Abstract:
This article presents 3 cases of immunocompromised patients for whom therapeutic drug monitoring of ganciclovir in combination with cytomegalovirus viral load measurement was used to guide treatment. The first patient is diagnosed with thymoma A, the second is a heart transplant recipient, and the third is an HIV-positive patient. These patients were all diagnosed with cytomegalovirus and treated with ganciclovir. Our case studies illustrate how therapeutic drug monitoring–guided dosing can be helpful in the management of these complex cases.
Key Words: ganciclovir, TDM, immunocompromised patients
CLINICIAN
Our patient is a 54-year-old man (90 kg) with type A thymoma, who was admitted to the intensive care unit because of respiratory failure and shock. Laboratory results showed severe hypogammaglobulinemia and absence of circulating B cells, and therefore, the diagnosis of Good syndrome was considered, and intravenous immunoglobulin was started.1,2 Based on chest imaging followed by bronchoscopic bronchoalveolar lavage, Pneumocystis jirovecii pneumonia (PCP) and cytomegalovirus (CMV) disease were diagnosed (CMV DNA viral load of 775,000 copies/mL in whole blood). The patient was CMV IgG positive thus suffering from a reactivation of CMV. As the patients' status deteriorated further, he was referred for extracorporeal membrane oxygenation. He was on sulfamethoxazole and trimethoprim (960 mg once daily) and ganciclovir [2 mg/kg once daily, intravenous (IV)] as well as continuous venovenous hemofiltration (CVVH, 50 mL/min).
TDM CONSULTANT
For CMV treatment, the standard dosing of ganciclovir is 900 mg every 12 hours for oral valganciclovir and 5 mg/kg every 12 hours for IV ganciclovir.3 In case of renal impairment and dialysis, dosages should be adjusted according to renal function to avoid serious side effects such as neutropenia and myelosuppression.4–7 Ganciclovir has a narrow therapeutic window, and higher concentrations have been connected to toxicity.8 Besides side effects, antiviral drug resistance may impact CMV treatment. Resistance to ganciclovir is caused by mutations in the genes UL97 kinase and UL54 polymerase.9 The risk for acquired resistance is increased in patients with high viral loads, that is, >100.000 copies/mL of blood.10 Adequate CMV treatment is of great importance, and low ganciclovir serum concentrations need to be avoided.10 To assure adequate ganciclovir drug exposure, therapeutic drug monitoring (TDM) is an easy-to-use tool to assess ganciclovir serum concentrations and to guide dosage adjustments.11 I suggest you start the TDM of ganciclovir for this patient. We can use a validated liquid chromatography–tandem mass spectrometry method. We have defined a ganciclovir target plasma trough concentration of 1–2 mg/L for CMV prophylaxis and 2–4 mg/L for CMV treatment. These are based on the inhibitory concentration of CMV (IC50) and pharmacokinetic parameters derived from available studies.6–8,12,13
CLINICIAN
I agree, to reduce the risks of ganciclovir treatment and to guide therapy using TDM would be a good idea. The viral load is now 283,000 copies/mL in plasma. The first measured trough concentration at steady state is 1.3 mg/L, and this is the 13th day of treatment. I think we should increase the dose.
TDM CONSULTANT
I agree, I think the dose should be increased to 1.7 mg/kg (intravenous) twice daily. We used a population pharmacokinetic model for ganciclovir dose estimation to which the individual patient data were fitted using Bayesian fitting. The model included following initial population pharmacokinetic parameters: volume of distribution (0.74 ± 0.15 L/kg), elimination rate constant (0.023 ± 0.1 h−1), bioavailability (0.6 ± 0.15), absorption rate constant (0.895 ± 0.464 h−1), lag time (0.825 ± 1.54 hours), and renal elimination constant [h−1/(mL/min/1.73 m2)].
CLINICIAN
This dose has resulted in a trough concentration of 2.2 mg/L.
On day 16, the viral load is still high (127,000 copies/mL in serum), and his condition has not improved. We suspect resistance to ganciclovir. This, however, was not confirmed by Sanger sequencing of UL54 and UL97, so we continued with ganciclovir while patient is still on CVVH. We have kept the patient on the same dose, and on day 27, we saw a reduction in viral load in serum to 1610 copies/mL. Now on day 29, however, we have obtained a trough concentration of 6.6 mg/L, what should our next step be?
TDM CONSULTANT
I suggest you continue with the 1.7 mg/kg once daily dose. The total of 6.6 mg/L is the concentration where we can see side effects and you have been having positive responses to the higher dose.
CLINICIAN
We reduced the dose as you suggested; however, today, on day 48, we see an increase in viral load and a measurement of 2280 copies/mL in whole blood. In the meantime, ganciclovir concentration has dropped to 0.4 mg/L. Do you have a suggestion for dosage change?
TDM CONSULTANT
I suggest you increase the dose back to 1.7 mg/kg twice daily.
CLINICIAN
We did increase the dose after your last suggestion; however, it is day 52, and ganciclovir concentrations have remained on the lower side.
TDM CONSULTANT
I suggest you increase the dose to 2.7 mg/kg twice daily, knowing that the patient is also still on CVVH.
CLINICIAN
We increased the dose, and ganciclovir trough concentrations have subsequently increased to 3.9 mg/L, and the patient remains on CVVH. It is day 62, and the viral load has dropped to 900 copies/mL (whole blood). We have obtained 2 consecutive negative viral load measurements (<100 copies/mL) on days 80 and 85. The patient is continued on valganciclovir tablet 900 mg twice daily.
I have another patient admitted with CMV. He is a 54-year-old heart transplant recipient (transplanted 6 months earlier; donor CMV IgG positive and recipient CMV IgG negative). He was admitted to the hospital with shortness of breath, nausea, and diarrhea that had lasted for 4 days. CMV DNA in whole blood was 120,000 copies/mL. Primary CMV infection has been diagnosed, and treatment is started with IV ganciclovir 2.6 mg/kg once a day [while the estimated glomerular filtration rate (eGFR) was 24 mL/min/1.73 m2]. Do you think we should also start TDM of ganciclovir for this patient?
TDM CONSULTANT
This would benefit the patient especially, as he has severe renal failure. Ganciclovir is mainly eliminated through the kidney with glomerular and tubular secretion, and it has been described that more than 80% of unchanged ganciclovir is found in urine.8 Thus, the clearance of ganciclovir during renal impairment and renal replacement therapy is changed, and in that case, the dosages should be adjusted accordingly, and TDM may be indicated.6
CLINICIAN
On day 9, ganciclovir trough concentration was 1.2 mg/L (eGFR 32 mL/min/1.73 m2), and the viral load had dropped to 27.800 copies/mL (in whole blood). However, the viral load has shown no further decline for the next 3 days (viral load 32,000 copies/mL in whole blood). We suspected that there is resistance to ganciclovir, and we decided to start with foscarnet treatment. After initiation of foscarnet treatment, an adverse drug reaction was noticed with vomiting, nausea, and exanthema, and therapy with foscarnet has now been stopped. The treatment with foscarnet lasted for 3 days. In the meantime, resistance against ganciclovir was not confirmed by Sanger sequencing of UL54 and UL97. We have decided to continue with ganciclovir.
TDM CONSULTANT
I suggest increasing the dose to 1.5 mg/kg three times daily.
CLINICIAN
We followed your suggestion, and the measured trough concentration was 3.7 mg/L, while the patient still suffered from severe kidney failure (eGFR 23 mL/min/1.73 m2). By day 22, the viral load has dropped to 415 copies/mL (in whole blood). We discharged the patient on day 32 with oral valganciclovir 450 mg twice daily (ganciclovir trough 3.1 mg/L, eGFR 31 mL/min/1.73 m2). Further TDM was performed during outpatient follow-up (day 36: 3 mg/L, day 39: 2.5 mg/L). On the 40th and 43rd day of treatment, we obtained 2 consecutive negative viral load measurements (<100 copies/mL in whole blood). This patient's dosing is presented in Figure 1.
FIGURE 1.
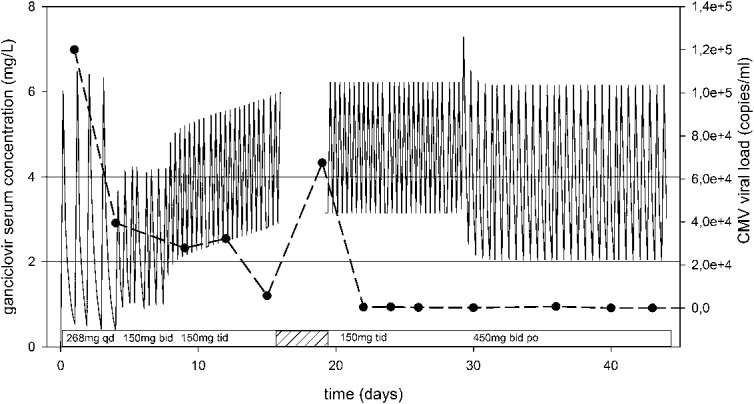
Bayesian-simulated ganciclovir serum concentrations during treatment and CMV DNA viral load in blood of the second presented patient. The shaded area presents the time patient was on foscarnet treatment. bid, twice daily; po, orally; qd, once daily; tid, three times daily.
CLINICIAN
We have a 43-year-old man, who was referred to us with newly diagnosed HIV infection. He has lost approximately 7 kg in the past 6 months and felt progressive shortness of breath with a cough. The patient has been tested for hepatitis B and C in serum (serology), and RT-PCR was performed on bronchoalveolar lavage fluid for CMV, PCP, herpes simplex virus types 1 and 2, and Toxoplasma gondii. PCP has been confirmed with polymerase chain reaction and Giemsa staining, and treatment has been started with sulfamethoxazole, trimethoprim (1920 mg three times daily), and prednisolone (40 mg twice daily). Herpes simplex virus type 1 (DNA) has also been found, with a cycle threshold (Ct-value) of 30. HIV is being treated with darunavir/cobicistat and emtricitabine/tenofovir. Now, 13 days after presentation, CMV viremia has been detected (15.900 copies/mL in whole blood, IgG positive). In addition, esophagitis grade D has been diagnosed for which he is being treated with omeprazole and fluconazole. We have started ganciclovir with a dose of 4 mg/kg twice daily (eGFR 115 mL/min/1.73 m2).
TDM CONSULTANT
This dose seems low, as his kidney function is normal, and I suggest increasing the dose and starting the TDM of ganciclovir.
CLINICIAN
The first 2 trough levels are on the low side (0.4 and 1.5 mg/L on days 2 and 4); we decided to increase the dose to 4 mg/kg four times daily. This resulted in a trough concentration of 1.9 mg/L on day 5 (eGFR 111 mL/min/1.73 m2). By day 8, the viral loads decreased to 241 copies/mL (in whole blood). We switched intravenous treatment to oral valganciclovir treatment on day 8 (900 mg once daily), and the patient was discharged from the hospital. We obtained 2 consecutive negative viral load measurements (<100 copies/mL) on days 16 and 21.
We presented 3 patients illustrating how the TDM of ganciclovir helped in their management. For these patients, the dosage changes are performed in a somewhat different pattern. This is because we do not have extensive experience with the TDM of ganciclovir, and there is no established dosing algorithm. However, the use of the population pharmacokinetic model in combination with Bayesian fitting of individual data allows for an individual TDM approach.
The third patient had a low starting dose of 4 mg/kg twice daily; in retrospect, we consider this dosing inadequate as reflected by the measured concentrations. However, for this patient, starting off with 5 mg/kg twice daily most likely would have resulted in low concentrations as well. Without TDM, these 3 patients would have received ganciclovir doses that were too low, which could have led to unnecessary switch of treatment (1 case) or to acquired resistance to ganciclovir.10
Although the diagnosis and management of CMV has improved in recent years, it is still considered a common complication for immunocompromised patients, especially for solid organ and stem cell transplant recipients.3,14 The prophylaxis and preemptive therapy approaches have shown to be beneficial in avoiding severe infection. TDM for ganciclovir is useful; however, the necessity of routine TDM for ganciclovir has been a subject of debate.8,15,16 There are currently insufficient data for confirming the need for routine TDM of ganciclovir; only case studies have suggested its wider use.17–19
The published case studies are unique and differ from the 3 cases we described here. Peredo et al17 investigated mainly the feasibility of using brain extracellular fluid (using microdialysis) to measure ganciclovir concentrations and concluded that concentrations in brain tissue are comparable with those from serum. On the other hand, Stockmann et al18 only briefly described a pediatric case where they used the area under the concentration–time curve over 24 h (AUC24) values to determine exposure to ganciclovir in a patient with confirmed ganciclovir resistance. Another case study by Nunez-Nunez described how continuous renal replacement therapy affects the elimination of ganciclovir.19 Applicability and use in patient care was not discussed in detail in these cases. Moreover, any consensus about an appropriate concentration for ganciclovir was not reached. With our 3 case studies, we showed how TDM may be of help to guide treatment in hospitalized patients.
Furthermore, another important reason why TDM should be performed is the necessity for dosage alterations in kidney dysfunction, as it is mainly excreted trough the kidneys.20 Also, for CMV treatment, higher doses and often intravenous administration of ganciclovir are used, which are adjusted to body weight. This can increase the variability in drug concentrations because both exposure and clearance can change.7,8,19 These patients can also be critically ill, thus require continuous hemofiltration, which makes dose estimation without TDM difficult.7 These criteria suggest that TDM can be a useful tool in monitoring ganciclovir treatment.
Our case studies illustrate how TDM-guided dosing can be helpful in the management of these complex cases.
Footnotes
The authors declare no conflict of interest.
REFERENCES
- 1.Kelleher P, Misbah SA. What is Good's syndrome? Immunological abnormalities in patients with thymoma. J Clin Pathol. 2003;56:12–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelesidis T, Yang O. Good's syndrome remains a mystery after 55 years: a systematic review of the scientific evidence. Clin Immunol. 2010;135:347–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotton CN, Kumar D, Caliendo AM, et al. The third international consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation. 2018;102:900–931. [DOI] [PubMed] [Google Scholar]
- 4.Gane E, Saliba F, Valdecasas GJ, et al. Randomised trial of efficacy and safety of oral ganciclovir in the prevention of cytomegalovirus disease in liver-transplant recipients. The Oral Ganciclovir International Transplantation Study Group [corrected]. Lancet. 1997;350:1729–1733. [DOI] [PubMed] [Google Scholar]
- 5.Paya C, Humar A, Dominguez E, et al. Efficacy and safety of valganciclovir vs. oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2004;4:611–620. [DOI] [PubMed] [Google Scholar]
- 6.Czock D, Scholle C, Rasche FM, et al. Pharmacokinetics of valganciclovir and ganciclovir in renal impairment. Clin Pharmacol Ther. 2002;72:142–150. [DOI] [PubMed] [Google Scholar]
- 7.Horvatits T, Kitzberger R, Drolz A, et al. Pharmacokinetics of ganciclovir during continuous venovenous hemodiafiltration in critically ill patients. Antimicrob Agents Chemother. 2014;58:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perrottet N, Decosterd LA, Meylan P, et al. Valganciclovir in adult solid organ transplant recipients: pharmacokinetic and pharmacodynamic characteristics and clinical interpretation of plasma concentration measurements. Clin Pharmacokinet. 2009;48:399–418. [DOI] [PubMed] [Google Scholar]
- 9.Biron KK. Antiviral drugs for cytomegalovirus diseases. Antivir Res. 2006;71:154–163. [DOI] [PubMed] [Google Scholar]
- 10.Lurain NS, Chou S. Antiviral drug resistance of human cytomegalovirus. Clin Microbiol Rev. 2010;23:689–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wiltshire H, Hirankarn S, Farrell C, et al. Pharmacokinetic profile of ganciclovir after its oral administration and from its prodrug, valganciclovir, in solid organ transplant recipients. Clin Pharmacokinet. 2005;44:495–507. [DOI] [PubMed] [Google Scholar]
- 12.Wiltshire H, Paya CV, Pescovitz MD, et al. Pharmacodynamics of oral ganciclovir and valganciclovir in solid organ transplant recipients. Transplantation. 2005;79:1477–1483. [DOI] [PubMed] [Google Scholar]
- 13.Mylonakis E, Kallas WM, Fishman JA. Combination antiviral therapy for ganciclovir-resistant cytomegalovirus infection in solid-organ transplant recipients. Clin Infect Dis. 2002;34:1337–1341. [DOI] [PubMed] [Google Scholar]
- 14.Griffiths PD, Emery VC. Taming the transplantation troll by targeting terminase. N Engl J Med. 2014;370:1844–1846. [DOI] [PubMed] [Google Scholar]
- 15.Scott JC, Partovi N, Ensom MHH. Ganciclovir in solid organ transplant recipients: is there a role for clinical pharmacokinetic monitoring? Ther Drug Monit. 2004;26:68–77. [DOI] [PubMed] [Google Scholar]
- 16.Perrottet N, Beguin A, Meylan P, et al. Determination of aciclovir and ganciclovir in human plasma by liquid chromatography-spectrofluorimetric detection and stability studies in blood samples. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;852:420–429. [DOI] [PubMed] [Google Scholar]
- 17.Peredo I, Hellden A, Wolmer-Solberg N, et al. Ganciclovir concentrations in the cerebral extracellular space after valganciclovir treatment; a case study. BMJ Case Rep. 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stockmann C, Sherwin CMT, Knackstedt ED, et al. Therapeutic drug monitoring of ganciclovir treatment for cytomegalovirus infections among immunocompromised children. J Pediatr Infect Dis Soc. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nunez-Nunez M, Bellapart J, O'Donoghue S, et al. Variable ganciclovir concentrations in a critically ill patient receiving continuous renal replacement therapy and plasma exchange? Int J Antimicrob Agents. 2014;43:572–573. [DOI] [PubMed] [Google Scholar]
- 20.McGavin JK, Goa KL. Ganciclovir: an update of its use in the prevention of cytomegalovirus infection and disease in transplant recipients. Drugs. 2001;61:1153–1183. [DOI] [PubMed] [Google Scholar]


