Stomatal responses to water status in ferns are highly predictable and not functionally regulated by the hormone abscisic acid.
Abstract
Stomatal responses to changes in leaf water status are important for the diurnal regulation of gas exchange and the survival of plants during drought. These stomatal responses in angiosperm species are well characterized, yet in species of nonseed plants, an ongoing debate surrounds the role of metabolism, particularly the role of the hormone abscisic acid (ABA), in functionally regulating stomatal responses to changes in leaf water status. Here, we measured the stomatal response to changes in vapor pressure difference (VPD) in two natural forms of the fern species Athyrium filix-femina, recently suggested to have stomata that are regulated by ABA. The two forms measured had considerable differences in key hydraulic traits, including leaf hydraulic conductance and capacitance, as well as the kinetics of stomatal response to changes in VPD. In both forms, the stomatal responses to VPD could be accurately predicted by a dynamic, mechanistic model that assumes guard cell turgor changes in concert with leaf turgor in the light, and not via metabolic processes including the level of ABA. During drought, endogenous ABA did not play a role in stomatal closure, and exogenous ABA applied to live, intact leaves did not induce stomatal closure. Our results indicate that functional stomatal responses to changes in leaf water status in ferns are regulated by leaf hydraulics and not metabolism. With ferns being sister to seed plants, this result has implications for the evolutionary reconstruction of functional stomatal responses across vascular land plant lineages.
The stomatal responses to environmental and endogenous signals in vascular plants are critical for regulating plant gas exchange with the atmosphere. In addition, stomatal closure is vital for minimizing water loss and preventing lethal embolism during drought (Martin-St Paul et al., 2017). These responses have fascinated researchers for over 150 years (von Mohl, 1856; Darwin, 1898), with a strong emphasis placed on characterizing stomatal behavior in the most economically important group of land plants, the angiosperms. To this end we now have a considerable array of data describing: (1) the behavior of angiosperm stomata (Ziegler, 1987), (2) the influence of angiosperm stomatal responses on leaf and canopy level gas exchange (Burrows and Milthorpe, 1976; Ewers and Oren, 2000), and (3) the fundamental molecular regulators controlling these responses (Maierhofer et al., 2014; Brandt et al., 2015; Hiyama et al., 2017). Given the importance of stomatal responses for terrestrial productivity, hydrological cycles, and plant survival during challenging abiotic events (Hetherington and Woodward, 2003), when and how the stomatal responses evolved, and the nature of gas exchange responses in nonangiosperms, are critical questions that remain comparatively understudied.
Stomata are among the most recognizable anatomical features of the oldest fossilized land plants, being present in very low numbers near the sporangia of Rhyniophytes, vascular plants that grew >410 million years ago (Edwards and Axe, 1992; Edwards et al., 1998). The advent of stomata, however, predates the evolution of these first vascular land plants, with stomates found on the sporophytes of some extant moss and hornwort species (Paton and Pearce, 1957). Whether stomata evolved once in the common ancestor of all land plants (being lost more than four times in the bryophyte lineages) or evolved independently in each stomatal-bearing lineage of land plants, utilizing a similar and ancestral set of developmental genes, remains a mystery (Pressel et al., 2014; Chater et al., 2017). Bryophyte stomata, however, appear to open once at a set developmental stage causing intercellular airspaces to dry out to aid the release of spores (Renzaglia et al., 2017; Duckett and Pressel, 2018). This behavior has been confirmed in bryophyte mutant plants, with delayed spore release being the only modification to a physiological trait observed in Physcomitrella patens mutant plants that do not form stomata (Chater et al., 2016). Opening to aid the desiccation of intercellular air space is the opposite function of vascular land plant stomata, which close to prevent desiccation. This major evolutionary transition in stomatal function between bryophytes and seed plants makes stomatal responses in the most basal extant lineages of vascular plants, the lycophytes and ferns, critical for reconstructing the evolutionary history of stomatal regulation in land plants.
In comparison to angiosperms, there have been a very small number of studies documenting stomatal responses in species from the earliest diverging lineages of vascular plants. Species from all major lineages of vascular land plants have a stomatal opening response to an increased fluence of red light (McAdam and Brodribb, 2012b). In addition, and possibly via a photosynthetic signal from the mesophyll (Wong et al., 1979; Farquhar and Wong, 1984; Mott et al., 2008), all species have a stomatal opening response when exposed to low CO2 in the light (Doi and Shimazaki, 2008). Similarly, species from all lineages of vascular land plants have a stomatal response to blue light, except the Leptosporangiate ferns, which appear to have lost this response, possibly because of a chimeric photoreceptor as an adaptation to low light environments (Doi et al., 2015). Species from all major lineages of vascular land plants also have a stomatal response to the vapor pressure difference (VPD) between the leaf and the atmosphere and leaf water status, closing when leaf water status declines or VPD increases and opening when VPD is low or leaf water status is high (Lösch and Tenhunen, 1981; McAdam and Brodribb, 2015). The mechanism regulating this last set of stomatal responses has been the center of an ongoing, recent debate (Cai et al., 2017; Sussmilch et al., 2017).
The simplest explanation for stomatal responses to changes in leaf water status is that guard cell turgor changes in concert with leaf turgor, resulting in stomatal closure as the leaf experiences desiccation (Lange et al., 1971). This mechanistic explanation cannot easily account for the stomatal responses to changes in leaf water status in angiosperms, many of which have an epidermis that exerts a mechanical advantage over the guard cells, such that stomatal aperture is a function of both guard and epidermal cell turgor (Raschke, 1970; Franks et al., 1998). To overcome the effects of this epidermal mechanical advantage, angiosperm stomata require a metabolically active control of stomatal responses to a change in leaf water status (Buckley et al., 2003; Buckley, 2016; although compare with Mott and Peak, 2013; Sweet et al., 2017). It has been proposed that abscisic acid (ABA) provides this metabolic signal because it closes stomata (Mittelheuser and van Steveninck, 1969), is synthesized when leaf turgor declines (during drought and at high VPD; Pierce and Raschke, 1981; Bauerle et al., 2004; McAdam and Brodribb, 2015; Qiu et al., 2017), and ABA biosynthetic and signaling mutants have highly dysfunctional stomatal responses to changes in leaf water status (Xie et al., 2006). These mutant plants all have a higher stomatal conductance (gs) than wild-type plants, and either a reduced stomatal sensitivity to changes in leaf water potential (Ψl), a lack of stomatal response to VPD, or an inability to restrict water loss when desiccated (Tal and Nevo, 1973; Koornneef et al., 1982; Xie et al., 2006; McAdam et al., 2016b). These observations suggest that a metabolic regulation, either via ABA directly or signaling through ABA perception proteins, is essential for normal stomatal responses to changes in leaf water status (Pantin and Blatt, 2018).
Two opposing views have been presented about whether ABA, or the canonical ABA signaling pathway, plays a central role in the regulation of functional stomatal responses to changes in leaf water status in species from the earliest diverging lineages of land plants. In early work, Brodribb and McAdam (2011) fed exogenous ABA, at levels that corresponded to those measured in drought-stressed plants, into the transpiration stream of a number of species and found no functionally significant closure of stomata measured by gas exchange. In addition, when fern and lycophyte species are drought-stressed and naturally synthesize ABA, this endogenous ABA has no effect on closing stomata, with a recovery in leaf hydration causing stomata to instantaneously reopen (McAdam and Brodribb, 2012a). This stomatal behavior is also not observed in seed plants, which have stomata that are functionally responsive to ABA, even in gymnosperm species which, like fern species, lack evidence of epidermal mechanical advantage (McAdam and Brodribb, 2014). An explanation for this absence of a functional stomatal response to endogenous ABA in fern species has been attributed to the key genes in the ABA signaling pathway regulating other processes, not the guard cells, of species from the earliest diverging lineages of vascular land plants (McAdam et al., 2016a).
In contrast to these findings, a recent report conducted a bioinformatic screening of ABA-related genes across land plants as well as an experiment using isolated epidermis in which the stomatal aperture of two fern species closed by 10% to 20% when floated on very high levels of exogenous ABA (Cai et al., 2017). An additional recent article observed stomatal closure by <15% when measuring gas exchange in leaves that were sprayed with high levels of exogenous ABA in the fern species Athyrium filix-femina (Hõrak et al., 2017). However, this response was only observed in plants acclimated to low VPD in a growth cabinet and not in plants of this species grown under high VPD or to any significant degree in two Dryopteris species (Hõrak et al., 2017). These two reports support an alternative theory that guard cell turgor in species of nonseed plants is functionally regulated by the hormone ABA or its signaling pathway (Chater et al., 2011; Ruszala et al., 2011).
Although these recent reports present an argument for functional regulation of stomatal aperture by ABA in fern species, many studies find little evidence of this hormone influencing functional stomatal responses in fern and lycophyte species. In studies spanning the observation of stomatal aperture in isolated epidermis (Lange et al., 1971; Lösch, 1979), leaf gas exchange in intact plants (Brodribb and McAdam, 2011) and the coupled recording of water flux into and transpiration from excised leaves (Martins et al., 2016), rapid and nonhysteretic stomatal responses to VPD have been observed across many fern and lycophyte species. This type of response is not observed in angiosperm species (McAdam and Brodribb, 2015). The predictable nature of these responses suggests that guard cell turgor changes in concert with leaf turgor in fern and lycophyte species, and not via a metabolic intermediary such as ABA or the ABA-signaling pathway. Here, we directly tested whether metabolism plays a functionally relevant role in regulating the stomatal responses to changes in leaf water status in a fern species. We comprehensively examined the stomatal responses to changes in leaf water status as well as ABA application in two varieties of A. filix-femina, the same species reported in a recent study to have stomata that are sensitive to ABA (Hõrak et al., 2017). These two varieties have considerable variation in foliar anatomy and consequently leaf hydraulic properties, meaning similar changes in VPD should have very different effects on leaf water status. If metabolism functionally regulates stomatal responses to changes in leaf water status in fern species (Cai et al., 2017; Hõrak et al., 2017), then the stomatal responses to changes in leaf water status in A. filix-femina should not be able to be predicted by the simple assumption that guard cell turgor changes in concert with leaf turgor.
RESULTS AND DISCUSSION
Leaf Hydraulic Properties Influence Stomatal Responses to VPD in A. filix-femina
The foliar morphology of the two forms of A. filix-femina, the wild-type and Frizelliae forms, are highly divergent. A. filix-femina variety Frizelliae, discovered growing in the wild near Castlekevin, County Wicklow, Ireland in 1857, has pinnae that are reduced to circular lobes, making this one of the most unusual of the more than 300 described naturally occurring varieties of this species (Lowe, 1867; Fig. 1A). This major difference in foliar morphology is associated with considerable variation in the hydraulic properties of the leaf. The Frizelliae form had a maximum leaf hydraulic conductance (Kleaf) more than double the Kleaf of the wild-type form, 14.7 (±0.6) and 6.5 (±1.0) mmol m−2 s−1 MPa−1, respectively (Fig. 1B). This intraspecific variation in maximum Kleaf between the two forms of A. filix-femina is large. It is greater than the reported interspecific variation in Kleaf between groups of angiosperms native to habitats that vary by >1,500 mm of annual rainfall (Blackman et al., 2010) and rivals the intrageneric variation in maximum Kleaf measured across the ecologically diverse genus Viburnum (Scoffoni et al., 2016). Similarly, bulk leaf capacitance (Cbulk) in the Frizelliae form was approximately four times the Cbulk measured in the wild-type form, 4.46 (±1.1) to 1.12 (±0.4) mol m−2 MPa−1, respectively (Fig. 1C). This variation in Cbulk also spans a considerable range in Cbulk reported across a wide ecological and phylogenetic diversity of angiosperms and ferns (Blackman and Brodribb, 2011; McAdam and Brodribb, 2013).
Figure 1.
Variation in anatomy and leaf hydraulic traits between two forms of A. filix-femina. A, Images of a representative leaf taken from the wild-type and Frizelliae forms of A. filix-femina (scale bar = 40 mm). B, Mean maximum leaf hydraulic conductance (n = 4 ± se). C, Leaf capacitance (n = 4 ± se) for each form. Stars denote a significant difference between means (**P < 0.01, t test).
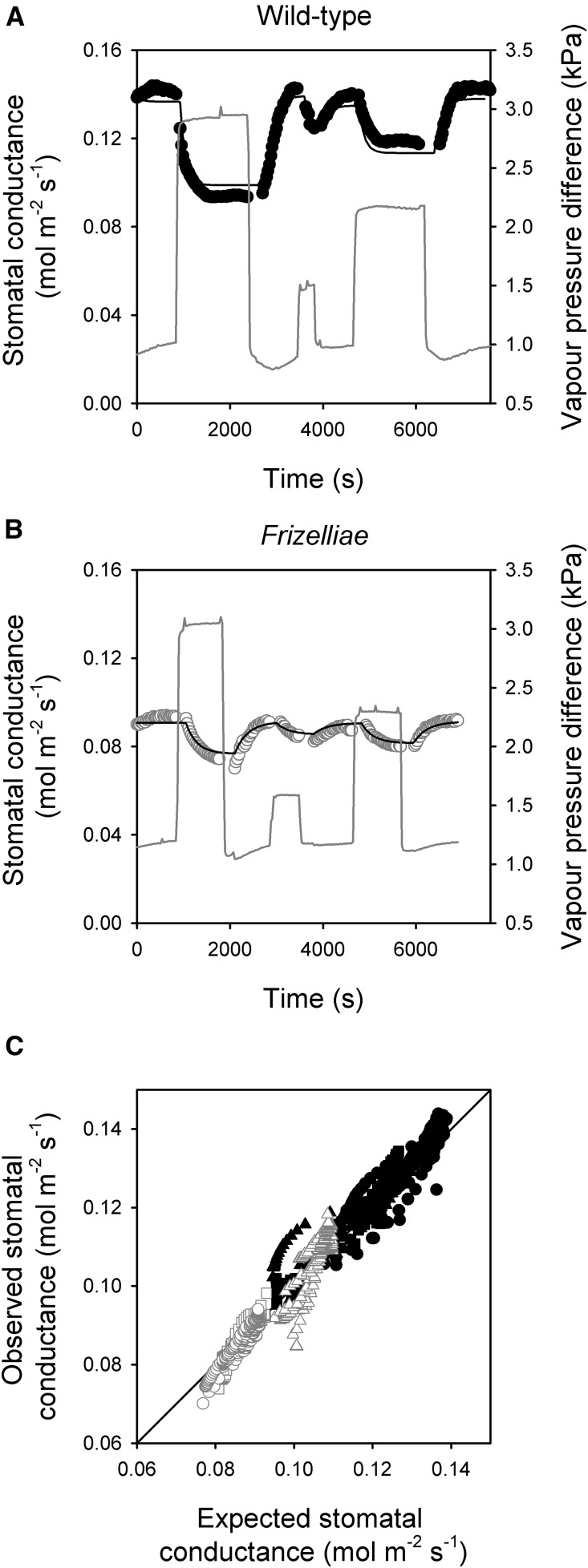
The kinetics of the stomatal response to a similar series of step changes in VPD were very different between the two forms of A. filix-femina (Fig. 2). Stomata of the Frizelliae form had a significantly greater half-time (P < 0.001, analysis of variance) and less magnitude in response to changes in VPD than stomata of the wild-type form (Fig. 2). Despite the highly contrasting kinetics, the stomatal response to changes in VPD could be accurately predicted by a simple mechanistic model that assumes that guard cell turgor changes with leaf turgor (Brodribb and McAdam, 2011), in both forms (Fig. 2). This model could also accurately predict the stomatal responses to VPD at low CO2 (Supplemental Fig. S1). The assumptions of this dynamic, mechanistic model have been comprehensively described by Brodribb and McAdam (2011) and Deans et al. (2017). Briefly, the model assumes that guard cell turgor changes instantaneously and in concert with leaf turgor in the light, that there is a negligible resistance to water flow between the guard cell and the leaf, and that evaporation from the leaf occurs primarily in the substomatal cavity. The model dynamically predicts changes in leaf turgor by assuming leaf water status is a simple function of evaporative demand, Kleaf and Cbulk. In both varieties, the hydraulic model accurately predicted stomatal responses to changes in VPD without factoring in any dynamic changes in Kleaf.
Figure 2.
Highly predictable stomatal responses to VPD in A. filix-femina. A and B, Representative traces of the response of stomatal conductance; circles, black in the wild-type form (A) and open in the Frizelliae form (B) to changes in VPD (gray line) and the modeled predictions for these stomatal responses (black line). C, The relationship between observed and expected stomatal conductance based on a hydraulic model during a series of transitions in a vapor pressure deficit in three independent replicates (each represented by a different symbol) of the wild-type (black symbols) and Frizelliae (gray symbols) forms of A. filix-femina. The solid line represents a 1:1 relationship between observed and expected stomatal conductance.
Like many fern and lycophyte species observed to date (McAdam and Brodribb, 2013), significant, linear relationships between gs and Ψl until stomata were closed were found in both forms of A. filix-femina (Fig. 3). Despite variation in maximum gs due to differences in stomatal anatomy and density (Table 1), the slopes of these relationships were the same for the two varieties (P < 0.001, analysis of covariance). ABA levels did not change during the foliar excision and rehydration experiments used to establish the relationship between gs and Ψl (Fig. 3), implying that ABA biosynthesis, catabolism, or changes in levels are unlikely drivers of these responses to leaf water status. In angiosperm species, the wrong-way responses of stomata due to epidermal mechanical advantage and the regulation of stomatal aperture by ABA biosynthesis and catabolism (which takes many minutes) makes it impossible to establish similar relationships using the method employed here. Key features of the stomatal response to VPD in fern species are an absence of wrong-way responses and no significant hysteresis that cannot be accounted for by dynamic changes in leaf turgor (Brodribb and McAdam, 2011; McAdam and Brodribb, 2015). The stomatal responses to VPD in both varieties of A. filix-femina reflected this (Fig. 2). Under saturating light, photosynthesis (A) in fern species is limited by gs (McAdam and Brodribb, 2012b), and during the VPD transitions we found that A responded similarly to gs, displaying no evidence of a wrong-way response or significant hysteresis in either variety (Supplemental Fig. S2).
Figure 3.
Stomatal responses to leaf water potential (Ψl) are not driven by foliar ABA levels in A. filix-femina. A, The relationship between stomatal conductance (gs) and Ψl in the wild-type (black) and Frizelliae (open gray) forms of A. filix-femina. Lines depict linear regressions. B, The relationship between gs and foliar ABA levels (expressed in terms of dry weight) in the same leaves.
Table 1. Mean stomatal density, pore length, pore width, pore depth, maximum stomatal pore area, and theoretical maximum stomatal conductance (n = 4 ± sd) for the wild-type and Frizelliae forms of A. filix-femina.
| Variety | Stomatal density | Pore length | Pore width | Pore depth | Maximum pore area | Maximum conductance |
|---|---|---|---|---|---|---|
| mm2 | μm | μm | μm | μm2 | mol m−2 s−1 | |
| Wild type | 33.7 ± 9.6 | 21.5 ± 2.6 | 3.5 ± 0.2 | 10.9 ± 1.5 | 58.8 ± 7.6 | 0.122 ± 0.03 |
| Frizelliae | 37.3 ± 5.0 | 14.9 ± 1.7 | 3.2 ± 0.5 | 10.8 ± 0.8 | 37.6 ± 10.1 | 0.096 ± 0.03 |
The most important traits governing the nature of stomatal responses to changes in leaf water status in fern species include the evaporative demand (which is a function of VPD and gs), Kleaf and Cbulk (Brodribb and McAdam, 2011). All of these components determine how rapidly Ψl changes during a VPD transition. Hõrak et al. (2017) recently claimed that faster stomatal responses to VPD should be observed in species with higher gs if stomata were controlled by leaf water status alone. However, this statement focused on only one of the key components that determine the half-times of the stomatal responses to changes in leaf water status. Faster stomatal responses can also be observed in fern or lycophyte species (or genotypes, as observed here) with lower Kleaf and/or lower Cbulk (Brodribb and McAdam, 2011).
In angiosperms, there is a current debate surrounding the role of ABA in regulating stomatal responses to VPD (McAdam et al., 2016b; Merilo et al., 2017; Pantin and Blatt, 2018). In angiosperm species, ABA levels are known to change in response to changes in VPD (Bauerle et al., 2004; McAdam et al., 2016b; Qiu et al., 2017). In addition, there are reports of limited stomatal responses to changes in VPD in ABA biosynthetic mutants, which are characteristically wilty (Tal and Nevo, 1973; Koornneef et al., 1982; Xie et al., 2006; McAdam et al., 2016b). However, this lack of a response in ABA biosynthetic mutants may not be universal (Assmann et al., 2000; Merilo et al., 2017). A minimal or absent stomatal response to VPD in mutants of the Arabidopsis (Arabidopsis thaliana) ABA signaling gene OPEN STOMATA1 (OST1) has been used as evidence for a new theory that OST1 (and not ABA levels per se) is a fundamental metabolic regulator of the stomatal responses to VPD in angiosperms (Merilo et al., 2017). This OST1 governed regulation of stomatal responses to VPD; however, it is most likely unique to seed plants, with mutants in the only functional homolog of OST1 of the fern species Ceratopteris richardii having normal, wild-type stomatal responses to changes in VPD (McAdam et al., 2016a).
Endogenous ABA Does Not Close Stomata during Soil Drought in A. filix-femina
To test whether A. filix-femina synthesizes endogenous ABA, we exposed both varieties to soil drought until plants showed incipient signs of leaf death and monitored foliar ABA levels during the drought (Fig. 4). In the wild-type form, as drought progressed, foliar ABA levels were only found to increase considerably when leaves reached −1.1 MPa (Fig. 4A), a Ψl that was 0.2 MPa less than turgor loss point determined by pressure-volume curve analysis (−0.88 MPa ± 0.13). In seed plants, the loss of cell turgor is the primary trigger for ABA biosynthesis when leaf water status declines (Pierce and Raschke, 1980, 1981). A similar disconnection between the turgor loss point and the Ψl threshold at which foliar ABA levels increase during drought has been observed previously in lycophyte, fern, and conifer species (McAdam and Brodribb, 2016). This is believed to be due to temporal delays in ABA biosynthesis in nonangiosperm species due to an absence of the key gene encoding the ABA2 enzyme in nonangiosperm species (Sussmilch et al., 2017).
Figure 4.
Xylem vulnerability to embolism determines whether foliar ABA levels are synthesized during drought in A. filix-femina. A, The relationship between foliar ABA level (expressed in terms of dry weight) and leaf water potential (Ψl) in the wild-type (black circles) and Frizelliae (open gray circles) forms of A. filix-femina exposed to soil water deficit. The mean Ψl at which 50% of xylem in the stipe is embolized (P50) is marked by a vertical dashed line for each form. B, Optical stipe vulnerability curves used to determine mean P50 for the wild-type (black lines) and Frizelliae (open gray circles) forms of A. filix-femina. C, Representative montages of embolism events observed in the xylem of the stipe of both forms of A. filix-femina (white scale bars in top left = 1 mm). Color scale bars in the wild-type form indicate the Ψl at which each embolism event occurred. Color scale bars in the Frizelliae form indicate the time after leaf excision, as the vulnerability curve for this form was constructed from a series of individual leaves dried until a certain percentage of embolism was observed, at which point Ψl was measured once; see “Materials and Methods.”
Unlike in the wild-type form, foliar ABA levels did not increase during soil drought in the Frizelliae form of A. filix-femina (Fig. 4B). During soil drought incipient leaf death occurred at −0.8 MPa in the Frizelliae form, and this Ψl corresponded to the water potential at which 50% of the xylem of the stipe had embolized (P50; Fig. 4B). Extensive embolism in the xylem is a nonreversible event, triggering lethal declines in xylem K; being a primary elicitor of plant death during drought (Brodribb and Cochard, 2009; Urli et al., 2013). There was a considerable difference in the P50 between the two forms of A. filix-femina, with the wild-type form having a P50 and Ψl of incipient leaf death of −1.2 MPa (±0.04; Fig. 4B). This considerable interspecific variation in P50 in A. filix-femina likely explains the differences in ABA accumulation during soil drought, with leaf death caused by embolism and hydraulic failure occurring in the Frizelliae form at a very similar water potential as the turgor loss point (−0.75 MPa ± 0.09).
Although ABA dynamics were highly contrasting between the two forms of A. filix-femina during drought, stomata closed in both forms as drought progressed (Fig. 5). Because foliar ABA levels did not increase during this drought in the Frizelliae form, stomatal closure cannot be attributed to the biosynthetic production or accumulation of foliar ABA, and is most likely due to low Ψl. In contrast, foliar ABA levels increased before leaf death in the wild-type form of A. filix-femina (Fig. 5). This endogenous foliar ABA, however, was found to play no role in closing stomata during drought stress in the wild-type form, with stomata rapidly reopening when water-stressed leaves were instantaneously rehydrated (Fig. 5). A similar inability of high levels of endogenous foliar ABA to close stomata in drought-stressed plants has been observed in other fern and lycophyte species including the lycophyte species Selaginella kraussiana and S. uncinata and fern species Dicksonia antarctica and Pteridium esculentum (McAdam and Brodribb, 2012a; Brodribb et al., 2017). In seed plants, including gymnosperms, because high levels of foliar ABA actively close stomata, when drought-stressed leaves are instantaneously rehydrated in similar experiments, stomata do not reopen (McAdam and Brodribb, 2012a).
Figure 5.
Endogenous ABA does not close stomata of A. filix-femina during drought. The response of stomatal conductance (gs) to instantaneous rehydration (marked by a vertical dashed line) through the stipe (wild-type form; solid circles) or intact plant through the roots (Frizelliae form; shaded circles) in drought-stressed individuals of A. filix-femina. Leaf water potential (Ψl) in each form before rehydration is noted in the bottom left of the panel. Horizontal dotted lines denote gs measured in the same leaves of both forms before drought stress. Foliar ABA levels (expressed in terms of dry weight) and Ψl, measured after gs stability after rehydration, are shown.
Exogenous ABA Does Not Close Live Stomata in Intact Leaves of A. filix-femina
If fern stomatal responses to changes in leaf water status in the light do not require any metabolic input or molecular signaling, then an important unanswered question for evolutionary biologists is: What is the adaptive relevance of reported stomatal responses to ABA in nonseed plants? All studies documenting a stomatal sensitivity to ABA in lycophyte or fern species have shown very small responses to exceptionally high levels of exogenous ABA compared to the results of numerous studies in diverse seed plant species (Fig. 6). Cai et al. (2017), for instance, found that ABA levels as high as 15 mm could only elicit a 10% to 20% reduction in aperture in two fern species. Unlike ferns, stomatal aperture in angiosperms can be reduced by at least 80% after the application of only 1 μm of ABA (Raschke, 1987; Trejo et al., 1993), reflecting the role of ABA as a plant hormone eliciting responses at very low concentrations. Observation of stomatal aperture in isolated epidermis as a technique has received considerable criticism for more than 150 years because of an inability to mimic stomatal responses in intact leaves (Darwin, 1898; Mott, 2009; Hubbard et al., 2012). To overcome the well-documented artifacts induced by measuring apertures in stomata from isolated epidermis, we monitored the stomatal response to high levels of exogenous ABA applied directly to the stomata in intact leaves of both forms of A. filix-femina and a representative angiosperm species, Rheum rhabarbarum. Unlike stomata from floating epidermis, which require multiple hours to close in angiosperms, when ABA was applied directly to the leaf of the representative angiosperm R. rhabarbarum, stomata completely closed within 30 min (Fig. 7). This speed of stomatal closure mirrors the speed of stomatal closure when ABA is feed into the transpiration stream of angiosperm species (Brodribb and McAdam, 2011), as well the speed of stomatal responses to changes in VPD that are hypothesized to be driven by the synthesis of ABA in the leaf in angiosperm species (McAdam et al., 2016b). In contrast to R. rhabarbarum, the stomata of both forms of A. filix-femina did not close after 30 min in the presence of 10−4 M ABA (Fig. 7), an ABA level 25-fold higher than the level of ABA synthesized by the wild-type form during lethal soil drought (Fig. 4A). The lack of a stomatal response was not because the stomata of A. filix-femina are unresponsive, or that direct observation of stomatal aperture in an intact leaf of this species was unable to detect changes in aperture, because stomata in both forms were observed to rapidly close completely upon dehydration after leaf excision (Fig. 7).
Figure 6.
Stomata of seeds plants are highly sensitive to exogenous ABA in comparison to ferns and lycophytes. The sensitivity of stomatal aperture (as a percentage of maximum aperture) in isolated epidermis of seed plant species (solid circles) and fern and lycophyte species (open circles) exposed to exogenous ABA concentrations. See Supplemental Dataset.
Figure 7.
Unlike seed plants, the stomata of A. filix-femina in live and intact leaves do not respond to exogenous ABA application. A to C, Mean stomatal aperture (n = 5 ± se) in continuously monitored stomata from a live, intact, photosynthesizing leaf of (A) the angiosperm R. rhabarbarum, (B) the wild-type form, and (C) the Frizelliae form of A. filix-femina. Stomata were continuously monitored for 30 min before and after an application of exogenous, aqueous 10−4 M ABA (marked by a vertical dashed line and denoted by solid circles) or a control solution of water (denoted by open circles) directly to the stomata on the surface of the leaf. In the two forms of A. filix-femina, stomata were also monitored in a leaf that was excised in air (triangle symbols; the time of excision is also marked by the vertical dashed line). D to H, Representative images of a monitored open stomata are shown immediately before the application of exogenous ABA and 30 min after the application, in (D) R. rhabarbarum and the (E) wild-type and (F) Frizelliae forms of A. filix-femina. Representative image of a monitored stomata immediately before and 30 min after leaf excision in air is also shown for the (G) wild-type and (H) Frizelliae forms of A. filix-femina. Scale bars = 25 µm.
Very small responses of gs to extremely high levels of applied ABA in fern and lycophyte species have been reported by Hõrak et al. (2017), Brodribb and McAdam (2011), and Ruszala et al. (2011). These very small stomatal responses to exogenous ABA, if they are not artifacts, could possibly be the result of a nonstomatal reduction in photosynthetic activity caused by very high levels of this hormone (Raschke and Hedrich, 1985). There is a strong correlation between stomatal aperture and photosynthetic rate in fern species (Doi and Shimazaki, 2008; McAdam and Brodribb, 2012b). In any case, any possible stomatal responses to ABA still lack an adaptive explanation given the requirement of unnaturally high levels of ABA applied to induce them. The most parsimonious explanation is that functional stomatal regulation by ABA evolved in the common ancestor of the seed plants.
CONCLUSION
We found that a simple mechanistic model that assumes guard cell turgor changes in concert with leaf turgor can accurately predict the stomatal responses to changes in VPD in a fern species, and that during soil drought endogenous ABA plays no role in closing stomata in this species. This hydraulic-based regulation of stomatal responses to changes in leaf water status occurs in A. filix-femina, which is reported to have a small stomatal response to exogenous ABA application (Hõrak et al., 2017). Direct observations of stomatal aperture in intact leaves, however, could not detect a stomatal response to exogenous ABA application. The assumption that stomatal responses to changes in leaf water status in the light are regulated by the simple linkage of guard cell turgor with leaf turgor provides the most parsimonious explanation for the regulation of these stomatal responses in this, and other, fern species. The ability to predict the response of plant gas exchange to changes in water status in species from the earliest diverging lineages of vascular land plants by this exceptionally simple model could greatly enhance our predictive power of terrestrial water fluxes over the past 400 million years.
MATERIALS AND METHODS
Four Athyrium filix-femina individuals per variety were grown in a 2:1 mix of IN Miami topsoil and ground pine bark in a glasshouse at Purdue University. Conditions in the glasshouse were maintained according to the growth cabinet conditions reported by Hõrak et al. (2017), because these conditions may have induced a slight stomatal sensitivity to ABA in this species (although growth temperatures were not reported by Hõrak et al. (2017)). A misting fan was used to maintain relative humidity at or above 70% during the day, day/night temperatures were regulated at 23°/16°C, and plants were grown under a 75% light reducing shade cloth resulting in ∼150 μmol m−2 s−1 of natural sunlight reaching pot level at midday. Plants received daily watering and a weekly application of liquid fertilizer. All experiments were conducted on the newest flush of leaves that emerged and expanded over a period of 4 weeks after plant establishment in the greenhouse.
Cbulk was quantified in four leaves from each variety by pressure-volume curve analysis according to the methods of Blackman and Brodribb (2011). Kleaf was measured in four leaves of each variety by the two-point rehydration method described by Brodribb and Holbrook (2003). Theoretical maximum gs used for model predictions was anatomically determined in four leaves from each variety according to the method of Parlange and Waggoner (1970). Maximum stomatal apertures were taken from direct observations of stomata in irradiated and fully hydrated leaves; stomatal density was quantified from bleached leaves stained with aqueous toluene blue and guard cell and stomatal pore dimensions were taken from leaf paradermal- and cross-sections.
In both varieties three leaves were exposed to a series of transitions in VPD while leaf gas exchange was measured using an infrared gas analyzer (LI-6800). Environmental conditions in the leaf cuvette were controlled for the duration of the experiment; light intensity was set at 500 μmol m−2 s−1 (to ensure maximum gs), temperature to 23°C, and CO2 concentration at 400 μmol mol−1. VPD in the leaf cuvette was regulated by the LI-6800 that controlled the amount of intake air passing through either a desiccant or humidifying column. In a leaf of the wild-type form, the stomatal response to VPD was repeated in a leaf that was acclimated to a low CO2 concentration of 100 μmol mol−1 in the cuvette. The relationship between gs and Ψl was measured in at least 12 leaves that were excised from the plant and exposed to cycles of slow dehydration on the bench and rehydration through the cut end of the stipe, during which pinnae were collected when gs had reached specific values for the measurement of Ψl using a Scholander pressure chamber. After the measurement of Ψl in these experiments, pinnae were then harvested for foliar ABA analysis by weighing and covering in cold (−30°C) 80% methanol in water (v v−1) and storing immediately at −30°C. In the Frizelliae form, whole leaves were taken for these experiments because of a lack of pinnae. ABA was extracted, purified, and quantified by physicochemical methods with an added internal standard using an Agilent 6400 Series Triple Quadrupole liquid chromatograph tandem mass spectrometer according to the methods of McAdam (2015). Foliar ABA levels were expressed in terms of dry weight (DW), which was quantified after ABA determination by weighing the dry mass of the sample harvested and extracted for analysis.
The response of stomata to changes in VPD was predicted using the mechanistic model described by Brodribb and McAdam (2011) and Deans et al. (2017). This dynamic model is based on the assumption that guard cell turgor in the light changes in concert with leaf turgor when leaf water status changes. The model predicts the dynamic response of Ψl, or turgor, to a change in VPD using an Ohm’s law analog whereby Ψl is a function of the evaporative demand, Kleaf and Cbulk (Eq. 1)
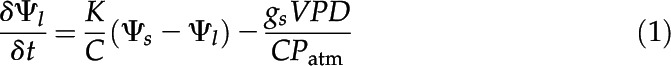 |
where Ψs is the source water potential in the stipe and Patm is the atmospheric pressure (Pa).
Four plants from both forms were drought-stressed by withholding water under glasshouse conditions; foliar ABA level and midday Ψl was recorded until incipient leaf death. In one plant from each form gs was measured on a leaf before the drought stress; this leaf was tagged for remeasuring. Once plants began to show signs of incipient leaf death in the wild-type form, the same leaf was enclosed in the cuvette of the LI6800, a pinnae was collected for Ψl and foliar ABA quantification, after which the stipe of the leaf was excised under water and gs was continuously recorded until stable. In the Frizelliae form, like in the wild-type form, when the plants began to show signs of incipient leaf death three neighboring leaves were harvested for Ψl and foliar ABA quantification after which the depotted roots of the plant in soil was immersed in water, with gs continuously recorded until stability.
Stipe vulnerability curves in both forms of A. filix-femina were determined optically according to the methods of Brodribb et al. (2016). The stipes of excised leaves were imaged under an Olympus Research Stereo S2H10 microscope equipped with an Omax A3550S camera, with images captured every 180 s. For details regarding image capturing and post‐image processing, see http://www.opensourceov.org. Ψl was recorded during dehydration in the wild-type form by periodically sampling pinnae until the xylem was fully embolized. In the Frizelliae form, because pinnae were too small to sample Ψl through time, an alternative method similar to the construction of hydraulic vulnerability curves was employed. The terminal end of the imaged leaf was taken for Ψl determination at a varied stage of embolism visualized in the xylem. After Ψl determination, the stipe was continuously imaged until all of the xylem in the stipe had embolized. The percentage embolism at the point of Ψl determination relative to 100% embolism was subsequently quantified. Color montages of stipe embolism over time (in the Frizelliae form) or Ψl (in the wild-type form) until fully embolized were constructed for each form according to the methods explained in detail at http://www.opensourceov.org.
The aperture response of five live stomata from intact leaves of A. filix-femina (one or two stomata per leaf, n = 3–5 leaves per form) to both the application of exogenous ABA and the excision of the leaf in air was determined using an Axio Imager A2 microscope (Zeiss) and a long working distance 20× objective combined with a 1.6× spacer. The same stomata were imaged every 10 min for the duration of the experiment. The response of stomatal aperture from five live stomata, continuously monitored in an intact leaf, accurately mirrors gas exchange responses to CO2, VPD, and light (Kaiser and Kappen, 2001; Mott et al., 2008). A small (50 µL) droplet of exogenous aqueous ABA (10−4 M, containing <0.1% methanol [v/v]) was applied directly to the stomata. To image stomata after ABA application, the droplet was wicked away with tissue paper, stomata were imaged, and the droplet immediately reapplied. Control samples in which water (containing <0.1% methanol [v/v]) was applied were also imaged in each form. The response of stomata to exogenous ABA application and a water control was monitored according to the same method in a representative angiosperm species, Rheum rhabarbarum, grown under identical glasshouse conditions. This species was selected because it has a comparable stomatal size (Cardoso et al., 2018).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Predictable stomatal responses to VPD at low CO2.
Supplemental Figure S2. Response of photosynthesis to changes in vapor pressure difference.
Supplemental Dataset. Stomatal aperture responses to exogenous ABA from literature.
Acknowledgments
We acknowledge the use of the facilities of the Bindley Bioscience Center, a core facility of the National Institute of Health–funded Indiana Clinical and Translational Sciences Institute. We thank two reviewers and the editor for helpful comments on an earlier version of this manuscript.
Footnotes
This work was supported by the USDA National Institute of Food and Agriculture (Hatch project no. 1014908 to S.A.M.M.).
Articles can be viewed without a subscription.
References
- Assmann SM, Snyder JA, Lee Y-RJ (2000) ABA-deficient (aba1) and ABA-insensitive (abi1-1, abi2-1) mutants of Arabidopsis have a wild-type stomatal response to humidity. Plant Cell Environ 23: 387–395 [Google Scholar]
- Bauerle WL, Whitlow TH, Setter TL, Vermeylen FM (2004) Abscisic acid synthesis in Acer rubrum L. leaves—a vapor-pressure-deficit-mediated response. J Am Soc Hortic Sci 129: 182–187 [Google Scholar]
- Blackman CJ, Brodribb TJ (2011) Two measures of leaf capacitance: insights into the water transport pathway and hydraulic conductance in leaves. Funct Plant Biol 38: 118–126 [DOI] [PubMed] [Google Scholar]
- Blackman CJ, Brodribb TJ, Jordan GJ (2010) Leaf hydraulic vulnerability is related to conduit dimensions and drought resistance across a diverse range of woody angiosperms. New Phytol 188: 1113–1123 [DOI] [PubMed] [Google Scholar]
- Brandt B, Munemasa S, Wang C, Nguyen D, Yong T, Yang PG, Poretsky E, Belknap TF, Waadt R, Alemán F, et al. (2015) Calcium specificity signaling mechanisms in abscisic acid signal transduction in Arabidopsis guard cells. eLife 4: e03599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Cochard H (2009) Hydraulic failure defines the recovery and point of death in water-stressed conifers. Plant Physiol 149: 575–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Holbrook NM (2003) Stomatal closure during leaf dehydration, correlation with other leaf physiological traits. Plant Physiol 132: 2166–2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, McAdam SAM (2011) Passive origins of stomatal control in vascular plants. Science 331: 582–585 [DOI] [PubMed] [Google Scholar]
- Brodribb TJ, Bienaimé D, Marmottant P (2016) Revealing catastrophic failure of leaf networks under stress. Proc Natl Acad Sci USA 113: 4865–4869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, McAdam SAM, Carins Murphy MR (2017) Xylem and stomata, coordinated through time and space. Plant Cell Environ 40: 872–880 [DOI] [PubMed] [Google Scholar]
- Buckley TN. (2016) Stomatal responses to humidity: Has the “black box” finally been opened? Plant Cell Environ 39: 482–484 [DOI] [PubMed] [Google Scholar]
- Buckley TN, Mott KA, Farquhar GD (2003) A hydromechanical and biochemical model of stomatal conductance. Plant Cell Environ 26: 1767–1785 [Google Scholar]
- Burrows FJ, Milthorpe FL (1976) Stomatal conductance in the control of gas exchange. Water Def Plant Growth 4: 103–152 [Google Scholar]
- Cai S, Chen G, Wang Y, Huang Y, Marchant DB, Wang Y, Yang Q, Dai F, Hills A, Franks PJ, et al. (2017) Evolutionary conservation of ABA signaling for stomatal closure. Plant Physiol 174: 732–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso AA, Randall JM, Jordan GJ, McAdam SAM (2018) Extended differentiation of veins and stomata is essential for the expansion of large leaves in Rheum rhabarbarum. Am J Bot 105: 1967–1974 [DOI] [PubMed] [Google Scholar]
- Chater CC, Kamisugi Y, Movahedi M, Fleming A, Cuming AC, Gray JE, Beerling DJ (2011) Regulatory mechanism controlling stomatal behavior conserved across 400 million years of land plant evolution. Curr Biol 21: 1025–1029 [DOI] [PubMed] [Google Scholar]
- Chater CC, Caine RS, Tomek M, Wallace S, Kamisugi Y, Cuming AC, Lang D, MacAlister CA, Casson S, Bergmann DC, et al. (2016) Origin and function of stomata in the moss Physcomitrella patens. Nat Plants 2: 16179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chater CC, Caine RS, Fleming AJ, Gray JE (2017) Origins and evolution of stomatal development. Plant Physiol 174: 624–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin F. (1898) Observations on stomata. Proc R Soc Lond 63: 413–417 [Google Scholar]
- Deans RM, Brodribb TJ, McAdam SAM (2017) An integrated hydraulic-hormonal model of conifer stomata predicts water stress dynamics. Plant Physiol 174: 478–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi M, Shimazaki K (2008) The stomata of the fern Adiantum capillus-veneris do not respond to CO2 in the dark and open by photosynthesis in guard cells. Plant Physiol 147: 922–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi M, Kitagawa Y, Shimazaki K (2015) Stomatal blue light response is present in early vascular plants. Plant Physiol 169: 1205–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckett JG, Pressel S (2018) The evolution of the stomatal apparatus: Intercellular spaces and sporophyte water relations in bryophytes—two ignored dimensions. Philos Trans R Soc Lond B Biol Sci 373: 1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D, Axe L (1992) Stomata and mechanics of stomatal functioning in some early land plants. Cour Forschungsinst Senckenberg 147: 59–73 [Google Scholar]
- Edwards D, Kerp H, Hass H (1998) Stomata in early land plants: An anatomical and ecophysiological approach. J Exp Bot 49: 255–278 [Google Scholar]
- Ewers BE, Oren R (2000) Analyses of assumptions and errors in the calculation of stomatal conductance from sap flux measurements. Tree Physiol 20: 579–589 [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Wong SC (1984) An empirical model of stomatal conductance. Aust J Plant Physiol 11: 191–209 [Google Scholar]
- Franks PJ, Cowan IR, Farquhar GD (1998) A study of stomatal mechanics using the cell pressure probe. Plant Cell Environ 21: 94–100 [Google Scholar]
- Hetherington AM, Woodward FI (2003) The role of stomata in sensing and driving environmental change. Nature 424: 901–908 [DOI] [PubMed] [Google Scholar]
- Hiyama A, Takemiya A, Munemasa S, Okuma E, Sugiyama N, Tada Y, Murata Y, Shimazaki KI (2017) Blue light and CO2 signals converge to regulate light-induced stomatal opening. Nat Commun 8: 1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hõrak H, Kollist H, Merilo E (2017) Fern stomatal responses to ABA and CO2 depend on species and growth conditions. Plant Physiol 174: 672–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard KE, Siegel RS, Valerio G, Brandt B, Schroeder JI (2012) Abscisic acid and CO2 signalling via calcium sensitivity priming in guard cells, new CDPK mutant phenotypes and a method for improved resolution of stomatal stimulus-response analyses. Ann Bot 109: 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser H, Kappen L (2001) Stomatal oscillations at small apertures: Indications for a fundamental insufficiency of stomatal feedback-control inherent in the stomatal turgor mechanism. J Exp Bot 52: 1303–1313 [PubMed] [Google Scholar]
- Koornneef M, Jorna ML, Brinkhorst-van der Swan DLC, Karssen CM (1982) The isolation of abscisic acid (ABA) deficient mutants by selection of induced revertants in non-germinating gibberellin sensitive lines of Arabidopsis thaliana (L.) heynh. Theor Appl Genet 61: 385–393 [DOI] [PubMed] [Google Scholar]
- Lange OL, Lösch R, Schulze ED, Kappen L (1971) Responses of stomata to changes in humidity. Planta 100: 76–86 [DOI] [PubMed] [Google Scholar]
- Lösch R. (1979) Responses of stomata to environmental factors—experiments with isolated epidermal strips of Polypodium vulgare: II. Leaf bulk water potential, air humidity, and temperature. Oecologia 39: 229–238 [DOI] [PubMed] [Google Scholar]
- Lösch R, Tenhunen JD (1981) Stomatal responses to humidity—phenomenon and mechanism. In Jarvis PG and Mansfield TA, eds, Stomatal Physiology. Cambridge University Press, Cambridge, UK [Google Scholar]
- Lowe EJ. (1867) Our Native Ferns, or A History of the British Species and Their Varieties. Groombridge and Sons, London [Google Scholar]
- Maierhofer T, Diekmann M, Offenborn JN, Lind C, Bauer H, Hashimoto K, S Al-Rasheid KA, Luan S, Kudla J, Geiger D, et al. (2014) Site- and kinase-specific phosphorylation-mediated activation of SLAC1, a guard cell anion channel stimulated by abscisic acid. Sci Signal 7: ra86. [DOI] [PubMed] [Google Scholar]
- Martins SCV, McAdam SAM, Deans RM, DaMatta FM, Brodribb TJ (2016) Stomatal dynamics are limited by leaf hydraulics in ferns and conifers: Results from simultaneous measurements of liquid and vapour fluxes in leaves. Plant Cell Environ 39: 694–705 [DOI] [PubMed] [Google Scholar]
- Martin-St Paul N, Delzon S, Cochard H (2017) Plant resistance to drought depends on timely stomatal closure. Ecol Lett 20: 1437–1447 [DOI] [PubMed] [Google Scholar]
- McAdam SAM. (2015) Physicochemical quantification of abscisic acid levels in plant tissues with an added internal standard by ultra-performance liquid chromatography. Bio Protoc 5: e1599 [Google Scholar]
- McAdam SAM, Brodribb TJ (2012a) Fern and lycophyte guard cells do not respond to endogenous abscisic acid. Plant Cell 24: 1510–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam SAM, Brodribb TJ (2012b) Stomatal innovation and the rise of seed plants. Ecol Lett 15: 1–8 [DOI] [PubMed] [Google Scholar]
- McAdam SAM, Brodribb TJ (2013) Ancestral stomatal control results in a canalization of fern and lycophyte adaptation to drought. New Phytol 198: 429–441 [DOI] [PubMed] [Google Scholar]
- McAdam SAM, Brodribb TJ (2014) Separating active and passive influences on stomatal control of transpiration. Plant Physiol 164: 1578–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam SAM, Brodribb TJ (2015) The evolution of mechanisms driving the stomatal response to vapor pressure deficit. Plant Physiol 167: 833–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam SAM, Brodribb TJ (2016) Linking turgor with ABA biosynthesis: Implications for stomatal responses to vapour pressure deficit across land plants. Plant Physiol 171: 2008–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam SAM, Brodribb TJ, Banks JA, Hedrich R, Atallah NM, Cai C, Geringer MA, Lind C, Nichols DS, Stachowski K, et al. (2016a) Abscisic acid controlled sex before transpiration in vascular plants. Proc Natl Acad Sci USA 113: 12862–12867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam SAM, Sussmilch FC, Brodribb TJ (2016b) Stomatal responses to vapour pressure deficit are regulated by high speed gene expression in angiosperms. Plant Cell Environ 39: 485–491 [DOI] [PubMed] [Google Scholar]
- Merilo E, Yarmolinsky D, Jalakas P, Parik H, Tulva I, Rasulov B, Kilk K, Kollist H (2017) Stomatal VPD response: There is more to the story than ABA. Plant Physiol 176: 851–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelheuser CJ, van Steveninck RFM (1969) Stomatal closure and inhibition of transpiration induced by (RS)-abscisic acid. Nature 221: 281–282 [Google Scholar]
- Mott KA. (2009) Opinion: Stomatal responses to light and CO2 depend on the mesophyll. Plant Cell Environ 32: 1479–1486 [DOI] [PubMed] [Google Scholar]
- Mott KA, Peak D (2013) Testing a vapour-phase model of stomatal responses to humidity. Plant Cell Environ 36: 936–944 [DOI] [PubMed] [Google Scholar]
- Mott KA, Sibbernsen ED, Shope JC (2008) The role of the mesophyll in stomatal responses to light and CO2. Plant Cell Environ 31: 1299–1306 [DOI] [PubMed] [Google Scholar]
- Pantin F, Blatt MR (2018) Stomatal response to humidity: Blurring the boundary between active and passive movement. Plant Physiol 176: 485–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlange JY, Waggoner PE (1970) Stomatal dimensions and resistance to diffusion. Plant Physiol 46: 337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JA, Pearce JV (1957) The occurrence, structure and functions of the stomata in British bryophytes. Trans Brit Bryol Soc 3: 228–259 [Google Scholar]
- Pierce M, Raschke K (1980) Correlation between loss of turgor and accumulation of abscisic acid in detached leaves. Planta 148: 174–182 [DOI] [PubMed] [Google Scholar]
- Pierce M, Raschke K (1981) Synthesis and metabolism of abscisic acid in detached leaves of Phaseolus vulgaris L. after loss and recovery of turgor. Planta 153: 156–165 [DOI] [PubMed] [Google Scholar]
- Pressel S, Goral T, Duckett JG (2014) Stomatal differentiation and abnormal stomata in hornworts. J Bryol 36: 87–103 [Google Scholar]
- Qiu C, Ethier G, Pepin S, Dubé P, Desjardins Y, Gosselin A (2017) Persistent negative temperature response of mesophyll conductance in red raspberry (Rubus idaeus L.) leaves under both high and low vapour pressure deficits: A role for abscisic acid? Plant Cell Environ 40: 1940–1959 [DOI] [PubMed] [Google Scholar]
- Raschke K. (1970) Leaf hydraulic system: Rapid epidermal and stomatal responses to changes in water supply. Science 167: 189–191 [DOI] [PubMed] [Google Scholar]
- Raschke K. (1987) Action of abscisic acid on guard cells. In Zeiger E, Farquhar GD, Cowan IR, eds, Stomatal Function. Stanford University Press, Stanford, CA, pp 253–279 [Google Scholar]
- Raschke K, Hedrich R (1985) Simultaneous and independent effects of abscisic acid on stomata and the photosynthetic apparatus in whole leaves. Planta 163: 105–118 [DOI] [PubMed] [Google Scholar]
- Renzaglia KS, Villarreal JC, Piatkowski BT, Lucas JR, Merced A (2017) Hornwort stomata: Architecture and fate shared with 400-million-year-old fossil plants without leaves. Plant Physiol 174: 788–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruszala EM, Beerling DJ, Franks PJ, Chater C, Casson SA, Gray JE, Hetherington AM (2011) Land plants acquired active stomatal control early in their evolutionary history. Curr Biol 21: 1030–1035 [DOI] [PubMed] [Google Scholar]
- Scoffoni C, Chatelet DS, Pasquet-Kok J, Rawls M, Donoghue MJ, Edwards EJ, Sack L (2016) Hydraulic basis for the evolution of photosynthetic productivity. Nat Plants 2: 16072. [DOI] [PubMed] [Google Scholar]
- Sussmilch FC, Brodribb TJ, McAdam SAM (2017) What are the evolutionary origins of stomatal responses to abscisic acid in land plants? J Integr Plant Biol 59: 240–260 [DOI] [PubMed] [Google Scholar]
- Sweet KJ, Peak D, Mott KA (2017) Stomatal heterogeneity in responses to humidity and temperature: Testing a mechanistic model. Plant Cell Environ 40: 2771–2779 [DOI] [PubMed] [Google Scholar]
- Tal M, Nevo Y (1973) Abnormal stomatal behavior and root resistance, and hormonal imbalance in three wilty mutants of tomato. Biochem Genet 8: 291–300 [DOI] [PubMed] [Google Scholar]
- Trejo CL, Davies WJ, Ruiz L (1993) Sensitivity of stomata to abscisic acid (an effect of the mesophyll). Plant Physiol 102: 497–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urli M, Porté AJ, Cochard H, Guengant Y, Burlett R, Delzon S (2013) Xylem embolism threshold for catastrophic hydraulic failure in angiosperm trees. Tree Physiol 33: 672–683 [DOI] [PubMed] [Google Scholar]
- von Mohl H. (1856) What mechanism causes the enlargement and narrowing of the stomata? [in German] Botanischer Zeitung 14: 697–720 [Google Scholar]
- Wong S-C, Cowan IR, Farquhar GD (1979) Stomatal conductance correlates with photosynthetic capacity. Nature 282: 424–426 [Google Scholar]
- Xie X, Wang Y, Williamson L, Holroyd GH, Tagliavia C, Murchie E, Theobald J, Knight MR, Davies WJ, Leyser HMO, et al. (2006) The identification of genes involved in the stomatal response to reduced atmospheric relative humidity. Curr Biol 16: 882–887 [DOI] [PubMed] [Google Scholar]
- Ziegler H. (1987) Stomatal Function. Stanford University Press, Stanford, CA [Google Scholar]