolicidin under different solution conditions, which highlights the ability of IMS-MS to monitor populations of conformers present in systems classified as “disordered”. IMS-MS analysis is also sensitive to conformers present in low abundances (e.g., A) that are harder to detect with current structural techniques. For disordered systems that can sample many structures, the structural characterization of multiple coexisting structures, including those present in low abundance, will be important to understand how these dynamic structures facilitate various biological functions. While we have focused on the ability to ascertain structural elements in what is nominally a disordered peptide, this peptide is also known to have antimicrobial properties; thus, while not stressed in detail, IMS-MS may emerge as a powerful means of characterizing the structures and mechanistic activity of antimicrobial peptides that exhibit broad activity against various pathogens despite the lack of secondary structure. Insight into the conformational preferences of such peptides, which is not easily ascertained by other techniques, may guide the development of better therapeutics.
Acknowledgments
Partial support of this work is from a NIH grant (R01 GM117207-01). NK was supported by Robert & Marjorie Mann Fellowship from Indiana University.
References
- 1.Theillet FX, Kalmar L, Tompa P, Han KH, Selenko P, Dunker AK, Daughdrill GW, Uversky VN. The alphabet of intrinsic disorder: I. Act like a Pro: on the abundance and roles of proline residues in intrinsically disordered proteins. Intrinsically Disord Proteins. 2013;1:e24360. doi: 10.4161/idp.24360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iakoucheva LM, Brown CJ, Lawson JD, Obradović Z, Dunker AK. Intrinsic disorder in cell-signaling and cancer-associated proteins. J Mol Biol. 2002;323:573–584. doi: 10.1016/s0022-2836(02)00969-5. [DOI] [PubMed] [Google Scholar]
- 3.Dunker AK, Cortese MS, Romero P, Iakoucheva LM, Uversky VN. Flexible nets. FEBS J. 2005;272:5129–5148. doi: 10.1111/j.1742-4658.2005.04948.x. [DOI] [PubMed] [Google Scholar]
- 4.Uversky VN, Oldfield CJ, Dunker AK. Showing your ID: intrinsic disorder as an ID for recognition, regulation and cell signaling. J Mol Recogn. 2005;18:343–384. doi: 10.1002/jmr.747. [DOI] [PubMed] [Google Scholar]
- 5.Xie H, Vucetic S, Iakoucheva LM, Oldfield CJ, Dunker AK, Obradovic Z, Uversky VN. Functional anthology of intrinsic disorder. III. Ligands, postranslational modifications and diseases associated with intrinsically disordered proteins. J Proteome Res. 2007;6:1917. doi: 10.1021/pr060394e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uversky VN, Oldfield CJ, Dunker AK. Intrinsically disordered proteins in human diseases: introducing the D2 concept. Annu Rev Biophys. 2008;37:215–246. doi: 10.1146/annurev.biophys.37.032807.125924. [DOI] [PubMed] [Google Scholar]
- 7.Shi H, Pierson NA, Valentine SJ, Clemmer DE. Conformation types of ubiquitin [M+8H] 8+ ions from water: methanol solutions: evidence for the N and A states in aqueous solution. J Phys Chem B. 2012;116:3344. doi: 10.1021/jp210797x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pierson NA, Valentine SJ, Clemmer DE. Evidence for a quasi-equilibrium distribution of states for bradykinin [M+3H] 3+ ions in the gas phase. J Phys Chem B. 2010;114:7777. doi: 10.1021/jp102478k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glover MS, Bellinger EP, Radivojac P, Clemmer DE. Penultimate proline in neuropeptides. Anal Chem. 2015;87:8466–8472. doi: 10.1021/acs.analchem.5b01889. [DOI] [PubMed] [Google Scholar]
- 10.Wyttenbach T, Bowers MT. Structural stability from solution to the gas phase: native solution structure of ubiquitin survives analysis in a solvent-free ion mobility–mass spectrometry environment. J Phys Chem B. 2011;115:12266–12275. doi: 10.1021/jp206867a. [DOI] [PubMed] [Google Scholar]
- 11.Wang SC, Politis A, Di Bartolo N, Bavro VN, Tucker SJ, Booth PJ, Barrera NP, Robinson CV. Ion mobility mass spectrometry of two tetrameric membrane protein complexes reveals compact structures and differences in stability and packing. J Am Chem Soc. 2010;132:15468–15470. doi: 10.1021/ja104312e. [DOI] [PubMed] [Google Scholar]
- 12.Silveira JA, Fort KL, Kim D, Servage KA, Pierson NA, Clemmer DE, Russell DH. From solution to the gas phase: stepwise dehydration and kinetic trapping of Substance P reveals the origin of peptide conformations. J Am Chem Soc. 2013;135:19147–19153. doi: 10.1021/ja4114193. [DOI] [PubMed] [Google Scholar]
- 13.Lee SW, Freivogel P, Schindler T, Beauchamp J. Freeze-dried biomolecules: FT-ICR studies of the specific solvation of functional groups and clathrate formation observed by the slow evaporation of water from hydrated peptides and model compounds in the gas phase. J Am Chem Soc. 1998;120:11758–11765. [Google Scholar]
- 14.Beveridge R, Chappuis Q, Macphee C, Barran P. Mass spectrometry methods for intrinsically disordered proteins. Analyst. 2013;138:32–42. doi: 10.1039/c2an35665a. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein SL, Liu D, Wyttenbach T, Bowers MT, Lee JC, Gray HB, Winkler JR. α-Synuclein: stable compact and extended monomeric structures and pH dependence of dimer formation. J Am Soc Mass Spectrom. 2004;15:1435–1443. doi: 10.1016/j.jasms.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Maurizio E, Cravello L, Brady L, Spolaore B, Arnoldo L, Giancotti V, Manfioletti G, Sgarra R. Conformational role for the C-terminal tail of the intrinsically disordered high mobility group A (HMGA) chromatin factors. J Proteome Res. 2011;10:3283–3291. doi: 10.1021/pr200116w. [DOI] [PubMed] [Google Scholar]
- 17.Canon F, Ballivian R, Chirot F, Antoine R, Sarni-Manchado P, Lemoine J, Dugourd P. Folding of a salivary intrinsically disordered protein upon binding to tannins. J Am Chem Soc. 2011;133:7847–7852. doi: 10.1021/ja200534f. [DOI] [PubMed] [Google Scholar]
- 18.Knapman TW, Valette NM, Warriner SL, Ashcroft AE. Ion mobility spectrometry-mass spectrometry of intrinsically unfolded proteins: trying to put order into disorder. Curr Anal Chem. 2013;9:181–191. doi: 10.2174/1573411011309020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jurneczko E, Cruickshank F, Porrini M, Nikolova P, Campuzano ID, Morris M, Barran PE. Intrinsic disorder in proteins: a challenge for (un) structural biology met by ion mobility–mass spectrometry. Biochem Soc Trans. 2012;40:1021–1026. doi: 10.1042/BST20120125. [DOI] [PubMed] [Google Scholar]
- 20.Jurneczko E, Cruickshank F, Porrini M, Clarke DJ, Campuzano ID, Morris M, Nikolova PV, Barran PE. Probing the conformational diversity of cancer-associated mutations in p53 with ion-mobility mass spectrometry. Angew Chem Int Ed. 2013;52:4370–4374. doi: 10.1002/anie.201210015. [DOI] [PubMed] [Google Scholar]
- 21.Saikusa K, Kuwabara N, Kokabu Y, Inoue Y, Sato M, Iwasaki H, Shimizu T, Ikeguchi M, Akashi S. Characterisation of an intrinsically disordered protein complex of Swi5–Sfr1 by ion mobility mass spectrometry and small-angle X-ray scattering. Analyst. 2013;138:1441–1449. doi: 10.1039/c2an35878f. [DOI] [PubMed] [Google Scholar]
- 22.Selsted ME, Novotny MJ, Morris WL, Tang YQ, Smith W, Cullor JS. Indolicidin, a novel bactericidal tridecapeptide amide from neutrophils. J Biol Chem. 1992;267:4292–4295. [PubMed] [Google Scholar]
- 23.Ahmad I, Perkins WR, Lupan DM, Selsted ME, Janoff AS. Liposomal entrapment of the neutrophil-derived peptide indolicidin endows it with in vivo antifungal activity. Biochim Biophys Acta. 1995;1237:109–114. doi: 10.1016/0005-2736(95)00087-j. [DOI] [PubMed] [Google Scholar]
- 24.Robinson WE, McDougall B, Tran D, Selsted ME. Anti-HIV-1 activity of indolicidin, an antimicrobial peptide from neutrophils. J Leukoc Biol. 1998;63:94–100. doi: 10.1002/jlb.63.1.94. [DOI] [PubMed] [Google Scholar]
- 25.Aley SB, Zimmerman M, Hetsko M, Selsted ME, Gillin FD. Killing of Giardia lamblia by cryptdins and cationic neutrophil peptides. Infect Immun. 1994;62:5397–5403. doi: 10.1128/iai.62.12.5397-5403.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marchand C, Krajewski K, Lee HF, Antony S, Johnson AA, Amin R, Roller P, Kvaratskhelia M, Pommier Y. Covalent binding of the natural antimicrobial peptide indolicidin to DNA abasic sites. Nucleic Acids Res. 2006;34:5157–5165. doi: 10.1093/nar/gkl667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu CH, Chen C, Jou ML, Lee AYL, Lin YC, Yu YP, Huang WT, Wu SH. Structural and DNA-binding studies on the bovine antimicrobial peptide, indolicidin: evidence for multiple conformations involved in binding to membranes and DNA. Nucleic Acids Res. 2005;33:4053–4064. doi: 10.1093/nar/gki725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lukasik SM, Cierpicki T, Borloz M, Grembecka J, Everett A, Bushweller JH. High resolution structure of the HDGF PWWP domain: a potential DNA binding domain. Protein Sci. 2006;15:314–323. doi: 10.1110/ps.051751706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiu C, Sawada K, Zhang X, Cheng X. The PWWP domain of mammalian DNA methyltransferase Dnmt3b defines a new family of DNA-binding folds. Nat Struct Biol. 2002;9:217–224. doi: 10.1038/nsb759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Falla TJ, Karunaratne DN, Hancock RE. Mode of action of the antimicrobial peptide indolicidin. J Biol Chem. 1996;271:19298–19303. doi: 10.1074/jbc.271.32.19298. [DOI] [PubMed] [Google Scholar]
- 31.Rozek A, Friedrich CL, Hancock RE. Structure of the bovine antimicrobial peptide indolicidin bound to dodecylphosphocholine and sodium dodecyl sulfate micelles†. Biochemistry. 2000;39:15765–15774. [PubMed] [Google Scholar]
- 32.Koeniger SL, Merenbloom SI, Valentine SJ, Jarrold MF, Udseth HR, Smith RD, Clemmer DE. An IMS- IMS analogue of MS- MS. Anal Chem. 2006;78:4161–4174. doi: 10.1021/ac051060w. [DOI] [PubMed] [Google Scholar]
- 33.Merenbloom SI, Koeniger SL, Valentine SJ, Plasencia MD, Clemmer DE. IMS- IMS and IMS- IMS- IMS/MS for separating peptide and protein fragment ions. Anal Chem. 2006;78:2802–2809. doi: 10.1021/ac052208e. [DOI] [PubMed] [Google Scholar]
- 34.Mason EA, McDaniel EW. Transport Properties of Ions in Gases. New York: 1988. [Google Scholar]
- 35.Mesleh M, Hunter J, Shvartsburg A, Schatz GC, Jarrold M. Structural information from ion mobility measurements: effects of the long-range potential. J Phys Chem. 1996;100:16082–16086. [Google Scholar]
- 36.Wyttenbach T, von Helden G, Batka JJ, Carlat D, Bowers MT. Effect of the long-range potential on ion mobility measurements. J Am Soc Mass Spectrom. 1997;8:275–282. [Google Scholar]
- 37.Revercomb H, Mason EA. Theory of plasma chromatography/gaseous electrophoresis. Review. Anal Chem. 1975;47:970–983. [Google Scholar]
- 38.Shvartsburg AA, Jarrold MF. An exact hard-spheres scattering model for the mobilities of polyatomic ions. Chem Phys Lett. 1996;261:86–91. [Google Scholar]
- 39.Tang K, Shvartsburg AA, Lee HN, Prior DC, Buschbach MA, Li F, Tolmachev A, Anderson GA, Smith RD. High-sensitivity ion mobility spectrometry/mass spectrometry using electrodynamic ion funnel interfaces. Anal Chem. 2005;77:3330. doi: 10.1021/ac048315a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clemmer DE, Jarrold MF. Ion mobility measurements and their applications to clusters and biomolecules. J Mass Spectrom. 1997;32:577–592. [Google Scholar]
- 41.Hoaglund CS, Valentine SJ, Sporleder CR, Reilly JP, Clemmer DE. Three-dimensional ion mobility/TOFMS analysis of electrosprayed biomolecules. Anal Chem. 1998;70:2236–2242. doi: 10.1021/ac980059c. [DOI] [PubMed] [Google Scholar]
- 42.Hess B, Kutzner C, Van Der Spoel D, Lindahl E. GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J Chem Theory Comp. 2008;4:435–447. doi: 10.1021/ct700301q. [DOI] [PubMed] [Google Scholar]
- 43.Kaminski GA, Friesner RA, Tirado-Rives J, Jorgensen WL. Evaluation and reparametrization of the OPLS-AA force field for proteins via comparison with accurate quantum chemical calculations on peptides. J Phys Chem B. 2001;105:6474–6487. [Google Scholar]
- 44.DeLano W. The PyMOL Molecular Graphics System, Version 1.5. 0.4. Schrödinger, LLC; 2016. [Google Scholar]
- 45.Hess B, Bekker H, Berendsen HJ, Fraaije JG. LINCS: a linear constraint solver for molecular simulations. J Comput Chem. 1997;18:1463–1472. [Google Scholar]
- 46.Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J Mol Graph. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 47.Seo J, Warnke S, Gewinner S, Schöllkopf W, Bowers MT, Pagel K, von Helden G. The impact of environment and resonance effects on the site of protonation of aminobenzoic acid derivatives. Phys Chem Chem Phys. 2016;18:25474–25482. doi: 10.1039/c6cp04941a. [DOI] [PubMed] [Google Scholar]
- 48.Chitta RK, Gross ML. Electrospray ionization-mass spectrometry and tandem mass spectrometry reveal self-association and metal-ion binding of hydrophobic peptides: a study of the gramicidin dimer. Biophys J. 2004;86:473–479. doi: 10.1016/S0006-3495(04)74125-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iizuka E, Yang JT. Effect of salts and dioxane on the coiled conformation of poly-L-glutamic acid in aqueous solution. Biochemistry. 1965;4:1249–1257. [Google Scholar]
- 50.Roccatano D, Colombo G, Fioroni M, Mark AE. Mechanism by which 2, 2, 2-trifluoroethanol/water mixtures stabilize secondary-structure formation in peptides: a molecular dynamics study. Proc Natl Acad Sci U S A. 2002;99:12179–12184. doi: 10.1073/pnas.182199699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hirota N, Goto Y, Mizuno K. Cooperative α-helix formation of β-lactoglobulin and melittin induced by hexafluoroisopropanol. Protein Sci. 1997;6:416–421. doi: 10.1002/pro.5560060218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Glover MS, Shi L, Fuller DR, Arnold RJ, Radivojac P, Clemmer DE. On the split personality of penultimate proline. J Am Soc Mass Spectrom. 2015;26:444–452. doi: 10.1007/s13361-014-1049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Masson A, Kamrath MZ, Perez MA, Glover MS, Rothlisberger U, Clemmer DE, Rizzo TR. Infrared spectroscopy of mobility-selected H+-Gly-Pro-Gly–Gly (GPGG) J Am Soc Mass Spectrom. 2015;26:1444–1454. doi: 10.1007/s13361-015-1172-4. [DOI] [PubMed] [Google Scholar]
- 54.Counterman AE, Clemmer DE. Cis- trans signatures of proline-containing tryptic peptides in the gas phase. Anal Chem. 2002;74:1946–1951. doi: 10.1021/ac011083k. [DOI] [PubMed] [Google Scholar]