PGR5-dependent CET protects PSI from photodamage under fluctuating light via the regulation of electron transport at both donor and acceptor sides of PSI.
Abstract
In response to a sudden increase in light intensity, plants must cope with absorbed excess photon energy to protect photosystems from photodamage. Under fluctuating light, PSI is severely photodamaged in the Arabidopsis (Arabidopsis thaliana) proton gradient regulation5 (pgr5) mutant defective in the main pathway of PSI cyclic electron transport (CET). Here, we aimed to determine how PSI is protected by two proposed regulatory roles of CET via transthylakoid ΔpH formation: (1) reservation of electron sink capacity by adjusting the ATP/NADPH production ratio (acceptor-side regulation) and (2) down-regulation of the cytochrome b6f complex activity called photosynthetic control for slowing down the electron flow toward PSI (donor-side regulation). We artificially enhanced donor- and acceptor-side regulation in the wild-type and pgr5 backgrounds by introducing the pgr1 mutation conferring the hypersensitivity of the cytochrome b6f complex to luminal acidification and moss Physcomitrella patens flavodiiron protein genes, respectively. Enhanced photosynthetic control partially alleviated PSI photodamage in the pgr5 mutant background but restricted linear electron transport under constant high light, suggesting that the strength of photosynthetic control should be optimized. Flavodiiron protein-dependent oxygen photoreduction formed a large electron sink and alleviated PSI photoinhibition, accompanied by the induction of photosynthetic control. Thus, donor-side regulation is essential for PSI photoprotection but acceptor-side regulation also is important to rapidly induce donor-side regulation. In angiosperms, PGR5-dependent CET is required for both functions.
Light reactions of photosynthesis convert solar energy into chemical energy in the forms of NADPH and ATP, which are required for CO2 fixation by the Calvin-Benson cycle. Linear electron transport (LET) from water to NADP+ generates both NADPH and ATP, whereas cyclic electron transport (CET) around PSI preferentially contributes to ATP synthesis. In LET, electrons derived from water splitting in PSII are transported to PSI via plastoquinone (PQ), the cytochrome b6f (Cyt b6f) complex, and plastocyanin. PSI reduces ferredoxin (Fd), an iron-sulfur electron carrier protein, which donates electrons to NADP+ via Fd:NADP+ oxidoreductase. Coupled with the electron transport, protons (H+) are concentrated in the thylakoid lumen by H+ translocation from the stroma to the thylakoid lumen via the quinone (Q) cycle in the Cyt b6f complex and by water splitting in PSII, resulting in the generation of the proton motive force (pmf) composed of a transthylakoid proton gradient (ΔpH) and a membrane potential (ΔΨ; Kramer et al., 2003). Both ΔpH and ΔΨ components contribute equivalently to ATP synthesis as the driving force of ATP synthase (Soga et al., 2017). However, the ATP/NADPH production ratio in LET is calculated as 1.29, based on the structure of the c-rotor ring of chloroplast ATP synthase (Hahn et al., 2018). Theoretically, LET does not satisfy the ATP/NADPH ratio of 1.5 required by the Calvin-Benson cycle for CO2 assimilation (Allen, 2002; Shikanai, 2007). In contrast to LET, CET is driven solely by PSI. In angiosperms, CET operates through two routes of electron transport, namely a PROTON GRADIENT REGULATION5 (PGR5)/PGR5-like Photosynthetic Phenotype1 (PGRL1) protein complex-dependent, antimycin A-sensitive pathway and an NADH dehydrogenase-like complex (NDH)-dependent, antimycin A-insensitive pathway (Munekage et al., 2002, 2004; DalCorso et al., 2008). Both protein complexes backflow electrons from Fd to the PQ pool as Fd-dependent PQ reductases without reducing NADP+ (Yamamoto et al., 2011; Hertle et al., 2013; Yamamoto and Shikanai, 2013), and the Cyt b6f complex moves H+ into the thylakoid lumen via the Q cycle, although the exact molecular function of the PGR5/PGRL1 complex is still a topic of debate. Furthermore, the NDH complex pumps H+ from the stroma to the thylakoid lumen (Strand et al., 2017). Consequently, CET generates ΔpH without net accumulation of NADPH. ΔpH formed by CET contributes to additional ATP production to satisfy the ATP/NADPH production ratio for CO2 fixation. Consistent with this idea, CO2 assimilation in rice (Oryza sativa) mutants defective in the PGR5/PGRL1-dependent CET or both CET pathways is disturbed significantly (Nishikawa et al., 2012; Wada et al., 2018).
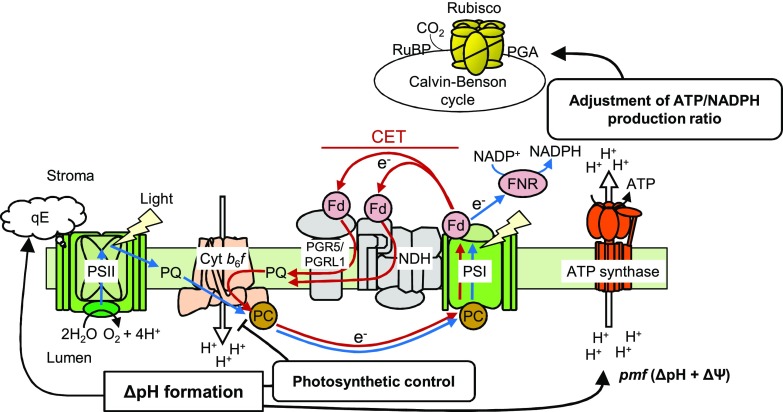
Furthermore, several photoprotective regulations of electron transport via CET-dependent ΔpH formation have been proposed (Fig. 1; Chaux et al., 2015; Yamori and Shikanai, 2016; Shikanai and Yamamoto, 2017). (1) Alleviation of PSI acceptor-side limitation of electron transport via adjustment of the ATP/NADPH production ratio for CO2 assimilation and photorespiration. This PSI acceptor-side regulation by CET sustains electron sinks downstream of PSI and prevents overreduction of the PSI primary electron donor (P700) from generating reactive oxygen species (Munekage et al., 2002; Takagi et al., 2016). (2) Acidification of the thylakoid lumen down-regulates the Cyt b6f complex to slow down the electron transport rate from PSII toward PSI and prevents the overreduction of P700. This enhancement of the PSI donor-side limitation of electron transport via luminal acidification is called photosynthetic control (Rumberg and Siggel, 1969; Tikhonov et al., 1981; Nishio and Whitmarsh, 1993). This is the PSI donor-side regulation by CET for PSI photoprotection (Joliot and Johnson, 2011; Suorsa et al., 2012). (3) Luminal acidification also induces the thermal dissipation of absorbed excess photon energy from PSII antennae, which can be monitored as the energy-dependent quenching (qE) component of nonphotochemical quenching (NPQ) of chlorophyll fluorescence (Müller et al., 2001). qE protects PSII from photoinhibition (Li et al., 2002). Compared with the Arabidopsis (Arabidopsis thaliana) wild type and chlororespiratory reduction mutants, which lack chloroplast NDH activity, the size of pmf and NPQ induction upon high-light illumination are reduced significantly in the pgr5 mutant, indicating that the contribution of the PGR5/PGRL1-dependent pathway to ΔpH formation is more significant than that of the NDH complex-dependent pathway in C3 plants (Munekage et al., 2002, 2004; DalCorso et al., 2008; Wang et al., 2015; Yamamoto et al., 2016). The same trend is observed in ATP synthesis in ruptured chloroplasts (Wang et al., 2018).
Figure 1.
Proposed mechanisms for the regulation of photosynthetic electron transport under high light by PGR5/PGRL1-dependent CET. LET and CET are indicated by blue and red arrows, respectively. In angiosperms, CET consists of two pathways: the PGR5/PGRL1- and NDH-dependent pathways. In response to a sudden increase in light intensity, PGR5/PGRL1-dependent CET backflows electrons from PSI to the PQ pool without net NADPH production and generates ΔpH across the thylakoid membrane via the Q cycle in the Cyt b6f complex. In the PSI acceptor-side regulation, pmf composed of ΔpH and ΔΨ drives ATP synthesis via ATP synthase and adjusts the ATP/NADPH production ratio, which is required for operating the Calvin-Benson cycle and photorespiration. This mechanism alleviates the PSI acceptor-side limitation of electron transport by increasing electron sink capacity downstream of PSI. In the PSI donor-side regulation, luminal acidification slows plastoquinol oxidation at the Cyt b6f complex to prevent excess electron flow toward PSI. This mechanism is called photosynthetic control. In addition, the luminal acidification induces qE quenching in the PSII antenna to discard excess photon energy as heat. FNR, Fd:NADP+ oxidoreductase; PC, plastocyanin; PGA, 3-phosphoglycerate; RuBP, ribulose 1,5-bisphosphate.
In nature, plants continuously experience extreme fluctuations of light intensity caused by sunflecks and shading (Pearcy et al., 1996). Long-standing studies of the photoinhibition of photosystems conducted under constant high light have indicated that PSII is preferentially photodamaged, but photodamaged PSII is quickly repaired by the sophisticated PSII repair cycle (Nixon et al., 2010; Järvi et al., 2015). In contrast, PSI photodamage under constant high light has been observed specifically in chilling-sensitive plants (Sonoike, 2011). Furthermore, photodamaged PSI cannot be repaired efficiently and is frequently fatal (Teicher et al., 2000). Recent works on Arabidopsis and rice have reported crucial roles of PGR5 in PSI photoprotection under fluctuating light (Suorsa et al., 2012; Yamori et al., 2016). The Arabidopsis pgr5 mutant cannot survive under artificial fluctuating light and also in natural conditions (Grieco et al., 2012; Suorsa et al., 2012). In contrast to constant high light, fluctuating light preferentially damages PSI even in wild-type plants (Kono et al., 2014). The pgr5 mutant suffers more severely from PSI photodamage than the wild type, whereas both npq1 and npq4 mutants defective in qE induction are not sensitive to fluctuating light (Grieco et al., 2012; Suorsa et al., 2012), indicating that PGR5/PGRL1 complex-dependent CET safeguards PSI against photodamage under fluctuating light. PSI photoprotection by PGR5 is considered to be associated with the alleviation of PSI acceptor-side limitation via adjustment of the ATP/NADPH production ratio via CET (acceptor-side regulation; Munekage et al., 2002). PSI photoinhibition is severe in the pgr5 mutant under CO2-limiting conditions. Furthermore, methyl viologen treatment relieves the strong reduction of P700 in the pgr5 mutant in strong light, suggesting a shortage of efficient electron acceptors downstream of PSI (Munekage et al., 2002). However, PSI photoinhibition in the pgr5 mutant under fluctuating light is strongly suppressed with 3-(3,4-dichlorophenyl)-1,1-dimethylurea and further in a mutant background with reduced PSII activity (Suorsa et al., 2012, 2016). Thus, Suorsa et al. (2012, 2013) proposed that PSI photoprotection by PGR5 is based on the slowdown of electron transport from PSII toward PSI via photosynthetic control by luminal acidification (donor-side regulation).
Here, we applied synthetic biological approaches to evaluate the contribution of PSI donor- and acceptor-side regulation via the PGR5/PGRL1-dependent CET to PSI photoprotection. We produced plants whose electron-sink sizes downstream of PSI and photosynthetic control responses were artificially enhanced in the Arabidopsis wild-type and pgr5 mutant backgrounds by introducing moss Physcomitrella patens flavodiiron protein (PpFlv) genes (Yamamoto et al., 2016) and a mutation in the Rieske subunit of the Cyt b6f complex, which confer hypersensitivity of the complex to luminal acidification, respectively (Munekage et al., 2001). Both the artificial enhancements of PSI donor- and acceptor-side regulation significantly suppressed PSI photoinhibition under fluctuating light in the pgr5 mutant background, indicating that both regulations via CET contribute to PSI photoprotection. We discuss the tradeoff between the photoprotection of two photosystems and the reason why balance is necessary between the CET-dependent luminal acidification and the pH sensitivity of the Cyt b6f complex.
RESULTS
Combining pgr1 and pgr5 Mutations Did Not Affect Plant Growth under Constant Low Light
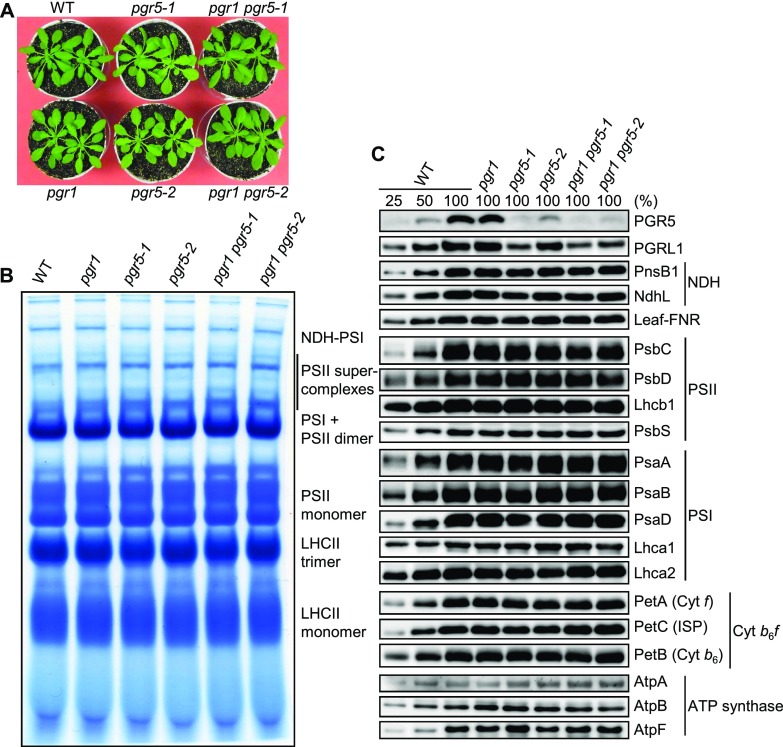
To evaluate the contribution of PSI donor-side regulation via CET to PSI photoprotection under fluctuating light, we analyzed six genotypes: wild type, pgr1, pgr5-1, pgr5-2, pgr1 pgr5-1, and pgr1 pgr5-2. The pgr1 mutant has an amino acid alteration (Pro-194Leu) in the Rieske subunit of the Cyt b6f complex, resulting in hypersensitivity of the complex to luminal acidification with a shift of acid dissociation constant or redox potential (Munekage et al., 2001; Jahns et al., 2002). In the pgr1 mutant, down-regulation of PQ oxidation by the Cyt b6f complex is observed at about 1 pH unit more alkaline than in the wild type, indicating that the response of photosynthetic control is enhanced by the single amino acid alteration in the Rieske subunit (Jahns et al., 2002). This hypersensitivity to low luminal pH restricts electron transport through the Cyt b6f complex at high light intensity. Consequently, the luminal pH does not decrease below the threshold required for the NPQ (qE) induction in the pgr1 mutant (Munekage et al., 2001). As the Cyt b6f complex is involved in both LET and CET, the pgr1 mutation likely slows down LET and CET to the same extent and, thus, is unlikely to affect the ATP/NADPH production ratio. Consistently, P700 is more oxidized in the pgr1 mutant, which contrasts with the severe reduction of P700 in the pgr5 mutant specifically defective in CET (Munekage et al., 2001, 2002). The strong mutant allele of pgr5 used in the previous studies is called pgr5-1 here to distinguish it from the weak allele pgr5-2 also used in this study. The pgr5-2 mutant was identified as a high-chlorophyll-fluorescence mutant in CO2-free air containing 5% (v/v) oxygen at 50 µmol photons m−2 s−1 (Wang et al., 2017). We characterized both alleles of pgr5 defective in the main CET pathway to assess the contribution of PGR5/PGRL1-dependent CET to PSI photoprotection under fluctuating light intensity. We also characterized two double mutants, pgr1 pgr5-1 and pgr1 pgr5-2, to quantitatively investigate the effect of enhanced photosynthetic control on CET defects. Under constant growth light at 50 to 60 μmol photons m−2 s−1, no obvious difference in growth was observed among the six genotypes (Fig. 2A). Blue native (BN)-PAGE and immunoblot analyses of thylakoid proteins indicated that neither the pgr1 nor pgr5 mutation affected the accumulation of the major protein complexes PSII, PSI, ATP synthase, and the NDH complex in the thylakoid membrane (Fig. 2, B and C). An amino acid alteration in the Rieske subunit in the pgr1 mutant did not affect the accumulation of the Rieske protein and other subunits of the Cyt b6f complex, as reported previously (Fig. 2C; Munekage et al., 2001). Accumulation of the PGR5 protein was more severely affected in the pgr5-1 allele (Gly-130Ala) than in the pgr5-2 allele (Ser-98Phe). The reduction of PGR5 accumulation in the pgr5-1 and pgr5-2 mutant backgrounds was accompanied by the reduced accumulation of PGRL1, which is proposed to form a protein complex with PGR5 in the thylakoid membrane (DalCorso et al., 2008; Hertle et al., 2013).
Figure 2.
Growth and thylakoid protein accumulation in wild-type (WT), pgr1, pgr5, pgr1 pgr5-1, and pgr1 pgr5-2 plants. A, Plants were grown in a growth chamber at 50 to 60 μmol photons m−2 s−1 under short-day conditions (9 h of light/15 h of dark) for 40 d. B, BN-PAGE analysis of thylakoid protein complexes. The gel was stained with Bio-Safe Coomassie stain. The positions of photosynthetic complexes are indicated on the right. C, Immunoblot analysis of photosynthetic proteins in the chloroplast membrane fractions. Antibodies used are indicated on the right.
Artificially Enhanced Photosynthetic Control Alleviates P700 Reduction during Steady-State Photosynthesis in the pgr5 Mutant Backgrounds
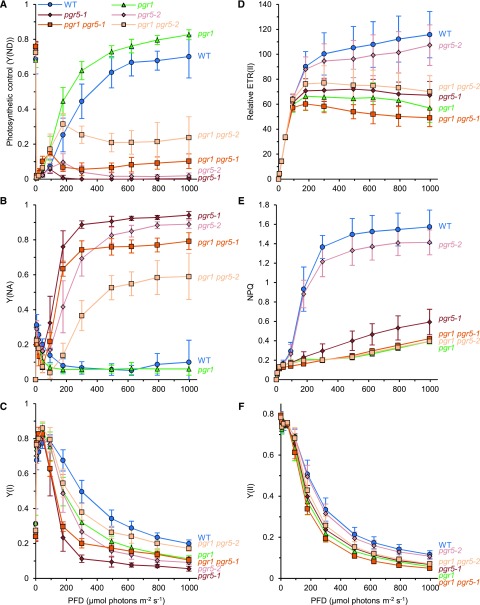
To investigate the effect of the hypersensitivity of the Cyt b6f complex to luminal pH on steady-state photosynthesis, we analyzed the light intensity dependence of both PSII and PSI photochemistry by measuring chlorophyll fluorescence and absorption changes in P700 (Fig. 3). The Y(ND) parameter of P700 absorbance change represents the PSI donor-side limitation in electron transport and was used to estimate the operation of photosynthetic control (Fig. 3A). In the pgr1 mutant, photosynthetic control was induced steeply in response to increased light intensity and reached a higher level than that in the wild type. Consistent with the previous conclusion (Munekage et al., 2001; Jahns et al., 2002), the sensitivity of photosynthetic control to the luminal acidification was enhanced in the pgr1 mutant. On the contrary, both alleles of the pgr5 mutant did not induce photosynthetic control via the Cyt b6f complex, because of its defect in the CET-dependent luminal acidification. Introduction of the pgr1 mutation into both pgr5 mutant alleles enhanced the PSI donor-side limitation. The level of photosynthetic control was higher in the pgr5-2 background than that in the pgr5-1 background, reflecting the different impacts of the alleles on PGR5/PGRL1-dependent CET (Fig. 3A). The Y(NA) parameter represents the acceptor-side limitation of PSI but cannot distinguish between excess electrons on the donor side and shortage of electron acceptors on the acceptor side. The high Y(NA) and the relatively high reduced levels of P700 were alleviated by the introduction of the pgr1 mutation into both pgr5 mutant alleles (Fig. 3B; Supplemental Fig. S1A).
Figure 3.
Impact of the enhanced photosynthetic control response by the pgr1 mutation on electron transport during steady-state photosynthesis. The light intensity dependence of PSII and PSI photosynthetic parameters was monitored in the wild type (WT) and pgr1, pgr5, and pgr1 pgr5 mutant alleles. A to C, PSI parameters donor-side limitation of PSI [Y(ND)], acceptor-side limitation of PSI [Y(NA)], and quantum yield of PSI [Y(I)], respectively. D to F, PSII parameters relative ETR(II), NPQ, and quantum yield of PSII [Y(II)], respectively (biological replicates n = 8–9 ± sd).
The maximum quantum yield of PSII (Fv/Fm) reflecting the intactness of PSII did not differ among the six genotypes (wild type, 0.79 ± 0.02; pgr1, 0.79 ± 0.01; pgr5-1, 0.79 ± 0.02; pgr5-2, 0.77 ± 0.03; pgr1 pgr5-1, 0.79 ± 0.01; and pgr1 pgr5-2, 0.78 ± 0.02; biological replicates n = 8–9 ± sd). The relative rate of electron transport through PSII [relative ETR(II)], estimated from chlorophyll fluorescence, was not affected in any mutants at light intensities below 100 μmol photons m−2 s−1, consistent with the same growth rate among the six genotypes at 50 to 60 µmol photons m−2 s−1 (Figs. 2 and 3D). As compared with the wild type, however, relative ETR(II) was saturated at lower levels in the pgr1 and pgr5-1 mutants. In the pgr5-2 mutant, relative ETR(II) was only slightly lower than that in the wild type, in contrast to its strong impact on photosynthetic control and Y(NA) (Fig. 3, A and B). In the pgr1 pgr5-2 double mutant, relative ETR(II) decreased to a similar level to the pgr1 single mutant, indicating that the pgr1 mutation slowed the rate of electron transport through the Cyt b6f complex at high light intensities (Fig. 3D). In the pgr1 pgr5-2 double mutant, the defect in relative ETR(II) was no more significantly enhanced than that in each single mutant. This is probably because of the mutual interaction between the higher sensitivity of photosynthetic control to luminal acidification in the pgr1 mutant and the higher luminal pH in the pgr5-1 mutant.
The defect of CET in the pgr5-1 mutant severely suppressed NPQ (qE) induction (Fig. 3E), as reported previously (Munekage et al., 2002). Although the pgr5-2 mutant induced NPQ close to the wild-type level, its photosynthetic control was not induced at high light intensities, as in the pgr5-1 mutant (Fig. 3, A and E). qE is not essential for the down-regulation of LET toward PSI, and lower luminal pH is required for the induction of photosynthetic control than for qE induction, as reported previously (Takizawa et al., 2007). Furthermore, in the pgr1 single mutant and both pgr1 pgr5 double mutants, the qL parameter, representing the oxidation of the PQ pool, was reduced more than in the other genotypes (Supplemental Fig. S1B). This phenotype is simply explained by the enhanced photosynthetic control in the pgr1 mutant background. Because the qE defect in the Arabidopsis npq1 and npq4 mutants also leads to reduction of the QA redox state (Niyogi et al., 1998; Li et al., 2002), we could not eliminate the possibility that low NPQ also partly affected qL.
The comparison of PSII and PSI photochemistry during steady-state photosynthesis indicates that enhanced photosynthetic control oxidizes P700 under high light in the pgr1 mutant, but it is accompanied by the strong reduction of electron carriers in the interelectron transport chain between PSII and the Cyt b6f complex. However, the pgr5-1 defect severely reduced electron carriers in the entire electron transport chain. Although the pgr5-2 mutation only slightly affected Y(II) and Y(I), the limitation of PSI photochemistry shifted significantly from the donor side to the acceptor side. Even the weak defect in PGR5/PGRL1-dependent CET in the pgr5-2 mutant significantly disturbed the regulation of electron transport through PSI. Notably, the pgr1 defect observed in both yields was partially alleviated by the pgr5-2 mutation (Fig. 3, C and F). The mild reduction in the size of ∆pH in the pgr5-2 mutant alleviated the hypersensitivity of photosynthetic control to luminal acidification in the pgr1 mutant background. CET and photosynthetic control should be balanced to maximize LET during steady-state photosynthesis, which is likely achieved in the wild-type plants.
Artificial Enhancement of Photosynthetic Control Alleviates PSI Photoinhibition in the pgr5 Mutant Background under Fluctuating Light
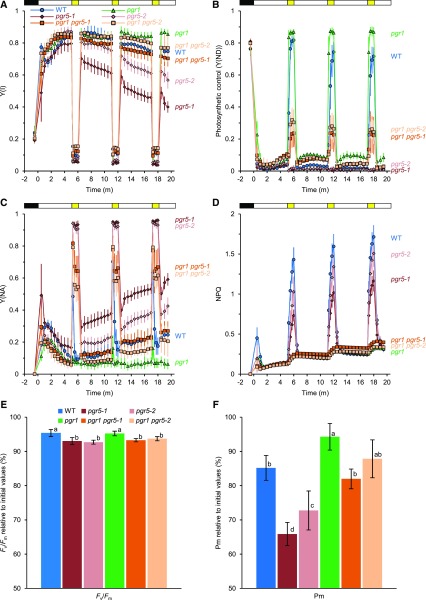
We then analyzed the effect of artificial enhancement of photosynthetic control on PSI photoprotection. Leaf discs from the six genotypes were exposed to fluctuating light consisting of three repetitions of a high-light phase (1,529 or 1,886 µmol photons m−2 s−1 for 1 min) sandwiched by two low-light phases (47 µmol photons m−2 s−1 for 5 min) using the DUAL-PAM system (Fig. 4). After the second high-light phase, the pgr1 mutant sustained higher PSII and PSI yields than the wild type (Fig. 4A; Supplemental Fig. S2A), suggesting that PSI was more resistant to the fluctuating light in the pgr1 mutant. As reported previously (Suorsa et al., 2012), the two pgr5 mutant alleles were sensitive to fluctuating light and the yield was decreased steeply in both photosystems (Fig. 4A; Supplemental Fig. S2A). Enhanced photosynthetic control in the pgr1 pgr5-1 and pgr1 pgr5-2 double mutants conferred stronger resistance of Y(II) and Y(I) to fluctuating light than the corresponding pgr5 single mutant allele (Fig. 4A; Supplemental Fig. S2A). Stronger photosynthetic control resulted in the accumulation of fewer electrons on the donor side and also was reflected by lower Y(NA) in the pgr1 mutant than in the wild type (Fig. 4, B and C). After the shift to the high-light phase, P700 also was oxidized more quickly in the pgr1 mutant than in the wild type, probably because of enhanced photosynthetic control (Fig. 4B; Supplemental Fig. S3A). P700 also was more oxidized in low-light phases in the pgr1 mutant than in the wild type. In contrast, in both pgr5 mutant alleles, P700 was reduced severely, especially after the first high-light phase. However, introduction of the pgr1 mutation into both pgr5 mutant alleles facilitated the partial oxidation of P700 in the high-light phases (Supplemental Figs. S2B and S3A). These results indicate that enhanced photosynthetic control in the pgr1 mutant backgrounds suppressed electron flow toward PSI. Consistent with the phenotype during steady-state photosynthesis (Fig. 3), the pgr1 and two pgr1 pgr5 mutant alleles strongly suppressed NPQ induction in the high-light phases (Fig. 4D). This contrasts with the responses of NPQ in the high-light phases in the pgr5 single mutant alleles, suggesting that ∆pH formation depends on LET or NDH-dependent CET in the pgr5 mutant backgrounds.
Figure 4.
Enhanced photosynthetic control response by the pgr1 mutation protects PSI from photodamage under fluctuating light. The effect of fluctuating light on photosynthetic parameters was monitored in the wild type (WT) and pgr1, pgr5, and pgr1 pgr5 mutant alleles. A to D, Y(I), Y(ND), Y(NA), and NPQ, respectively (biological replicates n = 7–9 ± sd). Leaf discs from plants dark adapted for 20 min were exposed to fluctuating light: black in the top bar, darkness; white, low light (47 µmol photons m−2 s−1); yellow, 1 min of high light (1,529 µmol photons m−2 s−1). E and F, PSII and PSI photoinhibition, respectively, in the wild type and pgr1, pgr5, and pgr1 pgr5 mutant alleles under fluctuating light. After the fluctuating light treatment, the leaf discs were sandwiched between wet tissue paper and incubated in the dark for 25 min, and then Fv/Fm and Pm were measured. Their relative values are shown against the values before the treatment (biological replicates n = 7–9 ± sd). Columns with the same letters are not significantly different between genotypes (Tukey-Kramer test, P < 0.05).
After the fluctuating light treatment, PSII and PSI photoinhibition were evaluated by measuring Fv/Fm and Pm, representing the oxidizable fraction of P700, respectively (Fig. 4, E and F). In our fluctuating light conditions, PSI photoinhibition was more evident than PSII photoinhibition. The Fv/Fm level was over 93% of that before the stress treatment in all the genotypes. In contrast, Pm was decreased to 85%, even in the wild type. Compared with the wild type, PSI was severely photodamaged in both pgr5 single mutant alleles. Pm decreased to 66% and 73% of that in the wild type in the pgr5-1 and pgr5-2 mutants, respectively. In contrast, the pgr1 single mutant sustained higher PSI activity than the wild type. The pgr1 pgr5 double mutants also sustained higher PSI activity than the corresponding pgr5 single mutants. These results indicate that the suppression of electron flow toward PSI via photosynthetic control is crucial for PSI photoprotection under fluctuating light and that PGR5/PGRL1-dependent CET is necessary for the operation of photosynthetic control via ΔpH formation.
To analyze PSI photoinhibition in the absence of the CET-dependent regulation of electron transport, we exposed leaves to repetitive saturation pulses of illumination (rSP) in the dark, as reported previously (Supplemental Fig. S4;http://www.plantphysiol.org/cgi/content/full/pp.18.01343/DC1Sejima et al., 2014). The rSP treatment preferentially decreases Y(I) rather than Y(II) (Supplemental Fig. S4, A and B). Under the rSP conditions, plants did not form sufficient ΔpH to increase NPQ or photosynthetic control (Supplemental Fig. S4, C and D). Initial levels of Y(NA) were similarly low in all the genotypes (Supplemental Fig. S4E), because the effect of disturbed regulation of the ATP/NADPH ratio was canceled in the dark by inactivating the Calvin-Benson cycle. The rSP treatment also preferentially photodamaged PSI (Supplemental Fig. S4, F and G). However, in contrast to the results under fluctuating light conditions (Fig. 4), no significant differences in the susceptibility of PSI to rSP between the six genotypes were observed (Supplemental Fig. S4G). This result also supports the idea that the ΔpH-dependent regulation of electron transport via PGR5/PGRL1-dependent CET is crucial for PSI photoprotection against a sudden increase in light intensity.
Artificial Enhancement of Electron Sink Capacity by Flv Suppressed PSI Photoinhibition under Fluctuating Light
Flv forms a large family of enzymes catalyzing the reduction of oxygen or nitric oxide to water or nitrous oxide, respectively (Allahverdiyeva et al., 2015; Folgosa et al., 2018). Phototrophs from cyanobacteria up to gymnosperms conserve the Flv genes, but angiosperms like Arabidopsis lack these genes (Allahverdiyeva et al., 2015; Gerotto et al., 2016; Yamamoto et al., 2016; Ilík et al., 2017). Flv significantly contributes to photoprotection as an electron sink in cyanobacteria, green algae, liverworts, and mosses (Allahverdiyeva et al., 2013; Gerotto et al., 2016; Chaux et al., 2017; Shimakawa et al., 2017). We previously showed that the introduction of P. patens FlvA and FlvB genes significantly alleviates PSI photodamage in fluctuating light in Arabidopsis (Yamamoto et al., 2016) and rice (Wada et al., 2018).
In our model, PGR5/PGRL1-dependent CET supplies sufficient electron sinks downstream of PSI by balancing the ATP/NADPH production ratio (Fig. 1; Munekage et al., 2002; Shikanai, 2007). To mimic this acceptor-side regulation via PGR5/PGRL1-dependent CET, we reevaluated the transgenic lines, in which the electron sink capacity was enhanced artificially by the expression of P. patens FlvA and FlvB genes in the Arabidopsis pgr5-1 mutant background (Yamamoto et al., 2016). In this study, we used the same conditions of fluctuating light used in the characterization of enhanced photosynthetic control depending on the pgr1 mutation (1 min of high light of 1,529 µmol photons m−2 s−1 and 5 min of low light of 47 µmol photons m−2 s−1 for the pgr5 mutant background) and finally quantitatively characterized the extent of photodamage in both photosystems (Fig. 5). In the pgr5-1 mutant background, up to 25% of electrons derived from water splitting in PSII were used for Flv-dependent oxygen photoreduction under steady-state photosynthesis, indicating that Flv-dependent electron transport formed a large electron sink downstream of PSI (Yamamoto et al., 2016). To address the contribution of this electron sink capacity to PSI photoprotection, we exposed wild-type, pgr5-1, and transgenic plants, WT+35S;PpFlv no. 13 and pgr5-1+35S;PpFlv no. 13, which accumulated PpFlv in the wild-type and pg5-1 mutant backgrounds, respectively, to fluctuating light (Fig. 5; Supplemental Fig. S5). During the fluctuating light treatment, pgr5-1 steeply decreased Y(II) and Y(I) (Supplemental Fig. S5, A and B). Flv accumulation in the pgr5-1 mutant significantly suppressed the decline in both Y(II) and Y(I) similar to the wild-type level. In the pgr5-1 mutant, Y(NA) was increased strongly in high-light phases. This is probably because of excess electron supply from the donor-side and the acceptor-side limitations. Flv accumulation suppressed an increase in Y(NA) in the pgr5-1 mutant background, as reported previously (Yamamoto et al., 2016), and kept P700 oxidized during high-light phases (Supplemental Fig. S5, D and E). Although the rate of NPQ development upon high-light illumination was similar between the wild type and WT+35S;PpFlv, the transient increase in Y(NA) upon the onset of high light was observed only in the wild type, indicating the function of Flv as a safety valve to immediately remove electrons from PSI (Supplemental Fig. S5D). Consistent with its safety valve function, Flv-dependent oxygen photoreduction oxidized P700 immediately upon the onset of high light in the wild-type background (Supplemental Fig. S3B). These results indicate that the Flv-dependent electron sink alleviates PSI acceptor-side limitation under fluctuating light in both the pgr5-1 and wild-type backgrounds.
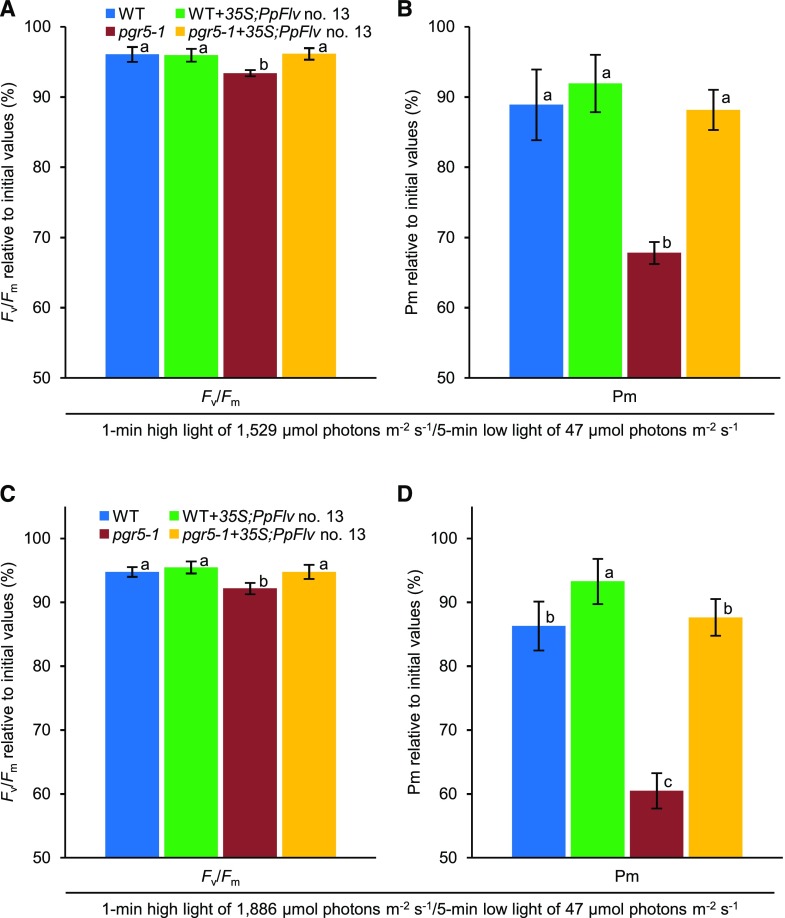
Figure 5.
Enhanced electron sink capacity downstream of PSI by PpFlv protects PSI from photodamage under fluctuating light. A and B, PSII and PSI photoinhibition, respectively, in the wild type (WT), pgr5-1, WT+35S;PpFlv no.13, and pgr5-1+35S;PpFlv no.13 under fluctuating light (1 min of high light of 1,529 µmol photons m−2 s−1 and 5 min of low light of 47 µmol photons m−2 s−1; biological replicates n = 8–9 ± sd). C and D, PSII and PSI photoinhibition, respectively, in the wild type, pgr5-1, WT+35S;PpFlv no.13, and pgr5-1+35S;PpFlv no.13 under fluctuating light (1 min of high light of 1,886 µmol photons m−2 s−1 and 5 min of low light of 47 µmol photons m−2 s−1; biological replicates n = 9–10 ± sd). Leaf discs from plants dark adapted for 20 min were exposed to fluctuating light (1 min of high light of 1,529 or 1,886 µmol photons m−2 s−1 and 5 min of low light of 47 µmol photons m−2 s−1). After the fluctuating light treatment, the leaf discs were sandwiched between wet tissue paper and incubated in the dark for 25 min, and then Fv/Fm and Pm were measured. Their relative values are shown against the values before the treatment. Columns with the same letters are not significantly different between genotypes (Tukey-Kramer test, P < 0.05).
After the fluctuating light treatment, we analyzed PSI photoinhibition. Flv expression significantly suppressed PSI photoinhibition in the pgr5-1 mutant background (Fig. 5B). By further increasing light intensity in high-light phases from 1,529 to 1,886 μmol photons m−2 s−1, Flv alleviated PSI photoinhibition even in the wild-type background (Fig. 5D). These results indicate that the alleviation of PSI acceptor-side limitation also is effective for PSI photoprotection under fluctuating light.
DISCUSSION
The artificial enhancement of photosynthetic control by the pgr1 mutation alleviated PSI photoinhibition under fluctuating light in two pgr5 mutant alleles and also in the wild-type background. Slowing down the electron flow toward PSI via photosynthetic control is crucial for PSI photoprotection under fluctuating light (Figs. 1 and 4). Although the induction of NPQ in the high-light phases was strongly suppressed in the pgr1 mutant backgrounds (pgr1, pgr1 pgr5-1, and pgr1 pgr5-2), P700 in these mutants was more oxidized than in the wild type and the corresponding pgr5 single mutants (Fig. 4). Furthermore, PSI was more severely photodamaged in the pgr5 single mutants than in the corresponding double mutants with the pgr1 background (Fig. 4). These results indicate that the contribution of NPQ to the suppression of electron flow toward PSI is much smaller than that of photosynthetic control via PGR5/PGRL1-dependent CET, as reported previously (Tikkanen et al., 2015). This is consistent with the fact that Y(II) is unaffected in the Arabidopsis npq1 and npq4 mutants defective in qE induction (Niyogi et al., 1998; Li et al., 2000). Even in the weak allele of pgr5-2, the induction of photosynthetic control was impaired severely (Fig. 3A). PGR5/PGRL1-dependent CET is essential for inducing photosynthetic control via luminal acidification, as suggested previously (Joliot and Johnson, 2011; Suorsa et al., 2012).
Both pgr1 and pgr5 mutants were identified based on the reduced size of NPQ at high light intensity (Shikanai et al., 1999), but NPQ induction was affected differently in the low light-to-high light shift. After the shift, transient NPQ was partially induced in both pgr5 mutant alleles but not in the pgr1 mutant background (Fig. 4D). In the pgr5 mutant alleles, qE is likely induced by the transient unbalance between ∆pH formation and relaxation. When the relaxation of ∆pH is not fully activated immediately after the low light-to-high light transition, LET or NDH-dependent CET is sufficient to partially induce transient NPQ. In contrast, all the electron transport through the Cyt b6f complex is strictly dependent on the luminal pH, resulting in the complete inhibition of transient NPQ in the pgr1 mutant background.
Even in the pgr5-1 mutant backgrounds, the introduction of the pgr1 mutation alleviated PSI photodamage in fluctuating light (Fig. 4). Is the function of PGR5/PGRL1-dependent CET dispensable with the enhanced photosynthetic control? During steady-state photosynthesis, relative ETR(II) was saturated at lower light intensity because of the stronger photosynthetic control in the pgr1 mutant than in the wild type (Fig. 3; Munekage et al., 2001). PSII is more severely photoinhibited in the pgr1 mutant than in the wild type at 500 µmol photons m−2 s−1 in 130 µL L−1 CO2 (Munekage et al., 2001). Furthermore, the strong photosynthetic control reduces the PQ pool even under low light (qL; Supplemental Fig. S1B; Munekage et al., 2001). The overreduction of the PQ pool promotes the formation of triplet P680 in PSII, which readily reacts with molecular oxygen, generating singlet oxygen. Singlet oxygen production damages the D1 protein of PSII (Vass, 2011) and the repair process of photodamaged PSII (Nishiyama et al., 2004). The suppression of luminal acidification due to limited LET also directly accelerates PSII photodamage under high light with unknown mechanisms (Takahashi et al., 2009). Therefore, photosynthetic control should be optimized to avoid PSII photoinhibition. Because of this tradeoff between the strength of photosynthetic control and PSII photoprotection, it is difficult to protect PSI from photodamage in fluctuating light simply depending on the higher sensitivity of photosynthetic control to luminal acidification (donor-side regulation). Consequently, it is also important to maintain a sufficient electron sink size from PSI (acceptor-side regulation).
A critical question is how does the acceptor-side regulation contribute to the alleviation of PSI photoinhibition under fluctuating light? Because PGR5/PGRL1-dependent CET balances the ATP/NADPH production ratio, it likely contributes to the regulation by providing sufficient acceptors, Fd and NADP+. Our strategy was the artificial enhancement of the acceptor-side capacity by introducing the P. patens FlvA and FlvB genes. However, the contribution of the acceptor-side regulation to the alleviation of PSI photodamage should be discussed with caution, because the modification of the acceptor-side limitation also affects the donor-side regulation. Flv-dependent pseudo-CET also acidifies the thylakoid lumen, as CET does (Yamamoto et al., 2016). The same level (0.87) of photosynthetic control indicated by Y(ND) was induced between the pgr1 mutant and the WT+35S;PpFlv line (Fig. 4B; Supplemental Fig. S5C). Even higher levels of Y(ND) were induced in the pgr5-1+35S;PpFlv line (0.64–0.78) than in the pgr1 pgr5-1 double mutant (0.2–0.24). With the constant Y(I), lower Y(NA) should associate with higher Y(ND), and it is theoretically difficult to evaluate the contribution of each mechanism on PSI photodamage independently. However, some careful comparisons among genotypes suggest the contribution of acceptor-side regulation in PSI photoprotection. (1) Faster oxidation of P700 upon the low light-to-high light transition and the suppression of the transient increase of Y(NA) in the WT+35S;PpFlv line compared with the wild type indicate that Flv-dependent oxygen photoreduction serves as a safety valve on the acceptor side of PSI (Supplemental Figs. S3B and S5D). (2) In the wild-type background, in which photosynthetic control was operating, Flv further alleviated PSI photoinhibition in fluctuating light (Fig. 5D). We could not eliminate the possibility that this Flv-dependent movement of electrons was accompanied by the further induction of photosynthetic control. In the WT+35S;PpFlv line, the alleviation of PSI photoinhibition was accompanied by the more rapid induction of higher levels of photosynthetic control than in the wild type (Supplemental Fig. S5). Based on the pgr5 phenotype in P700 reduction, it is probably necessary to move electrons through the CET pathway to protect PSI from photodamage. This CET immediately acidifies the thylakoid lumen to induce photosynthetic control. This process can be monitored in the transient induction of Y(NA), which was substituted immediately by Y(ND) during the high-light phase under fluctuating light conditions (Fig. 4; Supplemental Fig. S5). The extra electron sink for moving electrons was likely generated by PGR5/PGRL1-dependent CET. Its function can be substituted by the operation of Flv-dependent pseudo-CET more effectively.
In conclusion, we propose that balancing mechanisms protect PSI from photoinhibition under fluctuating light conditions. (1) The strength of photosynthetic control cannot be too high to optimize the tradeoff present between the photoinhibition of both photosystems. (2) CET activity should be optimized with the strength of photosynthetic control. Weak defects in CET, such as in the pgr5-2 mutant, can be partly complemented by the enhanced photosynthetic control, but this results in the photoinhibition of PSII. (3) Photosynthetic control is essential for protecting PSI in fluctuating light. But to induce photosynthetic control via the Cyt b6f complex, it is necessary to move electrons via the PGR5/PGRL1-dependent CET pathway. This would be the physiological function of the acceptor-side regulation. Even in wild-type plants, PSI acceptor-side limitation was increased transiently, which was linked to PSI photoinhibition (Fig. 4; Supplemental Fig. S5). In the presence of Flv, this dangerous transient increase in PSI acceptor-side limitation was not induced, highlighting the importance of the acceptor-side regulation.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis (Arabidopsis thaliana) wild type, mutants, and transgenic plants accumulating Physcomitrella patens Flvs in chloroplasts were grown in soil in a growth chamber (50–60 μmol photons m–2 s–1, 9-h photoperiod, 23°C, 55% humidity) for 6 to 8 weeks after germination. Fully expanded leaves were used for experiments. pgr5-2 was isolated from an Arabidopsis M2 generation mutagenized by ethylmethane sulfonate by its higher chlorophyll fluorescence in CO2-free air containing 5% oxygen in an N2 background (Wang et al., 2017). The pgr5-2 allele was identified by map-based cloning. In the pgr5-2 allele, a missense mutation (C293T) in the coding sequence changes the 98th amino residue from Ser to Phe. Primers used for genotyping by the derived cleaved-amplified polymorphic sequences assay are listed in Supplemental Table S1. In the transgenic plants accumulating Flv, P. patens FlvA and FlvB were overexpressed in the wild-type and pgr5-1 mutant backgrounds by the cauliflower mosaic virus 35S promoter (Yamamoto et al., 2016).
SDS-PAGE and Immunoblot Analyses
Intact chloroplasts were purified from leaves of 3- to 4-week-old plants, as described previously (Munekage et al., 2002). The purified chloroplasts were ruptured in a buffer (20 mm HEPES-KOH [pH 7.6], 5 mm MgCl2, and 2.5 mm EDTA). The insoluble fraction containing thylakoids and envelopes was separated from the soluble fraction by centrifugation for 10 min at 15,000g. The concentration of chlorophyll was determined as described previously (Porra et al., 1989).
Chloroplast thylakoid and membrane proteins were solubilized in 2× SDS-PAGE sample buffer. Proteins solubilized from the thylakoid membrane corresponding to 2 to 4 μg of chlorophyll were separated by 12.5% (w/v) SDS-PAGE or 16% (w/v) 4 m urea-Tricine-SDS-PAGE (for PGR5 detection; Schägger, 2006) and electrotransferred onto polyvinylidene fluoride membranes. The antibodies were added, and the protein-antibody complexes were labeled using the ECL Prime western-blotting detection system (GE Healthcare). The chemiluminescence was detected with a lumino-image analyzer (LAS4000; GE Healthcare).
BN-PAGE Analysis
BN-PAGE was performed as described previously (Shimizu et al., 2008). Solubilized thylakoid membranes with 1% (v/v) β-dodecyl-maltoside corresponding to 10 μg of chlorophyll were subjected to 5% to 12% BN-PAGE. The image of the BN-PAGE gel was captured with an image scanner after staining with Bio-Safe Coomassie stain (Bio-Rad).
In Vivo Measurements of Chlorophyll Fluorescence and P700 Absorption Changes
Chlorophyll fluorescence and chlorophyll P700 absorption changes of the PSI reaction center were measured simultaneously using a portable chlorophyll fluorometer (DUAL-PAM-100 MODULAR version chlorophyll fluorescence and P700 absorption analyzer equipped with a P700 dual-wavelength emitter at 830 and 870 nm; Walz). Plants were kept in the dark for 20 min before measurements, and detached leaves were used for the measurements. Minimal fluorescence in the dark-adapted state (Fo) was excited by a weak measuring light (620 nm) at a photon flux density of 0.05 to 0.1 µmol photons m−2 s−1. A saturating pulse of light (SP; 300 ms, 20,000 µmol photons m−2 s−1) was applied to determine the maximal fluorescence in the dark-adapted state (Fm) and during actinic light (AL) illumination (Fm′). The steady-state fluorescence level (Fs) was recorded during AL illumination (635 nm, 6–996 µmol photons m−2 s−1). The maximum quantum yields of PSII and NPQ were calculated as Fv/Fm and (Fm – Fm′)/Fm′, respectively. Y(II) was calculated as (Fm – Fs)/Fm′. Relative ETR(II) was calculated as Y(II) × light intensity (µmol photons m−2 s−1). The proportion of open PSII centers that reflects the redox level of the PQ pool (qL) was calculated as (Fm′ – F)/(Fm′ – Fo′) × Fo′/F (Kramer et al., 2004). Fo′ was calculated as Fo/(Fv/Fm + Fo/Fm′) (Oxborough and Baker, 1997).
The redox change of P700 was assessed by monitoring the absorbance changes of transmission light at 830 and 875 nm. Pm was determined by the application of an SP in the presence of far-red light (720 nm). The maximal level of oxidized P700 during AL illumination (Pm′) was determined by SP application. The P700 signal P was recorded just before an SP. Y(I) was calculated as (Pm′ – P)/Pm. Y(NA) was calculated as (Pm – Pm′)/Pm. Y(ND) was calculated as P/Pm. Three complementary quantum yields were defined: Y(I) + Y(NA) + Y(ND) = 1 (Klughammer and Schreiber, 1994). The relative level of reduced P700 was calculated as 1 – Y(ND). The value can vary between 0 (P700 fully oxidized) and 1 (P700 fully reduced) in a given state.
To analyze the effect of fluctuating light on photosynthetic electron transport and photoinhibition, three repetitions of fluctuating light composed of 5 min of low light (47 µmol photons m−2 s−1) and 1 min of high light (1,529 or 1,886 µmol photons m−2 s−1) were mimicked by changing the AL intensity, as described before (Yamamoto et al., 2016). Leaf discs (13 mm in diameter) were taken from plants dark adapted for 20 min. After the measurement of initial Fv/Fm and Pm, the leaf discs were subjected to fluctuating light treatment. The changes in photosynthetic parameters of the leaf discs under three repetitions of fluctuating light were monitored. After fluctuating light treatment, the leaf discs were sandwiched between wet tissue paper and incubated in the dark for 25 min, and then Fv/Fm and Pm were measured to evaluate PSII and PSI photoinhibition.
The rSP treatment in the dark was performed as described by Sejima et al. (2014). Leaf discs were taken from plants dark adapted for 20 min. After the measurement of initial Fv/Fm and Pm, the leaf discs were exposed to SP (300 ms, 20,000 µmol photons m−2 s−1) every 10 s in the dark for 18 min. The changes in photosynthetic parameters of the leaf discs during the 18-min rSP treatment were monitored. After the treatment, the leaf discs were sandwiched between wet tissue paper and incubated in the dark for 25 min, and then Fv/Fm and Pm were measured to evaluate PSII and PSI photoinhibition.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Impact of the enhanced photosynthetic control response by the pgr1 mutation on the redox state of P700 and the PQ pool during steady-state photosynthesis.
Supplemental Figure S2. Impact of the enhanced photosynthetic control response by the pgr1 mutation on photosynthetic parameters under fluctuating light.
Supplemental Figure S3. Representative data of P700 oxidation upon the low light-to-high light transition under fluctuating light.
Supplemental Figure S4. Effect of rSP illumination in the dark on PSII and PSI parameters.
Supplemental Figure S5. Impact of the enhanced electron sink capacity downstream of PSI by PpFlv on photosynthetic parameters under fluctuating light.
Supplemental Table S1. List of derived cleaved-amplified polymorphic sequence primers used for genotyping.
Acknowledgments
We thank Nozomi Sato (Kyoto University) and Sayaka Horiguchi (Nara Institute of Science and Technology) for their skilled technical support. We also thank Drs. Amane Makino (Tohoku University), Toru Hisabori (Tokyo Institute of Technology), and Toshiharu Hase (Osaka University) for kindly providing us with the anti-Cyt f, anti-PGRL1, and anti-leaf Fd:NADP+ oxidoreductase antibodies, respectively.
Footnotes
This work was supported by grants from the Japan Science and Technology Agency (CREST program) and the Japanese Society for the Promotion of Science KAKENHI (16H06553).
References
- Allahverdiyeva Y, Mustila H, Ermakova M, Bersanini L, Richaud P, Ajlani G, Battchikova N, Cournac L, Aro EM (2013) Flavodiiron proteins Flv1 and Flv3 enable cyanobacterial growth and photosynthesis under fluctuating light. Proc Natl Acad Sci USA 110: 4111–4116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allahverdiyeva Y, Isojärvi J, Zhang P, Aro EM (2015) Cyanobacterial oxygenic photosynthesis is protected by flavodiiron proteins. Life (Basel) 5: 716–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J. (2002) Photosynthesis of ATP-electrons, proton pumps, rotors, and poise. Cell 110: 273–276 [DOI] [PubMed] [Google Scholar]
- Chaux F, Peltier G, Johnson X (2015) A security network in PSI photoprotection: Regulation of photosynthetic control, NPQ and O2 photoreduction by cyclic electron flow. Front Plant Sci 6: 875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaux F, Burlacot A, Mekhalfi M, Auroy P, Blangy S, Richaud P, Peltier G (2017) Flavodiiron proteins promote fast and transient O2 photoreduction in Chlamydomonas. Plant Physiol 174: 1825–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DalCorso G, Pesaresi P, Masiero S, Aseeva E, Schünemann D, Finazzi G, Joliot P, Barbato R, Leister D (2008) A complex containing PGRL1 and PGR5 is involved in the switch between linear and cyclic electron flow in Arabidopsis. Cell 132: 273–285 [DOI] [PubMed] [Google Scholar]
- Folgosa F, Martins MC, Teixeira M (2018) Diversity and complexity of flavodiiron NO/O2 reductases. FEMS Microbiol Lett 365: fnx267. 10.1093/femsle/fnx267 [DOI] [PubMed] [Google Scholar]
- Gerotto C, Alboresi A, Meneghesso A, Jokel M, Suorsa M, Aro EM, Morosinotto T (2016) Flavodiiron proteins act as safety valve for electrons in Physcomitrella patens. Proc Natl Acad Sci USA 113: 12322–12327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco M, Tikkanen M, Paakkarinen V, Kangasjärvi S, Aro EM (2012) Steady-state phosphorylation of light-harvesting complex II proteins preserves photosystem I under fluctuating white light. Plant Physiol 160: 1896–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Vonck J, Mills DJ, Meier T, Kühlbrandt W (2018) Structure, mechanism, and regulation of the chloroplast ATP synthase. Science 360: eaat4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertle AP, Blunder T, Wunder T, Pesaresi P, Pribil M, Armbruster U, Leister D (2013) PGRL1 is the elusive ferredoxin-plastoquinone reductase in photosynthetic cyclic electron flow. Mol Cell 49: 511–523 [DOI] [PubMed] [Google Scholar]
- Ilík P, Pavlovič A, Kouřil R, Alboresi A, Morosinotto T, Allahverdiyeva Y, Aro EM, Yamamoto H, Shikanai T (2017) Alternative electron transport mediated by flavodiiron proteins is operational in organisms from cyanobacteria up to gymnosperms. New Phytol 214: 967–972 [DOI] [PubMed] [Google Scholar]
- Jahns P, Graf M, Munekage Y, Shikanai T (2002) Single point mutation in the Rieske iron-sulfur subunit of cytochrome b6/f leads to an altered pH dependence of plastoquinol oxidation in Arabidopsis. FEBS Lett 519: 99–102 [DOI] [PubMed] [Google Scholar]
- Järvi S, Suorsa M, Aro EM (2015) Photosystem II repair in plant chloroplasts: Regulation, assisting proteins and shared components with photosystem II biogenesis. Biochim Biophys Acta 1847: 900–909 [DOI] [PubMed] [Google Scholar]
- Joliot P, Johnson GN (2011) Regulation of cyclic and linear electron flow in higher plants. Proc Natl Acad Sci USA 108: 13317–13322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klughammer C, Schreiber U (1994) An improved method, using saturating light pulses, for the determination of photosystem I quantum yield via P700+-absorbance changes at 830 nm. Planta 192: 261–268 [Google Scholar]
- Kono M, Noguchi K, Terashima I (2014) Roles of the cyclic electron flow around PSI (CEF-PSI) and O2-dependent alternative pathways in regulation of the photosynthetic electron flow in short-term fluctuating light in Arabidopsis thaliana. Plant Cell Physiol 55: 990–1004 [DOI] [PubMed] [Google Scholar]
- Kramer DM, Cruz JA, Kanazawa A (2003) Balancing the central roles of the thylakoid proton gradient. Trends Plant Sci 8: 27–32 [DOI] [PubMed] [Google Scholar]
- Kramer DM, Johnson G, Kiirats O, Edwards GE (2004) New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosynth Res 79: 209–218 [DOI] [PubMed] [Google Scholar]
- Li XP, Björkman O, Shih C, Grossman AR, Rosenquist M, Jansson S, Niyogi KK (2000) A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403: 391–395 [DOI] [PubMed] [Google Scholar]
- Li XP, Muller-Moule P, Gilmore AM, Niyogi KK (2002) PsbS-dependent enhancement of feedback de-excitation protects photosystem II from photoinhibition. Proc Natl Acad Sci USA 99: 15222–15227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller P, Li XP, Niyogi KK (2001) Non-photochemical quenching: A response to excess light energy. Plant Physiol 125: 1558–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munekage Y, Takeda S, Endo T, Jahns P, Hashimoto T, Shikanai T (2001) Cytochrome b6f mutation specifically affects thermal dissipation of absorbed light energy in Arabidopsis. Plant J 28: 351–359 [DOI] [PubMed] [Google Scholar]
- Munekage Y, Hojo M, Meurer J, Endo T, Tasaka M, Shikanai T (2002) PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 110: 361–371 [DOI] [PubMed] [Google Scholar]
- Munekage Y, Hashimoto M, Miyake C, Tomizawa K, Endo T, Tasaka M, Shikanai T (2004) Cyclic electron flow around photosystem I is essential for photosynthesis. Nature 429: 579–582 [DOI] [PubMed] [Google Scholar]
- Nishikawa Y, Yamamoto H, Okegawa Y, Wada S, Sato N, Taira Y, Sugimoto K, Makino A, Shikanai T (2012) PGR5-dependent cyclic electron transport around PSI contributes to the redox homeostasis in chloroplasts rather than CO2 fixation and biomass production in rice. Plant Cell Physiol 53: 2117–2126 [DOI] [PubMed] [Google Scholar]
- Nishio JN, Whitmarsh J (1993) Dissipation of the proton electrochemical potential in intact chloroplasts (II. The pH gradient monitored by cytochrome f reduction kinetics). Plant Physiol 101: 89–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama Y, Allakhverdiev SI, Yamamoto H, Hayashi H, Murata N (2004) Singlet oxygen inhibits the repair of photosystem II by suppressing the translation elongation of the D1 protein in Synechocystis sp. PCC 6803. Biochemistry 43: 11321–11330 [DOI] [PubMed] [Google Scholar]
- Nixon PJ, Michoux F, Yu J, Boehm M, Komenda J (2010) Recent advances in understanding the assembly and repair of photosystem II. Ann Bot 106: 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyogi KK, Grossman AR, Björkman O (1998) Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 10: 1121–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxborough K, Baker NR (1997) Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components: Calculation of qP and Fv′/Fm′ without measuring Fo′. Photosynth Res 54: 135–142 [Google Scholar]
- Pearcy RW, Krall JP, Sassenrath-Cole GF (1996) Photosynthesis in fluctuating light environments. In Baker NR, ed, Photosynthesis and the Environment. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 321–346 [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975: 384–394 [Google Scholar]
- Rumberg B, Siggel U (1969) pH changes in the inner phase of the thylakoids during photosynthesis. Naturwissenschaften 56: 130–132 [DOI] [PubMed] [Google Scholar]
- Schägger H. (2006) Tricine-SDS-PAGE. Nat Protoc 1: 16–22 [DOI] [PubMed] [Google Scholar]
- Sejima T, Takagi D, Fukayama H, Makino A, Miyake C (2014) Repetitive short-pulse light mainly inactivates photosystem I in sunflower leaves. Plant Cell Physiol 55: 1184–1193 [DOI] [PubMed] [Google Scholar]
- Shikanai T. (2007) Cyclic electron transport around photosystem I: Genetic approaches. Annu Rev Plant Biol 58: 199–217 [DOI] [PubMed] [Google Scholar]
- Shikanai T, Yamamoto H (2017) Contribution of cyclic and pseudo-cyclic electron transport to the formation of proton motive force in chloroplasts. Mol Plant 10: 20–29 [DOI] [PubMed] [Google Scholar]
- Shikanai T, Munekage Y, Shimizu K, Endo T, Hashimoto T (1999) Identification and characterization of Arabidopsis mutants with reduced quenching of chlorophyll fluorescence. Plant Cell Physiol 40: 1134–1142 [DOI] [PubMed] [Google Scholar]
- Shimakawa G, Ishizaki K, Tsukamoto S, Tanaka M, Sejima T, Miyake C (2017) The liverwort, Marchantia, drives alternative electron flow using a flavodiiron protein to protect PSI. Plant Physiol 173: 1636–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu H, Peng L, Myouga F, Motohashi R, Shinozaki K, Shikanai T (2008) CRR23/NdhL is a subunit of the chloroplast NAD(P)H dehydrogenase complex in Arabidopsis. Plant Cell Physiol 49: 835–842 [DOI] [PubMed] [Google Scholar]
- Soga N, Kimura K, Kinosita K Jr, Yoshida M, Suzuki T (2017) Perfect chemomechanical coupling of FoF1-ATP synthase. Proc Natl Acad Sci USA 114: 4960–4965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoike K. (2011) Photoinhibition of photosystem I. Physiol Plant 142: 56–64 [DOI] [PubMed] [Google Scholar]
- Strand DD, Fisher N, Kramer DM (2017) The higher plant plastid NAD(P)H dehydrogenase-like complex (NDH) is a high efficiency proton pump that increases ATP production by cyclic electron flow. J Biol Chem 292: 11850–11860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suorsa M, Järvi S, Grieco M, Nurmi M, Pietrzykowska M, Rantala M, Kangasjärvi S, Paakkarinen V, Tikkanen M, Jansson S, et al. (2012) PROTON GRADIENT REGULATION5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant Cell 24: 2934–2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suorsa M, Grieco M, Järvi S, Gollan PJ, Kangasjärvi S, Tikkanen M, Aro EM (2013) PGR5 ensures photosynthetic control to safeguard photosystem I under fluctuating light conditions. Plant Signal Behav 8: e22741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suorsa M, Rossi F, Tadini L, Labs M, Colombo M, Jahns P, Kater MM, Leister D, Finazzi G, Aro EM, et al. (2016) PGR5-PGRL1-dependent cyclic electron transport modulates linear electron transport rate in Arabidopsis thaliana. Mol Plant 9: 271–288 [DOI] [PubMed] [Google Scholar]
- Takagi D, Takumi S, Hashiguchi M, Sejima T, Miyake C (2016) Superoxide and singlet oxygen produced within the thylakoid membranes both cause photosystem I photoinhibition. Plant Physiol 171: 1626–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Milward SE, Fan DY, Chow WS, Badger MR (2009) How does cyclic electron flow alleviate photoinhibition in Arabidopsis? Plant Physiol 149: 1560–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa K, Cruz JA, Kanazawa A, Kramer DM (2007) The thylakoid proton motive force in vivo: Quantitative, non-invasive probes, energetics, and regulatory consequences of light-induced pmf. Biochim Biophys Acta 1767: 1233–1244 [DOI] [PubMed] [Google Scholar]
- Teicher HB, Møller BL, Scheller HV (2000) Photoinhibition of photosystem I in field-grown barley (Hordeum vulgare L.): Induction, recovery and acclimation. Photosynth Res 64: 53–61 [DOI] [PubMed] [Google Scholar]
- Tikhonov AN, Khomutov GB, Ruuge EK, Blumenfeld LA (1981) Electron transport control in chloroplasts: Effects of photosynthetic control monitored by the intrathylakoid pH. Biochim Biophys Acta 637: 321–333 [Google Scholar]
- Tikkanen M, Rantala S, Aro EM (2015) Electron flow from PSII to PSI under high light is controlled by PGR5 but not by PSBS. Front Plant Sci 6: 521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vass I. (2011) Role of charge recombination processes in photodamage and photoprotection of the photosystem II complex. Physiol Plant 142: 6–16 [DOI] [PubMed] [Google Scholar]
- Wada S, Yamamoto H, Suzuki Y, Yamori W, Shikanai T, Makino A (2018) Flavodiiron protein substitutes for cyclic electron flow without competing CO2 assimilation in rice. Plant Physiol 176: 1509–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Yamamoto H, Shikanai T (2015) Role of cyclic electron transport around photosystem I in regulating proton motive force. Biochim Biophys Acta 1847: 931–938 [DOI] [PubMed] [Google Scholar]
- Wang C, Yamamoto H, Narumiya F, Munekage YN, Finazzi G, Szabo I, Shikanai T (2017) Fine-tuned regulation of the K+/H+ antiporter KEA3 is required to optimize photosynthesis during induction. Plant J 89: 540–553 [DOI] [PubMed] [Google Scholar]
- Wang C, Takahashi H, Shikanai T (2018) PROTON GRADIENT REGULATION 5 contributes to ferredoxin-dependent cyclic phosphorylation in ruptured chloroplasts. Biochim Biophys Acta 1859: 1173–1179 [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Shikanai T (2013) In planta mutagenesis of Src homology 3 domain-like fold of NdhS, a ferredoxin-binding subunit of the chloroplast NADH dehydrogenase-like complex in Arabidopsis: A conserved Arg-193 plays a critical role in ferredoxin binding. J Biol Chem 288: 36328–36337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Peng L, Fukao Y, Shikanai T (2011) An Src homology 3 domain-like fold protein forms a ferredoxin binding site for the chloroplast NADH dehydrogenase-like complex in Arabidopsis. Plant Cell 23: 1480–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Takahashi S, Badger MR, Shikanai T (2016) Artificial remodelling of alternative electron flow by flavodiiron proteins in Arabidopsis. Nat Plants 2: 16012. [DOI] [PubMed] [Google Scholar]
- Yamori W, Shikanai T (2016) Physiological functions of cyclic electron transport around photosystem I in sustaining photosynthesis and plant growth. Annu Rev Plant Biol 67: 81–106 [DOI] [PubMed] [Google Scholar]
- Yamori W, Makino A, Shikanai T (2016) A physiological role of cyclic electron transport around photosystem I in sustaining photosynthesis under fluctuating light in rice. Sci Rep 6: 20147. [DOI] [PMC free article] [PubMed] [Google Scholar]