Chlororespiration consumes photo-generated reductants in the green alga Chlamydomonas reinhardtii during periods without illumination, thus regulating the redox state of the chloroplast and preparing photosynthesis for the onset of light.
Abstract
Whereas photosynthetic function under steady-state light conditions has been well characterized, little is known about its changes that occur in response to light fluctuations. Chlororespiration, a simplified respiratory chain, is widespread across all photosynthetic lineages, but its role remains elusive. Here, we show that chlororespiration plays a crucial role in intermittent-light conditions in the green alga Chlamydomonas reinhardtii. Chlororespiration, which is localized in thylakoid membranes together with the photosynthetic electron transfer chain, involves plastoquinone reduction and plastoquinol oxidation by a Plastid Terminal Oxidase (PTOX). We show that PTOX activity is critical for growth under intermittent light, with severe growth defects being observed in a mutant lacking PTOX2, the major plastoquinol oxidase. We demonstrate that the hampered growth results from a major change in the kinetics of redox relaxation of the photosynthetic electron transfer chain during the dark periods. This change, in turn, has a dramatic effect on the physiology of photosynthesis during the light periods, notably stimulating cyclic electron flow at the expense of the linear electron flow.
Plants need to adapt to large variations in light irradiance to maintain efficient photosynthesis. Multiple regulatory processes in the chloroplast contribute to the optimization of carbon fixation via linear electron flow (LEF) from PSII to the Calvin-Benson-Bassham (CBB) cycle. These processes also provide protection against overreduction of the electron transport chain, which otherwise would lead to the production of deleterious reactive oxygen species (Krieger-Liszkay, 2005; Eberhard et al., 2008; Rutherford et al., 2012). Examples of these major processes include (1) down-regulation of PSII photochemical activity either through quenching of its excitonic energy (energy-dependent nonphotochemical quenching; Peers et al., 2009) or through a decrease in its absorption cross section (state transitions; Goldschmidt-Clermont and Bassi, 2015; Nawrocki et al., 2016) and (2) rerouting of part of the electron flow from PSII to alternative sinks, mostly oxygen (water-to-water cycles such as the Mehler reaction, photorespiration, or flavodiiron protein activity; Curien et al., 2016). Cyclic electron flow (CEF) around PSI, which reinjects electrons from reduced ferredoxin back to plastoquinone (PQ) and thereby increases the proton gradient across the thylakoid membrane, also contributes to the down-regulation of PSII and PSI activities and to the optimization of LEF by providing extra ATP to the CBB cycle (Johnson, 2011; Yamori and Shikanai, 2016).
Light-independent regulatory processes also exist, and these use part of the photosynthetic electron transport chain, such as chlororespiration. This pathway was identified originally as a process involving light-independent oxygen consumption by chloroplasts (Bennoun, 1982), and it is widely conserved in the photosynthetic lineage (Nawrocki et al., 2015). It involves two types of enzymatic activities that act in series on the redox state of the PQ/plastoquinol (PQH2) pool. An NAD(P)H:PQ oxidoreductase (NDA2 in algae and NDH in higher plants) reduces PQ using stromal reductants as electron donors (Peltier et al., 2016), and a PQH2:oxygen oxidoreductase termed PTOX (for Plastid Terminal Oxidase) oxidizes PQH2 and produces water (Nawrocki et al., 2015). Altogether, the in-series activity of NDA2/NDH and PTOX transfers electrons from stromal reductants to oxygen (Houille-Vernes et al., 2011). In vascular plants, PTOX also is involved in carotenoid biosynthesis during chloroplast development, but its preservation in fully differentiated tissues suggests that it has some additional physiological role (Carol et al., 1999; Wu et al., 1999).
Thus, chlororespiration is intertwined with the photosynthetic electron transfer chain in that it shares with it PQ and some stromal electron carriers. Furthermore, as an antagonist of the light reactions of photosynthesis, it consumes the primary products oxygen and NADPH (Nawrocki et al., 2015). However, the low rates of PTOX and NDA2 suggest that chlororespiratory fluxes cannot compete with the main PQ reductase (PSII) or oxidase (cytochrome b6f complex [cyt. b6f]) in the light. Indeed, we confirm here that PTOX2 plays no significant role during steady-state illumination in Chlamydomonas reinhardtii. However, in marked contrast, its activity becomes critical for growth under intermittent-light conditions. We provide evidence that chlororespiration not only oxidizes the PQ pool but also extensively oxidizes stromal reductants in darkness. By doing so, it modifies the nature and efficiency of photosynthesis in the light periods and controls the balance between LEF and CEF. Therefore, this balance can be altered easily by light treatment in vivo, providing an on-demand switch for studies of CEF regulation.
RESULTS
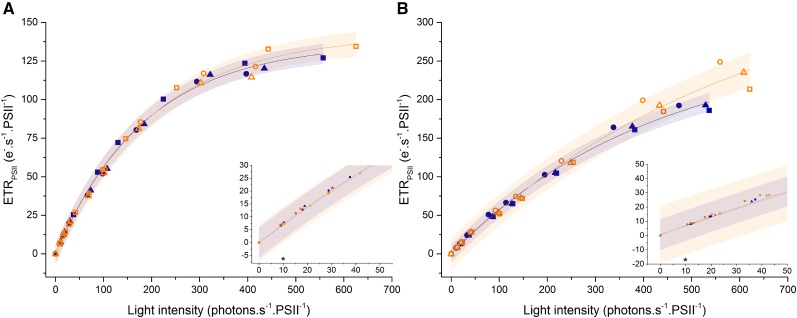
PTOX2 Cannot Compete with cyt. b6f-PSI for Oxidation of the PQ Pool in Continuous Light
The low rate of electron flow sustained by PTOX ranges from 0.1 to 5 e− PSII−1 s−1 in the chloroplasts of plants and C. reinhardtii (Houille-Vernes et al., 2011; Trouillard et al., 2012), which does not qualify PTOX as an efficient electron sink. Despite its low reaction rate, PTOX has been suggested to play a photoprotective role when the light absorption by PSII exceeds the capacity of the downstream reactions (Carol et al., 1999; Niyogi, 2000; Ort and Baker, 2002; Krieger-Liszkay and Feilke, 2016; Stepien and Johnson, 2018). We first examined the participation of PTOX in a pathway that reroutes electrons from PSII by monitoring the electron transfer rate (ETRPSII) in the wild type and the ptox2 mutant over a wide range of light intensities. If PTOX indeed was the terminal electron acceptor for a significant proportion of the electron flux from PSII, then the ETRPSII should be higher in the wild type than in the mutant. However, as shown in Figure 1, the presence of the major PQH2:oxygen oxidoreductase PTOX2 in C. reinhardtii does not significantly modify the ETRPSII under steady-state illumination, even at low intensities of about 10 µE m−2 s−1 (insets in Fig. 1). This holds true in both autotrophic and mixotrophic conditions. Thus, PTOX activity has no significant functional contribution to photosynthesis under continuous illumination; therefore, its putative photoprotective role is questionable (Nawrocki et al., 2015).
Figure 1.
PTOX2 is not an electron sink in steady-state photosynthesis. The light dependency of the ETRPSII is shown in wild-type (closed blue symbols) and ptox2 (open orange symbols) plants grown in mixotrophic (A) and autotrophic (B) conditions. Triangles, circles, and squares correspond to three independent biological samples. Cells were adapted for 30 s to each light intensity (for details, see “Materials and Methods”). The shaded area corresponds to the 95% confidence band of the fit (for the fit, see “Materials and Methods”). Insets show the values for low light intensities. Asterisks indicate the growth light intensity for both strains in both conditions.
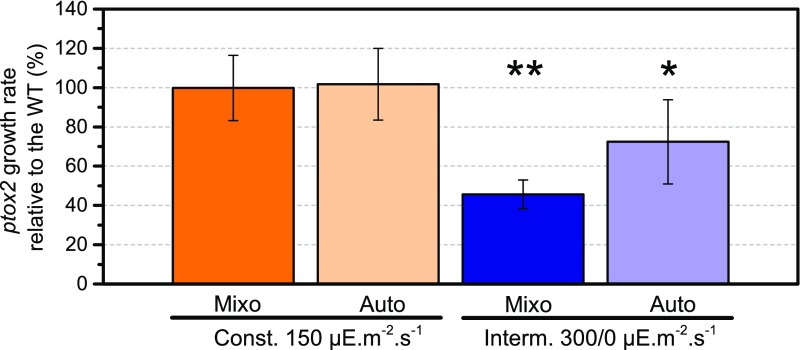
The ptox2 Mutant Exhibits Severe Growth Defects and Altered Photosynthesis in Intermittent Light
The ptox2 mutant does not exhibit an aberrant growth phenotype when placed in darkness or under continuous illumination (Houille-Vernes et al., 2011); however, we observed that its growth was severely hampered when exposed to an intermittent-light regime. As shown in Figure 2, the growth rate of the ptox2 strain decreased markedly relative to that of the wild type, which points to a crucial contribution of chlororespiration to chloroplast physiology. This proved to be the case in both mixotrophic and autotrophic conditions, although the differences were less pronounced in the latter.
Figure 2.
Growth of the ptox2 mutant is hampered in intermittent light. The growth rate of the ptox2 mutant relative to the wild type (WT) is presented for constant-light (orange) and intermittent-light (blue) conditions, in mixotrophy (dark) and autotrophy (light). The asterisks indicate significant differences in growth rate from the wild type, with P = 0.05 (*) and P = 0.001 (**), according to a paired Student’s t test (n = 5 ± sd).
The hampered growth of the mutant could result from the loss of light-controlled reversible binding of PTOX to the thylakoid membranes, which has been suggested to occur in thylakoids of land plants (Feilke et al., 2016). The authors found a pH-dependent difference in association between PTOX and the thylakoid membrane by following a fractionation protocol in illuminated and dark-adapted tobacco (Nicotiana tabacum) leaves (Feilke et al., 2016). One of the possible explanations for this association is that PTOX performs a protective safety valve function. However, as shown in Supplemental Figure S1, the wild type of C. reinhardtii showed only minute amounts of PTOX2 in a soluble form in both light and darkness. We noted that the ferredoxin-NADP+ reductase (FNR) binding to the thylakoid membrane increased in the light, as shown by its amount in the membrane fraction relative to that of PetD in the wild type and the ptox2 mutant (Supplemental Fig. S1). The major change in PTOX2 was its increased content in wild-type cells in intermittent-light conditions (Supplemental Fig. S2).
To examine whether the decrease in growth rate resulted from an altered photosynthesis in the mutant, we measured the photochemical yield of PSII (ΦPSII; Genty et al., 1989) directly in cells grown in intermittent-light conditions, which were thus acclimated to this light regime. As shown in Figure 3D, a marked decrease in ΦPSII in the ptox2 mutant occurred together with the decrease in its growth rate (Fig. 2). This decrease recovered rapidly when the cells were placed under continuous illumination (Fig. 3F).
Figure 3.
Photosynthesis is reversibly slowed down in the ptox2 mutant in the intermittent-light regime. The intermittent light corresponds to cycles of 1 min of dark and 1 min of light (340 μE m−2 s−1) in heterotrophic conditions. A, Kinetics of ΦPSII changes in the wild type (WT; blue squares) and the ptox2 mutant (orange circles) during 30 min of intermittent light (black bar; measured at the end of the light period) and a subsequent continuous-light (50 μE m−2 s−1) recovery (gray bar). B, Initial value of the ΦPSII after the first illumination period. C, ΦPSII after the last illumination period of the 30-min treatment. D, ΦPSII in cultures acclimated to a 0/340 μE m−2 s−1 intermittent light for several generations. E, ΦPSII after a 15-min continuous-light recovery after fluctuating-light treatment. F, ΦPSII after a 15-min continuous-light recovery of the cells acclimated to intermittent light for multiple generations. n = 4 ± sd for B to F.
We further aimed to establish whether the observed drop of photosynthetic efficiency was a long-term or a short-term acclimation by subjecting cells to intermittent-light treatment directly inside the spectrophotometer. This allowed measurements of photosynthetic parameters during each of the dark/light and light/dark transitions. Given the role of the redox state of the PQ pool in the regulation of state transitions (Houille-Vernes et al., 2011), wild-type and mutant cells were first adapted to low light intensity under strong oxygenation, setting both strains in the same state, close to state I, prior to the intermittent-light treatment (Fig. 3, A and B; Supplemental Fig. S3), a feat that would not be achievable in total darkness (Houille-Vernes et al., 2011).
We detected a drop in ΦPSII, similar to the one observed in acclimated cells, after only a few minutes of intermittent-light treatment of cells grown in standard, continuous-light conditions (Fig. 3, A and C). These differences also recovered rapidly when continuous-illumination treatment was resumed (Fig. 3, A and E). The latter observation shows that the growth phenotype is indeed due to a hampered ability to respond to fluctuating light in the mutant and not to irreversible photoinhibitory processes or changes in the overall protein composition of the photosynthetic membranes during growth.
We used strains with different genetic backgrounds (Supplemental Fig. S4, A and B) and varied the light intensities (Supplemental Fig. S4, C and D) and frequencies of intermittence (Supplemental Fig. S4, E and F). We consistently observed a drop in ΦPSII in the ptox2 mutant. Thus, the ΦPSII decrease in the mutant in the intermittent-light regime is a robust phenotype. Provided that this phenotype results from the absence of a chlororespiratory activity during the dark periods, we reasoned that it should be lost if the dark periods were short enough, compared with the time scale of PTOX activity. This was indeed the case, since the two strains no longer differed in ΦPSII as well as in the Fv/Fm (normalized variable PSII fluorescence) in an intermittent 1-s-dark/1-s-light regime (Supplemental Fig. S5). Similar observations were recorded when cells were grown in autotrophic conditions, although to a lesser extent than in heterotrophy (Supplemental Fig. S6), findings that are consistent with the lower impact of light fluctuations on photoautotrophic growth rate (Fig. 2).
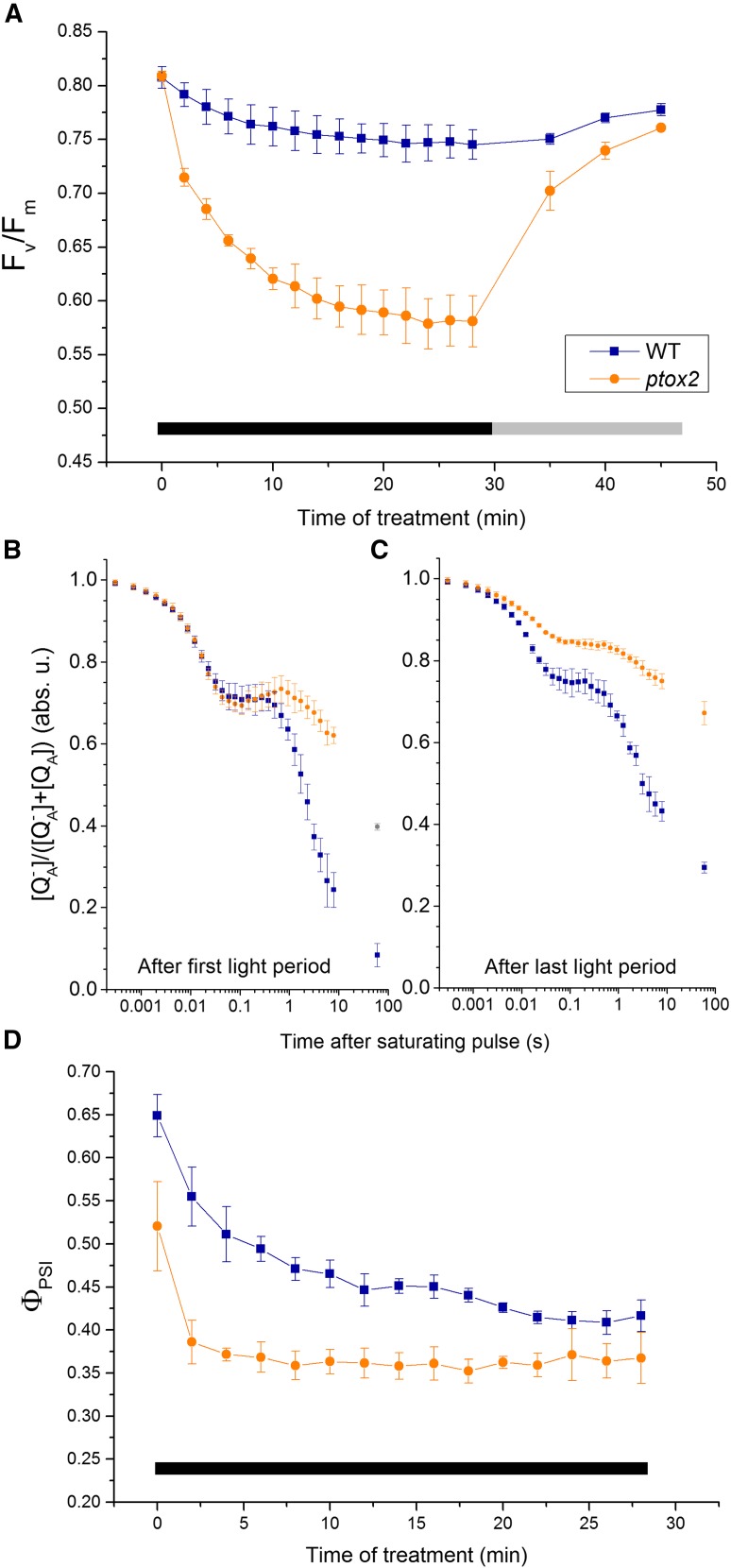
Chlororespiration Mediates the Redox State of Both the PQ Pool and PSI Acceptors in Darkness
To understand how processes occurring in the dark impact photosynthetic electron flow during the light period, we investigated the redox relaxation of the photosynthetic electron transfer chain during the dark periods. We simultaneously recorded the evolution of Fv/Fm and Fm (maximal PSII fluorescence) parameters during a 30-min exposure to intermittent light (Fig. 4A; Supplemental Fig. S3A). Fm decreased in a similar manner in the two strains, reflecting the similar amplitudes of state transitions, which is consistent with the increase in the far-red emission peak in the low-temperature emission spectra in both strains (Supplemental Fig. S3, B and C). However, the variable fluorescence, Fv/Fm, decreased markedly in the mutant but not in the wild type (Fig. 4A). Given that (1) this decrease is reversible if the mutant is transferred to continuous illumination (Fig. 4A) and (2) the Fm decrease was similar in the mutant and the wild type, the specific decline in Fv/Fm in the mutant reflects an increase in initial (minimum) PSII fluorescence (F0), which suggests an overreduction of the PQ pool during the experiment.
Figure 4.
Lack of chlororespiration induces reversible overreduction of the photosynthetic apparatus in the periods of darkness. A, Fv/Fm of the wild type (WT) and the ptox2 mutant at the end of each dark period during the 30-min 0/340 μE m−2 s−1 intermittent-light treatment (black bar) and a 15-min 150 μE m−2 s−1 continuous-light recovery (gray bar). n = 4 ± sd. B and C, Relative concentrations of the reduced form of the QA quinone in the wild type and the ptox2 mutant after the first (B) and last (C) illumination of the 30-min intermittent-light treatment. n = 3 ± sd. D, ΦPSI in the wild type and the ptox2 mutant at the end of each dark period during the 30-min 0/340 μE m−2 s−1 intermittent-light treatment. n = 4 ± sd. The cells used in all experiments were grown in heterotrophic conditions.
To further test this hypothesis, we measured QA reoxidation kinetics before and after the 30-min treatment. We applied the Stern-Volmer relationship to quantify changes in the concentration of QA− from the fluorescence data (see “Materials and Methods”) after applying a saturating pulse to reduce 100% of QA (Schreiber, 2004). The kinetics of QA reoxidation, which reflects the redox state of the PQ pool that is in equilibrium with QA in the dark, were strikingly different in the two strains (Fig. 4, B and C). The fraction of QA (and the PQ pool) which was oxidized within 100 ms by downstream electron transfer through cyt. b6f was similar in the two strains at the onset of fluctuating light (but not at the end of the 30-min treatment); however, this was not observed in the case of slower oxidation of the remaining PQ pool (in the range above 500 ms). This reflects a mainly chlororespiratory process: 40% of PSII centers remained in a reduced state at the end of the 1-min dark period in the mutants. Most notably, QA reoxidation was even slower after the 30-min light/dark treatment in the two strains, suggesting an accumulation of stromal reductants over time under such a light regime that would translate into a higher level of reduction of the PQ pool in the dark.
To test this assumption, we assessed the acceptor side limitation of PSI at the end of the dark periods throughout the 30-min treatment using the protocol of Klughammer and Schreiber (1994); Fig. 4D). However, this procedure is only semiquantitative and may underestimate the actual fraction of photooxidizable P700, which is probed by a saturating pulse allowing several PSI turnovers, thereby leading to a possible underestimation of ΦPSI and overestimation of the acceptor side limitation. Nonetheless, the amount of photooxidizable P700 decreased in both strains, indicating an increasing reduction of the PSI acceptors in the dark as the intermittent-light regime extends. This accumulation of stromal reductants (Fig. 4D) should translate into a higher rate of PQ reduction via the NDA2 enzyme, which uses stromal NAD(P)H as an electron donor to the PQ pool. Indeed, an increased reduction of the PQ pool explains the decreased rates of QA reoxidation during the dark periods from the onset to the end of the 30-min intermittent-light regime in the two strains (Fig. 4, B and C). Eventually, we observed a much steeper and larger decrease in the amount of photooxidizable P700 in the ptox2 mutant than in the wild type, which suggests a stronger PSI acceptor side limitation in the mutant at the end of the dark period (Fig. 4D). Thus, chlororespiration not only regulates the redox state of the PQ pool but also the redox state of the PSI acceptors, which are connected to the former via the activity of the NDA2 oxidoreductase. In the ptox2 mutant, the higher level of PQ pool reduction due to the absence of the oxidase prevents reoxidation of the PSI acceptors by NDA2. Therefore, we conclude that chlororespiration is critical to reoxidize, during the dark periods, both the PQ pool and PSI acceptors, which are reduced during the light periods.
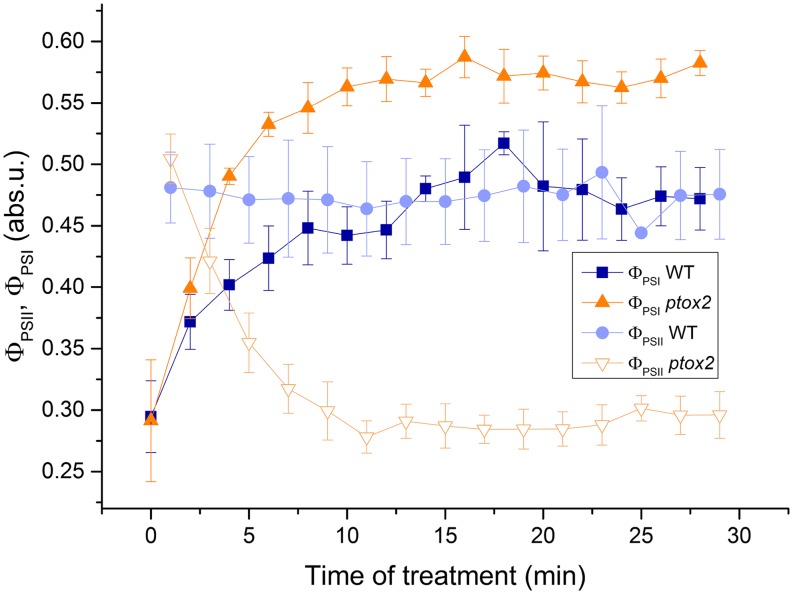
CEF around PSI Is Up-Regulated in the Absence of Chlororespiration
To determine by which process the photochemical rate of PSII is decreased in intermittent light in the absence of PTOX2, we compared the evolution of ΦPSII and ΦPSI at the end of each light period during the 30-min treatment. As discussed above, ΦPSII remained unchanged in the wild type while it dropped markedly in the ptox2 strain (Figs. 3, A and B, and 5); these results are consistent with our fluorescence measurements in intermittent-light-grown cells (Fig. 3D). In contrast, ΦPSI increased in both strains during the same treatment (Fig. 5). It reached the same value as ΦPSII in the wild type but increased further in the ptox2 mutant, reaching a value about 2 times larger than that of ΦPSII at the end of the treatment. Thus, at the end of the intermittent-light treatment in the ptox2 mutant, there was a severe imbalance between the photochemical yields of PSII and PSI. The most plausible explanation for this is an up-regulation of CEF in the mutant (Fan et al., 2016). Strictly speaking, ratios of PSII-to-PSI photochemical yields can only be interpreted as a ratio of PSII-to-PSI activities if the antenna sizes of PSII and PSI behave the same in the wild type and the mutant. Since the decrease in Fm and the amplitude of state transitions were similar in the two strains (Supplemental Figs. S3 and S4), we conclude that the higher ratio of ΦPSI to ΦPSII in the mutant reflects an increase in the CEF-LEF ratio. This increase was not accompanied by the formation of a CEF supercomplex that would be detected using sucrose density gradient fractionation experiments, nor did the light intermittence significantly alter the FNR recruitment to the thylakoid membrane (Supplemental Fig. S7).
Figure 5.
Chlororespiration in fluctuating-light conditions modifies the balance between LEF and CEF. Evolution of ΦPSII and ΦPSI during the fluctuating-light treatment is shown for the wild type (WT) and the ptox2 mutant grown in heterotrophic conditions. n = 4 ± sd.
Finally, we measured the evolution of the acceptor and donor side limitations for PSI charge separation (Supplemental Fig. S8) to establish whether the overreduction of the PSI acceptor side observed at the end of the dark periods persisted throughout the light periods. In contrast to the wild type, the ptox2 mutant accumulated a significant proportion of P700+ in the light [Y(ND)] together with a higher amount of photooxidizable P700 (Fig. 5; Supplemental Fig. S8). Thus, in the absence of PTOX2 and during the up-regulation of CEF within 10 min of exposure to intermittent light, the PSI donor side limitation increased while the acceptor side limitation decreased considerably [Supplemental Fig. S8; Y(NA)]. Therefore, the high CEF efficiency in the absence of PTOX2 cannot be explained merely by a higher degree of reduction of the PSI acceptors in the light but must reflect a reorganization of the electron transfer chain in response to intermittent light, a dynamic process occurring within a few minutes.
DISCUSSION
Although chlororespiration was discovered decades ago, its physiological relevance has not yet been understood. To date, all investigations aimed at understanding the role of chlororespiration have been performed exclusively in constant light or in darkness (for review, see Nawrocki et al., 2015). We found that the ptox2 mutant exhibits a marked decrease in growth rate relative to a wild-type control strain in intermittent light but not in continuous light or in darkness. Thus, the PTOX enzyme resembles several other auxiliary proteins involved in photosynthesis whose physiological roles become prominent under fluctuating-light conditions but not in standard laboratory conditions, such as constant light (Allahverdiyeva et al., 2015). In particular, flavodiiron proteins seem to perform a similar function in cyanobacteria and C. reinhardtii, where they serve as a typical overflow device when a saturating light is superimposed on growth light (Allahverdiyeva et al., 2013; Chaux et al., 2017). Similarly, the regulation of electron flux mediated by state transitions is important for growth in fluctuating-light or overreducing conditions (Bellafiore et al., 2005; Cardol et al., 2009). In cyanobacteria, multiple mutants of terminal oxidases exhibit a growth phenotype when exposed to fluctuating light at high light intensity (Lea-Smith et al., 2013), but the photosynthetic parameters that may be affected were not measured in these cultures. Here, in contrast to CEF regulation mutants showing impaired photosynthesis in fluctuating-light conditions (Suorsa et al., 2012) due to PSI photoinhibition, the total amount of PSI did not decrease in our study, despite PSI acceptor side overreduction upon illumination.
Growth differences under intermittent-light conditions correlated with changes in ΦPSII and Fv/Fm chlorophyll fluorescence parameters. Thus, PSII-driven electron transfer is decreased strongly in the ptox2 mutant, and this impedes algal growth. Importantly, we observed that chlororespiration markedly affects not only the PQ pool but also the pool of PSI acceptors. Therefore, it regulates the entire ambient redox potential of the photosynthetic electron transfer chain due to the NDA2-mediated connection between the PQ pool and NAD(P)H/ferredoxin pools. Interestingly, the overreduction of the PSI acceptor side in the ptox2 mutant at the onset of light was alleviated during the 1-min light period, as evidenced by an increased quantity of P700+ accumulating in the light and a larger photooxidizable fraction of P700 in the PTOX mutant than in the wild type at the end of the light interval.
Several hypotheses could be put forward to explain the observed decrease in ETRPSII during fluctuations in the chlororespiration mutant: (1) PTOX2 plays a significant quinol oxidase (safety valve) role in intermittent-light conditions; (2) the absence of PTOX2 leads to an overreduction of the downstream acceptors of PSII, which promotes PSII and/or PSI photoinhibition; (3) the absence of PTOX2 results in a decreased rate of oxidation of PSI acceptors by the CBB cycle; and (4) the stromal redox state during the dark periods, regulated by chlororespiration, controls some reorganization of the photosynthetic electron chain that favors CEF over LEF. The first hypothesis can be ruled out because the maximal rate of PTOX is too low to produce significant change in ETRPSII, the flux it sustains in the light being negligible with regard to LEF (Houille-Vernes et al., 2011; Trouillard et al., 2012; this work). The photoinhibition hypothesis also can be excluded since the Fm decreases similarly in the mutant and control strains. In addition, the decrease in Fv/Fm and ΦPSII throughout the treatment is rapidly reversible, and no changes in the concentration of the PSI special pair P700 were detected after the treatment (Supplemental Fig. S9). For the third hypothesis to apply, the CBB cycle must become less efficient, thus explaining the lower ETRPSII in the ptox2 mutant only in intermittent light. This is unlikely since (1) it requires a specific inactivation of the CBB cycle of unknown origin in the ptox2 mutant in the dark and (2) this state of inactivation should continue over the subsequent 1-min illumination period. These hypotheses are poorly consistent with our observations that the pool of PSI acceptors is more oxidized in the mutant than in the wild type in the light period.
The hypothesis of a supramolecular reorganization response requires some functional heterogeneity or compartmentation within thylakoid membranes and/or in the chloroplast stroma. Such a response is consistent with the most striking feature of the ptox2 mutant phenotype: the severe imbalance in PSI-to-PSII activity under intermittent light. Indeed, at the end of the fluctuating-light treatment, ΦPSI is twice as large as ΦPSII in the mutant, whereas these values are virtually identical in the wild type. This cannot be explained by changes in the PSII and PSI antenna sizes, since we detected no differences at this level between the two strains regarding either their initial state or their evolution due to the state transitions (Supplemental Fig. S3). Despite the drawbacks of this method (see “Results”), the trend is very clear: the intermittent-light regime increases the ratio of PSI-to-PSII activities in the ptox2 mutant relative to the ratio in the wild type. We interpret this as a higher CEF:LEF ratio. This explanation is further supported by the independent observation that the first phase of reoxidation of QA (Fig. 4C, before 100 ms), which corresponds to the reoxidation of the PQ pool through cyt. b6f activity, is smaller in the mutant. This is consistent with a rapid flux of reducing power from the stroma, via the CEF process, outcompeting the reoxidation of the PQ pool by cyt. b6f. It is noteworthy that this occurs at the end of the intermittent-light regime in the mutant but not at the beginning of the fluctuating-light treatment, nor is it observed in the wild type. Thus, a reorganization of the photosynthetic apparatus should occur under intermittent light mediated by the extensive reduction of the photosynthetic chain in darkness. Furthermore, the increase of the PSI-to-PSII activity concomitant with a strong reduction of the PQ pool demonstrates that cyt. b6f can mediate highly efficient CEF even when the PQ pool is largely reduced (for details, see Nawrocki et al., 2017). We note that this response also develops to a more limited extent in the wild type, since it shows some increased CEF:LEF (Fig. 5), which correlates with the increased reduction of QA and PSI acceptors (Fig. 4) during the intermittent-light treatment.
The regulation of CEF is an emerging topic in functional photosynthesis, and there are few reports of dynamic modification of the CEF:LEF ratio. Moreover, modulation of this ratio had not been achieved previously exclusively by light treatment. Indeed, high rates of CEF have been observed experimentally in C. reinhardtii under anoxia, and CEF has been found to be converted progressively into LEF upon the transition from dark anoxic conditions to light (Godaux et al., 2015); however, such a system is irreversible and laborious to control (Clowez et al., 2015), and straightforward interpretations of the observations are hampered by variations of both the light and oxygen concentrations throughout the treatment. Supercomplexes formed between PSI and cyt. b6f have been proposed to sustain rapid CEF in anoxia (Iwai et al., 2010; Alric, 2014), yet there are currently no data about their dynamics, and the kinetic rationale for supercomplex formation itself can be challenged (Nawrocki et al., 2017). Part of the biochemical evidence for supercomplexes can finally be accounted for by a lateral displacement of cyt. b6f toward stroma lamellae in reducing conditions (Vallon et al., 1991). Alternatively, as proposed for land plants (Hald et al., 2008; Joliot and Johnson, 2011), a reversible association of FNR with either PSI or cyt. b6f could modify the CEF:LEF ratio (Andersen et al., 1992; Zhang et al., 2001), but no instances of a dynamic control in C. reinhardtii have been presented to date, with current lines of research focusing on anoxia acclimation time scales on the order of gene expression (Mosebach et al., 2017). We found no biochemical evidence for these types of regulation in our experimental conditions (Supplemental Figs. S1 and S7). However, it should be noted that the currently available methods of thylakoid fractionation from C. reinhardtii alter the labile protein-protein interactions when following the regular Suc density gradient-assisted isolation approach. For instance, cyt. b6f from C. reinhardtii disintegrates into its monomeric form within 4 h at elevated detergent concentrations (Hovers et al., 2011). It is noteworthy that the use of detergent also has been shown to drastically influence cyt. b6f stability in ultrastructural studies on isolated thylakoids (Johnson et al., 2014), which hampers the reliable quantification of cyt. b6f. Thus, large-scale supramolecular rearrangements leading to CEF regulation may be better observed using emerging techniques such as cryoelectron microscopy in situ (Engel et al., 2015) and by developing new membrane solubilization procedures and new methods for preserving hydrophobic interactions (for review, see Zoonens and Popot, 2014).
Nonetheless, the method used in this study, placing ptox2 under intermittent-light conditions, serves as an unparalleled tool for the functional assessment of CEF. The simplicity and robustness of this light-dependent protocol reveals a regulation that requires more than a passive redox pressure on the electron transport chain and that relaxes only after a few minutes in continuous light (Fig. 3, E and F). The change in the CEF:LEF ratio is maintained on the scale of minutes to days in intermittent light (Fig. 3, A, C, and D), allowing convenient in vivo measurements using spectroscopic techniques. Thus, this on-demand, reversible switch provides a starting point for future biochemical and structural studies of novel CEF regulation mechanisms.
The influence of the dark state of the photosynthetic chain on the CEF:LEF ratio highlights the critical role of chlororespiration in maintaining the chloroplast redox state to secure short-term flexibility of the electron transport chain as a response to overreduction. To date, studies on CEF regulation were hindered by anoxic conditions, which impeded LEF from water to CO2. Since the ptox2 mutant showed an increased CEF in intermittent light in oxic conditions, which still allows LEF activity, the redox state of the chloroplast appears to be a signal that induces CEF in physiologically relevant conditions.
MATERIALS AND METHODS
Strain and Growth Conditions
For all experiments, a wild-type strain of Chlamydomonas reinhardtii cc124 was used. Before the experiments, the ptox2 mutant (Houille-Vernes et al., 2011) was backcrossed with the cc124 strain to keep both genetically close. The strains were grown at 25°C in Tris-acetate-phosphate (mixotrophic) or minimum (autotrophic) medium (Harris et al., 2009) in continuous ∼10 μE m−2 s−1 light (apart from the growth measurements; see below). The two strains used for the verification presented in Supplemental Figure S4B are genetically close descendants of the cc137 strain (Merchant et al., 2007).
Growth Measurements
The strains were grown simultaneously in autotrophic and mixotrophic conditions in a culture chamber (Infors HT) at 25°C with a custom-controlled, white light-emitting diode illumination system (BeamBio). During the 1-min/1-min intermittent-light periods, light of 300 µE m−2 s−1 was provided, whereas in the continuous-light control, the intensity was set at 150 µE m−2 s−1, so that the total number of photons per unit of time remained the same in both conditions. The cells were grown in glass jars and were bubbled continuously with air.
The concentration of the cells growing for at least 72 h was measured in three repetitions using a cell counter (Beckman Coulter). The growth rate was fitted with an exponential function (OriginLab software), and the τ values were compared. Because the scatter of absolute growth rates between biological replicates was large while the relative decrease in the growth of the mutant was highly reproducible, the growth of the latter is expressed as relative to that of the wild type. Statistical significance was assessed with a paired Student’s t test using the OriginLab software.
Functional Measurements
Before the experiments, apart from obtaining the ΦPSII measurements directly from the intermittent-light cultures, the cells were harvested by centrifugation (3,000 rpm for 5 min) and the resulting pellet was resuspended in minimal medium supplied with 10% (w/v) Ficoll to avoid excessive sedimentation. They were then adapted for 1 h to low light intensity (∼10 µE m−2 s−1) to set both of the strains in state I.
All fluorescence kinetics and P700 redox measurements were performed using the JTS-10 spectrophotometer (BioLogic) as described before (Nawrocki et al., 2016), except that they were conducted in an open cuvette setup, with regular air bubbling of the sample to avoid hypoxia and sedimentation of the cells during the 45-min treatments. The F0 was collected with pulses of only the detecting light (520 nm), while the Fm was recorded in the presence of strong actinic light (saturating pulse; 3,000 µE m−2 s−1 for 200 ms). The Fv/Fm [(Fm − F0)/Fm] was recorded at the end of the dark periods, and the ΦPSII [(Fm − Fs)/Fm, where Fs is the stationary fluorescence in the light] was recorded at the end of the light periods.
The ETRPSII was calculated as light intensity × ΦPSII × σPSII after a 30-s adaptation to a given light condition, where σPSII is the absorption cross section of PSII. The product light intensity × σPSII is expressed as e− s−1 PSII−1 and was measured as the area above the fluorescence induction curve in the presence of 3-(3,4-dichlorophenyl)-1,1-dimethylurea, where only the single electron (P680 to QA) transfer occurs (Lazár, 1999). We used the product light intensity × σPSII for the x axis of Figure 1 because it is more germane to photosynthesis than the light intensity expressed in µE m−2 s−1, which has no relevance unless the absorption spectrum of the cells and the emission spectrum of the source are known. The data were fitted with a monoexponential decay function in the OriginLab software.
The QA/QA− concentration was calculated according to the Stern-Volmer relationship, where (in this case, photochemical) quencher concentration influences the rate of fluorescence in the following manner:
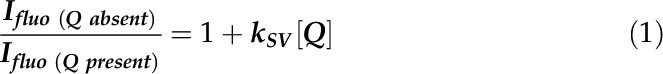 |
where 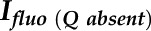 is the fluorescence intensity without a quencher,
is the fluorescence intensity without a quencher, 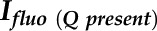 is that with the quencher,
is that with the quencher, 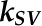 is the quenching coefficient, and
is the quenching coefficient, and 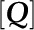 is the concentration of a quencher.
is the concentration of a quencher.
A transformation of this equation gives the following:
 |
One can change 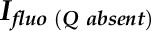 to
to 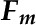 (the maximal level of fluorescence when the QA is in its reduced state after a saturating pulse),
(the maximal level of fluorescence when the QA is in its reduced state after a saturating pulse), 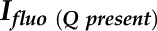 to
to  , and the
, and the 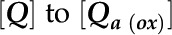 , which yields the following:
, which yields the following:
 |
or
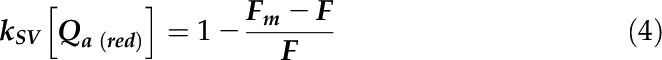 |
The PSI parameters were calculated using P700 redox values: in the dark with only the 705-nm detecting light present (P Dark), in the presence of actinic light (P Light), the minimal value upon a 200-ms, 3,000 µE m−2 s−1 saturating pulse (P Pulse), and with the total amount of P700 registered in the presence of 3-(3,4-dichlorophenyl)-1,1-dimethylurea upon a saturating pulse on the same batch of algae (P Total). The calculated values were as follows: ΦPSI = (P Pulse – P Dark)/P Total if probed in darkness (Fig. 4B) and ΦPSI = (P Pulse – P Light)/P Total if probed in the light (Fig. 5); Y(ND) = (P Light – P Dark)/P Total and Y(NA) = (P Total – P Pulse)/P Total.
To investigate whether a significant proportion of PTOX2 becomes stroma soluble in the darkness, as proposed before, we separated the soluble and membrane fractions of cells grown to the early exponential phase. The cells were harvested, concentrated, and exposed to 1 h of continuous moderate light (50 µE m−2 s−1) or oxic conditions under darkness; this was followed by cell disruption using a French press or a nebulizer. Unbroken cells were removed by spinning down for 5 min at 2,000 rpm, and the supernatant was ultracentrifuged for 20 min. The resulting supernatant was used as the soluble fraction and was precipitated in 10% (w/v) TCA at 4°C. The thylakoid membranes were isolated as described previously (Takahashi et al., 2013), separated by SDS-PAGE, and subjected to western blot. The precipitated protein was washed twice with 80% acetone and resuspended in an adjusted loading buffer volume for SDS-PAGE. For whole-cell protein and thylakoid membranes, the sample load was equivalent to 1 µg of chlorophyll.
To measure the accumulation of PTOX2 in different conditions, the cells were grown to early exponential phase in low light (∼10 µE m−2 s−1) and shifted to high-light or intermittent-light conditions (constant 300 µE m−2 s−1 or 1 min/1 min 0/300 µE m−2 s−1) for 5 or 24 h. They were then harvested, and whole-cell protein extracts were loaded on an identical chlorophyll basis and separated using 12% SDS-PAGE.
Thylakoid membranes were solubilized and separated in Suc density gradients as described (Takahashi et al., 2013), with a 1-h pretreatment using constant light, intermittent light (1 min/1 min), and anoxia in the dark by nitrogen bubbling. Cells were disrupted with a French press and processed as described previously.
Supplemental Data
The following supplemental materials are available
Supplemental Figure S1. Fractionation of broken C. reinhardtii cells.
Supplemental Figure S2. Accumulation of proteins in the wild type and the ptox2 mutant in various light conditions.
Supplemental Figure S3. Influence of the intermittent light on quenching and state transitions.
Supplemental Figure S4. The ΦPSII-decrease phenotype is robust in various intermittent-light conditions.
Supplemental Figure S5. Fluorescence parameters under 1-s/1-s intermittent-light treatment in the wild type and the ptox2 mutant.
Supplemental Figure S6. Fluorescence parameters of 60-s/60-s intermittent-light treatment in the wild type and the ptox2 mutant grown in autotrophic conditions.
Supplemental Figure S7. Membrane protein solubilization and fractionation using Suc density gradient ultracentrifugation.
Supplemental Figure S8. PSI redox state parameters at the end of the illumination periods during the intermittent-light treatment.
Supplemental Figure S9. PSI is not photoinhibited during the intermittent-light treatment.
Acknowledgments
We thank Stefano Santabarbara for experimental suggestions, Wojciech Wietrzyński and Suzanne Ferte for insightful discussions about the data and for help with article preparation, and Sandrine Bujaldon for performing a cross between the wild type and the ptox2 mutant.
Footnotes
This work was supported by the French Ministry of Education to W.J.N. and was further funded by the Centre National de la Recherche Scientifique, by Sorbonne University, and by the Agence Nationale de la Recherche Labex DYNAMO (ANR-11-LABX-0011-01).
References
- Allahverdiyeva Y, Mustila H, Ermakova M, Bersanini L, Richaud P, Ajlani G, Battchikova N, Cournac L, Aro EM (2013) Flavodiiron proteins Flv1 and Flv3 enable cyanobacterial growth and photosynthesis under fluctuating light. Proc Natl Acad Sci USA 110: 4111–4116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allahverdiyeva Y, Suorsa M, Tikkanen M, Aro EM (2015) Photoprotection of photosystems in fluctuating light intensities. J Exp Bot 66: 2427–2436 [DOI] [PubMed] [Google Scholar]
- Alric J. (2014) Redox and ATP control of photosynthetic cyclic electron flow in Chlamydomonas reinhardtii. II. Involvement of the PGR5-PGRL1 pathway under anaerobic conditions. Biochim Biophys Acta 1837: 825–834 [DOI] [PubMed] [Google Scholar]
- Andersen B, Scheller HV, Møller BL (1992) The PSI-E subunit of photosystem I binds ferredoxin:NADP+ oxidoreductase. FEBS Lett 311: 169–173 [DOI] [PubMed] [Google Scholar]
- Bellafiore S, Barneche F, Peltier G, Rochaix JD (2005) State transitions and light adaptation require chloroplast thylakoid protein kinase STN7. Nature 433: 892–895 [DOI] [PubMed] [Google Scholar]
- Bennoun P. (1982) Evidence for a respiratory chain in the chloroplast. Proc Natl Acad Sci USA 79: 4352–4356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardol P, Alric J, Girard-Bascou J, Franck F, Wollman FA, Finazzi G (2009) Impaired respiration discloses the physiological significance of state transitions in Chlamydomonas. Proc Natl Acad Sci USA 106: 15979–15984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carol P, Stevenson D, Bisanz C, Breitenbach J, Sandmann G, Mache R, Coupland G, Kuntz M (1999) Mutations in the Arabidopsis gene IMMUTANS cause a variegated phenotype by inactivating a chloroplast terminal oxidase associated with phytoene desaturation. Plant Cell 11: 57–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaux F, Burlacot A, Mekhalfi M, Auroy P, Blangy S, Richaud P, Peltier G (2017) Flavodiiron proteins promote fast and transient O2 photoreduction in Chlamydomonas. Plant Physiol 174: 1825–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowez S, Godaux D, Cardol P, Wollman FA, Rappaport F (2015) The involvement of hydrogen-producing and ATP-dependent NADPH-consuming pathways in setting the redox poise in the chloroplast of Chlamydomonas reinhardtii in anoxia. J Biol Chem 290: 8666–8676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curien G, Flori S, Villanova V, Magneschi L, Giustini C, Forti G, Matringe M, Petroutsos D, Kuntz M, Finazzi G (2016) The water to water cycles in microalgae. Plant Cell Physiol 57: 1354–1363 [DOI] [PubMed] [Google Scholar]
- Eberhard S, Finazzi G, Wollman FA (2008) The dynamics of photosynthesis. Annu Rev Genet 42: 463–515 [DOI] [PubMed] [Google Scholar]
- Engel BD, Schaffer M, Kuhn Cuellar L, Villa E, Plitzko JM, Baumeister W (2015) Native architecture of the Chlamydomonas chloroplast revealed by in situ cryo-electron tomography. eLife 4: e04889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan DY, Fitzpatrick D, Oguchi R, Ma W, Kou J, Chow WS (2016) Obstacles in the quantification of the cyclic electron flux around photosystem I in leaves of C3 plants. Photosynth Res 129: 239–251 [DOI] [PubMed] [Google Scholar]
- Feilke K, Streb P, Cornic G, Perreau F, Kruk J, Krieger-Liszkay A (2016) Effect of Chlamydomonas plastid terminal oxidase 1 expressed in tobacco on photosynthetic electron transfer. Plant J 85: 219–228 [DOI] [PubMed] [Google Scholar]
- Genty B, Briantais JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990: 87–92 [Google Scholar]
- Godaux D, Bailleul B, Berne N, Cardol P (2015) Induction of photosynthetic carbon fixation in anoxia relies on hydrogenase activity and proton-gradient regulation-like1-mediated cyclic electron flow in Chlamydomonas reinhardtii. Plant Physiol 168: 648–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt-Clermont M, Bassi R (2015) Sharing light between two photosystems: Mechanism of state transitions. Curr Opin Plant Biol 25: 71–78 [DOI] [PubMed] [Google Scholar]
- Hald S, Nandha B, Gallois P, Johnson GN (2008) Feedback regulation of photosynthetic electron transport by NADP(H) redox poise. Biochim Biophys Acta 1777: 433–440 [DOI] [PubMed] [Google Scholar]
- Harris EH, Stern DB, Witman GB (, editors2009) Chapter 8 - Chlamydomonas in the laboratory. In The Chlamydomonas Sourcebook, Ed 2, Vol 1 Academic Press, London, pp 241–302 [Google Scholar]
- Houille-Vernes L, Rappaport F, Wollman FA, Alric J, Johnson X (2011) Plastid terminal oxidase 2 (PTOX2) is the major oxidase involved in chlororespiration in Chlamydomonas. Proc Natl Acad Sci USA 108: 20820–20825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovers J, Potschies M, Polidori A, Pucci B, Raynal S, Bonneté F, Serrano-Vega MJ, Tate CG, Picot D, Pierre Y, et al. (2011) A class of mild surfactants that keep integral membrane proteins water-soluble for functional studies and crystallization. Mol Membr Biol 28: 171–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai M, Takizawa K, Tokutsu R, Okamuro A, Takahashi Y, Minagawa J (2010) Isolation of the elusive supercomplex that drives cyclic electron flow in photosynthesis. Nature 464: 1210–1213 [DOI] [PubMed] [Google Scholar]
- Johnson GN. (2011) Physiology of PSI cyclic electron transport in higher plants. Biochim Biophys Acta 1807: 384–389 [DOI] [PubMed] [Google Scholar]
- Johnson MP, Vasilev C, Olsen JD, Hunter CN (2014) Nanodomains of cytochrome b6f and photosystem II complexes in spinach grana thylakoid membranes. Plant Cell 26: 3051–3061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joliot P, Johnson GN (2011) Regulation of cyclic and linear electron flow in higher plants. Proc Natl Acad Sci USA 108: 13317–13322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klughammer C, Schreiber U (1994) An improved method, using saturating light pulses, for the determination of photosystem I quantum yield via P700+-absorbance changes at 830 nm. Planta 192: 261–268 [Google Scholar]
- Krieger-Liszkay A. (2005) Singlet oxygen production in photosynthesis. J Exp Bot 56: 337–346 [DOI] [PubMed] [Google Scholar]
- Krieger-Liszkay A, Feilke K (2016) The dual role of the plastid terminal oxidase PTOX: Between a protective and a pro-oxidant function. Front Plant Sci 6: 1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazár D. (1999) Chlorophyll a fluorescence induction. Biochim Biophys Acta 1412: 1–28 [DOI] [PubMed] [Google Scholar]
- Lea-Smith DJ, Ross N, Zori M, Bendall DS, Dennis JS, Scott SA, Smith AG, Howe CJ (2013) Thylakoid terminal oxidases are essential for the cyanobacterium Synechocystis sp. PCC 6803 to survive rapidly changing light intensities. Plant Physiol 162: 484–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant SS, Prochnik SE, Vallon O, Harris EH, Karpowicz SJ, Witman GB, Terry A, Salamov A, Fritz-Laylin LK, Maréchal-Drouard L, et al. (2007) The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 318: 245–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosebach L, Heilmann C, Mutoh R, Gäbelein P, Steinbeck J, Happe T, Ikegami T, Hanke G, Kurisu G, Hippler M (2017) Association of ferredoxin:NADP+ oxidoreductase with the photosynthetic apparatus modulates electron transfer in Chlamydomonas reinhardtii. Photosynth Res 134: 291–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrocki WJ, Tourasse NJ, Taly A, Rappaport F, Wollman FA (2015) The plastid terminal oxidase: Its elusive function points to multiple contributions to plastid physiology. Annu Rev Plant Biol 66: 49–74 [DOI] [PubMed] [Google Scholar]
- Nawrocki WJ, Santabarbara S, Mosebach L, Wollman FA, Rappaport F (2016) State transitions redistribute rather than dissipate energy between the two photosystems in Chlamydomonas. Nat Plants 2: 16031. [DOI] [PubMed] [Google Scholar]
- Nawrocki W, Bailleul B, Cardol P, Rappaport F, Wollman FA, Joliot P (2017) Cyclic electron flow in Chlamydomonas reinhardtii. bioRxiv [DOI] [PubMed] [Google Scholar]
- Niyogi KK. (2000) Safety valves for photosynthesis. Curr Opin Plant Biol 3: 455–460 [DOI] [PubMed] [Google Scholar]
- Ort DR, Baker NR (2002) A photoprotective role for O2 as an alternative electron sink in photosynthesis? Curr Opin Plant Biol 5: 193–198 [DOI] [PubMed] [Google Scholar]
- Peers G, Truong TB, Ostendorf E, Busch A, Elrad D, Grossman AR, Hippler M, Niyogi KK (2009) An ancient light-harvesting protein is critical for the regulation of algal photosynthesis. Nature 462: 518–521 [DOI] [PubMed] [Google Scholar]
- Peltier G, Aro EM, Shikanai T (2016) NDH-1 and NDH-2 plastoquinone reductases in oxygenic photosynthesis. Annu Rev Plant Biol 67: 55–80 [DOI] [PubMed] [Google Scholar]
- Rutherford AW, Osyczka A, Rappaport F (2012) Back-reactions, short-circuits, leaks and other energy wasteful reactions in biological electron transfer: Redox tuning to survive life in O2. FEBS Lett 586: 603–616 [DOI] [PubMed] [Google Scholar]
- Schreiber U. (2004) Pulse-amplitude-modulation (PAM) fluorometry and saturation pulse method: An overview. In Papageorgiou GC, Govindjee, eds, Chlorophyll a Fluorescence: A Signature of Photosynthesis. Springer, Dordrecht, The Netherlands, pp 279–319 [Google Scholar]
- Stepien P, Johnson GN (2018) Plastid terminal oxidase requires translocation to the grana stacks to act as a sink for electron transport. Proc Natl Acad Sci USA 115: 9634–9639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suorsa M, Järvi S, Grieco M, Nurmi M, Pietrzykowska M, Rantala M, Kangasjärvi S, Paakkarinen V, Tikkanen M, Jansson S, et al. (2012) PROTON GRADIENT REGULATION5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant Cell 24: 2934–2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Clowez S, Wollman FA, Vallon O, Rappaport F (2013) Cyclic electron flow is redox-controlled but independent of state transition. Nat Commun 4: 1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouillard M, Shahbazi M, Moyet L, Rappaport F, Joliot P, Kuntz M, Finazzi G (2012) Kinetic properties and physiological role of the plastoquinone terminal oxidase (PTOX) in a vascular plant. Biochim Biophys Acta 1817: 2140–2148 [DOI] [PubMed] [Google Scholar]
- Vallon O, Bulte L, Dainese P, Olive J, Bassi R, Wollman FA (1991) Lateral redistribution of cytochrome b6/f complexes along thylakoid membranes upon state transitions. Proc Natl Acad Sci USA 88: 8262–8266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Wright DA, Wetzel C, Voytas DF, Rodermel S (1999) The IMMUTANS variegation locus of Arabidopsis defines a mitochondrial alternative oxidase homolog that functions during early chloroplast biogenesis. Plant Cell 11: 43–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamori W, Shikanai T (2016) Physiological functions of cyclic electron transport around photosystem I in sustaining photosynthesis and plant growth. Annu Rev Plant Biol 67: 81–106 [DOI] [PubMed] [Google Scholar]
- Zhang H, Whitelegge JP, Cramer WA (2001) Ferredoxin:NADP+ oxidoreductase is a subunit of the chloroplast cytochrome b6f complex. J Biol Chem 276: 38159–38165 [DOI] [PubMed] [Google Scholar]
- Zoonens M, Popot JL (2014) Amphipols for each season. J Membr Biol 247: 759–796 [DOI] [PMC free article] [PubMed] [Google Scholar]