Abstract
Background
Use of topical NSAIDs to treat acute musculoskeletal conditions has become widely accepted because they can provide pain relief without associated systemic adverse events. This review is an update of 'Topical NSAIDs for acute pain in adults' originally published in Issue 6, 2010.
Objectives
To determine the efficacy and safety of topically applied NSAIDs in acute musculoskeletal pain in adults.
Search methods
We searched the Cochrane Register of Studies Online, MEDLINE, and EMBASE to February 2015. We sought unpublished studies by asking personal contacts and searching online clinical trial registers and manufacturers websites. For the earlier review, we also searched our own in‐house database and contacted manufacturers.
Selection criteria
We included randomised, double‐blind, active or placebo (inert carrier)‐controlled trials in which treatments were administered to adults with acute pain resulting from strains, sprains or sports or overuse‐type injuries (twisted ankle, for instance). There had to be at least 10 participants in each treatment arm, with application of treatment at least once daily.
Data collection and analysis
Two review authors independently assessed studies for inclusion, and extracted data. We used numbers of participants achieving each outcome to calculate the risk ratio and numbers needed to treat for an additional beneficial outcome (NNT) or additional harmful outcome (NNH) compared with placebo or other active treatment. We reported 95% confidence intervals (CI). We were particularly interested to compare different formulations (gel, cream, plaster) of individual NSAIDs.
Main results
For this update we added 14 new included studies (3489 participants), and excluded four studies. We also identified 20 additional reports of completed or ongoing studies that have not been published in full. The earlier review included 47 studies.
This update included 61 studies. Most compared topical NSAIDs in the form of a gel, spray, or cream with a similar topical placebo; 5311 participants were treated with a topical NSAID, 3470 with placebo, and 220 with an oral NSAID. This was a 63% increase in the number of included participants over the previous version of this review. We also identified a number of studies in clinical trial registries with unavailable results amounting to about 5900 participants for efficacy and 5300 for adverse events.
Formulations of topical diclofenac, ibuprofen, ketoprofen, piroxicam, and indomethacin demonstrated significantly higher rates of clinical success (more participants with at least 50% pain relief) than matching topical placebo (moderate or high quality data). Benzydamine did not. Three drug and formulation combinations had NNTs for clinical success below 4. For diclofenac, the Emulgel® formulation had the lowest NNT of 1.8 (95% CI 1.5 to 2.1) in two studies using at least 50% pain intensity reduction as the outcome. Diclofenac plasters other than Flector® also had a low NNT of 3.2 (2.6 to 4.2) based on good or excellent responses in some studies. Ketoprofen gel had an NNT of 2.5 (2.0 to 3.4), from five studies in the 1980s, some with less well defined outcomes. Ibuprofen gel had an NNT of 3.9 (2.7 to 6.7) from two studies with outcomes of marked improvement or complete remission. All other drug and formulation combinations had NNT values above 4, indicating lesser efficacy.
There were insufficient data to compare reliably individual topical NSAIDs with each other or the same oral NSAID.
Local skin reactions were generally mild and transient, and did not differ from placebo (high quality data). There were very few systemic adverse events (high quality data) or withdrawals due to adverse events (low quality data).
Authors' conclusions
Topical NSAIDs provided good levels of pain relief in acute conditions such as sprains, strains and overuse injuries, probably similar to that provided by oral NSAIDs. Gel formulations of diclofenac (as Emugel®), ibuprofen, and ketoprofen, and some diclofenac patches, provided the best effects. Adverse events were usually minimal.
Since the last version of this review, the new included studies have provided additional information. In particular, information on topical diclofenac is greatly expanded. The present review supports the previous review in concluding that topical NSAIDs are effective in providing pain relief, and goes further to demonstrate that certain formulations, mainly gel formulations of diclofenac, ibuprofen, and ketoprofen, provide the best results. Large amounts of unpublished data have been identified, and this could influence results in updates of this review.
Plain language summary
Topical non‐steroidal anti‐inflammatory drugs for acute musculoskeletal pain in adults
Acute musculoskeletal pain describes conditions like a sprained ankle or a muscle pull. These usually get better over two or three weeks without treatment, but can be very painful while they last.
Topical non‐steroidal anti‐inflammatory drugs (NSAIDs) are applied to unbroken skin where it hurts as gels, creams, sprays, or plasters. Topical NSAIDs penetrate the skin, enter tissues or joints, and reduce processes causing pain in the tissue. Drug levels in the blood with topical NSAIDs are very much lower than with the same drug taken by mouth. This minimises the risk of harmful effects.
We searched medical databases for clinical trials comparing topical NSAIDs with placebo (creams or gels that do not contain a medicine) or other medicines in adults aged 16 years or older with musculoskeletal pain (typically sports injuries). The evidence is current to February 2015.
This review is an update of 'Topical NSAIDs for acute pain in adults' originally published in Issue 6, 2010. We identified 14 new studies to add to the 47 studies included in the earlier review. We also identified 14 studies in a clinical trial registry that are completed and three short reports from meetings, for which we could not find full details (about 4500 participants). Three more studies are ongoing (almost 900 participants).
The 61 included studies, involving 8386 participants, were generally of high‐quality. They tested a number of different topical drugs, mostly against a topical placebo (carrier without the NSAID), with application at least once a day. We were interested in participants having good pain reduction (by about half) around seven days after treatment started. At later times, most people are expected to get better even without treatment.
We looked at particular formulations of individual drugs. Gel formulations of diclofenac and ketoprofen were among the most effective, along with ibuprofen gel and diclofenac plaster. For diclofenac and ketoprofen gels, 7 or 8 people out of 10 with a painful strain, sprain, or muscle pull had much reduced pain after seven days, compared with only 2 or 3 out of 10 with placebo (high quality data). Other NSAIDs and formulations were better than placebo, but not by as much. Because both topical NSAIDs and topical placebo are rubbed into the skin in these studies, we know that any effect is not just from rubbing.
About 1 in 20 people experienced a mild and short‐lived side effect like redness at the application site. This was the same for topical NSAID and topical placebo (high quality data). Side effects like a stomach upset or feeling sick were uncommon, with no difference between topical NSAID and topical placebo (high quality data). There were no serious side effects.
Summary of findings
Summary of findings for the main comparison. Topical NSAIDs compared with topical placebo for acute musculoskeletal pain in adults.
| Topical NSAIDs compared with topical placebo for acute musculoskeletal pain in adults | ||||||
|
Patient or population: adults with strains, sprains, or muscle pull Settings: community Intervention: topical NSAID (topical diclofenac, ibuprofen, and ketoprofen gels only shown here for efficacy) Comparison: topical placebo | ||||||
| Outcomes | Probable outcome with intervention | Probable outcome with comparator | RR, NNT, NNTp, or NNH (95% CI) | No of studies, participants | Quality of the evidence (GRADE) | Comments |
|
Topical diclofenac gel (as Emulgel) Clinical success (eg 50% reduction in pain) |
780 in 1000 | 200 in 1000 | RR 3.4 (2.7 to 55) NNT 1.8 (1.5 to 2.1) |
2 studies 314 participants |
High | Consistent results in 2 moderately sized recent studies of high quality |
|
Topical ibuprofen gel Clinical success (eg 50% reduction in pain) |
420 in 1000 | 160 in 1000 | RR 2.7 (1.7 to 4.2) NNT 3.9 (2.7 to 6.7) |
2 studies 241 participants |
Moderate | Modest effect size and numbers of participants |
|
Topical ketoprofen gel Clinical success (eg 50% reduction in pain) |
720 in 1000 | 330 in 1000 | RR 2.2 (1.7 to 2.8) NNT 2.5 (2.0 to 3.4) |
5 studies 348 participants |
Moderate | Modest effect size and numbers of participants, but studies small, with none recent |
|
All topical NSAIDs Local adverse events |
46 in 1000 | 50 in 1000 | RR 1.0 (0.80 to 1.2) NNH not calculated |
42 studies 6125 participants |
High | Large number of studies and participants with consistent results |
|
All topical NSAIDs Systemic adverse events |
32 in 1000 | 35 in 1000 | RR 1.0 (0.7 to 1.3) NNH not calculated |
38 studies 5372 participants |
High | Large number of studies and participants with consistent results |
|
All topical NSAIDs Withdrawals ‐ adverse events |
11 in 1000 | 11 in 1000 | RR 1.0 (0.7 to 1.7) NNH not calculated |
42 studies 5790 participants |
High | Large number of studies and participants with consistent results |
| Serious adverse events | 1 in total | 0 in total | Not calculated | All data | Low | Small numbers of events |
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
CI: confidence interval; RR: risk ratio; NNT: number needed to treat for an additional beneficial outcome; NNTp: number needed to treat to prevent an event happening; NNH: number needed to treat for an additional harmful outcome.
Background
This review is an update of a review originally published in Issue 6 2010 on 'Topical NSAIDs for acute pain in adults' (Massey 2010). We have changed the title to specify musculoskeletal pain because topical NSAIDs are not normally used to treat visceral pain or headache. We felt that the new title better reflected the content of the review.
The use of topical NSAIDs for pain relief has been a controversial subject in analgesic practice. In some parts of the world (including much of Western Europe) they have been available for many years, are widely available without prescription, widely advertised, used extensively, and evidence for their use is considered adequate. In other parts of the world they were regarded as little more than placebo, with any apparent effect attributed to the process of rubbing at the site of the affected area. In some places (for example the US) their use was almost unknown until the mid‐2010s. In England, 5.2 million prescriptions for topical NSAIDs were dispensed in 2013 (PACT 2014), mainly for formulations of ibuprofen (2.45 million), piroxicam (1.18 million), and diclofenac (1.27 million).
There is good evidence for the efficacy of topical NSAIDs in acute and chronic musculoskeletal pain (Mason 2004a; Mason 2004b; Moore 1998a). In the US, the Food and Drug Administration licensed topical nonsteroidal products in 2007, and in England, the National Institute for Health and Care Excellence (NICE) recommended topical therapies as first line treatment in its guidelines for osteoarthritis in 2008 (NICE 2008). Earlier reviews of topical analgesics covered studies investigating the underlying science to explain biological plausibility in addition to clinical trials (Anon 2005; Moore 2008a).
This review is one of a series on topical analgesics, including topical capsaicin at low and high doses (Derry 2012a; Derry 2013), and topical NSAIDs in chronic pain conditions (Derry 2012b), and salicylate‐containing rubefacients (Derry 2014).
Description of the condition
Acute pain is usually defined as pain of less than three months' duration. It is often associated with injury, including trauma; surgery; musculoskeletal injuries such as strains, sprains, and over‐use injuries; or soft tissue injuries such as muscle soreness or cramps.
Description of the intervention
Clinicians prescribe NSAIDs on a routine basis for a range of mild to moderate pain. NSAIDs are the most commonly prescribed analgesic medications worldwide, and their efficacy for treating acute pain has been well demonstrated (Moore 2003). They reversibly inhibit cyclooxygenase (prostaglandin endoperoxide synthase), the enzyme mediating production of prostaglandins and thromboxane A2 (FitzGerald 2001). Prostaglandins mediate a variety of physiological functions such as maintenance of the gastric mucosal barrier, regulation of renal blood flow, and regulation of endothelial tone. They also play an important role in inflammatory and nociceptive processes. However, relatively little is known about the mechanism of action of this class of compounds aside from their ability to inhibit cyclooxygenase‐dependent prostanoid formation (Hawkey 1999).
NSAIDs taken orally or intravenously are transported to all parts of the body in the blood, and relatively high blood concentrations are needed to achieve effective tissue concentrations at the site of the pain and inflammation. These high concentrations throughout the body can give rise to a number of adverse events that can be unpleasant (for example, dyspepsia) or potentially serious (for example, gastrointestinal bleeding).
A topical medication is a one applied to body surfaces such as the skin or mucous membranes to treat ailments. A large range of types of topical formulation may be used, including but not limited to creams, foams, gels, lotions, ointments, and plasters. The exact formulation of a topical medication is often determined by the speed of drug absorption required. The need may be for slow absorption into the circulation to maintain low drug concentrations, and, perhaps, avoiding extensive first pass metabolism in the liver; plasters containing drug reservoirs may be used for this, as with transdermal opioids or contraceptive steroids. For rapid absorption, the formulation is enhanced by substances to improve or assist skin penetration, perhaps only to generate high concentrations in tissues rather than in the blood; gel formulations are useful for this purpose, which is why they are sometimes used for topical NSAIDs.
Topical NSAIDs
Topical NSAIDs are formulated for direct application to the painful site, and to produce a local pain‐relieving effect while avoiding body‐wide distribution of the drug at physiologically active levels (McPherson 2013). This method of application (dosing) necessarily limits their use to more superficial painful conditions such as sprains, strains, and muscle or tendon soreness. They would not, for example, be indicated for deep visceral pain or headaches. They are also not appropriate for use on broken skin, so would not be used on open wounds (accidental or surgical).
How the intervention might work
For a topical formulation to be effective, it must first penetrate the skin. Only when the drug has entered the lower layers of the skin can it be absorbed by the blood and transported to the site of action, or penetrate deeper into areas where inflammation occurs. Individual drugs have different degrees of penetration. A balance between lipid and aqueous solubility is needed to optimise penetration, and use of prodrug esters has been suggested as a way of enhancing permeability. Formulation is also crucial to good skin penetration. Experiments with artificial membranes or human epidermis suggest that creams are generally less effective than gels or sprays, but newer formulations such as microemulsions may have greater potential.
Once the drug has reached the site of action, it must be present at a sufficiently high concentration to inhibit cyclooxygenase enzymes, thereby reducing prostaglandin synthesis. This in turn reduces inflammation and relieves pain. It is probable that in acute conditions, topical NSAIDs exert their action primarily by local reduction of symptoms, independent of any systemic uptake and delivery. Tissue levels of NSAIDs applied topically certainly reach levels high enough to inhibit cyclooxygenase‐2 (Anon 2005; Haroutiunian 2010; Moore 2008a). However, plasma concentrations found after topical administration are only a fraction (usually much less than 5%) of the levels found in plasma following oral administration. Topical application can potentially limit systemic adverse events by increasing local effects, and minimising systemic concentrations of the drug. We know that the incidence of upper gastrointestinal bleeding is low with chronic use of topical NSAIDs (Evans 1995), although it has been reported, particularly in people with risk factors (Zimmerman 1995). We have no certain knowledge of lower effects on cardiovascular events, or renal failure, both of which have been associated with oral NSAID use.
Why it is important to do this review
Since the last review in 2010, a number of new studies have been published, nearly all of which investigated various formulations of diclofenac. These new studies are generally of higher quality than many of the earlier ones in this review, and have the potential to influence the strength of its conclusions substantially. Moreover, the additional information allows for analysis based not only on a particular drug, but also on the formulation of that drug. This can provide better insight into whether formulation affects efficacy of topical NSAIDs in acute musculoskeletal pain.
An updated review of evidence for topical NSAIDs was needed to inform choices made by consumers, prescribers, and commissioners (purchasers of healthcare).
Objectives
To determine the efficacy and safety of topically applied NSAIDs in acute musculoskeletal pain in adults.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled double‐blind studies comparing topical NSAIDs with placebo (inert carrier) or other active treatment for acute pain, with at least 10 participants per treatment arm and outcomes close to seven days (minimum three days). We excluded studies published only as short abstracts (which report insufficient data to assess methods) or studying experimentally induced pain (which does not correlate well with clinical pain). Because a cross‐over design is not appropriate for self limiting conditions such as sprains, strains, and contusions, we only considered parallel‐group designs.
Types of participants
Adults (aged 16 years or more) with acute musculoskeletal pain of at least moderate intensity resulting mainly from strains, sprains, or sports injuries. Typically for sports injuries, the injury would have occurred within 24 or 48 hours.
Types of interventions
Included studies had at least one treatment arm using a topical NSAID and a comparator arm using placebo (inert carrier without NSAID or other active treatment). The topical NSAID had to be applied at least once daily. We did not include salicylates in this review as they are no longer classified as topical NSAIDs and are covered in a separate review (Derry 2014).
Types of outcome measures
We sought information on participant characteristics including age, sex, and condition treated.
Primary outcomes
The primary outcome was 'clinical success', defined as at least a 50% reduction in pain or equivalent measure, such as a 'very good' or 'excellent' global assessment of treatment, or 'none' or 'slight' pain on rest or movement, measured on a categorical scale (Moore 1998a). We used the following hierarchy of outcomes to extract data for the primary outcome.
Participant reported reduction in pain of at least 50%.
Participant reported global assessment of treatment.
Pain on movement.
Pain at rest or spontaneous pain.
Undefined 'improvement'.
We used only participant reported outcomes of efficacy, and not physician or investigator reported outcomes.
Secondary outcomes
Numbers of participants with adverse events: local and systemic.
Numbers of withdrawals: all cause, lack of efficacy, and adverse events.
We anticipated that outcomes would be reported after different durations of treatment, and extracted data reported as close to seven days as possible, with a minimum of three days. We also extracted data for outcomes reported after longer durations of treatment. We anticipated that reporting of adverse events would vary between studies with regard to the terminology used, method of ascertainment, and categories reported (for example, occurring in at least 5% of participants or where there is a statistically significant difference between treatment groups). We took care to identify these details where relevant.
Search methods for identification of studies
Electronic searches
We searched the following databases without language restriction:
CENTRAL (The Cochrane Library), Issue 4, 2009 for the original review, and the Cochrane Register of Studies Online (CRSO) to 3 February 2015 for this update;
MEDLINE (via Ovid), from inception to December 2009 for the original review, and from 2008 to 3 February 2015 for this update;
EMBASE (via Ovid), from inception to December 2009 for the original review, and from 2008 to 3 February 2015 for this update;
Oxford Pain Relief Database for the original review (Jadad 1996a). This resource is no longer being updated.
See Appendix 1 for the CENTRAL search strategy, Appendix 2 for the MEDLINE search strategy, and Appendix 3 for the EMBASE search strategy.
Searching other resources
We searched the reference lists of review articles and included studies. We have previously asked manufacturers for details of unpublished studies, but did not make new requests.
We searched two clinical trial registries (clinicaltrials.gov (clinicaltrials.gov/) and the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/)) and asked personal contacts about ongoing and unpublished studies.
Data collection and analysis
Selection of studies
We screened the titles and abstracts of studies identified by the searches to eliminate those that clearly did not satisfy the inclusion criteria, and obtained full reports of the remaining studies to determine inclusion in the review.
Data extraction and management
Review authors were not blinded to the authors' names and institutions, journal of publication, or study results at any stage of the review. Two review authors independently selected the studies for inclusion, assessed methodological quality and risk of bias, and extracted data. We resolved disagreements and uncertainties through discussion.
We abstracted information on participants, interventions, and outcomes from the original reports into a standard data extraction form. One review author entered data suitable for meta‐analysis into Review Manager 5 (RevMan 2014), and another review author checked it.
Assessment of risk of bias in included studies
We used the Oxford Quality Score as the basis for inclusion, limiting inclusion to studies that were randomised and double‐blind as a minimum (Jadad 1996b).
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions with any disagreements resolved by discussion (Higgins 2011). We assessed the following for each study.
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process, for example, random number table; computer random number generator); unclear risk of bias (method used to generate sequence was not clearly stated). We excluded studies using a non‐random process that were therefore at high risk of bias (for example, odd or even date of birth; hospital or clinic record number).
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions before assignment determines whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as: low risk of bias (for example, telephone or central randomisation; consecutively numbered sealed opaque envelopes); unclear risk of bias (method was not clearly stated). We excluded studies that did not conceal allocation and were therefore at high risk of bias (for example, open list).
Blinding of outcome assessment (checking for possible detection bias). We assessed the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study stated that it was blinded and described the method used to achieve blinding, for example, identical tubes containing gel, or identical plasters; matched in appearance and smell); unclear risk of bias (study stated that it was blinded but did not provide an adequate description of how blinding was achieved). We excluded studies that were not double‐blind and were therefore at high risk of bias.
Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as: low risk of bias (less than 10% of participants did not complete the study or used 'baseline observation carried forward' analysis, or both); unclear risk of bias (used 'last observation carried forward' analysis); or high risk of bias (used 'completer' analysis).
Size (checking for possible biases confounded by small size). Small studies have been shown to overestimate treatment effects, probably due to methodological weaknesses (Dechartres 2013; Nüesch 2010). We assessed studies as at low risk of bias if they had at least 200 participants, at unclear risk if they had 50 to 200 participants, and at high risk if they had fewer than 50 participants.
Measures of treatment effect
We used risk ratio (RR) to establish statistical difference and numbers needed to treat for an additional beneficial outcome (NNT) with 95% confidence intervals (CI). We pooled percentages as absolute measures of benefit or harm.
We used the following terms to describe adverse outcomes in terms of harm or prevention of harm.
When significantly fewer adverse outcomes occurred with treatment than with control (placebo or active), we used the term thenumber needed to treat to prevent one event (NNTp).
When significantly more adverse outcomes occurred with treatment compared with control (placebo or active), we used the term the number needed to treat for an additional harmful outcome or cause one event (NNH).
Unit of analysis issues
Randomisation was to the individual participant.
Dealing with missing data
Wherever possible we used intention‐to‐treat (ITT) analysis where the ITT population consists of participants who were randomised, applied at least one dose of the assigned study medication, and provided at least one post‐baseline assessment. We assigned missing participants zero improvement.
We also looked for information about methods of imputation for missing data.
Assessment of heterogeneity
We examined heterogeneity visually using L'Abbé plots (L'Abbé 1987), a visual method for assessing differences in results of individual studies, and with the I2 statistic.
Assessment of reporting biases
The aim of this review was to use dichotomous outcomes of known utility and of value to patients (Moore 2013). The review did not depend on what the authors of the original studies chose to report or not. Studies that did not report dichotomous results, but only average pain data, did not contribute to analyses.
We assessed publication bias using a method designed to detect the amount of unpublished data with a null effect required to make any result clinically irrelevant (usually taken to mean an NNT of 10 or higher; Moore 2008b).
Data synthesis
We pooled data only for comparisons and outcomes where there were at least two studies and 200 participants (Moore 1998b). When two active treatment arms were compared with a placebo arm, we took care to avoid double counting of participants in the placebo arm: if both active groups contributed to an analysis, we split the placebo group between them.
We calculated RRs with 95% CIs using the fixed‐effect model (Morris 1995). A statistically significant benefit of topical NSAID over control was assumed when the lower limit of the 95% CI of the RR was greater than one. A statistically significant benefit of control over active treatment was assumed when the upper limit of the 95% CI was less than one. We calculated NNTs with 95% CIs using the pooled number of events by the method of Cook and Sackett (Cook 1995).
Statistically significant differences between NNTs for different topical NSAIDs were tested using the z test (Tramer 1997), where there were sufficient data to do so, and where the studies were sufficiently similar in types of participant, outcome, and duration to make such comparisons sensible.
Subgroup analysis and investigation of heterogeneity
We carried out separate analyses for individual NSAIDs, and, where the data permitted, for different formulations of individual NSAIDs.
Sensitivity analysis
The earlier review included sensitivity analyses for various factors that are now covered by the assessment of risk of bias.
Results
Description of studies
Results of the search
New searches for this update identified 20 publications that were examined in further detail to determine inclusion status. We also identified 20 additional studies in clinical trial registries. See Figure 1.
1.
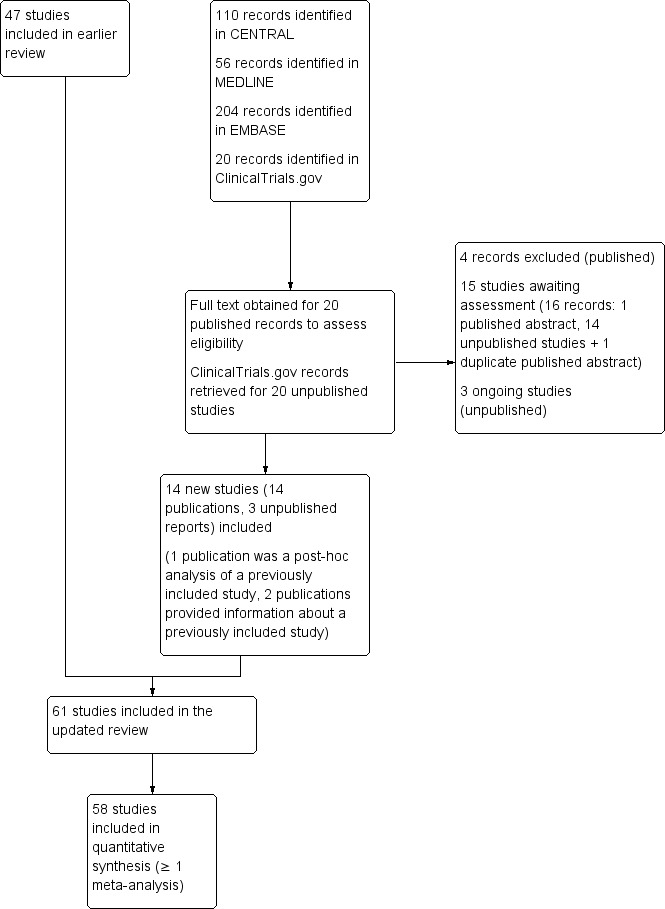
Study flow diagram.
We identified 14 new studies (11 publications, three unpublished reports) satisfying our inclusion criteria (Costantino 2011; Coudreuse 2010; González de Vega 2013; Hofman 2000; Klainguti 2010; Kuehl 2011; Li 2013; NCT01255423; NCT01272934; NCT01272947; Predel 2012; Predel 2013a; Predel 2013b; Saillant 1998), one post hoc analysis of a study that was included in the earlier review (Mueller 2010 in Predel 2004), and an additional publication and a pooled analysis that included new data for another study from the earlier review (Lionberger 2011 pooled analysis in Joussellin 2003).
Included studies
All except one of the new studies compared diclofenac with placebo. A number of different formulations were used, including diclofenac epolamine (DHEP) with or without heparin (Flectoparin Tissugel or Flector EP Tissugel) applied as a plaster (or patch), diclofenac diethylamine (DDEA) applied as a gel, and diclofenac with lethicin applied as a spray gel. The remaining study compared diclofenac gel with traumeel, a "fixed combination of plant and mineral extracts", applied as a gel or an ointment.
There were 47 studies in the original review; 14 new studies were included making a total of 61 studies in this updated review. All used a parallel group design. Forty‐four compared a topical NSAID with placebo, 13 a topical NSAID with an active comparator (a different topical NSAID, an oral NSAID, the same topical NSAID in a different formulation, or a compound of plant and mineral extracts), and four had both placebo and active comparators. In total, 5311 participants were treated with a topical NSAID, 3470 with placebo, and 220 with an oral NSAID. Topical NSAIDs used were benzydamine, diclofenac, etofenamate, felbinac, fentiazac, flunoxaprophen, flurbiprofen, ibuprofen, indomethacin, ketoprofen, ketorolac, lysine clonixinate, meclofenamic acid, naproxen, niflumic acid, and piroxicam. They were applied as creams, gels, sprays, foams, or plasters (patches). Topical placebos were the inert carriers, without the active NSAID. Oral NSAIDs used were ibuprofen (as tablets) and indomethacin (as capsules).
Most studies enrolled participants who had sprains, strains, and contusions, usually as a result of sports injuries, and treatment was started within a few hours or days. Other studies enrolled participants with overuse‐type injuries, such as tendinitis and acute low back pain, where pain had been present for days or weeks, but less than three months.
Participants were treated for at least five days, and up to three weeks, with most studies lasting seven to 14 days. Participants were usually assessed in clinic at intervals during treatment, and sometimes also at home using daily patient diaries. We used outcomes closest to seven days because many of these injuries are self limiting, with differences between active treatment and placebo being diminished or lost after longer intervals.
Most studies reported dichotomous outcomes suitable for a responder analysis, although group mean change (for pain or physical function, for example) was usually the primary outcomes. However, the definition of response varied both in the parameter measured (for example, pain, pain on movement, patient global evaluation of treatment), and in the scale used to measure it (for example, a 3‐, 4‐, or 5‐point scale for patient global evaluation).
Details of included studies are in the Characteristics of included studies table.
We identified 14 completed but apparently unpublished studies in a clinical trial registry for which no results have been posted (4403 participants, NCT00351104; NCT00352625; NCT00426985; NCT00640705; NCT00640939; NCT00680472; NCT00680784; NCT00765700; NCT00869063; NCT00869180; NCT00931866; NCT01874626; NCT01957215; NCT02324270). We have placed these under Characteristics of studies awaiting classification. We also identified three conference abstracts that relate to completed studies that do not appear to have been published, but that may satisfy our inclusion criteria. One is likely to be the same study as one of the included studies identified in a clinical trial registry (Pallay 2013 in NCT01272947), one relates to another study identified in the clinical trial registry that is awaiting classification (Ekman 2010 in NCT00765700), while we could find no published or unpublished reports of the other Sarzi‐Puttini 2014).
We also identified three ongoing studies with an estimated enrolment of 880 participants (NCT01945034; NCT02100670; NCT02290821). Details are in the Characteristics of ongoing studies table.
Excluded studies
For the original review, 25 studies were excluded after reading the full paper. For this update, we excluded four new studies (Cesarone 2008; Coulibaly 2009; Kuwabara 2013; Vinciguerra 2008). Details are in the Characteristics of excluded studies table.
Risk of bias in included studies
All studies were randomised and double‐blind. One study scored 2/5 (Sinniger 1981), 23 scored 3/5, 23 scored 4/5, and 14 scored 5/5 for methodological quality using the Oxford Quality Scale. A breakdown of the scores for individual studies is reported in the Characteristics of included studies table.
Comments on potential biases in individual studies are reported in the 'Risk of bias' section of the Characteristics of included studies table. The findings are displayed in Figure 2 and Figure 3.
2.
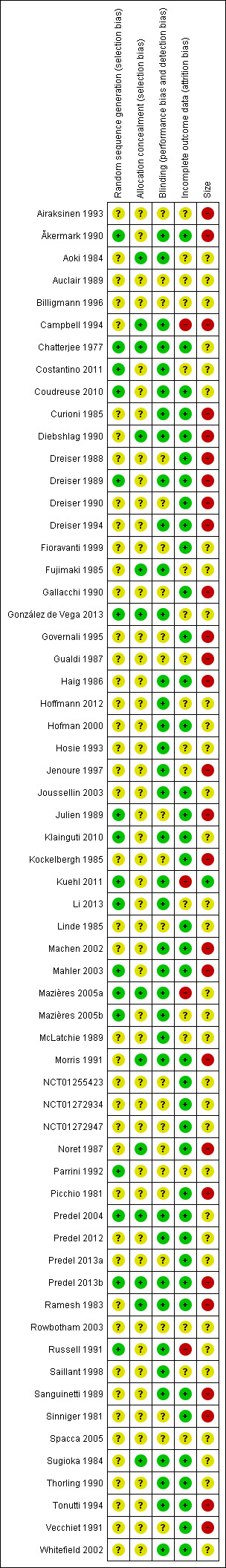
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
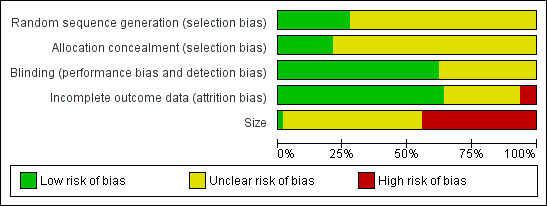
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Allocation
All the studies were randomised but only 17 adequately described the method used to generate the random sequence. Thirteen studies adequately described the method used to conceal allocation of the sequence. No studies were at high risk of bias for this item.
Blinding
All studies were double‐blind and 38 adequately described the method used to maintain the blinding. No studies were at high risk of bias for this item.
Incomplete outcome data
Thirty‐six studies included all participants in the primary analysis or provided sufficient data to allow missing participants to be included as non‐responders, and were judged at low risk of bias. We judged four studies to be at high risk of attrition bias (Campbell 1994; Kuehl 2011; Mazières 2005a; Russell 1991). Three unpublished studies contributed only to adverse event analyses and accounted for all participants for these outcomes (NCT01255423; NCT01272934; NCT01272947).
Other potential sources of bias
We judged one study that included more than 200 participants in each treatment arm to be at low risk of bias from size, but this study had a very high attrition rate (see 'Incomplete outcome data (attrition bias)') and did not report all the efficacy outcomes measured (Kuehl 2011). We judged 27 studies to be at high risk because they included fewer than 50 participants per treatment arm.
Effects of interventions
See: Table 1
Three studies did not contribute data suitable for analysis of at least one outcome (González de Vega 2013; Gualdi 1987; Hoffmann 2012).
1. Topical NSAID versus placebo
Details of efficacy outcomes in individual studies are in Appendix 4, and of adverse events and withdrawals in Appendix 5. Appendix 6 has details of the concentration of topical products, the amount applied, the frequency of application, and an estimation of the daily dose of topical NSAID applied. Not all studies provided sufficient information to allow calculation of daily dose applied. For example, for topical diclofenac, the estimated doses applied varied between about 60 and 280 mg; for topical ketoprofen 100 to about 450 mg; for topical ibuprofen 300 to 800 mg.
Participants with clinical success
Topical diclofenac versus placebo
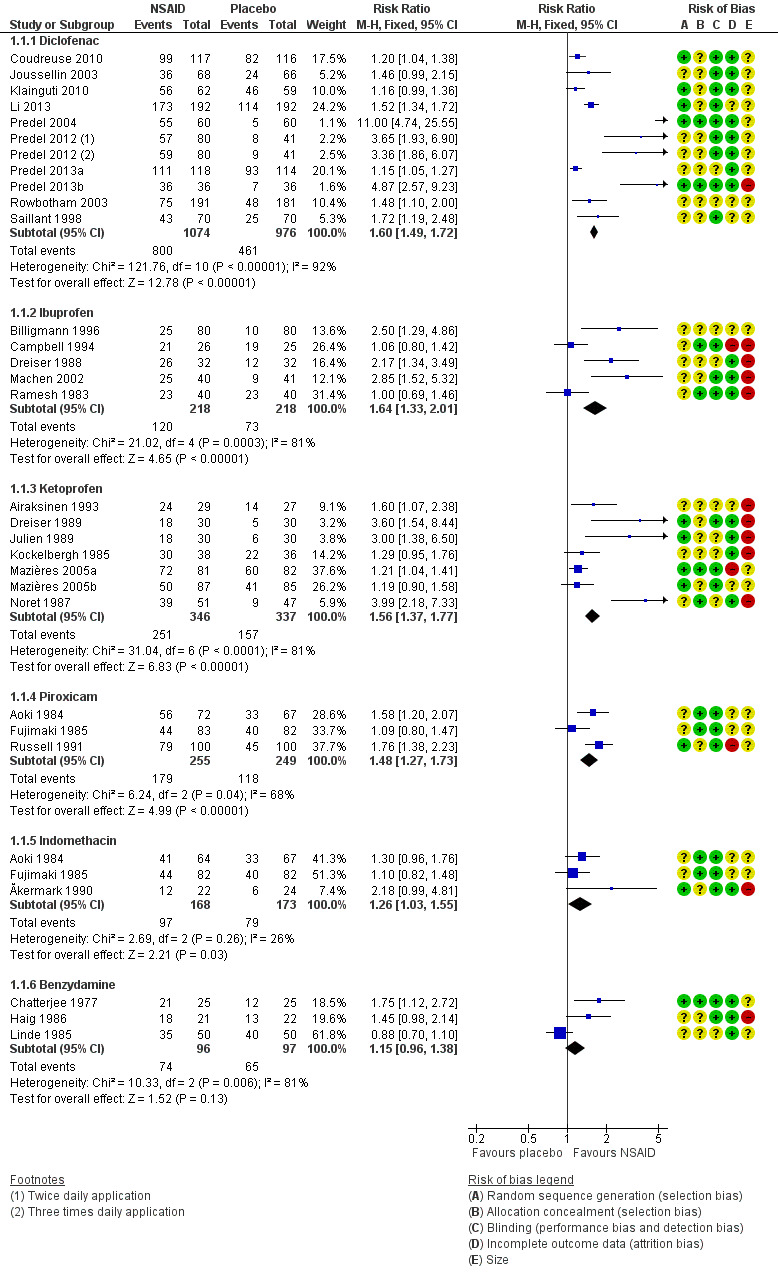
Ten studies contributed to this analysis (Coudreuse 2010; Joussellin 2003; Klainguti 2010; Li 2013; Predel 2004; Predel 2012; Predel 2013a; Predel 2013b; Rowbotham 2003), of which one (Predel 2012) had two active treatment arms. A total of 1074 participants were treated with topical diclofenac and 976 with placebo (Analysis 1.1; Figure 4).
1.1. Analysis.
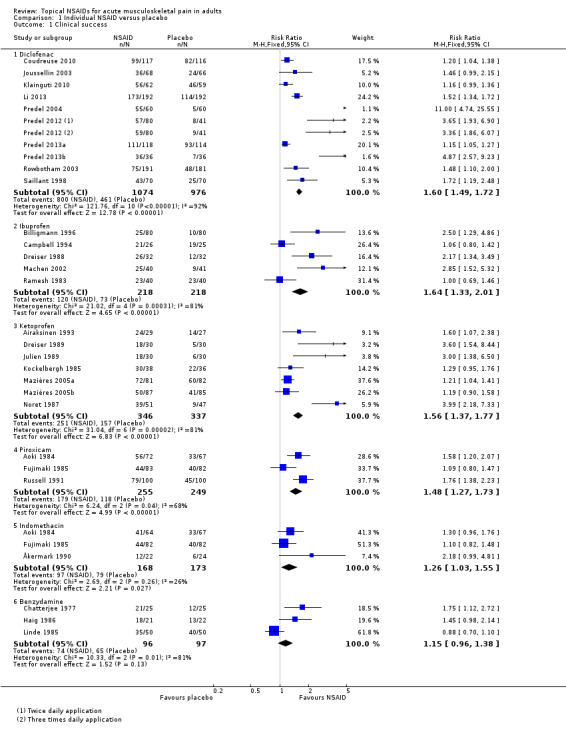
Comparison 1 Individual NSAID versus placebo, Outcome 1 Clinical success.
4.
Forest plot of comparison: 2 Individual NSAID versus placebo, outcome: 2.1 Clinical success.
The proportion of participants experiencing successful treatment with topical diclofenac was 74% (800/1074, range 39% to 100%).
The proportion of participants experiencing successful treatment with placebo was 47% (461/976, range 8% to 82%).
The RR for treatment compared with placebo was 1.6 (95% CI 1.5 to 1.7).
The NNT for successful treatment was 3.7 (3.2 to 4.3). For every four participants treated with topical diclofenac, one would experience successful treatment who would not have done so with placebo.
Effect of formulation
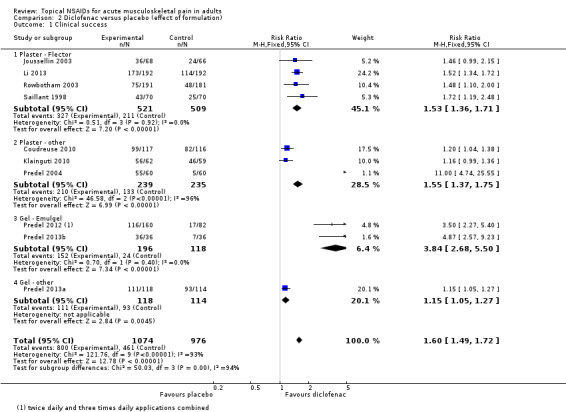
The effects of formulation are shown in Analysis 2.1 and Figure 5.
2.1. Analysis.
Comparison 2 Diclofenac versus placebo (effect of formulation), Outcome 1 Clinical success.
5.
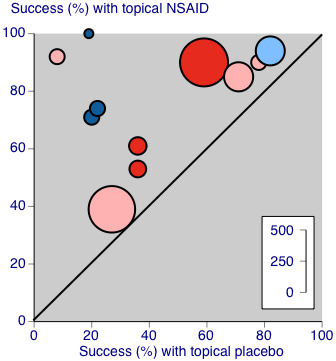
L'Abbé plot of clinical success in studies of topical diclofenac versus topical placebo. The size of the symbol is proportional to the size of the study (inset scale). Dark blue: Emulgel; light blue: spray/gel; red: Flector; pink: other patch or plaster.
Four studies used a Flector® plaster (1030 participants; Joussellin 2003; Li 2013; Rowbotham 2003; Saillant 1998). The RR for treatment compared with placebo was 1.5 (1.4 to 1.7), and the NNT was 4.7 (3.7 to 6.5).
Three studies used other makes of plaster (474 participants; Coudreuse 2010; Klainguti 2010; Predel 2004). The RR for treatment compared with placebo was 1.6 (1.4 to 1.8), and the NNT was 3.2 (2.6 to 4.2).
Two studies used Voltaren Emulgel (314 participants; Predel 2012; Predel 2013b). The RR for treatment compared with placebo was 3.8 (2.7 to 5.5), and the NNT was 1.8 (1.5 to 2.1).
One study used a spray gel (232 participants; Predel 2013a). The RR for treatment compared with placebo was 1.2 (1.05 to 1.3), and the NNT was 8.0 (4.8 to 24).
Diclofenac as the gel formulation Emulgel was statistically more efficacious than the plaster formulation as Flector plaster (z = 6.360; P value < 0.00001).
Topical ibuprofen versus placebo
Five studies contributed to this analysis (Billigmann 1996; Campbell 1994; Dreiser 1988; Machen 2002; Ramesh 1983). A total of 218 participants were treated with topical ibuprofen and 218 with placebo (Analysis 1.1).
The proportion of participants experiencing successful treatment with topical ibuprofen was 55% (120/218, range 31% to 81%).
The proportion of participants experiencing successful treatment with placebo was 33% (73/218, range 13% to 76%).
The RR of treatment compared with placebo was 1.6 (1.3 to 2.0).
The NNT for successful treatment was 4.6 (3.3 to 8.0). For every five participants treated with topical ibuprofen, one would experience successful treatment who would not have done so with placebo.
Effect of formulation
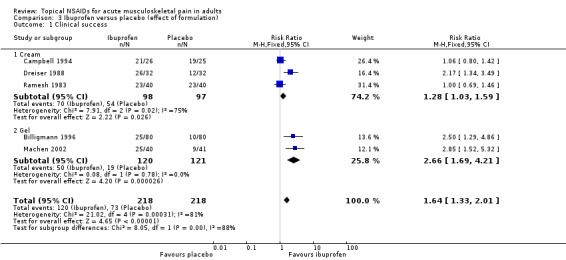
The effects of formulation are shown in Analysis 3.1.
3.1. Analysis.
Comparison 3 Ibuprofen versus placebo (effect of formulation), Outcome 1 Clinical success.
Three studies used cream formulations (195 participants; Campbell 1994; Dreiser 1988; Ramesh 1983). Although this is just below our threshold for pooled analysis, we have included this analysis for completeness and the results should be interpreted with caution. The RR for treatment compared with placebo was 1.3 (1.03 to 1.6), and the NNT was 6.4 (3.4 to 41).
Two studies used gel formulations (241 participants; Billigmann 1996; Machen 2002). The RR for treatment compared with placebo was 2.7 (1.7 to 4.2), and the NNT was 3.9 (2.7 to 6.7).
There was no statistically significant difference between the gel and cream formulations (z = 1.160, P value = 0.246).
Topical ketoprofen versus placebo
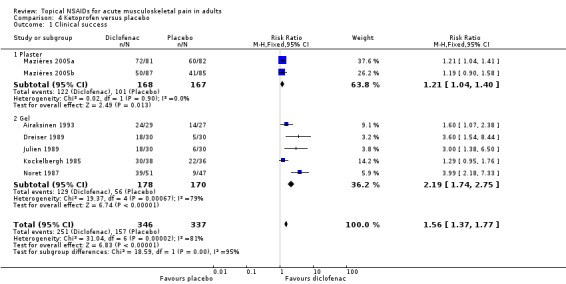
Seven studies contributed to this analysis (Airaksinen 1993; Dreiser 1989; Julien 1989; Kockelbergh 1985; Mazières 2005b; Mazières 2005a; Noret 1987). A total of 346 participants were treated with topical ketoprofen, and 337 with placebo (Analysis 1.1).
The proportion of participants experiencing successful treatment with topical ketoprofen was 73% (251/346, range 57% to 89%).
The proportion of participants experiencing successful treatment with placebo was 47% (157/337, range 17% to 73%).
The RR of treatment compared with placebo was 1.6 (1.4 to 1.8).
The NNT for successful treatment was 3.9 (3.0 to 5.3). For every four participants treated with topical ketoprofen, one would experience successful treatment who would not have done so with placebo.
Effect of formulation
The effects of formulation are shown in Analysis 4.1 and Figure 6.
4.1. Analysis.
Comparison 4 Ketoprofen versus placebo, Outcome 1 Clinical success.
6.
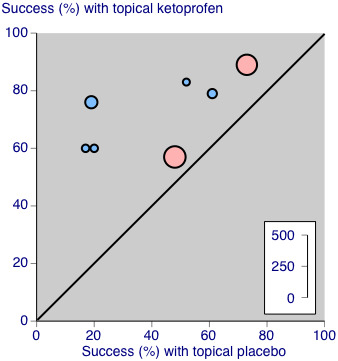
L'Abbé plot of clinical success in studies of topical ketoprofen versus topical placebo. The size of the symbol is proportional to the size of the study (inset scale). Light blue: ketoprofen gel; pink: ketoprofen plaster.
Two studies used a plaster formulation (335 participants; Mazières 2005b; Mazières 2005a). The RR for treatment compared with placebo was 1.2 (1.04 to 1.4), and the NNT was 8.2 (4.5 to 47).
Five studies used gel formulations (348 participants; Airaksinen 1993; Dreiser 1989; Julien 1989; Kockelbergh 1985; Noret 1987). The RR for treatment compared with placebo was 2.2 (1.7 to 2.8), and the NNT was 2.5 (2.0 to 3.4).
Ketoprofen as a gel formulation was statistically more efficacious than a plaster formulation (z = 3.860, P value = 0.00014).
Topical piroxicam versus placebo
Three studies contributed to this analysis (Aoki 1984; Fujimaki 1985; Russell 1991). A total of 255 participants were treated with topical piroxicam, and 249 with placebo (Analysis 1.1).
The proportion of participants experiencing successful treatment with topical piroxicam was 68% (179/255, range 53% to 79%).
The proportion of participants experiencing successful treatment with placebo was 47% (118/249, range 45% to 49%).
The RR of treatment compared with placebo was 1.5 (1.3 to 1.7).
The NNT for successful treatment was 4.4 (3.2 to 6.9). For every four participants treated with topical piroxicam, one would experience successful treatment who would not have done so with placebo.
Topical indomethacin versus placebo
Three studies contributed to this analysis (Ăkermark 1990; Aoki 1984; Fujimaki 1985). A total of 168 participants were treated with topical indomethacin, and 173 with placebo (Analysis 1.1).
The proportion of participants experiencing successful treatment with topical indomethacin was 58% (97/168, range 54% to 64%).
The proportion of participants experiencing successful treatment with placebo was 46% (79/173, range 25% to 49%).
The RR of treatment compared with placebo was 1.3 (1.03 to 1.6).
The NNT for successful treatment was 8.3 (4.4 to 65). For every eight participants treated with topical indomethacin, one would experience successful treatment who would not have done so with placebo.
Topical benzydamine versus placebo
Three studies contributed to this analysis (Chatterjee 1977; Haig 1986; Linde 1985). A total of 96 participants were treated with topical benzydamine, and 97 with placebo (Analysis 1.1). Although this is just below our threshold for pooled analysis, we have included this analysis for completeness and the results should be interpreted with caution.
The proportion of participants experiencing successful treatment with topical benzydamine was 77% (74/96, range 70% to 86%).
The proportion of participants experiencing successful treatment with placebo was 67% (65/97, range 48% to 80%).
The RR of treatment compared with placebo was 1.2 (0.96 to 1.4). There was no statistically significant difference between treatments (Figure 4).
Results for participants with clinical success with individual topical NSAIDs, where there were adequate data for analysis, are summarised below in 'Summary of results A' and Analysis 1.1, Analysis 2.1, Analysis 3.1, and Analysis 4.1.
| Summary of results A: Participants with clinical success | ||||||
| Comparison | Studies | Participants |
NSAID (%) |
Placebo (%) | Relative benefit (95% CI) | NNT (95% CI) |
| Diclofenac ‐ Flector plaster | 4 | 1030 | 63 | 41 | 1.5 (1.4 to 1.7) | 4.7 (3.7 to 6.5) |
| Diclofenac ‐ other plaster | 3 | 474 | 88 | 57 | 1.6 (1.4 to 1.8) | 3.2 (2.6 to 4.2) |
| Diclofenac ‐ Emulgel | 2 | 314 | 78 | 20 | 3.8 (2.7 to 5.5) | 1.8 (1.5 to 2.1) |
| Diclofenac ‐ other gel* | 1 | 232 | 94 | 82 | 1.2 (1.1 to 1.3) | 8.0 (4.8 to 24) |
| Ibuprofen ‐ cream* | 3 | 195 | 71 | 56 | 1.3 (1.03 to 1.6) | 6.4 (3.4 to 41) |
| Ibuprofen ‐ gel | 2 | 241 | 42 | 16 | 2.7 (1.7 to 4.2) | 3.9 (2.7 to 6.7) |
| Ketoprofen ‐ plaster | 2 | 335 | 73 | 60 | 1.2 (1.04 to 1.4) | 8.2 (4.5 to 47) |
| Ketoprofen ‐ gel | 5 | 348 | 72 | 33 | 2.2 (1.7 to 2.8) | 2.5 (2.0 to 3.4) |
| Piroxicam | 3 | 504 | 70 | 47 | 1.5 (1.3 to 1.7) | 4.4 (3.2 to 6.9) |
| Indomethacin | 3 | 341 | 58 | 46 | 1.3 (1.03 to 1.6) | 8.3 (4.4 to 65) |
| Benzydamine* | 3 | 193 | 77 | 67 | 1.2 (0.96 to 1.4) | not calculated |
* Results for these two comparisons are derived from very small amounts of data and are provided here for completeness. They should be interpreted with caution.
Local adverse events
Local adverse events were irritation of the area to which the topical NSAID was applied, including redness or erythema and itch or pruritus. Where reported, these were usually described as mild and transient.
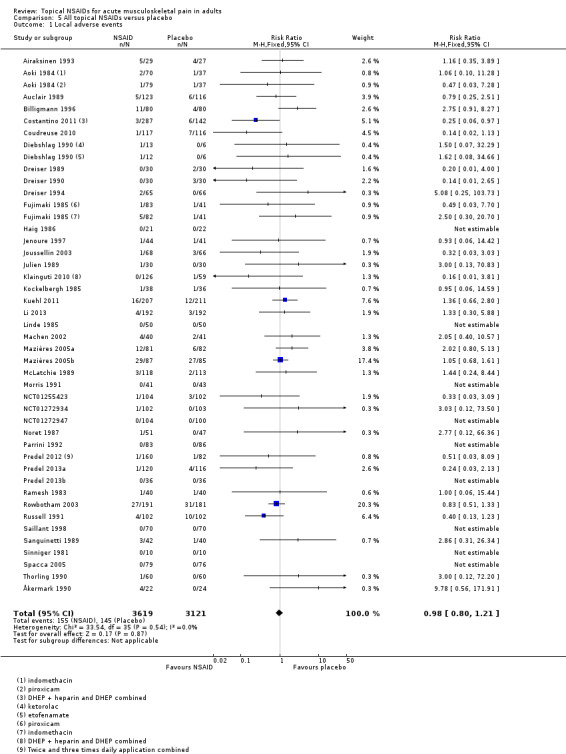
All topical NSAIDs versus placebo
Forty‐two studies contributed to this analysis, of which three compared two different drugs with placebo (Aoki 1984; Diebshlag 1990; Fujimaki 1985). Three studies had two treatment arms comparing different formulations or application regimens for diclofenac with placebo, which have been combined for this analysis (Costantino 2011; Klainguti 2010; Predel 2012). In total, 3619 participants were treated with topical NSAIDs and 3121 with placebo (Analysis 5.1).
5.1. Analysis.
Comparison 5 All topical NSAIDs versus placebo, Outcome 1 Local adverse events.
The proportion of participants experiencing a local adverse event with a topical NSAID was 4.3% (155/3619, range 0% to 33%).
The proportion of participants experiencing a local adverse event with placebo was 4.6% (145/3121, range 0% to 32%).
The RR of topical NSAID compared with placebo was 0.98 (0.80 to 1.2).
There was no significant difference between treatment groups so the NNH was not calculated.
Individual topical NSAIDs versus placebo
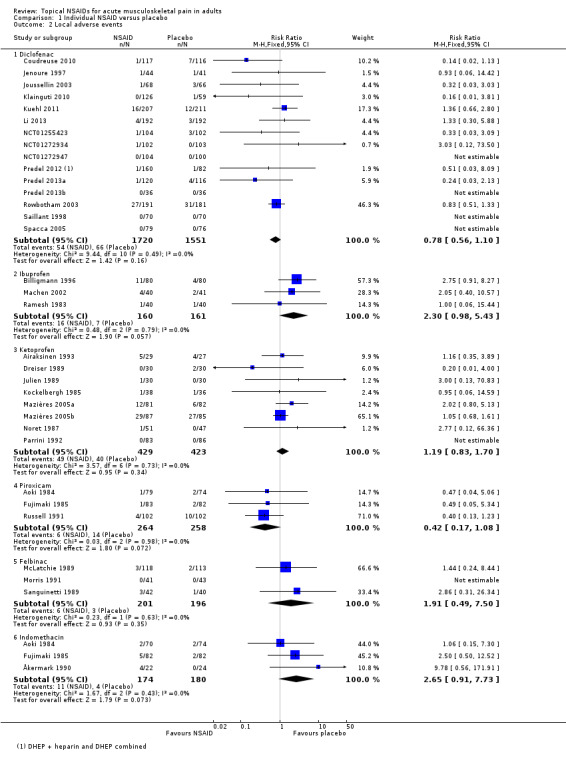
Results for local adverse events with individual topical NSAIDs, where there were adequate data for analysis, are in Summary of results B and Analysis 1.2.
1.2. Analysis.
Comparison 1 Individual NSAID versus placebo, Outcome 2 Local adverse events.
| Summary of results B: Participants with local adverse events | ||||||
| Comparison | Studies | Participants |
NSAID (%) |
Placebo (%) |
RR (95% CI) |
NNH (95% CI) |
| All NSAIDs | 42 | 6740 | 4.3 | 4.6 | 0.98 (0.80 to 1.2) | Not calculated |
| Diclofenac | 15 | 3271 | 3.1 | 4.3 | 0.78 (0.56 to 1.1) | Not calculated |
| Ketoprofen | 8 | 852 | 11 | 9.5 | 1.2 (0.83 to 1.7) | Not calculated |
| Piroxicam | 3 | 522 | 2.3 | 5.4 | 0.42 (0.17 to 1.1) | Not calculated |
| Felbinac | 3 | 397 | 3.0 | 1.5 | 1.9 (0.49 to 7.5) | Not calculated |
| Indomethacin | 3 | 354 | 6.3 | 2.2 | 2.7 (0.91 to 7.7) | Not calculated |
| Ibuprofen | 3 | 321 | 10 | 4.3 | 2.3 (0.98 to 5.4) | Not calculated |
Systemic adverse events
All topical NSAIDs versus placebo
Thirty‐six studies contributed data on systemic adverse events, of which three compared two different drugs with placebo (Aoki 1984; Diebshlag 1990; Fujimaki 1985). Two studies had two treatment arms comparing different formulations or application regimens for diclofenac with placebo, which have been combined for this analysis (Klainguti 2010; Predel 2012). In total, 2956 participants were treated with a topical NSAID and 2620 with placebo (Analysis 5.2).
5.2. Analysis.
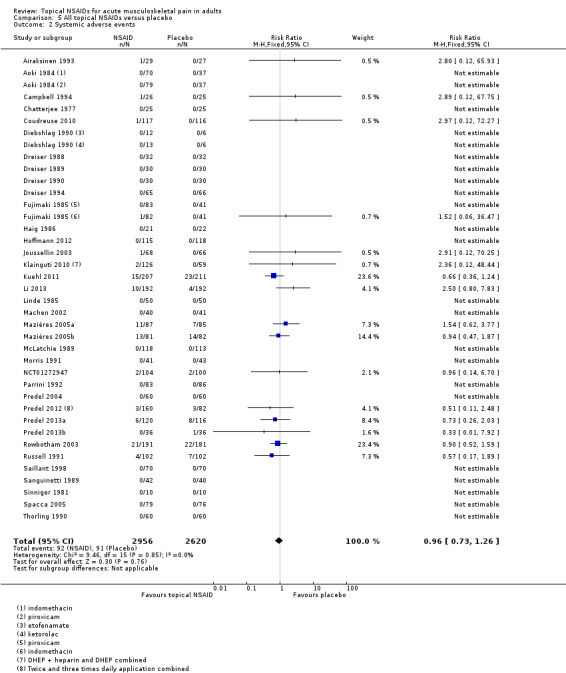
Comparison 5 All topical NSAIDs versus placebo, Outcome 2 Systemic adverse events.
Twenty‐three studies reported no systemic adverse events in any arm of the study.
The proportion of participants experiencing a systemic adverse event with a topical NSAID was 3.1% (92/2956).
The proportion of participants experiencing a systemic adverse event with placebo was 3.5% (91/2620).
The RR of topical NSAID compared with placebo was 0.96 (0.73 to 1.3).
There was no significant difference between treatment groups so the NNH was not calculated.
A further six studies did not report the occurrence or otherwise of systemic adverse events (Billigmann 1996; Julien 1989; Kockelbergh 1985; Noret 1987; Ramesh 1983; Vecchiet 1991), while two studies did not report numbers of participants with systemic adverse events (Ăkermark 1990; Auclair 1989). Costantino 2011 reported that there were no systemic gastrointestinal adverse events. Two studies reported only on total adverse events, without distinguishing between local and systemic events (NCT01255423; NCT01272934).
Serious adverse events
Two studies reported serious adverse events. In Hoffmann 2012, one participant experienced three serious adverse events, none of which was judged to be related to the study medication (diclofenac plaster). In NCT01272934, one participant using diclofenac gel ruptured the ligaments of the wrist. There was no statement about likely relationship to the study medication, but this seems unlikely.
Adverse event withdrawals
Forty‐two studies reported data relating to adverse event withdrawals, of which three compared two different drugs with placebo (Aoki 1984; Diebshlag 1990; Fujimaki 1985). Two studies had two treatment arms comparing different formulations or application regimens for diclofenac with placebo, which have been combined for this analysis (Klainguti 2010; Predel 2012). In total, 3365 participants received a topical NSAID and 3040 placebo (Analysis 5.3).
5.3. Analysis.
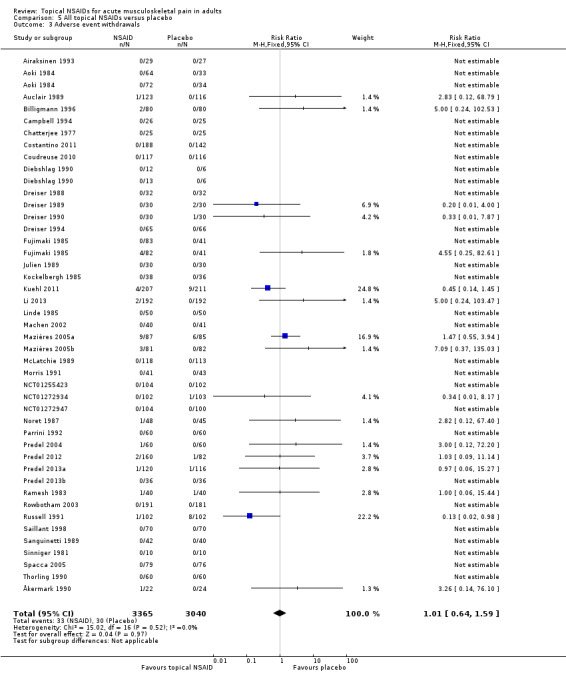
Comparison 5 All topical NSAIDs versus placebo, Outcome 3 Adverse event withdrawals.
Forty‐four comparisons reported no adverse event withdrawals.
The proportion of participants withdrawing from the study due to an adverse event after treatment with a topical NSAID was 0.98% (33/3365).
The proportion of participants withdrawing from the study due to an adverse event after treatment with placebo was 0.99% (30/3040).
The RR of topical NSAID compared to placebo was 1.0 (0.64 to 1.6).
There was no significant difference between treatment groups so the NNH was not calculated.
Four studies did not specifically mention adverse event withdrawals (Haig 1986; Hoffmann 2012; Klainguti 2010; Vecchiet 1991), while one reported that one participant withdrew with mild pruritus, but did not state the treatment arm (Joussellin 2003).
Ten studies specifically reported withdrawals due to lack of efficacy (Dreiser 1989; Dreiser 1994; Kuehl 2011; Machen 2002; Mazières 2005b; Mazières 2005a; Noret 1987; Predel 2013a; Russell 1991; Thorling 1990) (Appendix 5). Numbers of participants withdrawing were generally low, with rates of 6% or less, except in Kuehl 2011, where the rate was 10% with active treatment (diclofenac plaster) and 12% with placebo. We did not carry out any analysis because the outcome was inconsistently reported.
2. Topical NSAID versus active comparator
Details of efficacy outcomes in individual studies are in Appendix 4, and of adverse events and withdrawals in Appendix 5.
Participants with clinical success
Topical NSAID versus oral NSAID
Ăkermark 1990 compared indomethacin spray with indomethacin capsules, with response rates of 55% (12/22) with spray and 23% (5/22) with capsules.
Hosie 1993 compared felbinac foam with ibuprofen tablets, with response rates of 64% (81/127) with felbinac foam and 72% (96/133) with ibuprofen tablets.
Whitefield 2002 compared ibuprofen gel with ibuprofen tablets, with response rates of 60% (30/50) with gel and 54% (36/50) with tablets.
There were insufficient data for meta‐analysis for any one of these comparisons; felbinac is not known to be better than placebo.
Topical NSAID versus different formulation of the same topical NSAID
Fioravanti 1999 compared DHEP (diclofenac) gel formulated with and without lecithin, with response rates of 70% (35/50) in both treatment arms.
Mahler 2003 compared DHEP (diclofenac) gel formulated with and without lecithin, with response rates of 89% (82/92) with lecithin and 70% (62/88) without lecithin.
Gallacchi 1990 compared topical diclofenac formulated as Flector® gel and Emugel®, with response rates of 76% (19/25) in both treatment arms
Governali 1995 compared topical ketoprofen cream with gel, with response rates of 93% (14/15) with cream and 27% (4/15) with gel.
There were insufficient data for analysis.
Topical NSAID versus different topical NSAID
Eight studies compared one topical NSAID versus at least one other topical NSAID: piroxicam versus indomethacin (Aoki 1984; Fujimaki 1985; Sugioka 1984), ibuprofen versus ketoprofen (Curioni 1985; Picchio 1981), ketoprofen versus etofenamate (Curioni 1985; Tonutti 1994), ibuprofen versus etofenamate (Curioni 1985), ketorolac versus etofenamate (Diebshlag 1990), and diclofenac versus lysine clonixinate (Hofman 2000).
There were sufficient data to compare only piroxicam with indomethacin (Aoki 1984; Fujimaki 1985; Sugioka 1984; Analysis 6.1).
6.1. Analysis.
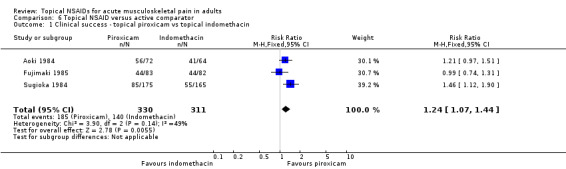
Comparison 6 Topical NSAID versus active comparator, Outcome 1 Clinical success ‐ topical piroxicam vs topical indomethacin.
The proportion of participants experiencing clinical success with topical piroxicam was 56% (185/330, range 49% to 78%).
The proportion of participants experiencing clinical success with topical indomethacin was 45% (140/311, range 33% to 64%).
The RR of piroxicam compared with indomethacin was 1.2 (1.1 to 1.4).
The NNT for successful treatment was 9.1 (5.3 to 30). For every nine participants treated with topical piroxicam, one would experience a clinical success who would not have experienced one with topical indomethacin.
Topical NSAID versus different topical intervention
One study compared diclofenac gel with a herbal product called Traumeel gel under double‐blind conditions, with response rates for being pain‐free at seven days of 8/137 (5.8%) with diclofenac gel and 7/140 (5.0%) with Traumeel gel (González de Vega 2013).
Local adverse events
Topical NSAID versus oral NSAID
Two studies comparing a topical NSAID with an oral NSAID provided data on local adverse events (Ăkermark 1990; Hosie 1993). There were five events with topical NSAID and three with oral NSAID, which were too few for analysis.
Topical NSAID versus different topical NSAID
All nine studies comparing one topical NSAID with at least one other reported on local adverse events, with a total of 48 events in 1005 participants (4.8%). There were sufficient data to compare only piroxicam with indomethacin (Aoki 1984; Fujimaki 1985; Sugioka 1984; Analysis 6.2).
6.2. Analysis.
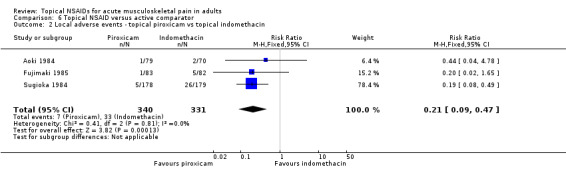
Comparison 6 Topical NSAID versus active comparator, Outcome 2 Local adverse events ‐ topical piroxicam vs topical indomethacin.
The proportion of participants experiencing local adverse events with topical piroxicam was 2.1% (7/340, range 1.2% to 2.8%).
The proportion of participants experiencing local adverse events with topical indomethacin was 10% (33/331, range 2.9% to 15%).
The RR of piroxicam compared with indomethacin was 0.21 (0.09 to 0.47).
The NNT to prevent a local adverse event was 13 (8.7 to 23). For every thirteen participants treated with topical piroxicam, one would not experience a local adverse event who would have experienced one with topical indomethacin.
Topical NSAID versus different topical intervention
González de Vega 2013 reported that adverse events were infrequent and mild to moderate in intensity, but did not distinguish between local and systemic events. Numbers of participants experiencing any adverse event were 8/147 with diclofenac gel and 14/148 with Traumeel gel.
Systemic adverse events
Ăkermark 1990 reported numbers of events, rather than numbers of participants with events, while Tonutti 1994 and Whitefield 2002 reported no adverse events attributable to the study medication, and Fioravanti 1999, Gallacchi 1990, Gualdi 1987, and Sugioka 1984 did not mention systemic adverse events. González de Vega 2013 did not distinguish between local and systemic events. In the remaining studies a total of 16 events were reported in topical NSAID treatment arms (797 participants, 2%) and 11 with ibuprofen tablets (134 participants, 8%).
Serious adverse events
No serious adverse events were reported in any treatment arm.
Withdrawals
The only withdrawals reported due to adverse events were in studies with placebo treatment arms (Ăkermark 1990; Fujimaki 1985), and have been reviewed.
Three studies reported withdrawals due to lack of efficacy (González de Vega 2013; Hofman 2000; Tonutti 1994) (Appendix 5). There were insufficient data for analysis.
Some studies reported exclusions from analysis (efficacy or safety, or both) following randomisation, mainly due to protocol violations or loss to follow‐up (Appendix 5). There is no reason to believe these exclusions would introduce systematic bias, and the numbers involved were not likely to influence results.
Discussion
This updated review of topical NSAIDs for acute musculoskeletal pain in adults differs from previous reviews. Previously, data allowed only for comparison of individual topical NSAIDs with placebo, irrespective of formulation. With a substantial amount of new data for diclofenac, it is possible to distinguish effects of formulation for individual NSAIDs. Because formulation chemistry can substantially affect the rate and total amount of drug accessing subcutaneous injured tissues, the effect of formulation may be as important as the individual NSAID used. Drug and formulation should thus be considered together when assessing efficacy, and this is now possible.
We have also included an assessment of the daily dose of NSAID applied to the skin. This involved having information on the concentration of NSAID in the preparation, the amount used, and the frequency of use. Not all studies reported all three, but an estimation of topical doses applied was possible. It varied by factors of three or four for each topical NSAID. However, for topical formulations, the key issue is less the dose applied but the amount that penetrates locally (producing analgesic effect) and the amount entering the systemic circulation (producing potential harms). Both will depend on the exact formulation of the topical agent, and whether there is occlusion (Moore 2008a).
Summary of main results
This review included 61 studies comparing a topical NSAID with placebo, another topical NSAID, or an oral NSAID. In total, 5311 participants were treated with a topical NSAID, 3470 with placebo, and 220 with an oral NSAID. There were 63% more participants than in the previous version of this review. Conditions treated were sprains, strains, and contusions, mainly resulting from sports injuries, and overuse injuries such as tendinitis.
Formulations of topical diclofenac, ibuprofen, ketoprofen, piroxicam, and indomethacin demonstrated significantly higher rates of clinical success than matching topical placebo lacking the NSAID; benzydamine did not. Three drug and formulation combinations had NNTs for clinical success below 4. For diclofenac, Emulgel® had the lowest NNT of 1.8 (1.5 to 2.1) in two studies using at least 50% pain intensity reduction as the outcome (high quality evidence). Diclofenac plasters other than Flector® also had a low NNT of 3.2 (2.6 to 4.2) based on good or excellent responses in relatively recent studies (high quality evidence). Ketoprofen gel had an NNT of 2.5 (2.0 to 3.4) from five studies in the 1980s, some with less well defined outcomes (moderate quality evidence). Ibuprofen gel had an NNT of 3.9 (2.7 to 6.7) from two studies with outcomes of marked improvement or complete remission (moderate quality evidence). All other drug and formulation combinations had NNT values above 4, indicating lesser efficacy.
These results are better than alternative topical products that might be used for acute musculoskeletal pain. There is no evidence to support the use of topical salicylate rubefacients (Derry 2014).
Treatment with a topical NSAID was not associated with an increase in local adverse events (skin reactions) compared with placebo (inert carrier), or in withdrawals due to adverse events (high quality evidence). The inert carrier was sometimes associated with mild skin irritation, but this rarely led to cessation of treatment, and quickly resolved. Systemic adverse events were uncommon and did not differ between topical NSAID and placebo (high quality evidence). Two participants experienced serious adverse events with diclofenac plaster and diclofenac gel, but it is unlikely that these were related to the study medications.
There were insufficient data directly comparing a topical NSAID with the same oral NSAID to draw conclusions about efficacy. Based on very limited data for oral NSAIDs, there were fewer systemic adverse events with topical than oral treatment. There were sufficient data only for topical piroxicam compared with topical indomethacin to compare one topical agent with another. These limited data suggested that piroxicam was more effective than indomethacin, and was less likely to cause local adverse events. It is worth noting here that topical indomethacin did not give significantly better pain relief than placebo in two of the three studies in this analysis.
Overall completeness and applicability of evidence
There is a tension between pooling studies to produce analyses with larger numbers and the subsequent large increases in clinical and statistical heterogeneity on the one hand, and using the approach of clinical homogeneity with subsequent smaller numbers of participants on the other hand. Previous reviews have taken the former approach; that is useful in demonstrating that topical NSAIDs 'work' by being significantly better than placebo. Because of the substantial increase in the amount of data available, in this review we have chosen to seek greater clinical homogeneity; this produces results that are more relevant to patient and prescriber choice.
There were too few studies comparing one topical NSAID versus another, or versus the same oral NSAID, to allow meaningful direct comparisons between individual drugs or routes of administration.
The conditions treated in these studies are representative of those likely to be suitable for acute treatment with topical NSAIDs. The mean age of participants in individual studies ranged from 25 to 57 years, and the nature of recruitment in many studies meant that participants were actively engaged in sporting activities. Nevertheless, older people in their 60s to 80s were also included in some studies, and the low levels of predominantly mild adverse events means that this route of administration of NSAIDs is suitable for all age groups able to manage the application process.
Information from other sources, mainly randomised studies lasting 12 weeks or more in older populations with arthritis, tend to confirm this. A systematic review of topical NSAIDs in older adults was difficult to interpret, but suggested that the range of withdrawal rates in these studies was similar with topical and oral NSAIDs (Makris 2010). It also claimed potentiation of warfarin effects, but that was with topical salicylate, not an NSAID. In contrast, a pooled safety analysis of topical diclofenac in people aged 75 years or older reported minimal changes, with a mean reduction in haemoglobin of less than 1 g/L with topical diclofenac, and a mean systolic blood pressure reduction of almost 4 mm Hg (Roth 2012). Two large randomised 12‐week studies comparing topical with oral diclofenac in arthritis reported lower rates of gastrointestinal adverse events with topical than oral, especially severe events, but larger reductions in haemoglobin with oral diclofenac (Simon 2009; Tugwell 2004).
The available evidence was limited by numbers to comment on rare but potentially serious adverse events. One example is the potential for photo‐sensitivity reactions with topical ketoprofen. Current advice from the Medicines and Healthcare products Regulatory Agency in the UK is to avoid direct sunlight, ultraviolet (UV) rays, sunlamps, and sunbeds while using topical ketoprofen, and to see a healthcare professional or go to hospital if they experience a skin reaction to sunlight, sunlamps, or sunbeds (MHRA 2009).
Quality of the evidence
All included studies were both randomised and double‐blind; none was considered at high risk of methodological bias. Many were carried out in the 1980s and 1990s when methodological rigor and detailed reporting were not given such high priority and studies did not always report details of the randomisation, treatment allocation, and blinding processes. More recent studies often did report methodological details, and tended to be larger (see Figure 4 for a comparison of quality of reporting for different dates and NSAIDs). Our primary outcome of clinical success was not always well‐defined, and was measured using different scales, but again more recent studies tended to report outcomes better.
The studies were conducted in different conditions, with somewhat different outcome definitions and duration, and with different topical NSAIDs and formulations. Moreover, the small size of many of the studies is likely to result in considerable chance variation (Counsell 1994; Moore 1998b). These factors would account for the high I2 values seen in several analyses. Despite these sources of potential clinical heterogeneity, most studies showed benefit of topical NSAID over placebo.
The design of studies to be able to demonstrate analgesic sensitivity is important in self limiting conditions such as strains and sprains. Too long a duration and the condition results in spontaneous resolution of painful symptoms, while too short a duration may be inadequate to show any effect. The decision by trialists to concentrate on outcomes closest to seven days of treatment appears to be prudent, and has been adopted in this and previous reviews. There are potential differences in response to treatment between strains and sprains and overuse‐type injuries such as tendinitis, and future reviews may examine this. At the present time, there are too few existing trials to explore any differences adequately.
Baseline pain may be a cause for concern. Seven studies did not report baseline pain levels (Billigmann 1996; Curioni 1985; Haig 1986; NCT01255423; NCT01272934; NCT01272947; Sinniger 1981), and a further 11 reported either mean levels of less than moderate pain or a significant proportion of individuals with less than moderate pain (Ăkermark 1990; Aoki 1984; Auclair 1989; Diebshlag 1990; Fujimaki 1985; Jenoure 1997; Linde 1985; Picchio 1981; Ramesh 1983; Sugioka 1984; Whitefield 2002), using recognised scales. Insufficient pain at baseline compromises the ability of a study to demonstrate any improvement. All the newly added studies reported baseline pain to be of at least moderate intensity.
Potential biases in the review process
There has been greater interest in topical NSAIDs in recent years, mainly because lower systemic drug levels reduce the risk of troublesome and severe adverse events, particularly in the gastrointestinal tract, and renal and cardiovascular systems. Most of the attention has been in chronic conditions such as osteoarthritis, with few studies in acute painful conditions. Low levels of serious adverse events with topical NSAIDs has been noted previously (Evans 1995), and the near absence of serious adverse events in this review is unlikely to be due to any biases in the review process.
One potential bias is that clinical trials for topical NSAIDs may not have been published. One previous review did find previously unpublished trials (Moore 1998a), but a subsequent attempt that included extensive contacts with pharmaceutical companies revealed no additional data (Mason 2004a). While some old unpublished studies of topical NSAIDs in acute painful conditions may exist, they constitute an unknown number of studies and participants whose results are unknown, and are likely to remain unknown. Furthermore, their relevance to current clinical practice may be limited as better formulations are developed. New systems of trial registration mean that we know what recent studies have been done or are ongoing; the number of studies and participants is known even if their results remain unknown. We identified in Clinicaltrials.gov three unpublished studies (612 participants) with adverse event data but no dichotomous efficacy data, 14 completed unpublished studies (4403 participants) with no results posted, and three ongoing studies (880 participants).
For the main topical NSAIDs of interest and where most information exists, about 4200 participants in this review provided data on efficacy for diclofenac, ibuprofen, ketoprofen, indomethacin, and benzydamine compared with placebo. For efficacy, there are unknown results from almost 5900 participants in studies known to have been done but essentially unpublished. Almost 6500 participants in this review provided information on local adverse events for topical NSAIDs compared with placebo. For local adverse events, the unknown results from known studies represents almost 5300 participants. It is clear that identified unpublished but unavailable study data amounts to a further potentially large increase in knowledge, over and above the 60% increase in numbers of participants already included in this updated review.
Based on efficacy data on known and available study results, unpublished trials showing no difference between any topical NSAID and topical placebo and involving 5500 participants would have to exist in order for the NNT to be as high as 9, at which point the effectiveness of topical NSAIDs would become clinically irrelevant (Moore 2006). This amount of unpublished negative data is obviously available, and while a negative result in all the identified studies is unlikely, knowledge would be greatly served by having these unpublished trial results available.
We have not yet attempted to obtain results from these clinical trials from the trial sponsors, because this takes a considerable amount of time and may not be successful. Moreover, the studies were spilt between nine different sponsors: Cerimon Pharmaceuticals (five studies), Novartis (four studies), Endo Pharmaceuticals (three studies), GlaxoSmithKline (two studies), Hisamatsu Pharmaceutical (two studies), and one each from Actavis, Pfizer, Imprimis Pharmaceutical, and Strategic Science & Technologies.
Agreements and disagreements with other studies or reviews
A review published in 2004 included some of the studies in this review and reported an NNT for all topical NSAIDs combined of 3.8 (3.4 to 4.4) for clinical success equivalent to half pain relief at seven days (Mason 2004a). That review found no difference between topical NSAID and placebo for local adverse events, as did this review. In turn, the Mason review was in broad agreement with the original systematic review on topical NSAIDs (Moore 1998a). To our knowledge, no previous review assembled sufficient trial data to analyse results by both drug and formulation, as was done here.
Authors' conclusions
Implications for practice.
For people with acute musculoskeletal pain
Topical NSAIDs can provide good levels of pain relief in acute conditions such as sprains, strains, and overuse injuries, probably similar to that provided by oral NSAIDs. Gel formulations of diclofenac (as Emulgel®), ibuprofen, and ketoprofen, and some diclofenac patches provide the best effects. Adverse events are usually minimal with topical NSAIDs.
For clinicians
Topical diclofenac, ibuprofen, or ketoprofen gels provide good pain relief for painful acute musculoskeletal conditions and are better tolerated than oral formulations. These drugs and formulations are more likely to be cost effective than alternative topical preparations such as topical rubefacients.
For policy makers
Topical NSAIDs are not associated with an increased incidence of local skin reactions compared with the inert carrier, and while the carrier may cause mild, transient irritation, it is rarely troublesome. Topical NSAIDs do not cause systemic (mainly gastrointestinal) problems commonly seen with oral NSAIDs, making them particularly useful for individuals unable to tolerate oral administration, or for whom it is contraindicated.
For funders
Topical diclofenac, ibuprofen, or ketoprofen gels should be considered for initial treatment of acute musculoskeletal painful conditions where there are no contraindications, such as damaged skin. These drugs and formulations are more likely to be cost effective than alternative topical preparations such as topical rubefacients.
Because formulations of topical NSAIDs are likely to change over time, the relevant trials performed and reported in or before the 1990s must be limited and may be questionable. Funders might wish to consider asking pharmaceutical companies without recent trial evidence for their products to produce it.
Implications for research.
General
The general thrust of these findings is that gel formulations of topical diclofenac, ibuprofen, and ketoprofen work best, but for some drugs (ketoprofen, for instance) studies were pre‐1990. These studies may not be relevant to products available now. Because formulation can have a significant effect on efficacy, formulation changes should be accompanied by relevant randomised trials.
Design
The design of the trials is generally good, and the sports injury model appears to be reliable and reproducible. Modern studies have ensured that participants entering the trials have at least moderate pain, and this helps sensitivity to detect an analgesic response. Major changes to the design of these trials would not appear to be needed.
Measurement (endpoints)
A major issue is not in the measurement of pain, as most studies, especially modern studies, have used standard pain intensity and pain relief scales. However, reporting of average pain changes is inadequate, and the use of responder analyses (at least 50% pain intensity reduction, or people experiencing mild or no pain) is preferred.
Comparison between active treatments
Indirect comparisons with placebo are probably as informative as use of an active comparator.
Feedback
Query on formulations of topical NSAIDs, particularly DMSO from Dr Chrubasik, 11 April 2012
Summary
Dr Chrubasik highlighted this letter to the Editor: postgradmed.org/doi/10.3810/pgm.2011.09.2482. DMSO [dimethyl sulphoxide] but also other additives, e.g. nonivamide (which is a capsaicinoid, added as drug enhancer) may contribute to the overall effect of topical NSAIDs. Nonivamide certainly contributes to the analgesic effect and to adverse events (heat sensation, burning, pruritus etc.). This has not been considered in the Cochrane review by: Massey T, Derry S, Moore RA, McQuay HJ. Topical NSAIDs for acute pain in adults, but Dr Chrubasik believes should be done, otherwise the effect size of the NSAID topicals is favoured.
Reply
We have been asked by Dr Chrubasik to comment on a letter (Roth 2011) about the formulations of topical NSAIDs, particularly how DMSO and other penetration enhancers can affect efficacy estimates or adverse event reporting in osteoarthritis. It was suggested that the review of Topical NSAIDs for acute pain in adults did not consider this, resulting in a bias towards the topical NSAID.
There are a number of points to be made here:
Penetration enhancers are used in formulations of topical products to encourage local absorption through the skin and produce a high local concentration. Topical NSAIDs use penetration enhancers, and the result is high local concentration in joints, for instance, but low systemic concentrations (Moore 2008). That is how they work. Formulation is an important part of medicinal chemistry as a whole, not just for topical agents.
In our analysis of topical NSAIDs we were aware that a range of properties are or have been ascribed to the analgesia resulting from application of topical agents, and which could contribute to overestimation of treatment effect of topical NSAID. These include feelings of heat or cold, and even the act of rubbing itself. For that reason we have chosen to include only double‐blind studies where the placebo agent is identical to the active, with the exception, of course, of the NSAID. So heat, cold, rubbing, and penetration enhancers should be identical, as best we can judge. That leaves only the NSAID itself to provide any additional analgesic effect, and it is that which we measure. This is analogous, for example, to use of acupuncture, say, where the better studies show no difference between “true” acupuncture and “sham” acupuncture performed at nonspecific sites, but better than non‐treatment controls. The argument that we should only use high quality studies to evaluate evidence about pain interventions is well made.
Overestimation of analgesic effect because of effects of enhancers themselves would be better made in direct comparisons of topical and oral NSAIDs, where local or even systemic effects would not be balanced in the oral study arm. However, our review concentrated on placebo‐controlled studies, and had few studies with active controls. Moreover, the real test would be in chronic rather than acute conditions, with long duration (12 week) outcomes using current best evidence rules (Moore 2010), including imputation (Moore 2012). In their response to Roth’s letter, the authors of the original review of products available in the USA show rather similar effect sizes of oral diclofenac and topical diclofenac with different penetration enhancers (Barthel 2011) in such studies.
The Roth letter sought to differentiate between topical diclofenac preparations based on the penetration enhancers used. That different formulations may have different effect sizes is a fair point to make. Two of the studies in our review of topical NSAIDs in acute conditions used diclofenac sodium 1% gel, comparing it with either diclofenac epolamine gel (Gallacchi 1990; 50 participants) or lysine clonixinate gel (Hofman 2000; 142 participants); no difference between formulations was demonstrated. It is difficult to make any judgement for topical NSAIDs in acute conditions due to the relatively small number and particularly the small size of studies. We did an analysis by drug, and this showed that some topical NSAIDs were consistently beneficial, irrespective of formulation, while others had little or no efficacy. This fits in with some theoretical considerations of molecular architecture and tissue penetration (Moore 2008). In chronic pain, where there are larger studies and much more data, we have considered formulation (Derry, in preparation).
References:
Roth SH. Letter to the editor: The importance of differentiating between topical NSAIDs. Postgraduate Medicine 2011;123:251‐2. [10.3810/pgm.2011.09.2482]
Moore RA, Derry S, McQuay HJ. Topical agents in the treatment of rheumatic pain. Rheumatic Diseases Clinics of North America 2008;34:415‐32.
Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al; ACTINPAIN Writing Group of the IASP Special Interest Group on Systematic Reviews in Pain Relief; Cochrane Pain, Palliative and Supportive Care Systematic Review Group Editors. "Evidence" in chronic pain ‐ establishing best practice in the reporting of systematic reviews. Pain 2010;150:386‐9.
Moore RA, Straube S, Eccleston C, Derry S, Aldington D, Wiffen P, et al. Estimate at your peril: imputation methods for patient withdrawal can bias efficacy outcomes in chronic pain trials using responder analyses. Pain 2012;153:265‐8.
Barthel HR, Axford‐Gatley RA. Response to Roth Letter to the Editor. Postgraduate Medicine 2011;123:253‐4. [10.3810/pgm.2011.09.2483]
Contributors
Feedback from Sigrun Chrubasik.
Authors involved with responding: Andrew Moore, Sheena Derry.
Feedback Editor: Kate Seers.
Query submitted by Peter C Gøtzsche, 3 November 2015
Summary
Date of Submission: 03‐Nov‐2015
Name: Peter C Gøtzsche
Email Address: pcg@cochrane.dk
Affiliation: Nordic Cochrane Centre
Role: Director
Comment: The authors found that the results were missing from 5900 patients. Furthermore, there was extreme heterogeneity in their meta‐analyses, e.g. I square was 92% for the diclofenac trials, which were the most common ones, and there was extreme funnel plot asymmetry, with the largest trials showing the smallest effects (the authors didn’t show funnel plots but I constructed one for diclofenac). Moreover, the trials were industry funded, of relatively poor quality, and the authors analysed published data, not data from clinical study reports, and did not try to obtain all the missing trials and data from the manufacturers.
The authors concluded that topical NSAIDs are effective in providing pain relief but also cautioned that the large amounts of unpublished data “could influence results in updates of this review.” They certainly could. I believe it is plain wrong to perform meta‐analyses on the authors’ data. When I most recently reviewed this area for the BMJ in 2010, I concluded that we don't know whether topical NSAIDs are beneficial (1).
1 Gøtzsche PC. NSAIDs. Clin Evid (Online). 2010 Jun 28.
I agree with the conflict of interest statement below:
I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Reply
Response submitted by authors Sheena Derry and Professor Andrew Moore on 4 November 2015.
Peter has made these same points elsewhere [PubMed Commons: PubPeer > Cochrane Database Syst Rev, October 2015; https://pubpeer.com/publications/A9E5BEA36549727357F9FD14CC2537].
As a preliminary, it is important to stress that the number of trials available in pain has increased over time, and newer trials are typically better and larger. This means we can move from answering the simple question of whether an intervention works to more important and relevant questions, such as how well the intervention works, and, for drugs particularly, to examine effects of dose and formulation. Formulation is now recognised to have profound effects. For oral analgesics, for example, fast acting formulations demonstrate up to double the analgesic effect for a given dose, as our recently updated overview points out (Cochrane Database Syst Rev. 2015 Sep 28;9:CD008659; see also Pain. 2014 Jan;155(1):14‐21).
What was true for oral analgesics in acute pain is now true also for topical NSAIDs in acute pain, where manufacturers have been putting a lot of effort into trying to produce new formulations that work better. This updated review sought to examine the influence of formulation and drug, although dose is somewhat more difficult. There are good reasons why formulation may be important (Rheumatic Disease Clinics 2008 Vol. 34, Issue 2, p 415–432).
Our searches did identify a large number of unpublished trials. We deplore this, but are powerless to change things. For a previous review of topical NSAIDs we contacted all identified manufacturers and asked for published or unpublished data. The yield was small (as others have found), but some unpublished studies were brought into the public domain. Waiting for all studies and clinical trial reports of all studies would take forever, and could probably never be achieved. We believe that many of these unpublished studies relate to drugs and/or formulations that have never been manufactured commercially. While these would be of interest in determining what does and doesn't work and to direct future research, they would probably have little clinical relevance because these formulations are unlikely to come to market.
Peter’s main issue appears to be heterogeneity. Tests for heterogeneity are problematical anyway (Pain. 2000 85:415‐24), and the I square of 92% that he quotes was for all topical diclofenac formulations combined. While we do give an overall summary for diclofenac, we demonstrated in the review that the different diclofenac formulations produced different results from one another using L’Abbé plots and in our detailed analyses, showing large variations in efficacy between them. In the circumstance, a high I square for all combined (clinical heterogeneity) is to be expected, but it is not relevant. The bulk of the studies on diclofenac were published in the last five years, were of decent quality, and moderate to large size. There were older data for ketoprofen, but again these showed major differences between formulations. Differences between formulations are highlighted throughout the review.
Trying to determine publication bias using funnel plots or other measures is something of a lost cause (Journal of Clinical Epidemiology 2000;53:207‐16). It is especially so with small numbers of trials (Journal of Clinical Epidemiology 2000 53: 477‐484), and making sense of funnel plots is anything but easy for most people (Journal of Clinical Epidemiology 2005 58: 894‐901). A useless method seems an odd choice to make to criticise our review.
There are very good scientific reasons why drug and formulation may play a big part in the efficacy of topical NSAIDs. This is also the case for oral analgesics used in acute pain, where formulation improvements generating rapid absorption confers greater efficacy. Simple lumping strategies may have been permissible in past systematic review methodology, but a more forensic approach is needed now and in the future. This is what we have attempted to do in this latest review.
Comparisons with Peter’s 2010 review seem inappropriate since that review was based on other reviews that are now out of date.
Contributors
Feedback from Peter C Gøtzsche.
Authors involved with responding: Andrew Moore, Sheena Derry.
Feedback Editor: Kate Seers.
Query on figures in Summary of findings table, 27 January 2016
Summary
Date of Submission: 27‐Jan‐2016 Name: Karen Pettersen Email Address: kpettersen@wiley.com Affiliation: Wiley Role: Editor Comment: I may have missed something but the figures quoted for Clinical success in your Summary of findings table do not seem to match Analysis 1.1 in the Review for the clinical success outcome I agree with the conflict of interest statement below: I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Reply
Response submitted by author Professor Andrew Moore on 2 February 2016.
The Summary of findings table for efficacy refers only to the best formulations for each drug. It specifies the gel formulation; the results for different formulations are shown in Analysis 2.1 for diclofenac, Analysis 3.1 for ibuprofen, and Analysis 4.1 for ketoprofen. We judged that as individuals will use a particular formulation, it made sense to provide in the Summary of findings table the information for the best formulation for each drug, since there was sufficient evidence available for that to make sense. The issue of formulation is up front and centre throughout the review, including the abstract, PLS, Results, and Discussion, and made possible by the very large (63%) increase in included participants in this [2015] update, from modern trials relevant to drugs available today. So there is no conflict between the Summary of findings table and Analysis 1.1 because they refer to different things. We have made no changes to the review.
Contributors
PaPaS staff and author team.
What's new
| Date | Event | Description |
|---|---|---|
| 29 May 2019 | Amended | Contact details updated. |
| 18 March 2019 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 4, 2008 Review first published: Issue 6, 2010
| Date | Event | Description |
|---|---|---|
| 9 July 2018 | Amended | Minor correction to included study reference. |
| 21 February 2017 | Review declared as stable | See Published notes. |
| 4 February 2016 | Feedback has been incorporated | Feedback submitted January 2016. See Feedback 3. |
| 4 November 2015 | Feedback has been incorporated | Feedback has been incorporated. See Feedback 2. |
| 13 October 2015 | Amended | Small errors found in Summary Table B for total number of participants with piroxicam and indomethacin, and in text for NNT with diclofenac (other gel). No change to conclusions |
| 6 October 2015 | Amended | Small error found in Summary Table B: percentages for NSAID and placebo were the wrong way round for comparison of all NSAIDs and placebo. RR and text were correct. No change to results or conclusions |
| 13 February 2015 | New citation required but conclusions have not changed | Conclusions not changed. Results remain essentially the same, but the focus has changed to examine drug and formulation combination. |
| 3 February 2015 | New search has been performed | Title changed from Topical NSAIDs for acute pain in adults to Topical NSAIDs for acute musculoskeletal pain in adults to increase specificity. New searches run and new studies identified. Fourteen new included studies using diclofenac (3489 new participants, a 63% increase); four new excluded studies. Fifteen studies awaiting classification (completed, no results available, but likely to satisfy inclusion criteria). |
| 23 May 2014 | Amended | Error in data reported for clinical success in Hosie 1993 was brought to our attention and has been corrected. |
| 12 June 2012 | Feedback has been incorporated | We have incorporated feedback received from Dr Sigrun Chrubasik and the author's response on DMSO and other additives. |
Notes
2019
In March 2019, this review was stabilised following discussion with the authors and editors. Restricted searches in March 2019 identified another two new studies (Bussin 2017; Lai 2017), but we judged that including them would not affect the conclusions of the review. If appropriate, we will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions. Bussin ER, Cairns B, Bovard J, Scott A. Randomised controlled trial evaluating the short‐term analgesic effect of topical diclofenac on chronic Achilles tendon pain: a pilot study. BMJ Open. 2017 May 4;7(4):e015126. doi:10.1136/bmjopen‐2016‐015126.
Lai PM, Collaku A, Reed K. Efficacy and safety of topical diclofenac/menthol gel for ankle sprain: A randomized, double‐blind, placebo‐ and active‐controlled trial. J Int Med Res. 2017 Apr;45(2):647‐661. doi: 10.1177/0300060517700322.
2017
In February 2017, this review was stabilised following discussion with the authors and editors. Restricted searches in February 2017 identified two new studies (Cheechareoan 2016; Predel 2016), but we judged that including them would not affect the conclusions of the review. If appropriate, we will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions. Cheechareoan S, Pathanawiriyasirikul T, Manmee C, Janpol K. Efficacy of Plai Cream in Adult Patients with Muscle Strain: A Randomized, Double‐Blind, Placebo‐Controlled Trial. Journal of the Medical Association of Thailand 2016;99(Suppl 2):S147‐52. No significant difference between groups in mean pain intensity at two weeks (N = 140). Predel HG, Pabst H, Schäfer A, Voss D, Giordan N. Diclofenac patch for the treatment of acute pain caused by soft tissue injuries of limbs: a randomized, placebo‐controlled clinical trial. The Journal of Sports Medicine and Physical Fitness. 2016:56(1‐2):92‐9. Statistically significant difference between groups in mean pain intensity at two days, and comparable adverse events between groups (N = 164).
Acknowledgements
The Oxford Pain Relief Trust, the NHS Cochrane Collaboration Programme Grant Scheme, and the NIHR Biomedical Research Centre Programme provided support for the earlier review.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pain, Palliative and Supportive Care Review Group. Disclaimer: the views and opinions expressed herein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS), or the Department of Health.
Appendices
Appendix 1. CENTRAL search strategy (via CRSO) for 2015 update
MESH DESCRIPTOR Anti‐Inflammatory Agents, Non‐Steroidal EXPLODE ALL TREES (13419)
(bufexamac OR bufexine OR calmaderm OR ekzemase OR dicoflenac OR solaraze OR pennsaid OR voltarol OR emugel OR voltarene OR voltarol OR optha OR voltaren OR etofenamate OR afrolate OR algesalona OR bayro OR deiron OR etofen OR flexium OR flogoprofen OR rheuma‐gel OR rheumon OR traumalix OR traumon OR zenavan OR felbinac OR dolinac OR flexfree OR napageln OR target OR traxam OR fentiazac OR domureuma OR fentiazaco OR norvedan OR riscalon OR fepradinol OR dalgen OR flexidol OR cocresol OR rangozona OR reuflodol OR pinazone OR zepelin OR flufenamic OR dignodolin OR rheuma OR lindofluid OR sastridex OR lunoxaprofen OR priaxim OR flubiprofen OR fenomel OR ocufen OR ocuflur OR "Trans Act LAT" OR tulip OR ibuprofen OR cuprofen OR "deep relief" OR fenbid OR ibu‐cream OR ibugel OR ibuleve OR ibumousse OR ibuspray OR "nurofen gel" OR proflex OR motrin OR advil OR radian OR ralgex OR ibutop OR indomethacin OR indocin OR indospray OR isonixin OR nixyn OR ketoprofen OR tiloket OR oruvail OR powergel OR solpaflex OR ketorolac OR acular OR trometamol OR meclofenamic OR naproxen OR naprosyn OR niflumic OR actol OR flunir OR niflactol topico OR niflugel OR nifluril OR oxyphenbutazone OR californit OR diflamil OR otone OR tanderil OR piketoprofen OR calmatel OR triparsean OR piroxicam OR feldene OR pranoprofen OR oftalar OR pranox OR suxibuzone OR danilon OR flamilon OR ufenamate OR fenazol OR flector OR benzydamine): TI,AB,KY (25220)
1 OR 2 (32484)
MESH DESCRIPTOR Administration, Topical EXPLODE ALL TREES (2169)
(topical* OR cutaneous OR dermal OR transcutaneous OR transdermal OR percutaneous OR skin OR massage OR embrocation OR gel OR ointment OR aerosol OR cream OR crème OR lotion OR mouse OR foam OR liniment OR spray OR rub OR balm OR salve OR emulsion OR oil OR patch OR plaster):TI,AB,KY (67940)
4 OR 5 (70486)
MESH DESCRIPTOR Athletic Injuries EXPLODE ALL TREES (411)
(strain OR sprain* OR contusion OR distortion OR compression OR "sports injur*" OR "soft tissue injur*" OR tend?nitis OR "muscle pain" OR periarthritis OR epicondylitis OR tenosynovitis):TI,AB,KY (9158)
7 OR 8 (9448)
MESH DESCRIPTOR pain EXPLODE ALL TREES (29943)
(pain* OR analgesi*):TI,AB,KY (74815)
10 OR 11 (80041)
3 AND 6 AND 9 AND 12 (110)
Appendix 2. MEDLINE search strategy via Ovid (for 2015 update)
exp Anti‐inflammatory Agents, non‐steroidal/ (162888)
(bufexamac OR bufexine OR calmaderm OR ekzemase OR diclofenac OR solaraze OR pennsaid OR voltarol OR emugel OR voltarene OR voltarol OR optha OR voltaren OR etofenamate OR afrolate OR algesalona OR bayro OR deiron OR etofen OR flexium OR flogoprofen OR rheuma‐gel OR rheumon OR traumalix OR traumon OR zenavan OR felbinac OR dolinac OR flexfree OR napageln OR target OR traxam OR fentiazac OR domureuma OR fentiazaco OR norvedan OR riscalon OR fepradinol OR dalgen OR flexidol OR cocresol OR rangozona OR reuflodol OR pinazone OR zepelin OR flufenamic OR dignodolin OR rheuma OR lindofluid OR sastridex OR lunoxaprofen OR priaxim OR flubiprofen OR fenomel OR ocufen OR ocuflur OR "Trans Act LAT" OR tulip OR ibuprofen OR cuprofen OR "deep relief" OR fenbid OR ibu‐cream OR ibugel OR ibuleve OR ibumousse OR ibuspray OR "nurofen gel" OR proflex OR motrin OR advil OR radian OR ralgex OR ibutop OR indomethacin OR indocin OR indospray OR isonixin OR nixyn OR ketoprofen OR tiloket OR oruvail OR powergel OR solpaflex OR ketorolac OR acular OR trometamol OR meclofenamic OR naproxen OR naprosyn OR niflumic OR actol OR flunir OR niflactol topico OR niflugel OR nifluril OR oxyphenbutazone OR californit OR diflamil OR otone OR tanderil OR piketoprofen OR calmatel OR triparsean OR piroxicam OR feldene OR pranoprofen OR oftalar OR pranox OR suxibuzone OR danilon OR flamilon OR ufenamate OR fenazol OR flector OR benzydamine).mp (558284)
1 OR 2 (664691)
exp Administration, Topical/ (69697)
(topical* OR cutaneous OR dermal OR transcutaneous OR transdermal OR percutaneous OR skin OR massage OR embrocation OR gel OR ointment OR aerosol OR cream OR crème OR lotion OR mouse OR foam OR liniment OR spray OR rub OR balm OR salve OR emulsion OR oil OR patch OR plaster).mp (1982395)
4 OR 5 (1997720)
exp Athletic Injuries/ (21464)
(strain OR sprain* OR contusion OR distortion OR compression OR "sports injur*" OR "soft tissue injur*" OR tend?nitis OR "muscle pain" OR periarthritis OR epicondylitis OR tenosynovitis).mp (420133)
7 OR 8 (439228)
Pain/ (113906)
(pain* OR analgesi*).mp (585249)
10 or 11 (585249)
randomized controlled trial.pt (401171)
randomized.ab (296222)
placebo.ab (155341)
drug therapy.fs (1789858)
randomly.ab (207517)
trial.ab (308477)
groups.ab (1318386)
OR/13‐19
3 AND 6 AND 9 AND 12 AND 20 (139)
limit 21 to yr="2008‐Current" (56)
Appendix 3. EMBASE search strategy via Ovid (for 2015 update)
exp Anti‐inflammatory Agents, non‐steroidal/ (452266)
(bufexamac OR bufexine OR calmaderm OR ekzemase OR dicoflenac OR solaraze OR pennsaid OR voltarol OR emugel OR voltarene OR voltarol OR optha OR voltaren OR etofenamate OR afrolate OR algesalona OR bayro OR deiron OR etofen OR flexium OR flogoprofen OR rheuma‐gel OR rheumon OR traumalix OR traumon OR zenavan OR felbinac OR dolinac OR flexfree OR napageln OR target OR traxam OR fentiazac OR domureuma OR fentiazaco OR norvedan OR riscalon OR fepradinol OR dalgen OR flexidol OR cocresol OR rangozona OR reuflodol OR pinazone OR zepelin OR flufenamic OR dignodolin OR rheuma OR lindofluid OR sastridex OR lunoxaprofen OR priaxim OR flubiprofen OR fenomel OR ocufen OR ocuflur OR "Trans Act LAT" OR tulip OR ibuprofen OR cuprofen OR "deep relief" OR fenbid OR ibu‐cream OR ibugel OR ibuleve OR ibumousse OR ibuspray OR "nurofen gel" OR proflex OR motrin OR advil OR radian OR ralgex OR ibutop OR indomethacin OR indocin OR indospray OR isonixin OR nixyn OR ketoprofen OR tiloket OR oruvail OR powergel OR solpaflex OR ketorolac OR acular OR trometamol OR meclofenamic OR naproxen OR naprosyn OR niflumic OR actol OR flunir OR niflactol topico OR niflugel OR nifluril OR oxyphenbutazone OR californit OR diflamil OR otone OR tanderil OR piketoprofen OR calmatel OR triparsean OR piroxicam OR feldene OR pranoprofen OR oftalar OR pranox OR suxibuzone OR danilon OR flamilon OR ufenamate OR fenazol OR flector OR benzydamine).mp (790435)
1 OR 2 (1108683)
exp Administration, Topical/ (68490)
(topical* OR cutaneous OR dermal OR transcutaneous OR transdermal OR percutaneous OR skin OR massage OR embrocation OR gel OR ointment OR aerosol OR cream OR crème OR lotion OR mouse OR foam OR liniment OR spray OR rub OR balm OR salve OR emulsion OR oil OR patch OR plaster).mp (3208844)
4 OR 5 (3208844)
exp Athletic Injuries/ (24675)
(strain OR sprain* OR contusion OR distortion OR compression OR "sports injur*" OR "soft tissue injur*" OR tend?nitis OR "muscle pain" OR periarthritis OR epicondylitis OR tenosynovitis).mp (804322)
7 OR 8 (825164)
Pain/ (216406)
(pain* OR analgesi*).mp (971593)
10 OR 11 (971593)
clinical trials.sh (841252)
controlled clinical trials.sh (389335)
randomized controlled trial.sh (357169)
double‐blind procedure.sh (118945)
(clin* adj25 trial*).ab (331002)
((doubl* or trebl* or tripl*) adj25 (blind* or mask*)).ab (141726)
placebo*.ab (203390)
random*.ab (906176)
OR/13‐20 (1731380)
3 AND 6 AND 9 AND 10 AND 19 (439)
limit 22 to yr="2008‐Current" (204)
Appendix 4. Summary of outcomes: efficacy
| Study ID | Treatment | Clinical response | Other response |
| Airaksinen 1993 | (1) Ketoprofen gel 2 x 5 g (125 mg) daily, n = 29 (2) Placebo gel, n = 27 |
PGE "improved" at 7 days (1) 24/29 (2) 14/27 |
No additional data |
| Ăkermark 1990 | (1) Indomethacin spray 1% (Elmetacin), 3‐5 x 0.5‐1.5 mL daily, n = 23 (2) Indomethacin capsules, 3 x 25 mg daily, n = 23 (3) Placebo spray and capsules, n = 24 |
No pain on palpation at 7 days (1) 12/22 (2) 5/22 (3) 6/24 |
Patient assessment of improvement at 7 days (scale 0 to 100) (1) 57 (2) 49 (3) 30 |
| Aoki 1984 | (1) Piroxicam gel 5%, 3 or 4 x 1 g daily, n = 84 (2) Indomethacin gel 1%, 3 or 4 x 1 g daily, n = 84 (3) Placebo gel, n = 84 |
PGE (5 point) "better or much better" at 7 days (1) 56/72 (2) 41/64 (3) 33/67 |
Pain on movement "reduced" or "disappeared" at 7 days (1) 48/61 (2) 38/60 (3) 35/63 |
| Auclair 1989 | (1) Niflumic acid gel 2.5%, 3 x 5 g daily, n = 117 (2) Placebo gel, n = 110 |
PGE (5 point) "good or very good" at 7 days (1) 69/117 (2) 54/110 |
Pain on palpation "improved" at 7 days (1) 69/117 (2) 53/110 |
| Billigmann 1996 | (1) Ibuprofen microgel 5% 3 x 200 mg daily, n = 80 (2) Placebo gel, n = 80 |
Complete remission (1) 25/80 (2) 10/80 |
Improvement in pain with movement of 20% at 7 days (1) 65/80 (2) 55/80 |
| Campbell 1994 | (1) Ibuprofen cream 5% (Proflex) 4 x 4" daily, n = 26 (2) Placebo cream, n = 25 |
Improvement in walking ability (4 point) at 7 days (1) 21/26 (2) 19/25 |
No additional data |
| Chatterjee 1977 | (1) Benzydamine HCl cream 3% 3 x daily, n = 25 (2) Placebo cream, n = 25 |
Pain on movement "absent/slight" at 6 days (1) 21/25 (2) 12/25 |
Tenderness with pressure "absent/slight" at 6 days (1) 21/25 (2) 12/25 |
| Costantino 2011 | (1) DHEP‐heparin plaster (Flectorparin) x 1 daily, n = 142 (2) DHEP plaster (Flector) x 1 daily, n = 146 (3) Placebo plaster, n = 142 |
No responder analysis for efficacy. Overall treatment efficacy not reported | Mean reduction from baseline for PI on movement at 3 days (from graph) (1) 24 mm (2) 19 mm (3) 14 mm |
| Coudreuse 2010 | (1) DHEP‐heparin plaster (Flectorparin), n = 120 (2) Placebo, n = 120 | PGE "good" or "excellent" at 7 days (1) 99/117 (2) 82/116 These are results for physician‐reported judgement ‐ not participant |
Mean reduction from baseline for PI on movement over 6 hours (1) about 30% (2) about 20% Overall greater in DHEP‐heparin group than placebo over 7 days |
| Curioni 1985 | (1) Ibuproxam, n = 20 (2) Ketoprofen, n = 20 (3) Etofenamate, n = 20 |
Resolution of symptoms by 7 days (1) 15/20 (2) 13/20 (3) 13/20 |
PGE "good" or "excellent" at 10 days (1) 19/20 (2) not reported (3) 16/20 |
| Diebshlag 1990 | (1) Ketorolac gel 2% 3 x 3 g daily, n = 13 (2) Etofenamate gel 5% 3 x 3 g daily, n = 12 (3) Placebo gel, n = 12 |
Improvement in pain at 7 days (1) 12/13 (2) 10/12 (3) 9/12 |
No additional data |
| Dreiser 1988 | (1) Ibuprofen cream 5%, 3 x 4 cm daily, n = 32 (3 x 10 cm for large joints) (2) Placebo cream, n = 32 |
PGE "improvement" or "complete relief" at 7 days (1) 26/32 (2) 12/32 |
(1) significantly better than (2) for mean improvement in spontaneous pain, movement pain, rest pain, tenderness to pressure (VAS) |
| Dreiser 1989 | (1) Ketoprofen gel 2.5%, 2 x 5 cm daily, n = 30 (2) Placebo gel, n = 30 |
PGE (3 point) "better" at 7 days (1) 18/30 (2) 5/30 |
(1) significantly better than (2) for mean improvement in pain (rest and movement) (VAS) |
| Dreiser 1990 | (1) Niflumic acid gel 2.5%, 3 x 5 g daily, n = 30 (2) Placebo gel, n = 30 |
PGE (4 point) "cured" or "improved" at 7 days (1) 23/30 (2) 10/30 |
(1) significantly better than (2) for mean improvement in pain (VAS) |
| Dreiser 1994 | (1) Flurbiprofen plaster 2 x 40 mg daily, n = 65 (2) Placebo plaster, n = 66 |
PGE (4 point) "good" or "very good" at 7 days (1) 48/65 (2) 41/66 |
(1) significantly better than (2) for mean improvement in spontaneous pain, but not pain on movement or palpation (VAS) |
| Fioravanti 1999 | (1) DHEP lecithin gel 3 x 5 g (= 65 mg) daily, n = 50 (2) DHEP gel 3 x 5 g (= 65 mg) daily, n = 50 |
PGE (4 point) "good" or "excellent" at 10 days (1) 35/50 (2) 35/50 |
(1) significantly better than (2) for mean improvement in spontaneous pain at 7 days, but not for pain on movement at 10 days (VAS) |
| Fujimaki 1985 | (1) Piroxicam gel 0.5% 3 or 4 x 1 g daily, n = 92 (2) Indomethacin gel 1% 3 or 4 x 1 g daily, n = 90 (3) Placebo gel, n = 89 |
PGE (5 point) "better" or "much better" at end of treatment at 14 days (1) 44/83 (2) 44/82 (3) 40/82 |
No additional data |
| Gallacchi 1990 | (1) Diclofenac hydroxyethylpyrrolidine gel 1%, 4 x 2 g daily, n = 25 (Flector gel) (2) Diclofenac sodium 1% 4 x 2 g daily, n = 25 (Voltaren Emugel) |
PGE (5 point) "good" or "excellent" at 14 days (1) 19/25 (2) 19/25 |
No significant difference between groups for pain on applied pressure at 7 and 14 days |
| González de Vega 2013 | (1) Traumeel gel 3 x 2 g daily, n = 140 (2) Traumeel ointment, n = 143 (3) Diclofenac gel 1% 3 x 2 g daily, n = 137 |
Pain‐free at 7 days
(1) 7/140
(3) 8/137 PGE (5 point) "good" or "very good" (1) 128/140 (3) 127/137 |
Normal function (5 point) score of 0 or 1 at 14 days (1) 133/140 (3) 131/137 |
| Governali 1995 | (1) Ketoprofen gel 5% 3 x 2‐3 g daily, n = 15 (2) Ketoprofen cream 1%, 3 x 2‐3 g daily, n = 15 |
PGE (5 point) "good" or "excellent" at 7 days (1) 14/15 (2) 4/15 |
No additional data |
| Gualdi 1987 | (1) Flunaxaprofen gel 2 x 3‐5 cm daily, n = 30 (2) Ketoprofen gel 2 x 3‐5 cm daily, n = 30 |
No dichotomous data | No significant difference between groups for pain on movement at 7 days |
| Haig 1986 | (1) Benzydamine cream 3%, 6 x daily, n = 21 (2) Placebo cream, n = 22 |
Pain on movement "improved" by 6 days (1) 18/21 (2) 13/22 |
No additional data |
| Hoffmann 2012 | (1) DHEP‐heparin plaster (Flectorparin) x 1 daily, n = 121 (2) DHEP (Flector) plaster x 1 daily, n = 115 (3) Placebo plaster, n = 118 | PGE (5 point) "good" or "excellent": over 80% in all groups | Mean reduction from baseline in PI (100 mm VAS) Day 3 (1) 19.1 mm (2) 11.4 mm (3) 5.2 mm Day 8 (1) 44.3 mm (2) 37.2 mm (3) 29.6 mm |
| Hofman 2000 | (1) Diclofenac sodium gel 1%, 4 x 2 cm daily, n = 69 (2) Lysine clonixinate gel 5%, 4 x 2 cm (22.5 mg) daily, n = 73 |
PGE (3 point) at 8 days: "good" (1) 38/69 (2) 36/73 |
No significant difference between treatments for any pain outcomes |
| Hosie 1993 | (1) Felbinac foam 3% 3 x 2 g daily + placebo tablets, 3 x 1 daily, n = 140 (127 analysed for efficacy) (2) Ibuprofen tablets 3 x 400 mg daily + placebo foam 3 x 2 g daily, n = 147 (134 analysed for efficacy) |
Pain on movement "none" or "mild" at 7 days (1) 81/127 (2) 96/133 |
Spontaneous pain "none" or "mild" at 7 days (1) 99/127 (2) 108/134 |
| Jenoure 1997 | (1) DHEP plaster (Tissugel), 2 x daily, n = 44 (2) Placebo plaster 2 x daily, n = 41 |
Baseline pain in two groups not balanced, and data in table and figure do not agree, so efficacy outcomes not used | No additional data |
| Joussellin 2003 | (1) DHEP plaster (Flector Tissugel 1%), 1 x daily, n = 68 (2) Placebo plaster 1 x daily, n = 66 |
PGE (4 point) "excellent" at 7 days (1) 36/68 (2) 24/66 ≥ 30% reduction in PI at 7 days (1) 25/68 (2) 11/66 |
(1) significantly better than (2) for mean pain on movement at 6 days |
| Julien 1989 | (1) Ketoprofen gel 2.5% 2 x 5 cm (= 50 mg) daily, n = 30 (2) Placebo gel, n = 30 |
PGE (4 point) "recovered" at 7 days (1) 18/30 (2) 6/30 |
PGE (4 point) "recovered" or "improved" at 7 days (1) 25/30 (2) 13/30 |
| Klainguti 2010 | (1) DHEP‐heparin plaster (Flectorparin) x 1 daily, n = 62 (2) DHEP plaster (Flector) x 1 daily, n = 61 (3) Placebo, n = 59 |
Overall treatment efficacy (participant and investigator) (5 point) "good" or "excellent" at 3 days (1) 56/62 (2) not reported (3) 46/59 |
Mean reduction in PI at day 3 (from graph)
(1) 33 mm (2) Not reported (3) 24 mm |
| Kockelbergh 1985 | (1) Ketoprofen gel 2.5% 2 x 5 cm (= 15 mg) daily, n = 38 (2) Placebo gel, n = 36 |
PGE (3 point) "good" at 7 days (1) 30/38 (2) 22/36 |
(1) and (2) slightly better than (3) for mean spontaneous pain at 7 days |
| Kuehl 2011 | (1) DETP 1.3% plaster 2 x daily, n = 207 (2) Placebo plaster, n = 211 | No dichotomous data | Percentage reduction from baseline at last application (1) 73% (2) 62% |
| Li 2013 | (1) DHEP plaster (Flector) 2 x daily, n = 192 (2) Placebo plaster 2 x daily, n = 192 | ≥ 50% pain reduction at day 7 (posthoc analysis) (1) 173/192 (2) 114/192 |
Overall treatment efficacy (5 point) "good" or "excellent" (1) 161/192 (2) 81/192 Participant and investigator rating Mean ± SD reduction in PI on movement at 7 days (1) 53.8 ± 17.0 (2) 37.0 ± 18.3 |
| Linde 1985 | (1) Benzydamine 3% cream 3 x daily, n = 50 (2) Placebo gel, n = 50 |
No pain on movement (walking) at 8 days (1) 35/50 (2) 40/50 |
No additional data |
| Machen 2002 | (1) Ibuprofen gel 5% 3 x daily, n = 40 (2) Placebo gel, n = 41 |
PGE: (5 point) "marked improvement" or "complete clearance" at 7 days (1) 25/40 (2) 9/41 |
Clinically meaningful (≥ 30 mm) pain relief at day 7 (1) 30/40 (2) 16/41 |
| Mahler 2003 | (1) DHEP + lethicin gel 3 x 5 g daily, n = 52 (2) DHEP gel 3 x 5 g daily, n = 48 |
PGE (4 point) "good" or "excellent" at 10 days (1) 49/52 (2) 39/48 |
Mean reduction in pain on movement at 3 and 10 days significantly greater with (1) than (2) |
| Mazières 2005b | (1) Ketoprofen plaster 100 mg, x 1 daily, n = 81 (2) Placebo plaster, n = 82 |
PGE (4 point) "good" or "excellent" at 14 days (1) 72/81 (2) 60/82 |
All mean efficacy measures improved more for (1) than (2), most were statistically significant |
| Mazières 2005a | (1) Ketoprofen plaster 100 mg, x 1 daily, n = 87 (2) Placebo plaster, n = 85 |
PGE (4 point) "good" or "excellent" at 14 days (1) 50/87 (2) 41/85 |
All mean efficacy measures improved more for (1) than (2), most were statistically significant |
| McLatchie 1989 | (1) Felbinac gel 3% 3 x 3 cm daily, n = 118 (2) Placebo gel, n = 113 |
No dichotomous data | Patient daily self‐assessment for mean pain on rest, movement, at night, interference with normal and leisure activities show better efficacy for (1) than (2) from day 2 (VAS) |
| Morris 1991 | (1) Felbinac gel 3% 3 x 1 cm daily, n = 41 (2) Placebo gel, n = 43 |
PGE (5 point) "good" or "very good" at 7 days (1) 23/41 (2) 27/43 |
(1) better than (2) for mean improvement in symptoms and sporting function at 7 days |
| NCT01255423 | (1) Diclofenac sodium gel 1% x 4 daily, n = 104 (2) Placebo gel x 4 daily, n = 100 | No dichotomous data | VAS (mean ± SD) at 72 hours (baseline PI not reported) (1) 37.4 ± 25.2 (2) 38.8 ± 24.1 |
| NCT01272934 | (1) Diclofenac sodium gel 1% x 4 daily, n = 104 (2) Placebo gel x 4 daily, n = 100 | No dichotomous data | VAS (mean ± SD) at 72 hours (baseline PI not reported) (1) 25.6 ± 15.9 (2) 61.2 ± 16.6 |
| NCT01272947 | (1) Diclofenac sodium gel 1% x 4 daily, n = 104 (2) Placebo gel x 4 daily, n = 100 | No dichotomous data | VAS (mean ± SD) at 24 hours (baseline PI not reported) (1) 33.1 ± 21.4 (2) 65.4 ± 16.9 |
| Noret 1987 | (1) Ketoprofen gel 2.5% 2 x 5 cm (7.5 mg) daily, n = 48 (2) Placebo gel, n = 45 |
PGE (4 point) "good" or "excellent" at 8 days (1) 39/48 (2) 9/45 |
Decrease in mean spontaneous pain significantly greater in (1) than (2) by 3 days |
| Parrini 1992 | (1) Ketoprofen foam 15% 3 x 2 g (200 mg) daily, n = 83 (2) Placebo foam, n = 86 |
No dichotomous data | Mean pain on movement and pressure significantly decreased by 7 days in (1) compared with (2) |
| Picchio 1981 | (1) Ibuprofen gel 10% 3 x daily , n = 20 (2) Ketoprofen gel 1% 3 x daily, n = 20 |
No pain on movement at 8 days (1) 3/20 (2) 0/20 |
Spontaneous pain "none" at 8 days (1) 6/20 (2) 0/20 |
| Predel 2004 | (1) Diclofenac sodium plaster, 2 x daily (140 mg/plaster), n = 60 (2) Placebo plaster, n = 60 |
PGE (4 point) "good" "excellent" at 7 days (1) 55/60 (2) 5/60 |
(1) better than (2) for reduction in tenderness, pain, and speed of pain reduction |
| Predel 2012 | (1) Diclofenac gel (Voltaren Emulgel 2.32%) 2 x 5 cm daily, n = 80 (2) Diclofenac gel (Voltaren Emulgel 2.32% ) 3 x 5 cm daily, n = 80 (3) Placebo gel, n = 82 | ≥ 50% red in PI on movement at 5 days (1) 57/80 (2) 59/80 (3) 17/82 |
PGE efficacy (5 point) "good" or "very good" at 8 days (1) 68/80 (2) 73/80 (3) 24/82 |
| Predel 2013a | (1) Diclofenac 4% spray gel 4 or 5 sprays 3 x daily (96‐120 mg diclofenac sodium), n = 118 (2) Placebo spray gel, n = 114 | None or slight PI on movement at 7‐8 days (1) 111/118 (2) 93/114 |
None or slight PI on movement at 3‐4 days (1) 76/118 (2) 58/114 |
| Predel 2013b | (1) Diclofenac gel (Voltaren Emulgel) 4 x 2 g daily, n = 36 (2) Placebo gel 4 x 2 g daily, n = 36 | PGE (5 point) "good" or "excellent" (1) 36/36 (2) 7/36 |
≥ 50% reduction in PI on movement after 48 hours (1) 34/36 (2) 3/36 |
| Ramesh 1983 | (1) Ibuprofen cream 5% 3 or 4 x 5‐10 cm daily, n = 40 (2) Placebo cream, n = 40 |
Pain on movement (4 point) "none" or "slight" at 7 days (1) 23/40 (2) 23/40 |
Physician global assessment at 10 days: "good" (1) 29/40 (2) 16/40 |
| Rowbotham 2003 | (1) Diclofenac epolamine plaster (Flector Tissuegel) 2 x daily, n = 191 (2) Placebo plaster, n = 181 |
Pain intensity ≤ 2/10 for 2 days or 4 consecutive evaluations, by 7 days (1) 75/191 (2) 48/181 |
Mean pain on rest significantly better with (1) than (2) after 7 days |
| Russell 1991 | (1) Piroxicam gel 0.5% 4 x 5 mg daily, n = 100 (2) Placebo gel, n = 100 |
PGE (4 point) "good" or "excellent" at 8 days (1) 79/100 (2) 45/100 |
Statistically greater reduction in mean pain on movement at 8 days with (1) than (2) |
| Saillant 1998 | (1) DHEP plaster (Flector Tissugel 1%) 1 x daily, n = 70 (2) Placebo plaster 1 x daily, n = 70 | PGE (4 point) "excellent" at 7 days (1) 43/70 (2) 25/70 |
≥ 30% decrease in PI at 7 days (1) 64/70 (2) 50/70 |
| Sanguinetti 1989 | (1) Felbinac gel 3% 3 x daily, n = 42 (2) Placebo gel, n = 40 |
PGE "good" or "very good" at 7 days (1) 34/42 (2) 11/40 |
(1) better than (2) by 2 days |
| Sinniger 1981 | (1) Fentiazac cream 5% 2 or 3 x daily, n = 10 (2) Placebo cream, n = 10 |
Complete pain relief within 10 days (1) 7/10 (2) 1/10 |
Improvement in active pain on movement at 5 days (1) 67% (2) 32% |
| Spacca 2005 | (1) DHEP lecithin gel (Effigel), 3 x 5 g, daily, n = 79 (2) Placebo gel, n = 76 |
No dichotomous data | Mean pain scores improved more rapidly in (1) than (2) ‐ statistically significant at 3 and 6 days |
| Sugioka 1984 | (1) Piroxicam gel 0.5% 3 or 4 x 1 g daily, n = 183 (2) Indomethacin gel 1% 3 or 4 x 1 g daily, n = 183 |
PGE (5 point) "better" or "much better" at 14 days (1) 85/175 (2) 55/165 |
Pain on movement "reduced" or "disappeared" at 7 days (1) 77/175 (2) 63/165 |
| Thorling 1990 | (1) Naproxen gel 10% 2‐6 x daily, n = 60 (2) Placebo gel, n = 60 |
PGE (5 point) "good" or "very good" at 7 days (1) 38/60 (2) 27/60 |
Participants using naproxen improved more rapidly and had significantly lower severity scores by day 3 |
| Tonutti 1994 | (1) Ketoprofen gel 5%, 3 x 2‐3 g daily, n = 15 (2) Etofenamate gel 5%, 3 x 2‐3 g, n = 15 |
PGE (4 point) "good" or "excellent" at 7 days (1) 10/15 (2) 11/15 |
Significant reductions in pain on movement by 7 days in both groups |
| Vecchiet 1991 | (1) Meclofenamic acid gel 5% 2 x 10 cm daily (2 g), n = 30 (2) Placebo, n = 30 |
PGE (4 point) "good" or "excellent" at 10 days (1) 30/30 (2) 19/30 |
(1) significantly better than (2) for mean improvement in spontaneous pain, movement pain, functional restriction |
| Whitefield 2002 | (1) Ibuprofen gel 5% + placebo tablet 3 x daily, n = 50 (2) Ibuprofen 400 mg tablet + placebo gel 3 x daily, n = 50 |
Participant satisfied at 7 days (1) 30/50 (2) 36/50 |
"Completely better" at 14 days (1) 24/50 (2) 30/50 |
| DHEP: diclofenac epolamine; HCl: hydrochloride; n: number; PGE: participant global evaluation; PI: pain intensity; SD: standard deviation; VAS: visual analogue scale. | |||
Appendix 5. Summary of outcomes: adverse events and withdrawals
| Study ID | Treatment | Local AEs | Systemic AEs | Serious AEs | Withdrawals |
| Airaksinen 1993 | (1) Ketoprofen gel 2 x 5 g (125 mg) daily, n = 29 (2) Placebo gel, n = 27 |
(1) 5/29 (2) 4/27 |
(1) 1/29 (nausea after paracetamol) (2) 0/27 |
None | AE: none Other: none reported |
| Ăkermark 1990 | (1) Indomethacin spray 1% (Elmetacin), 3‐5 x 0.5‐1.5 mL daily, n = 23 (2) Indomethacin capsules, 3 x 25 mg daily, n = 23 (3) Placebo spray and capsules, n = 24 |
(1) 4/22 (2) 0/22 (3) 0/24 |
No usable data ‐ reported for events not participants | None reported | AE: (1) 1, (2) 1, (3) 0 Lost to follow‐up: (1) 1, (2) 2, (3) 3 |
| Aoki 1984 | (1) Piroxicam gel 5%, 3 or 4 x 1 g daily, n = 84 (2) Indomethacin gel 1%, 3 or 4 x 1 g daily, n = 84 (3) Placebo gel, n = 84 |
(1) 1/79 (2) 2/70 (3) 2/74 |
None | None reported | AE: none 23 excluded for protocol violations: (1) 7, (2) 7, (3) 9 26 withdrew for reasons unrelated to treatment: (1) 5, (2) 13, (3) 8 |
| Auclair 1989 | (1) Niflumic acid gel 2.5%, 3 x 5 g daily, n = 117 (2) Placebo gel, n = 110 |
All AEs (1) 5/123 (2) 6/116 Most commonly cutaneous eruptions |
No usable data | None reported | AE: (1) 1/123, (2) 0/116 26 excluded from efficacy analysis for not meeting entry criteria and protocol violations |
| Billigmann 1996 | (1) Ibuprofen microgel 5% 3 x 200 mg daily, n = 80 (2) Placebo gel, n = 80 |
(1) 11/80 (2) 4/80 |
None reported | None reported | AE: (1) 2/80 (allergic rash, dermatitis) No reason given: (1) 3/80, (2) 5/80 Symptom‐free: (1) 1/80, (2) 1/80 |
| Campbell 1994 | (1) Ibuprofen cream 5% (Proflex) 4 x 4" daily, n = 26 (2) Placebo cream, n = 25 |
No data | (1) 1/26 (headache) (2) 0/25 |
No data | AE: none Exclusions 49: 3 presented late, 2 missing forms, 1 appeared twice, 43 did not return diaries |
| Chatterjee 1977 | (1) Benzydamine HCl cream 3% 3 x daily, n = 25 (2) Placebo cream, n = 25 |
None | None | None | AE: none 1 participant lost to follow‐up (group not reported) |
| Costantino 2011 | (1) DHEP‐heparin plaster (Flectorparin) x 1 daily, n = 142 (2) DHEP plaster (Flector) x 1 daily, n = 146 (3) Placebo plaster, n = 142 |
Possibly or probably drug‐related (1) 2/142 (2) 1/145 (3) 6/142 All mild and gone by 14 days, except 1 (moderate) in placebo group | Not reported No systemic GI events | None | AE: none Other: (1) 0/142 (2) 2/146 (protocol deviation, lost to follow‐up) (3) 2/142 (lost to follow‐up) |
| Coudreuse 2010 | (1) DHEP‐heparin plaster (Flectorparin), n = 120 (2) Placebo, n = 120 | (1) 1/117 (2) 7/116 All AEs mild or moderate, resolved spontaneously | (1) 1/117 (2) 0/116 | None | AE: (1) 1/117 (increased oedema) (2) 0/116 Other: (1) 6/117 (recovery 3, no reason 3) (2) 3/116 (recovery 1, no reason 2) |
| Curioni 1985 | (1) Ibuproxam, n = 20 (2) Ketoprofen, n = 20 (3) Etofenamate, n = 20 |
None | None | None | AE: none Other: none |
| Diebshlag 1990 | (1) Ketorolac gel 2% 3 x 3 g daily, n = 13 (2) Etofenamate gel 5% 3 x 3 g daily, n = 12 (3) Placebo gel, n = 12 |
(1) 1/13 (2) 1/12 (3) 0/12 |
None | None | AE: none 1 ketorolac participant did not attend 15 day follow‐up due to car accident |
| Dreiser 1988 | (1) Ibuprofen cream 5%, 3 x 4 cm daily, n = 32 (3 x 10 cm for large joints) (2) Placebo cream, n = 32 |
No usable data | None | Not reported | AE: none 4 placebo participants lost to follow‐up |
| Dreiser 1989 | (1) Ketoprofen gel 2.5%, 2 x 5 cm daily, n = 30 (2) Placebo gel, n = 30 |
(1) 0/30 (2) 2/30 |
None | None reported | AE: (2) 2/30 (intolerance) LoE: (1) 1/30, (2) 1/30 |
| Dreiser 1990 | (1) Niflumic acid gel 2.5%, 3 x 5 g daily, n = 30 (2) Placebo gel, n = 30 |
(1) 0/30 (2) 3/30 |
None | None | AE: (2) 1/30 (erythema) Exclusion: 1 from (2) from efficacy analysis for inadequate baseline pain |
| Dreiser 1994 | (1) Flurbiprofen plaster 2 x 40 mg daily, n = 65 (2) Placebo plaster, n = 66 |
(1) 2/65 (2) 0/66 |
None | None | AE: 0 (1) 1/65 excluded from efficacy analysis for protocol violation (2) 2/66 (1 LoE, 1 cured) |
| Fioravanti 1999 | (1) DHEP lecithin gel 3 x 5 g (= 65 mg) daily, n = 50 (2) DHEP gel 3 x 5 g (= 65 mg) daily, n = 50 |
(1) 0/50 (2) 1/50 |
No data | None reported | AE: none Other: none |
| Fujimaki 1985 | (1) Piroxicam gel 0.5% 3 or 4 x 1 g daily, n = 92 (2) Indomethacin gel 1% 3 or 4 x 1 g daily, n = 90 (3) Placebo gel, n = 89 |
(1) 1/83 (2) 5/82 (3) 2/82 |
(1) 0/83 (2) 1/82 (nausea and vomiting) (3) 0/82 |
None | AE: (1) 0, (2) 4, (3) 0 Unknown reasons: (1) 2, (2) 1 Did not return after 1st visit/irregular visits: (1) 6, (2) 6, (3) 7 |
| Gallacchi 1990 | (1) Diclofenac hydroxyethylpyrrolidine gel 1%, 4 x 2 g daily, n = 25 (Flector gel) (2) Diclofenac sodium 1% 4 x 2 g daily, n = 25 (Voltaren Emugel) |
No AEs | None | None | AE: none Other: none |
| González de Vega 2013 | (1) Traumeel gel 3 x 2 g daily, n = 140 (2) Traumeel ointment, n = 143 (3) Diclofenac gel 1% 3 x 2 g daily, n = 137 |
No usable data Infrequent, mild to moderate | No usable data | None | AE: none Other: (1) 11/140 (2) 12/137 |
| Governali 1995 | (1) Ketoprofen gel 5% 3 x 2‐3 g daily, n = 15 (2) Ketoprofen cream 1%, 3 x 2‐3 g daily, n = 15 |
No side effects | None | None | AE: none Other: none |
| Gualdi 1987 | (1) Flunaxaprofen gel 2 x 3‐5 cm daily, n = 30 (2) Ketoprofen gel 2 x 3‐5 cm daily, n = 30 |
(1) 1/30 (2) 3/30 |
No data | None reported | AE: none Other: none |
| Haig 1986 | (1) Benzydamine cream 3%, 6 x daily, n = 21 (2) Placebo cream, n = 22 |
No AEs reported | None | None reported | AE: none reported Other: no data |
| Hoffmann 2012 | (1) DHEP‐heparin plaster (Flectorparin) x 1 daily, n = 121 (2) DHEP (Flector) plaster x 1 daily, n = 115 (3) Placebo plaster, n = 118 | Most AEs were minor local reactions (e.g. pruritus and erythema) in area of plaster, of mild to moderate intensity and resolved without interrupting treatment | No treatment‐related systemic AEs recorded | (2) 1 participant had 3 SAEs, none judged related to study medication | AE: none Exclusions: (1) 5/121 (2) 10/115 (3) 7/118 Excluded from per protocol analysis due to poor compliance or personal decision |
| Hofman 2000 | (1) Diclofenac sodium gel 1%, 4 x 2 cm daily, n = 69 (2) Lysine clonixinate gel 5%, 4 x 2 cm (22.5 mg) daily, n = 73 |
(1) 1/58 (2) 1/61 |
None | None | AE: none LoE: (1) 9, (2) 8 |
| Hosie 1993 | (1) Felbinac foam 3% 3 x 2 g daily + placebo tablets, 3 x 1 daily, n = 140 (127 analysed for efficacy) (2) Ibuprofen tablets 3 x 400 mg daily + placebo foam 3 x 2 g daily, n = 147 (134 analysed for efficacy) |
(1) 1/127 (2) 3/134 |
GI events: (1) 14/127, (2) 11/134 For (1) more mild, none definitely drug related, for (2) definitely related to study drug |
None | AE: none Exclusions: (1) 13, (2) 13 did not return for 7 day follow‐up |
| Jenoure 1997 | (1) DHEP plaster (Tissugel), 2 x daily, n = 44 (2) Placebo plaster 2 x daily, n = 41 |
(1) 1/44 (2) 1/41 |
No data | None reported | AE: none reported Other: none reported |
| Joussellin 2003 | (1) DHEP plaster (Flector Tissugel 1%), 1 x daily, n = 68 (2) Placebo plaster 1 x daily, n = 66 |
(1) 1/68 (pruritus) (2) 3/66 (pruritus 2, burning 1) All AEs mild or moderate | (1) 1/68 (allergic reaction) (2) 0/66 | None reported | AE: (1) 0/66 (2) 1/66 Other: (1) 3/66 (2) 2/66 |
| Julien 1989 | (1) Ketoprofen gel 2.5% 2 x 5 cm (= 50 mg) daily, n = 30 (2) Placebo gel, n = 30 |
(1) 1/30 (2) 0/30 |
Not reported | None | AE: none Other: none |
| Klainguti 2010 | (1) DHEP‐heparin plaster (Flectorparin) x 1 daily, n = 62 (2) DHEP plaster (Flector) x 1 daily, n = 61 (3) Placebo, n = 59 | (1) 0/62 (2) 0/61 (3) 1/59 All AEs mild in nature and resolved spontaneously |
(1) 1/65 (facial infection) (2) 1/61 (abdominal pain) (3) 0/59 |
None | AE: none Other: (1) 3/65 (2) 1/61 (3) 3/59 |
| Kockelbergh 1985a | (1) Ketoprofen gel 2.5% 2 x 5 cm (= 15 mg) daily, n = 38 (2) Placebo gel, n = 36 |
(1) 1/38 (2) 1/26 |
Not reported | None | AE: none Other: none |
| Kuehl 2011 | (1) DETP 1.3% plaster 2 x daily, n = 207 (2) Placebo plaster, n = 211 | (1) 16/207 (2) 12/211 | (1) 15/207 (2) 23/211 | None | AE: (1) 4/207 (2) 9/211 LoE: (1) 21/207 (2) 25/211 Other: (1) 58/207 (2) 62/211 |
| Li 2013 | (1) DHEP plaster (Flector) 2 x daily, n = 192 (2) Placebo plaster 2 x daily, n = 192 | (1) 4/192 (2) 3/192 | (1) 10/192 (2) 4/192 | None | AE: (1) 2/192 (2) 0/192 Other: (1) 5/192 (2) 1/192 |
| Linde 1985 | (1) Benzydamine 3% cream 3 x daily, n = 50 (2) Placebo gel, n = 50 |
(1) 4/40 (2) 2/41 |
None | None | AE: none (1) 6, (2) 6 excluded from 1st assessment (1) 3, (2) 4 excluded from final assessment |
| Machen 2002 | (1) Ibuprofen gel 5% 3 x daily, n = 40 (2) Placebo gel, n = 41 |
(1) 4/40 (2) 2/41 |
None | None | AE: none (1) 1 LoE, 1 protocol violation (2) 4 LoE |
| Mahler 2003 | (1) DHEP + lethicin gel 3 x 5 g daily, n = 52 (2) DHEP gel 3 x 5 g daily, n = 48 |
(1) 1/52 (2) 0/48 |
(1) 1/52 (2) 0/48 |
None | AE: none 5 lost to follow‐up |
| Mazières 2005b | (1) Ketoprofen plaster 100 mg, x 1 daily, n = 81 (2) Placebo plaster, n = 82 |
At 21 days: (1) 12/81 (2) 6/82 |
(1) 13/81 (2) 14/82 |
None | AE: (1) 3/81 (1) 7/81 (1 LoE, 6 cured) (2) 7/82 (5 LoE, 2 cured) |
| Mazières 2005a | (1) Ketoprofen plaster 100 mg, x 1 daily, n = 87 (2) Placebo plaster, n = 85 |
At 21 days: (1) 29/87 (2) 27/85 |
(1) 11/87 (2) 7/85 |
None | AE: (1) 9/87, (2) 6/85 (1) 6/87 (2 LoE, 4 cured) (2) 5/85 (4 LoE, 1 cured) |
| McLatchie 1989 | (1) Felbinac gel 3% 3 x 3 cm daily, n = 118 (2) Placebo gel, n = 113 |
(1) 3/118 (2) 2/113 Mild transient local irritation |
None reported | None | AE: none Other: none |
| Morris 1991 | (1) Felbinac gel 3% 3 x 1 cm daily, n = 41 (2) Placebo gel, n = 43 |
None | None | None | AE: none (1) 4 (protocol violations) (2) 1 (lost to follow‐up) Exclusions: 11 from efficacy analysis because evaluated by 4 different investigators |
| NCT01255423 | (1) Diclofenac sodium gel 1% x 4 daily, n = 104 (2) Placebo gel x 4 daily, n = 100 | (1) 1/104 (2) 3/102 |
Total AE (1) 11/104 (2) 8/102 |
None | None |
| NCT01272934 | (1) Diclofenac sodium gel 1% x 4 daily, n = 104 (2) Placebo gel x 4 daily, n = 100 | (1) 1/102 (2) 0/103 |
Total AE (excluding SAE) (1) 6/102 (2) 3/103 |
(1) 0/102 (2) 1/103 (ruptured ligaments in wrist) |
AE: see SAE |
| NCT01272947 | (1) Diclofenac sodium gel 1% x 4 daily, n = 104 (2) Placebo gel x 4 daily, n = 100 | None | (1) 2/104 (2) 2/100 |
None | None |
| Noret 1987 | (1) Ketoprofen gel 2.5% 2 x 5 cm (7.5 mg) daily, n = 48 (2) Placebo gel, n = 45 |
(1) 1/51 (2) 0/47 |
None reported | Not reported | AE: (1) 1/51 (skin allergy) (1) 1 LoE, 1 unrelated to trial (2) 1 LoE, 1 unrelated to trial |
| Parrini 1992 | (1) Ketoprofen foam 15% 3 x 2 g (200 mg) daily, n = 83 (2) Placebo foam, n = 86 |
None | None | None | AE: none Other: none |
| Picchio 1981 | (1) Ibuprofen gel 10% 3 x daily , n = 20 (2) Ketoprofen gel 1% 3 x daily, n = 20 |
None | None | None | AE: none Other: not reported |
| Predel 2004 | (1) Diclofenac sodium plaster, 2 x daily (140 mg/plaster), n = 60 (2) Placebo plaster, n = 60 |
12 participants experienced 16 mild AEs with no differences between groups | None | None | AE: (1) 1/60 Other: none |
| Predel 2012 | (1) Diclofenac gel (Voltaren Emulgel 2.32%) 2 x 5 cm daily, n = 80 (2) Diclofenac gel (Voltaren Emulgel 2.32%) 3 x 5 cm daily, n = 80 (3) Placebo gel, n = 82 | (1) 0/80
(2) 1/80 (3) 1/82 All mild to moderate |
(1) 2/80 (2) 1/80 (3) 3/82 | None | AE: (1) 0/80 (2) 0/80 (3) 1/82 Other: (1) 1/80 (2) 2/80 (3) 2/82 (protocol violations, lost to follow‐up) |
| Predel 2013a | (1) Diclofenac 4% spray gel 4 or 5 sprays 3 x daily (96‐120 mg diclofenac sodium), n = 118 (2) Placebo spray gel, n = 114 | (1) 1/118 (2) 4/114 | (1) 5/118
(2) 4/114 All AEs mild, reversible |
None | AE: (1) 1/118 (2) 1/114 Other: (1) 3/118 (2) 43/114 |
| Predel 2013b | (1) Diclofenac gel (Voltaren Emulgel) 4 x 2 g daily, n = 36 (2) Placebo gel 4 x 2 g daily, n = 36 | None | (1) 0/36 (2) 1/36 | None | AE: none Other: none |
| Ramesh 1983 | (1) Ibuprofen cream 5% 3 or 4 x 5‐10 cm daily, n = 40 (2) Placebo cream, n = 40 |
(1) 1/40 (2) 1/40 |
None reported | Not reported | AE: (1) 1/40, (2) 1/40 Other: none |
| Rowbotham 2003 | (1) DHEP plaster (Flector Tissuegel) 2 x daily, n = 191 (2) Placebo plaster, n = 181 |
(1) 27/191 (pruritis 14) (2) 31/181 (pruritis 21) |
(1) 21/191 (2) 22/181 |
None reported ("vast majority mild") | AE: none (1) 3/191, (2) 4/181 (did not finish trial and complete daily diaries) |
| Russell 1991 | (1) Piroxicam gel 0.5% 4 x 5 mg daily, n = 100 (2) Placebo gel, n = 100 |
(1) 4/102 (2) 10/102 |
GI or CNS events: (1) 4, (2) 7 Any AE: (1) 7/102, (2) 15/102 |
None reported | AE: (1) 1/102, (2) 8/102 Other: (1) 6 LoE, 1 "other" (2) 42 LoE Exclusions: 7 did not comply with study medication schedule, 6 lost to follow‐up, 1 protocol violation |
| Saillant 1998 | (1) DHEP plaster (Flector Tissugel 1%) 1 x daily, n = 70 (2) Placebo plaster 1 x daily, n = 70 | None | None | None | AE: none Other: (1) 5/70 (2) 5/70 |
| Sanguinetti 1989 | (1) Felbinac gel 3% 3 x daily, n = 42 (2) Placebo gel, n = 40 |
(1) 3/42 (2) 1/40 | None | None reported | AE: none Other: none reported |
| Sinniger 1981 | (1) Fentiazac cream 5% 2 or 3 x daily, n = 10 (2) Placebo cream, n = 10 |
"No untoward side effects" | None | None | AE: none Other: none reported |
| Spacca 2005 | (1) DHEP lecithin gel (Effigel), 3 x 5 g, daily, n = 79 (2) Placebo gel, n = 76 |
"No signs of cutaneous irritation or sensitisation observed" | No AEs observed | None | AE: none Other: none reported |
| Sugioka 1984 | (1) Piroxicam gel 0.5% 3 or 4 x 1 g daily, n = 183 (2) Indomethacin gel 1% 3 or 4 x 1 g daily, n = 183 |
(1) 5/178 (2) 26/179 |
None reported | None reported | AE: none reported Exclusions due to protocol violations: (1) 8, (2) 18 Withdrawals: (1) 11, (2) 12 |
| Thorling 1990 | (1) Naproxen gel 10% 2‐6 x daily, n = 60 (2) Placebo gel, n = 60 |
(1) 1/60 (2) 0/60 |
None | None | AE: none (1) 1 LoE, 1 protocol violation (2) 1 participant request |
| Tonutti 1994 | (1) Ketoprofen gel 5%, 3 x 2‐3 g daily, n = 15 (2) Etofenamate gel 5%, 3 x 2‐3 g, n = 15 |
None | No AEs attributable to the medication | None | AE: None LoE: (1) 1, (2) 2 |
| Vecchiet 1991 | (1) Meclofenamic acid gel 5% 2 x 10 cm daily (2 g), n = 30 (2) Placebo, n = 30 |
Tolerability excellent or good in nearly all participants | No data | None | AE: none reported (2) 5 lost to follow‐up |
| Whitefield 2002 | (1) Ibuprofen gel 5% + placebo tablet 3 x daily, n = 50 (2) Ibuprofen 400 mg tablet + placebo gel 3 x daily, n = 50 |
No data | 6 AEs reported, none judged related to study medication | None reported | AE: none Recovered: (1) 3, (2) 2 LoE: (2) 1 Lost to follow‐up: (1) 1, (2) 1 |
| AE: adverse event; CNS: central nervous system; DHEP: diclofenac epolamine; GI: gastrointestinal; HCl: hydrochloride; LoE: lack of efficacy; n: number; SAE: serious adverse event. | |||||
Appendix 6. Concentration, amount, and frequency of dosing
| Study | Drug | Concentration | Quantity | Frequency | Estimated daily dose of topical NSAID |
| Joussellin 2003 | Diclofenac | 1% | Plaster | 1 | 180 mg epolamine salt, 140 mg Na salt |
| Li 2013 | Diclofenac | 1% | Plaster | 2 | 360 mg epolamine salt, 280 mg Na salt |
| Rowbotham 2003 | Diclofenac | 1% | Plaster | 2 | 360 mg epolamine salt, 280 mg Na salt |
| Saillant 1998 | Diclofenac | 1% | Plaster | 1 | 180 mg epolamine salt, 140 mg Na salt |
| Coudreuse 2010 | Diclofenac | 1% | Plaster | 1 | 180 mg epolamine salt, 140 mg Na salt |
| Klainguti 2010 | Diclofenac | 1% | Plaster | 1 | 180 mg epolamine salt, 140 mg Na salt |
| Predel 2004 | Diclofenac | ‐ | Plaster | 2 | 280 mg Na salt |
| Predel 2012 | Diclofenac | 2.32% | 5 cm ribbon/˜ 2 g | 2 or 3 | 92‐138 mg (?Na equiv) as diethylamine salt |
| Predel 2013b | Diclofenac | 1.16% | 2 g | 4 | 92 mg (?Na equiv) as diethylamine salt |
| Predel 2013a | Diclofenac | 4% | 4‐5 sprays | 3 | 96‐120 mg Na salt |
| Costantino 2011 | Diclofenac | 1% | Plaster | 1 | 180 mg epolamine salt, 140 mg Na salt |
| Fioravanti 1999 | Diclofenac | ‐ | 5 g | 3 | 195 mg epolamine salt |
| Gallacchi 1990 | Diclofenac | 1% | 2 g | 4 | 80 mg |
| González de Vega 2013 | Diclofenac | 1% | 6 cm, 2g | 3 | 60 mg |
| Hoffmann 2012 | Diclofenac | 1% | Plaster | 1 | 180 mg epolamine salt, 140 mg Na salt |
| Hofman 2000 | Diclofenac | 1% | 2 cm | 4 | probably 30‐40 mg Na salt |
| Jenoure 1997 | Diclofenac | 1% | Plaster | 2 | 360 mg epolamine salt, 280 mg Na salt |
| Mahler 2003 | Diclofenac | ‐ | 5 g | 3 | 195 mg epolamine salt |
| NCT01255423 | Diclofenac | 1% | ‐ | 4 | ‐ |
| NCT01272934 | Diclofenac | 1% | ‐ | 4 | ‐ |
| NCT01272947 | Diclofenac | 1% | ‐ | 4 | ‐ |
| Spacca 2005 | Diclofenac | ‐ | 5 g | 3 | 195 mg epolamine salt |
| Campbell 1994 | Ibuprofen | 5% | 4 inch ribbon | 4 | Assume 800 mg |
| Dreiser 1988 | Ibuprofen | 5% | 4 cm ribbon (10 cm for larger joints) | 4 | Assume up‐800 mg |
| Ramesh 1983 | Ibuprofen | 5% | 5‐10 cm ribbon | 3‐4 | Assume 300‐800 mg |
| Billigmann 1996 | Ibuprofen | 5% | 10 cm, 4 g gel | 3 | 600 mg |
| Machen 2002 | Ibuprofen | 5% | ‐ | 3 | ‐ |
| Picchio 1981 | Ibuprofen | 10% | ‐ | 3 | ‐ |
| Whitefield 2002 | Ibuprofen | 5% | ‐ | 3 | ‐ |
| Mazières 2005b | Ketoprofen | ‐ | Plaster | 1 | 100 mg |
| Mazières 2005a | Ketoprofen | ‐ | Plaster | 1 | 100 mg |
| Airaksinen 1993 | Ketoprofen | ‐ | 5 g | 2 | 125 mg |
| Dreiser 1989 | Ketoprofen | 2.5% | 5 cm | 2 | 100 mg |
| Julien 1989 | Ketoprofen | 2.5% | 5 cm | 2 | 100 mg |
| Noret 1987 | Ketoprofen | 2.5% | 5 cm, 7.5 g | 2 | 375 mg |
| Curioni 1985 | Ketoprofen | No details | 2 | ‐ | |
| Governali 1995 | Ketoprofen | 5% | 2‐3 g | 3 | 300‐450 mg |
| Governali 1995 | Ketoprofen | 1% | 2‐3 g | 3 | 60‐90 mg |
| Gualdi 1987 | Ketoprofen | ‐ | 3‐5 cm | 2 | ‐ |
| Kockelbergh 1985 | Ketoprofen | 2.5% | 5 cm | 2 | 100 mg |
| Parrini 1992 | Ketoprofen | 15.0% | 2 g | 3 | 600 mg |
| Picchio 1981 | Ketoprofen | 1% | ‐ | 3 | ‐ |
| Tonutti 1994 | Ketoprofen | 5% | 2‐3 g | 3 | 300‐450 mg |
| Ăkermark 1990 | Indomethacin | 1% | 0.5‐1.5 mL | 3‐5 | 12‐60 mg |
| Aoki 1984 | Indomethacin | 1% | 1 g | 3‐4 | 30‐40 mg |
| Fujimaki 1985 | Indomethacin | 1% | 1 g | 3‐4 | 30‐40 mg |
| Sugioka 1984 | Indomethacin | 1% | 1 g | 3‐4 | 30‐40 mg |
| Aoki 1984 | Piroxicam | 0.5% | 1 g | 3‐4 | 15‐20 mg |
| Fujimaki 1985 | Piroxicam | 0.5% | 1 g | 3‐4 | 15‐20 mg |
| Russell 1991 | Piroxicam | 0.5% | 5 mg | 4 | 20 mg |
| Sugioka 1984 | Piroxicam | 0.5% | 1 g | 3‐4 | 15‐20 mg |
| Chatterjee 1977 | Benzydamine | 3% | ‐ | 3 | ‐ |
| Haig 1986 | Benzydamine | 3% | ‐ | 6 | ‐ |
| Linde 1985 | Benzydamine | 3% | ‐ | 3 | ‐ |
| Auclair 1989 | Niflumic acid | 2.5% | 10 cm, 5 g | 3 | 375 mg |
| Dreiser 1990 | Niflumic acid | 2.5% | 10 cm, 5 g | 3 | 375 mg |
| Curioni 1985 | Ibuproxam gel | 10% | ‐ | 2 | ‐ |
| Curioni 1985 | Etofenamate | No details | ‐ | 2 | ‐ |
| Diebshlag 1990 | Etofenamate | 5% | 3 g | 3 | 450 mg |
| Tonutti 1994 | Etofenamate | 5% | 2‐3 g | 3 | 300‐450 mg |
| Diebshlag 1990 | Ketorolac | 2% | 3 g | 3 | 360 mg |
| Dreiser 1994 | Flurbiprofen | Patch | 2 | 80 mg | |
| Gualdi 1987 | Flunoxaprofen | ‐ | 3‐5 cm | 2 | ‐ |
| Hofman 2000 | Lysine clonixinate | 5% | 2 cm | 4 | 90 mg |
| Hosie 1993 | Felbinac | 3% | 2 g | 3 | 180 mg |
| McLatchie 1989 | Felbinac | 3% | 3 cm | 3 | ‐ |
| Morris 1991 | Felbinac | 3% | 1 cm | 3 | ‐ |
| Sanguinetti 1989 | Felbinac | 3% | ‐ | 3 | ‐ |
| Sinniger 1981 | Fentiazac | 5% | Varied according to involved areas | 2‐3 | ‐ |
| Thorling 1990 | Naproxen | 10% | ‐ | 2‐6 | ‐ |
| Vecchiet 1991 | Meclofenamic acid | 5% | 10 cm, 4 g | 2 | 400 mg |
| equiv: equivalent; Na: sodium. | |||||
Data and analyses
Comparison 1. Individual NSAID versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical success | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Diclofenac | 10 | 2050 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.49, 1.72] |
| 1.2 Ibuprofen | 5 | 436 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.33, 2.01] |
| 1.3 Ketoprofen | 7 | 683 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.37, 1.77] |
| 1.4 Piroxicam | 3 | 504 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.27, 1.73] |
| 1.5 Indomethacin | 3 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.03, 1.55] |
| 1.6 Benzydamine | 3 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.96, 1.38] |
| 2 Local adverse events | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Diclofenac | 15 | 3271 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.56, 1.10] |
| 2.2 Ibuprofen | 3 | 321 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [0.98, 5.43] |
| 2.3 Ketoprofen | 8 | 852 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.83, 1.70] |
| 2.4 Piroxicam | 3 | 522 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.17, 1.08] |
| 2.5 Felbinac | 3 | 397 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [0.49, 7.50] |
| 2.6 Indomethacin | 3 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.65 [0.91, 7.73] |
Comparison 2. Diclofenac versus placebo (effect of formulation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical success | 10 | 2050 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.49, 1.72] |
| 1.1 Plaster ‐ Flector | 4 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.36, 1.71] |
| 1.2 Plaster ‐ other | 3 | 474 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.55 [1.37, 1.75] |
| 1.3 Gel ‐ Emulgel | 2 | 314 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.84 [2.68, 5.50] |
| 1.4 Gel ‐ other | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.05, 1.27] |
Comparison 3. Ibuprofen versus placebo (effect of formulation).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical success | 5 | 436 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.33, 2.01] |
| 1.1 Cream | 3 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.03, 1.59] |
| 1.2 Gel | 2 | 241 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.66 [1.69, 4.21] |
Comparison 4. Ketoprofen versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical success | 7 | 683 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [1.37, 1.77] |
| 1.1 Plaster | 2 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.04, 1.40] |
| 1.2 Gel | 5 | 348 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.19 [1.74, 2.75] |
Comparison 5. All topical NSAIDs versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Local adverse events | 42 | 6740 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.80, 1.21] |
| 2 Systemic adverse events | 36 | 5576 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.73, 1.26] |
| 3 Adverse event withdrawals | 42 | 6405 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.64, 1.59] |
Comparison 6. Topical NSAID versus active comparator.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical success ‐ topical piroxicam vs topical indomethacin | 3 | 641 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.07, 1.44] |
| 2 Local adverse events ‐ topical piroxicam vs topical indomethacin | 3 | 671 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.09, 0.47] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Airaksinen 1993.
| Methods | R, DB, PC, parallel groups Gel applied to the painful area twice daily for 7 days Assessment at baseline, 3, 7 days |
|
| Participants | Minor soft tissue injuries (< 7 days) N = 56 M 45, F 11 Age not reported Mean baseline pain at rest 25‐26 mm |
|
| Interventions | Ketoprofen gel, 2 x 5 g (125 mg) daily, n = 29 Placebo gel, n = 27 Rescue medication paracetamol 500 mg No other treatment allowed |
|
| Outcomes | PGE: 5‐point scale but reported as "improved" or "same or worse" (responder = "improved") Improvement in pain with movement: 100 mm VAS, reported as group mean Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of early withdrawals or method of imputation |
| Size | High risk | < 50 participants per treatment group |
Aoki 1984.
| Methods | R, DB, PC, AC, parallel groups Gel applied to affected area 3 or 4 times daily, with no occlusion for 7 days Assessment at baseline, 3, 7 days |
|
| Participants | Acute orthopaedic trauma (contusion, distortion, fracture, < 7 days) N = 252 (203 analysed for efficacy) M 98, F 105 Age range 8 to 86 years, 13% younger than 20 years Baseline pain mild in 35% Exclusions: 23 protocol violations, 26 reasons "not related" to drug. Equally distributed between groups |
|
| Interventions | Piroxicam gel 0.5%, 1 g 3 to 4 x daily, n = 84 Indomethacin gel 1%, 1 g 3 to 4 x daily, n = 84 Placebo gel, n = 84 No other medication or initiation of physical therapy allowed |
|
| Outcomes | PGE: 5‐point scale (responder = "better" and "much better") Adverse events Withdrawals and exclusions |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | "key code sealed until end of study" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Gels in "identical tubes" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | > 10% withdrawals "unrelated to treatment" and for "protocol violations". No further details, but no significant differences between groups |
| Size | Unclear risk | 50 to 200 participants per treatment group |
Auclair 1989.
| Methods | R, DB, PC, parallel groups Gel massaged into skin over affected heel 3 times daily after cleaning with soap and water for up to 21 days Assessment at baseline, 7, 21 days |
|
| Participants | Acute Achilles heel tendinitis (not associated with continuous pain at rest or > 1 month history) N = 243 (227 analysed for efficacy) M/F not reported Mean age 29 years Baseline pain: ˜ 10% had < 26 mm on palpation of tendon, ˜ 30% had mild or no pain on dorsiflexion of foot Exclusions: failure to meet inclusion criteria, major protocol violations, failure to take study medication for full duration |
|
| Interventions | Niflumic acid gel 2.5%, 3 x 5 g daily, n = 117 Placebo gel, n = 110 No other analgesics and anti‐inflammatories, physiotherapy or supportive measures allowed |
|
| Outcomes | PGE: 5‐point scale (responder = "good" or "very good") Pain improved or disappeared on dorsiflexion Adverse events Withdrawals and exclusions |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 10% excluded for "failing to meet entry criteria and protocol violations". No further details |
| Size | Unclear risk | 50 to 200 participants per treatment group |
Billigmann 1996.
| Methods | R, DB, PC, parallel groups Gel applied 3 times daily with rubbing Assessed at baseline, 3, 5, 7 days |
|
| Participants | Distortion of ankle joint N = 160 M and F Age 18+ years Baseline pain not reported |
|
| Interventions | Ibuprofen microgel 5%, 3 x 10 cm (= 200 mg) daily, n = 80 Placebo gel, n = 80 |
|
| Outcomes | Pain with movement: VAS (responder = decreased by 20%) Complete remission Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient data to assess |
| Size | Unclear risk | 50 to 200 participants per treatment group |
Campbell 1994.
| Methods | R, DB, PC, parallel groups Cream applied 4 times daily for 7 days (up to 14 days optional) Self assessed using daily diary for 7 days, and up to 14 days |
|
| Participants | Acute ankle sprain (< 24 hours, no fracture) N = 100 (51 analysed) M 33, F 18 Mean age 29 years Baseline pain at rest > 35 mm, on walking 80 mm Exclusions: did not return diaries, protocol exclusions (25 ibuprofen, 24 placebo) |
|
| Interventions | Ibuprofen cream 5% (Proflex), 4 x 4" (10 cm) daily, n = 26 Placebo cream, n = 25 Advised to use rest and regular icing for 48 hours, then walking and exercise Rescue medication: paracetamol |
|
| Outcomes | Improvement in walking ability: 4‐point scale (responder = "improvement") Pain on walking: 100 mm VAS (mean data) Withdrawals and exclusions |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Randomisation carried out by sponsor. Tubes dispensed by hospital pharmacy who held the codes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "identical cream" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | > 40% lost to follow‐up. Approximately equal between groups |
| Size | High risk | < 50 participants per treatment arm as analysed |
Chatterjee 1977.
| Methods | R, DB, PC, parallel groups Cream applied to site of injury 3 times daily for 6 days Assessment at baseline, 2, 6 days |
|
| Participants | Soft tissue injuries (recent) N = 51 M/F not reported Age not reported Baseline pain on passive movement moderate or severe in all but 3 participants |
|
| Interventions | Benzydamine HCl cream 3%, 3 x daily, n = 25 Placebo cream, n = 25 (5 active, 6 placebo participants also received ultrasound) No other topical agent allowed |
|
| Outcomes | Pain on passive movement: 4‐point scale (responder = "absent" or "slight") Tenderness with pressure: 4‐point scale (responder = "absent" or "slight") Adverse events Withdrawals and exclusions |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "predetermined randomised schedule" |
| Allocation concealment (selection bias) | Low risk | Sealed copy of schedule held by investigator and duplicate copy kept by clinical trial co‐ordinator. Looked at only in event of adverse reaction (not necessary) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "indistinguishable .... in appearance and consistency" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2% lost to follow‐up, no withdrawals due to adverse events. Responder analysis |
| Size | Unclear risk | < 50 participants per treatment arm |
Costantino 2011.
| Methods | R, DB, PC, multicentre, parallel group Plaster applied daily for 7 days PI assessment daily, overall treatment efficacy and tolerability assessed at 3 and 7 days |
|
| Participants | Grade I or II ankle sprain (< 48 hours) with lateral external ligament involvement
PI on movement ≥ 50/100, oedema ≥ 20 mm difference between ankles N = 430 M 249, F 175 (for analysis) Mean age 35 years Baseline PI on movement 72/100 (SD 12) |
|
| Interventions | DHEP/hep, n = 142
DHEP, n = 146
Placebo, n = 142 Rescue medication: paracetamol to maximum 3 g daily No other treatments allowed |
|
| Outcomes | Mean change in PI on movement from baseline to 3 days Mean reduction in oedema at 3 days Tolerability at 3 days Rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | "sealed envelopes" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Shape, colour, size, and application method identical for all plasters |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Very few withdrawals, but used LOCF |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
Coudreuse 2010.
| Methods | R, DB, PC, multicentre, parallel group Plaster applied daily for 7 days PI assessed twice daily over 3 days, then day 7. Overall treatment efficacy and tolerability assessed on days 3, 7 |
|
| Participants | Ankle sprain (< 48 hours) with lateral external ligament involvement
PI on movement ≥ 50/100, oedema ≥ 20 mm difference between ankles N = 240 (233 for analysis) M 148, F 86, 6 unknown |
|
| Interventions | DHEP/hep, n = 120
Placebo, n = 120 Rescue medication: paracetamol to maximum 4 g daily No other treatments allowed |
|
| Outcomes | Global efficacy at 7 days: 4‐point scale (responder = "excellent" or "good") Mean change in PI on movement at 6 hours and 7 days Mean change in oedema at 3 and 7 days Tolerability Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | "sealed envelopes" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Appearance and odour identical for all plasters |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis, < 5% excluded for missing data, equal between groups |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
Curioni 1985.
| Methods | R, DB, PC, AC, parallel groups Gel rubbed into affected area until absorbed, twice daily for 10 days Assessed at baseline, and daily to 10 days |
|
| Participants | Acute soft tissue injuries N = 60 M 33, F 27 Median age 33 years Baseline pain not given |
|
| Interventions | Ibuproxam gel 10%, n = 20 Ketoprofen gel, n = 20 Etofenamate gel, n = 20 |
|
| Outcomes | PGE: 4‐point scale ("good" or "excellent") Resolution of symptoms Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Medication supplied in identical tubes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analysis |
| Size | High risk | < 50 participants per treatment arm |
Diebshlag 1990.
| Methods | R, DB, PC, AC, parallel groups Gel applied 3 times daily, without occlusion, for 14 days Assessment at baseline, 2, 3, 4, 8, 15 days |
|
| Participants | Ankle sprain (< 24 hours) N = 37 M 24, F 13 Mean age 28 years Baseline pain slight to moderate |
|
| Interventions | Ketorolac gel 2%, 3 x 3 g daily, n = 13 Etofenamate gel 5%, 3 x 3 g daily, n = 12 Placebo gel, n = 12 Rescue medication: paracetamol No other analgesic or anti‐inflammatory medication, ice packs, or physiotherapy allowed |
|
| Outcomes | Reduction in PI: 100 mm VAS and 4‐point scale (responder = "improved") Adverse events Withdrawals and exclusions |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | "Medication assignment ... supplied in a sealed envelope." Opened only if serious participant event necessitation treatment disclosure occurred (not necessary) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "identical appearance" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analysis |
| Size | High risk | < 50 participants per treatment arm |
Dreiser 1988.
| Methods | R, DB, PC, parallel groups Cream applied 3 times daily Assessment at baseline, 7 days |
|
| Participants | Acute tendinitis (< 1 month) N = 64 M 35, F 25 Mean age 36 years Baseline spontaneous pain ≥ 60 mm |
|
| Interventions | Ibuprofen cream 5%, 3 x 4 cm daily, n = 32 (3 x 10 cm for large joints) Placebo cream, n = 32 No other topical, systemic, or physical treatment allowed |
|
| Outcomes | PGE: scale not reported (responder = "improvement" or "complete relief") Improvement in pain: VAS (mean data) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 Oxford Validity Score: 10/16 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing participants added back using BOCF |
| Size | High risk | < 50 participants per treatment arm |
Dreiser 1989.
| Methods | R, DB, PC, parallel groups Gel applied twice daily to affected area with light massage, then covered with standard compress Assessed at baseline, 3, 7 days |
|
| Participants | Uncomplicated, recent ankle sprain N = 60 M 36, F 24 Mean age 33 years Mean baseline pain 54 mm |
|
| Interventions | Ketoprofen gel 2.5%, 2 x 5 cm daily, n = 30 Placebo gel, n = 30 No concomitant therapy other than simple oral analgesia allowed |
|
| Outcomes | PGE: 3‐point scale (responder = "better") Improvement in pain: VAS (mean data) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "drawing lots" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Treatments "identical in every way except that placebo did not contain active principle" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analysis |
| Size | High risk | < 50 participant per treatment arm |
Dreiser 1990.
| Methods | R, DB, PC, parallel groups Gel lightly massaged into skin over affected area 3 times daily, then covered with standard compress Assessed at baseline, 3, 7 days |
|
| Participants | Uncomplicated, ankle sprain (< 4 days) N = 60 (59 analysed) M 29, F 29 (not stated for 1 participant) Mean age 33 years Baseline pain ≥ moderately severe Exclusions: 1 participant had only moderate pain at baseline |
|
| Interventions | Niflumic acid gel 2.5%, 3 x 5 g daily, n = 30 Placebo gel, n = 30 Concomitant treatment with systemic NSAIDs, local therapies, or physiotherapy were not allowed |
|
| Outcomes | PGE: 4‐point scale (responder = "cured" or "improved") Improvement in pain: VAS (mean data) Adverse events Withdrawals and exclusions |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analysis |
| Size | High risk | < 50 participants per treatment arm |
Dreiser 1994.
| Methods | R, DB, PC, parallel groups Patch applied twice daily Assessed at baseline, 3, 7 days |
|
| Participants | Traumatic ankle sprain (< 2 days) N = 131 M 84, F 47 Mean age 34 years Baseline pain ≥ 50 mm |
|
| Interventions | Flurbiprofen patch, 2 x 40 mg daily, n = 65 Placebo patch, n = 66 Rescue medication: paracetamol. Ice or light restraint allowed Exclusions: 1 from flurbiprofen group for protocol violation |
|
| Outcomes | PGE: 4‐point scale (responder = "good" or "very good") Improvement in pain: VAS (mean data) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo patch was "non‐medicated (but otherwise identical)" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analysis |
| Size | High risk | < 50 participants per treatment arm |
Fioravanti 1999.
| Methods | R, DB, AC, parallel groups Gel lightly massaged into skin 3 times daily, and kept dry for 6 to 8 hours Assessed at baseline, 3, 10 days |
|
| Participants | Peri and extra‐articular inflammatory diseases N = 100 M 32, F 68 Mean age 49 years Baseline spontaneous pain ≥ 40 mm |
|
| Interventions | DHEP lecithin gel, 3 x 5 g (= 65 mg) daily, n = 50 DHEP gel, 3 x 5 g (= 65 mg) daily, n = 50 |
|
| Outcomes | PGE: 4‐point scale (responder = "good" or "excellent") Pain on movement: mean Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analysis |
| Size | Unclear risk | 50 participants per treatment arm |
Fujimaki 1985.
| Methods | R, DB, PC, AC, parallel groups Gel applied to affected area 3 or 4 times daily with no occlusion for up to 14 days Assessed at baseline, 7, 14 days |
|
| Participants | Muscle pain or inflammation in neck, shoulder, back, chest and upper and lower extremities, or a combination N = 271 (247 analysed) M 97, F 149 Age < 20 to 89 years Baseline pain mostly mild to moderate Exclusions: 24 due to protocol violations, loss to follow‐up |
|
| Interventions | Piroxicam gel 0.5% 1 g, 3 to 4 x daily, n = 92 Indomethacin gel 1% 1 g, 3 to 4 x daily, n = 90 Placebo gel, n = 89 No concomitant oral or topical analgesic or anti‐inflammatory medication allowed. No physical therapy initiated after start of study |
|
| Outcomes | PGE: 5‐point scale (responder = "better" or "much better") Physician rated improvement: 5‐point scale (responder = "marked improvement") Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total =4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Cartons numbered randomly and numbers held in a key code until study completion |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "identical tubes" packed in numbered carton. Gel bases slightly different in appearance, so dispensing physician did not have access to them |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ˜ 8% excluded from analysis for unknown reasons or lost to or inadequate follow‐up. Approximately equal between groups |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
Gallacchi 1990.
| Methods | R, DB, AC, parallel groups Gel applied to affected area 4 times daily, with light massage, for 14 days Assessment at baseline, 7, 14 days |
|
| Participants | Painful inflammatory conditions N = 50 M 20, F 30 Mean age 50 years Baseline pain ≥ moderate severity |
|
| Interventions | Diclofenac gel 1%, 2 g 4 x daily, n = 25 (Flector) Diclofenac sodium 1%, 2 g 4 x daily, n = 25 (Voltaren Emugel) No other medication that could interfere with test drugs allowed |
|
| Outcomes | PGE: 5‐point scale (responder = "good" or "excellent") Improvement in pain on pressure: 4‐point scale (mean data) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analysis |
| Size | High risk | < 50 participants per treatment arm |
González de Vega 2013.
| Methods | R, DB (for gels), SB (for ointment), AC, multicentre, parallel groups 2 g (˜ 6 cm of gel) applied over injured area x 3 daily for 14 days Assessments at 0, 4, 7, 14 days of treatment, and day 42 follow‐up |
|
| Participants | Grade I or II ankle sprain (< 24 hours) with lateral external ligament involvement, PI on weight bearing ≥ 30/100 N = 449 M = 308, F = 112 (for analysis) Mean age 28 years |
|
| Interventions | Traumeel gel, 3 x 2 g daily, n = 140 Traumeel ointment, n = 143 (not analysed in this review) Diclofenac gel 1%, 3 x 2 g daily, n = 137 Rescue medication: paracetamol to maximum 2 g daily |
|
| Outcomes | Pain‐free on day 7 Global efficacy: 5‐point scale (responder = "good" and "very good") on day 14 Normal function on day 14: yes or no (responder = "yes") Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2 (gels), W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Central allocation, kits assigned in order received, and used envelopes (no further details) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | For gel comparison: "identical containers" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Used LOCF. Most withdrawals due to early recovery (within 14 days), approximately equal between groups |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
Governali 1995.
| Methods | R, DB, AC, parallel groups Gel or cream applied 3 times daily for up to 14 days Assessed at baseline, 7, 14 days |
|
| Participants | Soft tissue injuries + 2 fractures N = 30 M = 21, F = 9 Median age 38 years Mean baseline pain on movement moderate to severe (2.8, scale 0 to 4) |
|
| Interventions | Ketoprofen gel 5%, 3 x 2 to 3 g daily, n = 15 Ketoprofen cream 1%, 3 x 2 to 3 g daily, n = 15 |
|
| Outcomes | PGE: 5‐point scale (responder = "good" and "excellent") Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total =3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Treatments were given in identical tubes and measurements made by blinded observers, but one was a cream and the other a gel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analysis |
| Size | High risk | < 50 participants per treatment arm |
Gualdi 1987.
| Methods | R, DB, AC, parallel groups Gel applied twice daily for 10 days Assessed at baseline, 4, 7, 10 days |
|
| Participants | Soft tissue injuries N = 60 M = 37, F = 23 Mean age 32 years (range 13 to 78) Mean baseline pain on movement moderate to severe (2.2, scale 0 to 3) |
|
| Interventions | Flunoxaprofen gel, 2 x 3 to 5 cm daily, n = 30 Ketoprofen gel, 2 x 3 to 5 cm daily, n = 30 |
|
| Outcomes | Improvement in pain on pressure (mean data) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient data |
| Size | High risk | < 50 participants per treatment arm |
Haig 1986.
| Methods | R, DB, PC, parallel groups Cream applied lightly to affected area 6 times daily for 6 days Assessed at baseline, 2, 4, 6 days |
|
| Participants | Soft tissue injuries (< 24 hours) N = 43 M/F not reported Age not reported Baseline pain not reported |
|
| Interventions | Benzydamine cream 3%, 6 x daily, n = 21 Placebo cream, n = 22 |
|
| Outcomes | Pain on movement: 4‐point scale (responder = "improved") Adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W0. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "matching placebo" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants apparently included in analysis |
| Size | High risk | < 50 participants per treatment arm |
Hoffmann 2012.
| Methods | R, DB, PC, multicentre, parallel group Plaster applied x 1 daily (minimum of 20 consecutive hours of application per day) for 14 days PI assessed daily, overall efficacy assessed at 7 and 14 days |
|
| Participants | Unilateral, mild to moderate muscle contusion of upper or lower limb (< 72 hours), PI ≥ 50/100, superficial haematoma ≤ 10 x 14 cm at injured site N = 354 M 126, F 228 Mean age 39 years |
|
| Interventions | DHEP/hep plaster, x 1 daily, n = 121
DHEP plaster, x 1 daily, n = 115
Placebo plaster, n = 118 Rescue medication: paracetamol 500 mg (no limit reported) |
|
| Outcomes | PGE: 5‐point scale (responder = excellent or good) at 14 days Mean reduction in PI at 3 and 8 days Rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported: "Medication packed and labelled in order to render all participants and personnel fully blinded to treatment administered" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Indistinguishable regarding appearance, shape, colour, size and odour" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No responder analysis reported, and imputation method not mentioned |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
Hofman 2000.
| Methods | R, DB, AC, parallel groups Gel applied to affected region 4 times daily, with gentle massage Assessed at baseline, 8 days in clinic and daily diary |
|
| Participants | Soft tissue articular pain (≤ 15 days) N = 142 M 19, F 123 Mean age 57 years Mean baseline PI moderate to severe |
|
| Interventions | Diclofenac sodium gel 1%, 4 x 2 cm daily, n = 69 Lysine clonixinate gel 5%, 4 x 2 cm daily, n = 73 (2 cm = 22.5 mg) No other analgesic, local treatment (including immobilisation, bandaging), or acupuncture Rescue mediation allowed after 2 applications, if needed |
|
| Outcomes | PGE: 3‐point scale ("good") PI: participant diary (mean data) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Diclofenac gel repackaged to maintain DB with lysine clonixinate gel. Minor differences between gels only apparent when directly compared |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analysis |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
Hosie 1993.
| Methods | R, DB, AC, parallel groups Foam (approximately the size of a golf ball) applied, and 1 tablet taken, 3 times daily for 7 days and up to 14 days Assessed at baseline, 7, 14 (if necessary) days |
|
| Participants | Acute lower back injury (< 1 month) N = 287 (261 analysed for efficacy) M 151, F 136 Mean age 37 years (range 18 to 63) Most participants had moderate to severe pain on movement, one had none Exclusions: 25 lost to follow up, one assessed at 14 days, but not 7 days |
|
| Interventions | Felbinac foam 3%, 3 x 2 g daily + placebo tablets, 3 x 1 daily, n = 140 (127 analysed for efficacy) Ibuprofen tablets, 3 x 400 mg daily + placebo foam, 3 x 2 g daily, n = 147 (134 analysed for efficacy, but one had no pain at baseline) No other oral, injectable, or topical analgesic or anti‐inflammatory medication. Ongoing physiotherapy to continue without change |
|
| Outcomes | Pain on movement: 5‐point scale (responder = "none" or "mild") Spontaneous pain: 5‐point scale (responder = "none" or "mild") Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double dummy" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Imputation method not mentioned |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
Jenoure 1997.
| Methods | R, DB, PC, parallel groups Plaster applied to skin over affected area twice daily, and kept in place with an elastic bandage Assessed at baseline, 7, 14 days, and after further 14 days without treatment |
|
| Participants | Humero‐radial epicondyl pain (tendinopathic) ‐ nearly all tennis elbow N = 85 M 54, F 31 Mean age 45 years Baseline pain: "mild" in ˜ 10% of placebo group and 29% of active group |
|
| Interventions | DHEP plaster (Tissugel), x 2 daily, n = 44 Placebo plaster x 2 daily, n = 41 |
|
| Outcomes | Pain on pressure: 5‐point scale (responder = "none" or "mild") Spontaneous pain: 5‐point scale (responder = "no pain") Adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W0. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "identical characteristics" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No useable data |
| Size | High risk | < 50 participants per treatment arm |
Joussellin 2003.
| Methods | R, DB, PC, parallel groups Plaster applied to skin over affected area once daily Assessed at baseline, 1, 2, 3, 7 days |
|
| Participants | Ankle sprain (< 48 hours) N = 134 M 72, F 62 Age range 18 to 65 years Baseline spontaneous pain ≥ 50 mm |
|
| Interventions | DHEP plaster (Flector Tissugel 1%), x 1 daily, n = 68 Placebo plaster, x 1 daily, n = 66 Rescue medication: paracetamol |
|
| Outcomes | PGE: 4‐point scale (responder = "excellent") Pain on movement: VAS (mean) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Identical in size, appearance and used same formula as active patch, without active ingredient" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in responder analysis |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
Julien 1989.
| Methods | R, DB, PC, parallel groups Gel applied to affected area twice daily, with light massage Assessed at baseline, 3, 7 days in clinic and daily diary |
|
| Participants | Tendinitis N = 60 M 29, F 31 Mean age 41 years Baseline pain > 50 mm |
|
| Interventions | Ketoprofen gel 2.5%, 2 x 5 cm (= 50 mg) daily, n = 30 Placebo gel, 2 x 5 cm daily, n = 30 No concomitant therapy other than simple analgesia |
|
| Outcomes | PGE: 4‐point scale (responder = "improved" or "recovered") Pain on movement: 4‐point scale (mean data) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB1, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Randomisation code supplied by Menarini laboratories, remote from allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in responder analysis |
| Size | High risk | < 50 participants per treatment arm |
Klainguti 2010.
| Methods | R, DB, PC, multicentre, parallel group Plaster applied x 1 daily (minimum 20 consecutive hours of application daily) for 10 days PI assessed daily, Overall treatment efficacy and tolerability assessed at days 3 and 7 |
|
| Participants | Unilateral, mild to moderate muscle contusion or strain of upper or lower limb (< 72 hours), superficial haematoma ≤ 140 cm2, PI ≥ 40/100 N = 185 M 90, F 95 Mean age 39 years |
|
| Interventions | DHEP/hep plaster, x 1 daily, n = 65 DHEP plaster, x 1 daily, n = 61 Placebo plaster, x 1 daily, n = 59 | |
| Outcomes | Overall treatment efficacy at 3 days: 5‐point scale (responder = "good" or "excellent") Resolution of haematoma at 10 days: yes or no Rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Identical in size, appearance and odour" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals < 5%. All participants included in analysis |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
Kockelbergh 1985.
| Methods | R, DB, PC, parallel groups Gel applied twice daily Assessed at baseline, 3, 7 days |
|
| Participants | Acute soft tissue trauma (< 24 hours) N = 74 M 60, F 14 Mean age 27 years Baseline pain > 65 mm |
|
| Interventions | Ketoprofen gel 2.5%, 2 x 5 cm (= 15 mg) daily, n = 38 Placebo gel, 2 x 5 cm daily, n = 36 No concomitant treatment Rescue medication: glafenine |
|
| Outcomes | PGE: 3‐point scale (responder = "good") Spontaneous pain: 100 mm VAS (mean data) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in responder analysis |
| Size | High risk | < 50 participants per treatment arm |
Kuehl 2011.
| Methods | R, DB, PC, multicentre, parallel groups Plaster applied x 2 daily for 14 days or until resolution PI assessed daily. Overall treatment efficacy assessed at 14 days |
|
| Participants | Mild or moderate sprain, strain or contusion (< 7 days), PI ≥ 5/10 N = 418 M 206, F 212 Mean age 39 years |
|
| Interventions | DHEP patch, x 2 daily, n = 207
Placebo patch, x 2 daily, n = 211 Rescue medication: no analgesic allowed (or ice/wrapping): use = discontinuation |
|
| Outcomes | Resolution of injury (VAS ≤ 2/10) Overall tolerability: 5‐point scale (responder = "good" or "excellent") Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "identical in appearance with same content except diclofenac" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Used LOCF, high withdrawal rate (43%). Did not report all efficacy outcomes measured |
| Size | Low risk | > 200 participants per treatment arm |
Li 2013.
| Methods | R, DB, PC, multicentre, parallel groups Plaster applied x 2 daily for 7 days PI assessed daily, overall treatment efficacy and tolerability assessed at 7 days |
|
| Participants | Mild or moderate ankle or knee sprain, muscle strain or contusion (< 72 hours), PI ≥ 50/100
N = 384 M 144, F 240 Mean age 42 years |
|
| Interventions | DHEP plaster, x 2 daily, n = 192
Placebo plaster, x 2 daily, n = 192 Rescue medication: paracetamol to maximum 2 g daily |
|
| Outcomes | ≥ 50% reduction in PI at 7 days Overall treatment efficacy: 5‐point scale (responder = "good" or "excellent") Overall tolerability: 5‐point scale (responder = "good" or "excellent") Rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Method not fully described: "sealed envelopes", "concealed from investigators and participants" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "identical in texture, size, color and odor" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawals 2%. Methods states LOCF, but appears to describe BOCF for withdrawals |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
Linde 1985.
| Methods | R, DB, PC, parallel groups Cream applied x 3 daily for 5 days, with elastic support for the first 3 days Assessed at baseline 4, 8 days |
|
| Participants | Sprained ankle (< 24 hours) N = 100 M 58, F 42 Mean age 28 years Baseline pain: all participants had "walking pain" |
|
| Interventions | Benzydamine 3% cream, x 3 daily, n = 50 Placebo gel, x 3 daily, n = 50 |
|
| Outcomes | Pain on movement: responder = "free of walking pain" Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1 Total = 3/5 *Paper describes a benzydamine cream and a placebo gel |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. Paper describes a benzydamine cream and a placebo gel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in responder analysis |
| Size | Unclear risk | 50 participants per treatment arm |
Machen 2002.
| Methods | R, DB, PC, parallel groups Gel gently ("minimal rub", not vigorously) massaged into skin over affected site until absorbed 3 times daily until symptoms disappeared or for maximum of 7 days Assessment at baseline and once daily using diary cards to 7 days |
|
| Participants | Soft tissue injury (< 2 weeks and untreated) N = 85 (81 analysed) M 42, F 39 Mean age 41 years Baseline pain > 50 mm 4 placebo participants lost to follow‐up |
|
| Interventions | Ibuprofen gel 5%, x 3 daily, n = 40 Placebo gel, x 3 daily, n = 41 Initiation of other medication or physiotherapy not allowed during study |
|
| Outcomes | PGE: 5‐point scale (responder = "marked improvement" or "complete clearance") Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Gels had similar physical characteristics and were supplied in identical tubes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 5% missing participants. Remaining participants included in responder analysis |
| Size | High risk | < 50 participants per treatment arm |
Mahler 2003.
| Methods | R, DB, AC, parallel groups Gel applied with gentle massage to affected area 3 times daily, without occlusion, for 10 days Assessed at baseline, 3, 10 days in clinic and daily patient diary |
|
| Participants | First‐degree ankle or knee sprains, first‐degree muscle strains and mild‐to‐moderate contusions n = 100 M 69, F 31 Mean age 32 years Mean baseline pain with activity ≥ 65 mm |
|
| Interventions | DHEP lethicin gel, 3 x 5 g (= 65 mg) daily, n = 52 DHEP gel, 3 x 5 g (= 65 mg) daily, n = 48 All participants treated with ice at site of inflammation for first 48 hours, but no immobilisation allowed Rescue medication: paracetamol 500 mg if strictly necessary |
|
| Outcomes | PGE: 4‐point scale (responder = "good" or "excellent") Pain on movement: 100‐mm VAS (mean data) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated randomization list" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Pharmaceutically inert colouring agents added to reference formulation so that gels were indistinguishable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in responder analysis |
| Size | High risk | < 50 participants in each treatment arm |
Mazières 2005a.
| Methods | R, DB, PC, parallel groups New patch applied directly to skin over painful area each morning Assessed at baseline, 3, 7, 14 days |
|
| Participants | Symptomatic tendonitis in upper or lower limbs, not requiring surgery (≤ 15 days) N = 172 M 72, F 100 Mean age 46 years Baseline pain with activity ≥ 40 mm |
|
| Interventions | Ketoprofen patch 100 mg, x 1 daily, n = 87 Placebo patch, x 1 daily, n = 85 No analgesic or steroid by any route or other topical medication or physical therapy allowed Rescue medication permitted, but not within 12 hours of assessment |
|
| Outcomes | PGE: 4‐point scale (responder = "good" or "excellent") Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated global randomization code" |
| Allocation concealment (selection bias) | Low risk | "The randomization list and code envelopes were prepared by the company appointed for clinical supplies packaging. The random code was disclosed only after study completion and database closure" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "the same indistinguishable patch with no ingredient" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | LOCF, withdrawals 12%. All participants included in analysis |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
Mazières 2005b.
| Methods | R, DB, PC, parallel groups New patch applied directly to skin over painful area each morning Assessed at baseline, 3, 7, 14 days |
|
| Participants | Painful, benign ankle sprain (≤ 48 hours) N = 163 M 83, F 80 Mean age 37 years Baseline spontaneous pain ≥ 50 mm |
|
| Interventions | Ketoprofen patch 100 mg, x 1 daily, n = 81 Placebo patch, x 1 daily, n = 82 No analgesic or steroid by any route or other topical medication or physical therapy allowed Rescue medication permitted, but not within 12 hours of assessment |
|
| Outcomes | PGE: 4‐point scale (responder = "good" or "excellent") Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated global randomization code" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The same transdermal patch with no active ingredient |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | LOCF, withdrawals 5% to 6%. All participants included in analysis |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
McLatchie 1989.
| Methods | R, DB, PC, parallel groups Gel applied to injured site 3 times daily for 7 days Assessment at baseline 4, 7 days at clinic, daily patient diary |
|
| Participants | Acute soft tissue injury (< 48 hours) N = 231 M 143, F 88 Mean age 33 years Baseline pain moderate to severe |
|
| Interventions | Felbinac gel 3%, 3 x 3 cm daily, n = 118 Placebo gel, 3 x 3 cm daily, n = 113 Rescue medication: paracetamol |
|
| Outcomes | Patient diary: mean change Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "tubes identical in all aspects" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No usable efficacy data |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
Morris 1991.
| Methods | R, DB, PC, parallel groups Gel applied to site of injury 3 times daily for 7 days Assessed at baseline, 7 days at clinic, and daily patient diary |
|
| Participants | Acute soft tissue injury (< 3 days) N = 100 (84 analysed for efficacy) M 70, F 14 Mean age 25 years Baseline pain moderate to severe Exclusions: 1 participant in placebo group lost to follow‐up, 15 protocol violations |
|
| Interventions | Felbinac gel 3%, 3 x 1 cm daily, n = 41 Placebo gel, n = 43 Ice, joint immobilisation, bandaging and compression allowed No concomitant oral NSAID, occlusive dressing, physiotherapy, or liniments allowed Rescue medication: paracetamol |
|
| Outcomes | PGE: 5‐point scale (responder = "good" and "very good") Change in PI: patient diary 10 cm VAS (mean data) Adverse events Withdrawals and exclusions |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | "Randomisation was undertaken at the production facility and a sealed copy of the list supplied to the investigator for reference, only in defined circumstances" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "identical tubes and outer boxes", "placebo was a similarly constituted gel" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All relevant participants included in analysis |
| Size | High risk | < 50 participants per treatment arm |
NCT01255423.
| Methods | R, DB, PC, parallel group study Gel applied 4 x daily |
|
| Participants | Acute lateral ankle sprain, Grade I to II, ≤ 12 hours
N = 206 Mean (± SD) age 31 years (± 13) M 87, F 119 Baseline PI not reported |
|
| Interventions | Diclofenac sodium gel 1% x 4 daily, n = 104 Placebo gel, n = 102 | |
| Outcomes | Mean PI on movement Adverse events |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Method of blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
NCT01272934.
| Methods | R, DB, PC, parallel group study Gel applied 4 x daily |
|
| Participants | Acute lateral ankle sprain, Grade I to II, ≤ 12 hours
N = 205 M 101, F 104 Mean (± SD) age 32 years (± 11) Baseline PI not reported |
|
| Interventions | Diclofenac sodium gel 1%, x 4 daily, n = 102 Placebo gel, x 4 daily, n = 103 | |
| Outcomes | Mean PI on movement Adverse events |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Method of blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
NCT01272947.
| Methods | R, DB, PC parallel groups Gel applied 4 x daily |
|
| Participants | Acute blunt soft tissue injuries/contusions of the limbs, < 3 hours
N = 204 M 101, F 103 Mean (± SD) age 30 years (± 11) Baseline PI not reported |
|
| Interventions | Diclofenac sodium gel 1%, x 4 daily, n = 104 Placebo gel, x 4 daily, n = 100 | |
| Outcomes | Mean PI on movement Adverse events |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Method of blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
Noret 1987.
| Methods | R, DB, PC parallel groups Gel applied twice daily for 7 days Assessment at baseline, 3, 8 days |
|
| Participants | Minor sports injuries (< 24 hours) N = 98 (93 analysed) M 71, F 27 Mean age 29 years Baseline pain > 60 mm |
|
| Interventions | Ketoprofen gel 2.5%, 2 x 5 cm daily (= 15 mg), n = 48 Placebo gel, n = 45 No other treatment given |
|
| Outcomes | PGE: 4‐point scale (responder = "good" and "excellent") Spontaneous pain: 100 mm VAS (mean data) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | "allocated according to a randomization list and a corresponding code in a sealed envelope" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analysis |
| Size | High risk | < 50 participants per treatment arm |
Parrini 1992.
| Methods | R, DB, PC, parallel groups Foam (the size of a walnut, or a 1‐second spray) applied with massage 3 x daily for 7 days |
|
| Participants | Articular trauma, strains, distortions N = 169 M 94, F 75 Mean age 37 years Mean baseline pain on movement 3.1 (scale 1 to 4) |
|
| Interventions | Ketoprofen foam 15%, 3 x 2 g (= 600 mg) daily, n = 83 Placebo foam, 3 x 2 g, n = 86 |
|
| Outcomes | Pain on movement: 4‐point scale (mean data) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB1, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "patients were randomised according to the method of random numbers" [translated] |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No usable efficacy data |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
Picchio 1981.
| Methods | R, DB, AC, parallel groups Cream applied with slight massage until completely absorbed, 3 times daily for up to 16 days Assessed at baseline, 4, 8, 12, 16 days |
|
| Participants | Acute sports injuries N = 40 M 24, F 16 Mean age 22 years (range 12 to 46) Most participants had mild to moderate baseline pain (12 and 9 with slight pain on movement) |
|
| Interventions | Ibuprofen gel 10%, 3 x daily, n = 20 Ketoprofen gel 1%, 3 x daily, n = 20 |
|
| Outcomes | Pain on movement (responder = "none") Adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W0. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "tubes were identical in appearance" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analysis |
| Size | High risk | < 50 participants per treatment arm |
Predel 2004.
| Methods | R, DB, PC, parallel groups New patch applied to injured area twice daily for 7 days. Contact of patch with humidity or water to be avoided Assessment at baseline 3, 7 days |
|
| Participants | Traumatic blunt soft tissue injuries (< 3 hours, no treatment) N = 120 M 73, F 47 Mean age 32 years Baseline pain > 60 mm |
|
| Interventions | Diclofenac sodium patch, 2 x daily (140 mg per patch), n = 60 Placebo patch, 2 x daily, n = 60 NSAIDs, analgesics, psychotropic agents, other topical preparations, and bandages not allowed |
|
| Outcomes | PGE: 4‐point scale (responder = "good" and "excellent") Pain on movement: 10 cm VAS (mean data) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated block randomisation list |
| Allocation concealment (selection bias) | Low risk | An independent statistician produced randomisation list, and an independent contract research organisation packaged medication according to list. Nobody else had access to the randomisation list until the database was closed |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The placebo patch was visually indistinguishable from the active patch." To avoid unblinding due to different smell, any study nurse involved with medication was not involved in outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals < 1%. All participants included in analysis |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
Predel 2012.
| Methods | R, DB, PC, parallel groups Treatment for 7 days Assessed at baseline, 1, 3, 5 and 8 days | |
| Participants | Grade I or II ankle sprain with lateral external ligament involvement, < 12 hours
PI on movement ≥ 50/100
N = 242 M 152, F 90 Mean age 32 years Mean baseline pain on movement = 75/100 |
|
| Interventions | Diclofenac gel (Voltaren Emulgel 2.32%), 2 x 5 cm + 1 x 5 cm placebo gel daily, n = 80
Diclofenac gel (Voltaren Emulgel 2.32%), 3 x 5 cm daily, n = 80
Placebo gel, 3 x 5 cm daily, n = 82 Rescue medication: paracetamol (to maximum 2 g daily) No ice or bandages after randomisation |
|
| Outcomes | ≥ 50% reduction in PI on movement at 5 days PGE: 5‐point scale (responder = "good" or 'very good") Patient satisfaction: 5‐point scale (responder = "good" or 'very good" or "excellent") Rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "identical in composition, appearance, texture and smell" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | LOCF, but withdrawals < 3% in all treatment arms |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
Predel 2013a.
| Methods | R, DB, PC, parallel groups Treatment for 14 days Assessed at baseline, 3, 7, 10 and 14 days (± 1 day) | |
| Participants | Uncomplicated, one‐sided ankle sprain with swelling ≥ 12 mm (2 to 18 hours)
PI on movement, tenderness, joint mobility (scale 0 to 3) summed as ≥ 5 and ≤ 7
N = 232 M 126, F 106 Mean age 29 years |
|
| Interventions | Diclofenac 4% spray gel, 3 x 4 to 5 sprays daily (96 to 120 mg diclofenac sodium), n = 118
Placebo spray gel, 3 x 4 to 5 sprays daily, n = 114 Rescue medication: paracetamol 500 mg (maximum 10 tablets per week) No ice or bandaging allowed |
|
| Outcomes | None or slight PI on movement at 3 and 7 days PGE: 4‐point scale (responder = "very good" at 14 days) > 50% reduction in swelling at 10 days Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "placebo (vehicle only, no active ingredient)" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | LOCF for full or early cure. Withdrawals < 2% |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
Predel 2013b.
| Methods | R, DB, PC, parallel groups 2 g gel applied with fingertips over affected area and massaged in for 1 minute. Treatment for 5 days Assessed at baseline, 2, 3, 5 days | |
| Participants | Uncomplicated neck pain originating from cervical joints and accompanying soft tissues (≥ 12 hours but < 3 months), PI ≥ 50/100
N = 72 M 33, F 39 Mean age 34 years |
|
| Interventions | Diclofenac gel (Voltaren Emulgel), 4 x 2 g daily, n = 36
Placebo gel, 4 x 2 g daily, n = 36 Rescue medication: paracetamol up to 2 g daily No concomitant therapies allowed |
|
| Outcomes | ≥ 50% decrease in PI on movement after 48 hours
PGE: 5‐point scale (responder = "good" or "excellent" at 5 days Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sponsor "produced randomisation list" |
| Allocation concealment (selection bias) | Low risk | Automated remote system |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "identical in packaging, labelling, schedule of administration, appearance and odour" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Size | High risk | < 50 participants per treatment arm |
Ramesh 1983.
| Methods | R, DB, PC, parallel groups Cream applied to painful area and rubbed into skin over a large area for up to 10 days Assessment at baseline, 3, 7, 10 days |
|
| Participants | Strains, sprains, contusions, compressions N = 80 M 42, F 38 Age 11 to 81 years Baseline pain: 5 ibuprofen, 2 placebo participants had no or slight pain |
|
| Interventions | Ibuprofen cream 5%, 3 to 4 x 5 to 10 cm daily, n = 40 Placebo cream, 3 to 4 x 5 to 10 cm daily, n = 40 Adjuvant therapy was not administered |
|
| Outcomes | Pain on movement: 4‐point scale (responder = "none" or "slight") Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Randomisation key in sealed envelope, available for emergencies, but opened only after completion |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "identical appearance and odour" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analysis |
| Size | High risk | < 50 participants per treatment arm |
Rowbotham 2003.
| Methods | R, DB, PC, parallel groups New patches applied to the affected painful area for 12 consecutive hours twice daily, for up to 14 days Assessed at baseline, 14 days in clinic and daily patient diary |
|
| Participants | Minor sports injuries (sprains, sprains, contusions, < 72 hours) N = 372 M 253, F 119 Mean age 33 years Baseline pain at rest ≥ 5/10 |
|
| Interventions | DHEP patch (Flector Tissuegel), 2 x daily (equivalent to diclofenac sodium 140 mg per patch), n = 191 Placebo patch, 2 x daily, n = 181 |
|
| Outcomes | PGE: 5‐point scale (responder = "good" and "excellent") Pain resolved: < moderate for 2 days Spontaneous pain: 10 cm VAS (mean data) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Système identique" without diclofenac |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information about imputation. All participants included in analysis |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
Russell 1991.
| Methods | R, DB, PC, parallel groups Affected area washed with soap and water and dried, then gel applied and carefully rubbed into skin, 4 times daily for at least 7 days Assessed at baseline, 4, 8, 15 (if necessary) days at clinic, and daily patient diary |
|
| Participants | Acute soft tissue injuries (recent, not recurrent) N = 214 (200 analysed) M 95, F 105 Mean age 40 years Baseline pain > 65 mm |
|
| Interventions | Piroxicam gel 0.5%, 4 x 5 mg daily, n = 100 Placebo gel, n = 100 No other NSAIDs or analgesic drugs, including liniments containing salicylates, allowed. Ancillary therapy at the discretion of the investigator |
|
| Outcomes | PGE: 4‐point scale (responder = "good" and "excellent") Spontaneous pain: mean reduction Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated randomization code" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "identical base formulation" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High withdrawal rate (8% with piroxicam, 50% with placebo). No information about imputation |
| Size | Unclear risk | 50 to 100 participants per treatment group |
Saillant 1998.
| Methods | R, DB, PC, parallel groups Treatment for 7 days Assessed at baseline, 1, 2, 3, and 7 days | |
| Participants | Ankle sprain (< 48 hours) N = 140 M 72, F 62 Age 18 to 65 years Baseline spontaneous pain ≥ 50 mm | |
| Interventions | (1) DHEP plaster (Flector Tissugel 1%), 1 x daily, n = 70
(2) Placebo plaster, 1 x daily, n = 70 Rescue medication: paracetamol (to maximum 3 g daily) Ice allowed |
|
| Outcomes | ≥ 30% decrease in PI at 7 days PGE: 4‐point scale (responder = "excellent") No or low pain on passive stretch Single foot leaning OK without pain Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical in size, appearance, and used same formula as active patch, without active ingredient |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Used LOCF for primary outcome. Withdrawals < 7% and equal between groups. All participants included in analysis |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
Sanguinetti 1989.
| Methods | R, DB, PC, parallel groups Gel applied 3 times daily for 7 consecutive days Assessment at baseline, 7 days |
|
| Participants | Soft tissue trauma (< 48 hours) N = 82 M = 47, F = 35 Mean age 34 years Baseline pain moderate to severe |
|
| Interventions | Felbinac* gel 3%, 3 x daily, n = 42 Placebo gel, 3 x daily, n = 40 No other NSAID, steroid, other topical application allowed Rescue medication: paracetamol * felbinac is an active metabolite of the NSAID fenbufen |
|
| Outcomes | PGE: scale not reported (responder = "good" and "very good") Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "indistinguishable in appearance, colour or odour" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analysis |
| Size | High risk | < 50 participants per treatment arm |
Sinniger 1981.
| Methods | R, DB, PC, parallel groups Cream applied 2 to 3 times daily, with gentle massage, or if massage not possible (too painful) with protective dressing Assessment at baseline, 5, 10 days |
|
| Participants | Minor soft tissue injuries N = 20 M 11, F 9 Mean age 40 years Baseline pain not reported |
|
| Interventions | Fentiazac cream 5%, 2 to 3 x daily, n = 10 Placebo cream, 2 to 3 x daily, n = 10 All participants told to rest No other local and systemic treatments allowed Rescue medication: analgesic if actually needed |
|
| Outcomes | Pain relief: scale not reported (responder = total pain relief) % improvement in pain on movement: pain scale not reported (mean data) Adverse events |
|
| Notes | Oxford Quality Score: R1, DB1, W0. Total = 2/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analysis |
| Size | High risk | < 50 participants per treatment arm |
Spacca 2005.
| Methods | R, DB, PC, parallel groups Gel applied 3 times daily, with gentle massage until complete absorption, for up to 10 days Assessment at baseline, 10 days in clinic, and daily patient diary |
|
| Participants | Shoulder periarthritis or lateral epicondylitis (< 5 days) N = 155 M 74, F 81 Mean age 51 years Baseline pain with activity > 70 mm |
|
| Interventions | DHEP lecithin gel (Effigel), 3 x 5 g daily, n = 79 Placebo gel, 3 x 5 g daily, n = 76 Rescue medication (paracetamol) allowed if pain unbearable No other analgesic or anti‐inflammatory drug allowed |
|
| Outcomes | Improvement in pain: 100 mm VAS (mean data) Adverse events |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No usable efficacy data |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
Sugioka 1984.
| Methods | R, DB, AC, parallel groups Gel applied to affected area 3 to 4 times daily, without occlusion, for 14 days Assessed at baseline, 7, 14 days |
|
| Participants | Non‐traumatic diseases of muscle or tendon N = 366 (340 analysed for efficacy) M 115, F 202 (completers) Age range 12 to 84 years (most 30 to 70) Baseline pain on movement "none" or "mild" in about 1/3 of participants Exclusions for protocol violations: 8 piroxicam, 18 indomethacin |
|
| Interventions | Piroxicam gel 0.5%, 3 to 4 x 1 g daily, n = 183 Indomethacin gel 1%, 3 to 4 x 1 g daily, n = 183 No concomitant anti‐inflammatory or analgesic drug, including steroids, or initiation of physical therapy allowed |
|
| Outcomes | PGE: 5‐point scale (responder = "better" or "much better") Pain on movement: 4‐point scale (responder = "reduced" or "disappeared") |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Key code sealed and retained until end of study |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "both packages were of the same appearance and indistinguishable", and investigators did not see contents |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All relevant participants included in analysis |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
Thorling 1990.
| Methods | R, DB, PC, parallel groups Participants given specific instructions on how to apply gel (not reported) to affected area 2 to 6 times daily as required Assessment at baseline, 3, 7 days in clinic |
|
| Participants | Soft tissue injuries (< 48 hours) N = 120 M 85, F 35 Mean age 27 years Baseline pain moderate to severe |
|
| Interventions | Naproxen gel 10%, 2 to 6 x daily, n = 60 Placebo gel, 2 to 6 x daily, n = 60 Rescue medication: paracetamol 500 mg |
|
| Outcomes | PGE: 5‐point scale (responder = "good" and "very good") Pain on passive movement: 4‐point scale (mean data) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "supplied in unmarked tubes" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analysis |
| Size | Unclear risk | 50 to 200 participants per treatment arm |
Tonutti 1994.
| Methods | R, DB, AC, parallel groups Gel applied 3 times daily for 2 to 3 weeks Assessed at baseline, and intervals of 7 days |
|
| Participants | Muscle or joint trauma N = 30 M 20, F 10 Mean age 34 years 1 participant had injury of mild severity. Mean baseline pain on active movement 2.8 (scale 0 to 4) |
|
| Interventions | Ketoprofen gel 5%, 3 x 2 to 3 g daily, n = 15 Etofenamate gel 5%, 3 x 2 to 3 g, n = 15 No concomitant treatment with NSAID, aspirin, steroid or physical therapy |
|
| Outcomes | PGE: 4‐point scale (responder = "good" and "excellent") Pain on movement: 5‐point scale (mean data) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "the two drugs were packed in indistinguishable tubes" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analysis |
| Size | High risk | < 50 participants per treatment arm |
Vecchiet 1991.
| Methods | R, DB, PC, parallel groups Gel applied to the skin on and around painful area and gently rubbed in until absorbed, twice daily for up to 10 days Assessed at baseline, 5, 10 days |
|
| Participants | Soft tissue trauma (minor sports injuries) N = 60 M 60 Mean age 25 years Mean baseline pain on active movement: moderate |
|
| Interventions | Meclofenamic acid gel 5%, 2 x 10 cm daily (= 4 g), n = 30 Placebo gel, 2 x 10 cm daily, n = 30 Both groups treated with ice, rest, and bandage for first 48 hours before starting test treatment Rescue medication: paracetamol |
|
| Outcomes | PGE: 4‐point scale (responder = "good" and "excellent") Pain on movement: 4‐point scale (mean data) Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analysis |
| Size | High risk | < 50 participants per treatment arm |
Whitefield 2002.
| Methods | R, DB (double dummy), AC, parallel groups Gel applied to affected site, with gentle massage, and 1 tablet taken 3 times daily for at least 7 days Assessed at baseline, 7, 14 (if necessary) days in clinic, and daily patient diary |
|
| Participants | Soft tissue injuries (< 24 hours) N = 100 M 95, F 5 Mean age 26 years (range 18 to 50) Mean baseline pain on movement 2.2 cm |
|
| Interventions | Ibuprofen gel 5% + placebo tablet 3 x daily, n = 50 Ibuprofen 400 mg tablet + placebo gel 3 x daily, n = 50 No other medication or physical therapy was prescribed and no other analgesics were allowed |
|
| Outcomes | PGE: 3‐point scale (responder = "excellent") Change in condition of injury site: 5‐point scale (responder = "completely better") Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo tablets Identical in appearance to active tablets. Active and placebo gels had similar physical characteristics and were supplied in identical tubes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analysis |
| Size | Unclear risk | 50 participants per treatment arm |
Ăkermark 1990.
| Methods | R, DB (double dummy), PC, AC, parallel groups Spray applied to affected area, and capsules taken 3 times daily for 2 weeks Assessment at baseline, 3 or 4, 7, and 14 days |
|
| Participants | Superficial overuse sports injuries (symptom onset 7.4 weeks) N = 70 M 44, F 18 (completers) Mean age 30 years Baseline pain on palpation mostly slight to moderate |
|
| Interventions | Elmetacin spray (indomethacin 1%), 3 to 5 x 0.5 to 1.5 ml daily + placebo capsules, n = 23 Indomethacin capsules, 3 x 25 mg daily + placebo spray, n = 23 Placebo spray and capsules, n = 24 Rescue medication: paracetamol |
|
| Outcomes | No pain on palpation (= responder) Participant improvement: 100 mm VAS (mean data) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5/5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random number code" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "identical in appearance" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Responder analysis, < 5% withdrawals |
| Size | High risk | < 50 participants per treatment group |
AC: active control; BOCF: baseline observation carried forward; DB: double‐blind; DHEP: diclofenac hydroxyethylpyrrolidine, or diclofenac epolamine; F: female; HCl: hydrochloride; hep: heparin; LOCF: last observation carried forward; M: male; N: number of participants in study; n: number of participants in treatment arm; PC: placebo control; PGE: Participant Global Evaluation; PI: pain intensity; R: randomised; VAS: visual analogue scale; W: withdrawals.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ambrus 1987 | No usable dichotomous data |
| Anon 1993 | Not double‐blind |
| Ascherl 1982 | No usable dichotomous data |
| Bagliani 1976 | Not an RCT |
| Baracchi 1982 | No usable data |
| Burnham 1998 | <10 participants/treatment arm in first period of crossover study |
| Böhmer 1995 | Active control invalid |
| Cesarone 2008 | Not an RCT |
| Coulibaly 2009 | Not double‐blind |
| Diebschlag 1985 | No usable dichotomous data |
| Diebschlag 1986 | Inappropriate randomisation |
| Diebschlag 1992 | No usable dichotomous data |
| Fantato 1971 | No usable dichotomous data |
| Galer 2000 | No usable data |
| Hallmeier 1986 | Not double‐blind |
| Hallmeier 1988 | Not double‐blind |
| Kaneko 1999 | Inappropriate randomisation ‐ quasi‐randomised |
| Kockelbergh 1985b | Treatment not applied daily |
| Kuwabara 2013 | Used NSAID‐lidocaine combination (conference abstract) |
| Lee 1991 | Not an RCT |
| Link 1996 | No usable dichotomous data |
| May 2007 | No usable dichotomous data |
| Oakland 1993 | Inappropriate comparator |
| Odaglia 1987 | Not an RCT |
| Picardi 1993 | Not an RCT |
| Taboada 1992 | Dose and duration of treatment unclear |
| Vanderstraeten 1990 | Not double‐blind |
| Vinciguerra 2008 | Not an RCT |
| Von Klug 1977 | Chronic and acute outcomes combined |
RCT: randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
NCT00351104.
| Methods | R, DB, PC, parallel groups |
| Participants | Ankle sprain or strain, Grade I or II. Age ≥ 18 years N = 220 |
| Interventions | Ketoprofen patch 20% x 1 daily Placebo patch x 1 daily Treatment for 21 days |
| Outcomes | Assessments days 3, 7, 21 PI (0‐10) at rest and during activities PGE Function Rescue medication |
| Notes | Sponsor: Endo Pharmaceuticals Estimated completion February 2007. No results |
NCT00352625.
| Methods | R, DB, PC, parallel groups |
| Participants | Shoulder, elbow, or knee tendonitis or bursitis. Age ≥ 18 years N = 330 |
| Interventions | Ketoprofen patch 20% x 1 daily Placebo patch x 1 daily Treatment for 21 days |
| Outcomes | Assessments days 3, 7, 21 PI (0‐10) at rest and during activities PGE Function Rescue medication |
| Notes | Sponsor: Endo Pharmaceuticals Estimated completion February 2007. No results |
NCT00426985.
| Methods | R, DB, PC, parallel groups |
| Participants | Shoulder, elbow, or knee tendonitis or bursitis. Age ≥ 18 years |
| Interventions | Ketoprofen patch 20% x 1 daily Placebo patch Treatment for 21 days |
| Outcomes | PI during activity
PI at rest
PGE
Rescue medication Safety |
| Notes | Study terminated May 2008, but "sufficient number of subjects accrued to conduct analysis". No results |
NCT00640705.
| Methods | R, DB, PC, parallel groups |
| Participants | Ankle sprain or strain, Grade I or II, ≤ 48 hours, PI 5/10 to 9/10. Age 18 to 75 years N = 170 |
| Interventions | Diclofenac sodium patch (15 mg) x 1 daily Placebo Treatment for 7 days |
| Outcomes | Efficacy Safety |
| Notes | Study terminated July 2008 (Sponsor decision ‐ no further details). No results posted |
NCT00640939.
| Methods | R, DB, PC, parallel groups |
| Participants | Shoulder, elbow, or knee tendonitis or bursitis. Age 18 to 75 years
Baseline pain mild to moderate, but states 5/10 to 9/10 N = 308 |
| Interventions | Diclofenac sodium patch 1% (15 mg) x 1 daily
Placebo patch x 1 daily Duration of treatment not reported, probably 14 days |
| Outcomes | Efficacy Safety |
| Notes | Sponsor: Cerimon Pharmaceuticals Estimated completion April 2008. No results |
NCT00680472.
| Methods | R, DB, PC, parallel groups |
| Participants | Acute unilateral shoulder pain, requiring treatment for ≥ 2 weeks. Age ≥ 18 years N = 368 |
| Interventions | Ketoprofen patch (HKT‐500) x 1 daily Placebo x 1 daily Treatment for 14 days |
| Outcomes | Pain Safety |
| Notes | Sponsor: Hisamitsu Pharmaceutical Co., Inc. Estimated completion October 2008. No results |
NCT00680784.
| Methods | R, DB, PC, parallel groups |
| Participants | Acute benign ankle sprain, Grade I‐II, ≤ 48 hours. Age ≥ 18 years N = 260 |
| Interventions | Ketoprofen patch (HKT‐500) x 1 daily Placebo patch x 1 daily Treatment for 14 days |
| Outcomes | Pain Safety |
| Notes | Sponsor: Hisamitsu Pharmaceutical Co., Inc. Estimated completion November 2008. No results |
NCT00765700.
| Methods | R, DB, PC, parallel groups |
| Participants | Acute sprain or strain of upper and lower extremities, mild to moderate injuries, ≤ 72 hours. Age 18 to 70 years N = 364 |
| Interventions | Ketoprofen cream 10% 1 g x 3 daily Placebo cream x 3 daily Treatment for 7 days |
| Outcomes | Pain Safety |
| Notes | Sponsor: Imprimis Pharmaceuticals, Inc. Estimated completion September 2009. No results |
NCT00869063.
| Methods | R, DB, PC, parallel groups |
| Participants | Wrist sprain, strain, or contusion (mild to moderate), "recent". Age 17 to 75 years N = 214 |
| Interventions | Diclofenac sodium patch 1% x 1 daily Placebo patch x 1 daily Treatment for 7 days |
| Outcomes | Change in PI during activity at 3 and 7 days Safety |
| Notes | Sponsor: Cerimon Pharmaceuticals Estimated completion September 2009. No results |
NCT00869180.
| Methods | R, DB, PC, parallel groups |
| Participants | Unilateral, ankle sprain (mild or moderate), "recent". Age 17 to 75 years N = 219 |
| Interventions | Diclofenac sodium patch x 1 daily Placebo patch x 1 daily Treatment for 7 days |
| Outcomes | Change in PI during activity at 3 and 7 days |
| Notes | Sponsor: Cerimon Pharmaceuticals Estimated completion August 2009. No results |
NCT00931866.
| Methods | R, DB, PC, parallel groups |
| Participants | Unilateral soft tissue injury between mid‐bicep to wrist or mid‐thigh to ankle (mild to moderate), "recent". Age 18 to 75 years N = 407 |
| Interventions | Diclofenac sodium patch x 1 daily Placebo patch x 1 daily Treatment for 14 days |
| Outcomes | Change in PI during activity at 7 and 14 days
PI
PGE
Rescue medication Safety |
| Notes | Sponsor: Cerimon Pharmaceuticals Estimated completion December 2009. No results |
NCT01874626.
| Methods | R, DB, PC, parallel groups |
| Participants | Acute ankle sprain, Grade I or II ≤ 24 hours. Age ≥ 16 years. Baseline PI ≥ 5/10 N = 305 |
| Interventions | Ibuprofen cream 200 mg in 2.7 g cream x 4 daily Placebo cream x 4 daily Treatment for 7 days |
| Outcomes | PI on movement daily to 7 days
PGE 7 days Systemic and local adverse events |
| Notes | Estimated completion January 2014. No results |
NCT01957215.
| Methods | R, DB, PC, parallel groups |
| Participants | Acute lateral ankle sprain, Grade I‐II, ≤ 24 hours, PI ≥ 5/10. Age 18 to 65 years N = 270 |
| Interventions | Indomethacin patch x 2 daily
Placebo patch x 2 daily Duration of treatment not reported, probably 7 days |
| Outcomes | PI on movement daily PR daily PGE Rescue medication |
| Notes | Estimated completion September 2014. No results |
NCT02324270.
| Methods | R, DB, PC and AC, parallel groups |
| Participants | Uncomplicated acute ankle sprain, Grade I or II, < 48 hours, PI > 50/100. Age 18 to 65 years N = 658 |
| Interventions | Diclofenac epolamine (Flector) 1.3% patch
Generic diclofenac epolamine 1.3% patch
Placebo patch Duration of treatment not reported |
| Outcomes | Bioequivalence study
Change from baseline in PI (VAS) at 3 days Application site reactions |
| Notes | Estimated completion December 2014. No results |
Sarzi‐Puttini 2014.
| Methods | R, DB, AC, parallel group, non‐inferiority study Duration 7 days |
| Participants | Acute musculoskeletal injury (mainly muscular, joint, tendon) N = 697 M 271, F 426 Mean age 52 years |
| Interventions | Ketoprofen (SKP‐021) patch, ketoprofen 30 mg Diclofenac (Voltadola), patch diclofenac sodium 140 mg Patches applied twice daily for 7 days |
| Outcomes | ≥ 50% reduction in PI from baseline to end of study (100 mm VAS) Patient overall rating Clinical symptoms (4‐point scale) Time to response Adverse events, skin reactions Serious adverse events |
| Notes | "The analysis of the data of this trial showed that the two formulations were equally effective and well tolerated in the treatment of acute musculoskeletal injuries." |
AC: active‐controlled; DB: double‐blind; F: female; M: male; N: number of participants in study; PC: placebo‐controlled; PGE: Patient Global Evaluation of treatment; PI: pain intensity; R: randomised; VAS: visual analogue scale.
Characteristics of ongoing studies [ordered by study ID]
NCT01945034.
| Trial name or title | Placebo‐controlled, double‐blind evaluation of the efficacy and safety of ibuprofen 5% topical gel for the treatment of ankle sprain |
| Methods | R, DB, PC, parallel groups |
| Participants | Ankle sprain, Grade I or II, age ≥ 12 years Estimated enrolment 280 |
| Interventions | Ibuprofen gel 5% x 2 daily Ibuprofen gel 5% x 3 daily Placebo gel Treatment for 7 days, with additional 3 days as needed |
| Outcomes | PI on weight bearing and rest at 7 days
PGE at 10 days
Rescue medication Safety |
| Starting date | November 2013 |
| Contact information | Pfizer |
| Notes | Estimated completion February 2015 Includes participants aged ≥ 12 years |
NCT02100670.
| Trial name or title | A clinical study to assess the efficacy and onset of pain relief of topical MFC51123 diclofenac‐menthol gel versus controls in ankle sprain |
| Methods | R, DB, PC, and AC, parallel groups |
| Participants | Acute lateral ankle sprain, Grade I or II, ≤ 24 hours, PI ≥ 5/10. Age 16 to 65 years Estimated enrolment 400 |
| Interventions | Diclofenac sodium 1% + methanol 3% gel Diclofenac sodium 1% + methanol 0.09% gel Methanol 3% gel Placebo + 0.09% methanol gel Treatment for 10 days with 4 g gel x 4 daily |
| Outcomes | PR
PID on movement (0 to 10 days)
PGE
Time to complete recovery Adverse events |
| Starting date | November 2013 |
| Contact information | GSKClinicalSupportHD@gsk.com |
| Notes | Estimated completion November 2014 |
NCT02290821.
| Trial name or title | A randomised, double‐blind, placebo‐controlled, parallel group study to evaluate the efficacy and safety of diclofenac sodium topical gel (DSG) 1% applied four times daily in subjects with acute blunt soft tissue injuries/contusions of the limbs |
| Methods | R, DB, PC, parallel groups |
| Participants | Acute blunt soft tissue injuries or contusions of the limbs, ≤ 6 hours ("fresh"). Age ≥ 16 years Estimated enrolment 200 |
| Interventions | Diclofenac sodium gel 1% x 4 daily
Placebo gel Duration of treatment not reported |
| Outcomes | Pain Safety |
| Starting date | December 2014 |
| Contact information | Novartis |
| Notes | Estimated completion August 2015 |
AC: active control; DB: double‐blind; PC: placebo‐controlled; PGE: Patient Global Evaluation of treatment; PI: pain intensity; PID: pain intensity difference; PR: pain relief; R: randomised
Differences between protocol and review
For this update in 2015, we have changed the title to specify musculoskeletal pain because topical NSAIDs are not normally used to treat visceral pain or headache. We felt that the new title better reflected the content of the review. We have also changed the focus of the review from pooled analysis of all topical NSAIDs and all studies of a particular NSAID to an examination of individual drug and its formulation. This makes the review much more relevant. We have expanded the 'Risk of bias' assessment, and added a 'Summary of findings' table and PRISMA flow chart. We have removed a number of sensitivity analyses because they were not appropriate given the current information on the impact of formulation on efficacy. The sensitivity analyses have been superseded by the 'Risk of bias' assessment and taken into account in the 'Summary of findings' tables.
An earlier review in 2004 chose to exclude studies using benzydamine, on the grounds that it was no longer considered to be an NSAID (Mason 2004a). Although the protocol for this review stated that we would not include benzydamine, after further consultation we now believe that it should be classified as an NSAID, albeit with a different mode of action, which is not fully understood (Quane 1998). Thus, we have reinstated studies using topical benzydamine.
Contributions of authors
For the earlier review, Tom Massey and SD identified studies, and carried out data extraction, analysis and drafting. RAM and HJM were involved in planning, acted as adjudicators, and were involved with writing.
For this update, SD and RAM carried out searches, data extraction, and analysis. All authors were involved with writing the review.
Sources of support
Internal sources
-
Oxford Pain Relief Trust, UK.
General institutional support
External sources
No sources of support supplied
Declarations of interest
SD has no conflicts relating to this review or any similar product.
RAM has no conflicts relating to this review or any similar product.
PW has no conflicts relating to this review or any similar product.
HG has no conflicts relating to this review or any similar product.
MM has no conflicts relating to this review or any similar product.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Airaksinen 1993 {published data only}
- Airaksinen O, Venäläinen J, Pietiläinen T. Ketoprofen 2.5% gel versus placebo gel in the treatment of acute soft tissue injuries. International Journal of Clinical Pharmacology, Therapy and Toxicology 1993;31(11):561‐3. [PubMed] [Google Scholar]
Ăkermark 1990 {published data only}
- Ăkermark C, Forsskåhl B. Topical indomethacin in overuse injuries in athletes. A randomized double blind study comparing Elmetacin® with oral indomethacin and placebo. International Journal of Sports Medicine 1990;11(5):393‐6. [DOI] [PubMed] [Google Scholar]
Aoki 1984 {published data only}
- Aoki T, Numajiri M, Yamamoto M. A well controlled comparative study of piroxicam gel, indomethacin gel and placebo gel in the treatment of trauma. Japanese Pharmacology and Therapeutics 1984;12(12):101‐17. [Google Scholar]
Auclair 1989 {published data only}
- Auclair J, Georges M, Grapton X, Gryp L, D'Hooghe M, Meisser RG, et al. A double‐blind controlled multi‐center study of percutaneous niflumic acid gel and placebo in the treatment of Achilles heel tendinitis. Current Therapeutic Research 1989;46(4):782‐8. [Google Scholar]
Billigmann 1996 {published data only}
- Billigmann PW. Treatment of ankle distortion with ibuprofen gel [Therapie von Sprunggelenks‐distosionen mit ibuprofen‐mikrogel]. Therapiewoche 1996;46(21):1187‐92. [Google Scholar]
Campbell 1994 {published data only}
- Campbell J, Dunn T. Evaluation of topical ibuprofen cream in the treatment of acute ankle sprains. Journal of Accident and Emergency Medicine 1994;11(3):178‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chatterjee 1977 {published data only}
- Chatterjee DS. A double‐blind clinical study with benzydamine 3% cream on soft tissue injuries in an occupational health centre. Journal of International Medical Research 1977;5(6):450‐8. [DOI] [PubMed] [Google Scholar]
Costantino 2011 {published data only}
- Costantino C, Kwarecki J, Samokhin AV, Mautone G, Rovati S. Diclofenac epolamine plus heparin plaster versus diclofenac epolamine plaster in mild to moderate ankle sprain: a randomized, double‐blind, parallel‐group, placebo‐controlled, multicentre, phase III trial. Clinical Drug Investigation 2011;31(1):15‐26. [DOI: 10.2165/11585890-000000000-00000] [DOI] [PubMed] [Google Scholar]
Coudreuse 2010 {published data only}
- Coudreuse JM, Vathaire F. Effect of a plaster containing DHEP and heparin in acute ankle sprains with oedema: a randomized, double‐blind, placebo‐controlled, clinical study. Current Medical Research and Opinion 2010;26(9):2221‐8. [DOI: 10.1185/03007995.2010.508020] [DOI] [PubMed] [Google Scholar]
Curioni 1985 {published data only}
- Curioni BG, Domenica F, Daolio P, Spignoli G. Evaluation of ibuproxam gel in traumatology [Valutazione dell'efficacia terapeutica di ibudros gel in traumatologia]. Clinica Europea 1985;24(3):456‐60. [Google Scholar]
Diebshlag 1990 {published data only}
- Diebschlag W, Nocker W, Bullingham R. A double‐blind study of the efficacy of topical ketorolac tromethamine gel in the treatment of ankle sprain, in comparison to placebo and etofenamate. Journal of Clinical Pharmacology 1990;30(1):82‐9. [DOI] [PubMed] [Google Scholar]
Dreiser 1988 {published data only}
- Dreiser RL. Clinical trial of efficacy and tolerability of topical ibuprofen in the treatment of tendinitis. Le Journal International De Médecine 1988;119:15‐31. [Google Scholar]
Dreiser 1989 {published data only}
- Dreiser RL. Clinical trial ‐ Fatsum gel FG‐6. Supplied by Menarini 1989.
Dreiser 1990 {published data only}
- Dreiser RE, Charlot J, Lopez A, Ditisheim A. Clinical evaluation of niflumic acid gel in the treatment of uncomplicated ankle sprains. Current Medical Research and Opinion 1990;12(2):93‐9. [DOI] [PubMed] [Google Scholar]
Dreiser 1994 {published data only}
- Dreiser RL, Roche R, Sahb R, Thomas F, Leutenegger E. Flurbiprofen local action transcutaneous (LAT tm): clinical evaluation in the treatment of acute ankle sprains. European Journal of Rheumatology and Inflammation 1994;14(4):9‐13. [PubMed] [Google Scholar]
Fioravanti 1999 {published data only}
- Fioravanti A, Cicero MR, Nerucci F, Manopulo R, Marcolongo R. Double‐blind controlled clinical study of the efficacy and tolerability of diclofenac‐N‐(2‐hydroxyethyl)‐pyrrolidine lecithin gel compared with diclofenac‐N‐(2‐hydroxyethyl)‐pyrrolidine gel in patients with peri and extraarticular inflammatory diseases. Drugs under Experimental and Clinical Research 1999;25(5):235‐40. [PubMed] [Google Scholar]
Fujimaki 1985 {published data only}
- Fujimaki E, et al. Clinical evaluation of piroxicam gel versus indomethacin gel and placebo in the treatment of muscle pain: a double‐blind, multicenter study. Japanese Pharmacology and Therapeutics 1985;12(12):119‐37. [Google Scholar]
Gallacchi 1990 {published data only}
- Gallacchi G, Mautone G, Lauldi P. Topical treatment with diclofenac hydroxyethylpyrrilidine (Flector gel 1%). Clinical Trials Journal 1990;27(1):58‐64. [Google Scholar]
González de Vega 2013 {published data only}
- González de Vega C, Speed C, Wolfarth B, González J. Traumeel vs. diclofenac for reducing pain and improving ankle mobility after acute ankle sprain: a multicentre, randomised, blinded, controlled and non‐inferiority trial. International Journal of Clinical Practice 2013;67(10):979‐89. [DOI: 10.1111/ijcp.12219] [DOI] [PMC free article] [PubMed] [Google Scholar]
Governali 1995 {published data only}
- Governali E, Casalini D. A controlled clinical study on ketoprofen gel 5% versus ketoprofen ointment 1% in patients with post‐traumatic lesions [Ricerda clinica controllata tra ketoprofene gel 5% e ketoprofene crema 1% in pazienti con postumi di lesioni traumatiche]. Riabilitazione 1995;28(1):61‐9. [Google Scholar]
Gualdi 1987 {published data only}
- Gualdi A, Bonollo L, Martini A, Forgione A. Non‐steroidal anti‐inflammatory drugs for topical therapy in traumatology: a double‐blind study with flunoxaprofen and ketoprofen [Antinflammatori no steroidi per uso topico in traumatologia: studio clinico con flunoxaprofene e chetoprofene]. Riforma Medica 1987;102(10):401‐4. [Google Scholar]
Haig 1986 {published data only}
- Haig G. Portable thermogram technique for topically applied benzydamine cream in acute soft‐tissue injuries. Internation Journal of Tissue Reactions 1986;8(2):145‐7. [PubMed] [Google Scholar]
Hoffmann 2012 {published data only}
- Hoffmann P, Kopačka P, Gugliotta B, Rovati S. Efficacy and tolerability of DHEP‐heparin plaster in reducing pain in mild‐to‐moderate muscle contusions: a double‐blind, randomized trial. Current Medical Research and Opinion 2012;28(8):1313‐21. [DOI: 10.1185/03007995.2012.709182] [DOI] [PubMed] [Google Scholar]
Hofman 2000 {published data only}
- Hofman J, Nasswetter G, Cayetti LM. Lysine clonixinate gel in soft tissue injuries. Controlled randomized prospective double‐blind clinical trial with diclofenac [Clonixinato de lisina gel en lesiones de tejidos blandos ensayo clinico prospectivo doble ciego al azar controlado con diclofenac]. Prensa Medica Argentina 2000;87(5):513‐20. [Google Scholar]
Hosie 1993 {published data only}
- Hosie GAC. The topical NSAID, felbinac, versus oral ibuprofen: a comparison of efficacy in the treatment of acute lower back injury. British Journal of Clinical Research 1993;4:5‐17. [Google Scholar]
Jenoure 1997 {published data only}
- Jenoure PJ, Rostan A, Gremion G, Meier JL, Grossen R, Bielinki R, et al. Multicentre, double‐blind, controlled clinical study on the efficacy of diclofenac epolamine Tissugel plaster in patients with epicondylitis [Studio multicentrico controllato in doppio cieco su diclofenac Tissugel plaster in pazienti con epicondilite]. Medicina Dello Sport 1977;50(3):285‐92. [Google Scholar]
Joussellin 2003 {published data only}
- Joussellin E. Flector Tissugel for the treatment of painful ankle sprains [Flector Tissugel dans le traitement des entorses douloureuses de la cheville]. Journal de Traumatologie du Sport 2003;20:1S5‐9. [Google Scholar]
- Lionberger DR, Joussellin E, Lanzarotti A, Yanchick J, Magelli M. Diclofenac epolamine topical patch relieves pain associated with ankle sprain. Journal of Pain Research 2011;4:47‐53. [DOI: 10.2147/JPR.S15380] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionberger DR, Joussellin E, Yanchick J, Magelli M, Lanzarotti A. Pooled analysis of clinical trial data evaluating the safety and effectiveness of diclofenac epolamine topical patch 1.3% for the treatment of acute ankle sprain. Open Access Journal of Sports Medicine 2011;2:75‐84. [DOI: 10.2147/OAJSM.S17048] [DOI] [PMC free article] [PubMed] [Google Scholar]
Julien 1989 {published data only}
- Julien D. Clinical trial ‐ Fastum gel FG‐8. Supplied by Menarini 1989.
Klainguti 2010 {published data only}
- Klainguti A, Forgacs A, Berkes I, Castellacci E. A plaster containing DHEP and heparin for mild to moderate contusions and sprains with haematoma: a double‐blind randomized study. Current Medical Research and Opinion 2010;26(9):2243‐51. [DOI: 10.1185/03007995.2010.508022] [DOI] [PubMed] [Google Scholar]
Kockelbergh 1985 {published data only}
- Kockelbergh M, Verspeelt P, Caloine R, Dermaux F. Local anti inflammatory treatment with a ketoprofen gel: current clinical findings. Journal Belge de Medecine Physique et de Rehabilitation 1985;8(4):205‐13. [PubMed] [Google Scholar]
Kuehl 2011 {published data only}
- Kuehl K, Carr W, Yanchick J, Magelli M, Rovati S. Analgesic efficacy and safety of the diclofenac epolamine topical patch 1.3% (DETP) in minor soft tissue injury. International Journal of Sports Medicine 2011;32(8):635‐43. [DOI: 10.1055/s-0031-1275359] [DOI] [PubMed] [Google Scholar]
Li 2013 {published data only}
- Li C, Frangione V, Rovati S, Zheng Q. Diclofenac epolamine medicated plaster in the treatment of minor soft tissue injuries: a multicenter randomized controlled trial. Current Medical Research and Opinion 2013;29(9):1137‐46. [DOI: 10.1185/03007995.2013.816669] [DOI] [PubMed] [Google Scholar]
Linde 1985 {published data only}
- Linde F, Hvass I, Jürgensen U, Madsen F. Treatment of sprained ankles with 5% benzydamine creme. A double‐blind study [Ankelforstuvninger behandlet med benzydamin 5% creme]. Ugeskrift for Laeger 1985;148(1):12‐3. [PubMed] [Google Scholar]
Machen 2002 {published data only}
- Machen J, Whitefield M. Efficacy of a proprietary ibuprofen gel in soft tissue injuries: a randomised, double blind placebo controlled study. International Journal of Clinical Practice 2002;56(2):102‐6. [PubMed] [Google Scholar]
Mahler 2003 {published data only}
- Mahler P, Mahler F, Duruz H, Ramazzina M, Liguori V, Mautone G. Double‐blind, randomized, controlled study on the efficacy and safety of a novel diclofenac epolamine gel formulated with lecithin for the treatment of sprains, strains and contusions. Drugs under Experimental and Clinical Research 2003;29(1):45‐52. [PubMed] [Google Scholar]
Mazières 2005a {published data only}
- Mazières B, Rouanet S, Velicy J, Scarsi C, Reiner V. Topical ketoprofen patch (100 mg) for the treatment of ankle sprain: a randomized, double‐blind, placebo‐controlled study. American Journal of Sports Medicine 2005;33(4):515‐23. [DOI: 10.1177/0363546504268135] [DOI] [PubMed] [Google Scholar]
Mazières 2005b {published data only}
- Mazières B, Rouanet S, Guillon Y, Scarsi C, Reiner V. Topical ketoprofen patch in the treatment of tendinitis: a randomized, double blind, placebo controlled study. Journal of Rheumatology 2005;32(8):1563‐70. [PubMed] [Google Scholar]
McLatchie 1989 {published data only}
- McLatchie GR, McDonald M, Lawrence GF, Rogmans D, Lisai P, Hibberd M. Soft tissue trauma: a randomised controlled trial of the topical application of felbinac, a new NSAID. British Journal of Clinical Practice 1989;43(8):277‐80. [PubMed] [Google Scholar]
Morris 1991 {published data only}
- Morris WD, Scott HV, Peters WA, Ketelbey JW. Felbinac topical gel for acute soft tissue sports injuries. New Zealand Journal of Sports Medicine 1991;19:45‐7. [Google Scholar]
NCT01255423 {unpublished data only}
- Novartis (Sponsors). A randomized, double‐blind, multi‐center, placebo‐controlled, parallel group study to evaluate the efficacy and safety of diclofenac sodium topical gel (DSG) 1% applied 4 times daily in subjects with acute ankle sprain. clinicaltrials.gov/ct2/show/NCT01255423 (accessed 3 February 2015) 2012. [CTG: NCT01255423]
NCT01272934 {unpublished data only}
- Novartis (Sponsors). A randomized, double‐blind, multi‐center, placebo‐controlled, parallel group study to evaluate the efficacy and safety of diclofenac sodium topical gel (DSG) 1% applied four times daily in subjects with acute ankle sprain. clinicaltrials.gov/ct2/show/results/NCT01272934 (accessed 3 February 2015) 2013. [CTG: NCT01272934]
NCT01272947 {unpublished data only}
- Novartis (Sponsors). A randomized, double‐blind, multi‐center, placebo‐controlled, parallel group study to evaluate the efficacy and safety of diclofenac sodium topical gel (DSG) 1% applied four times daily in subjects with acute blunt soft tissue injuries/contusions of the limbs. clinicaltrials.gov/ct2/show/results/NCT01272947 (accessed 3 February 2015) 2012. [CTG: NCT01272947]
- Pallay R, Pabst H, Giannetti B, Burnett I, Monnet J. Diclofenac sodium topical gel (DSG) 1% for acute soft tissue injuries/contusions of the limbs. 2013 Annual Assembly of the American Academy of Physical Medicine and Rehabilitation. National Harbor, MD United States, 2013; Vol. 5 (9 Suppl 1):S204.
Noret 1987 {published data only}
- Noret A, Roty V, Allington N, Hauters P, Zuinen C, Poels R. Ketoprofen gel as topical treatment for sport injuries. Acta Therapeutica 1987;13:367‐78. [Google Scholar]
Parrini 1992 {published data only}
- Parrini M, Cabitza P, Arrigo A, Vanasia M. Efficacy and tolerability of ketoprofen lysine salt foam for topical use in the treatment of traumatic pathologies of the locomotor apparatus [Efficacia e tollerabilita del ketoprofene sale di lisina schiuma per uso topico nel trattamento di alcune patologie traumatiche dell'apparato locomotore]. La Clinica Terapeutica 1992;141(9):199‐204. [PubMed] [Google Scholar]
Picchio 1981 {published data only}
- Picchio AA, Volta S, Longoni A. Controlled clinical trial of ibuprofen for topical use in sport injuries [Studio clinico controllato sull'impiego dell'ibuprofen per uso topico in traumatologica sportiva]. Medicina dello Sport 1981;34:403‐6. [Google Scholar]
Predel 2004 {published data only}
- Mueller EA, Kirch W, Reiter S. Extent and time course of pain intensity upon treatment with a topical diclofenac sodium patch versus placebo in acute traumatic injury based on a validated end point: post hoc analysis of a randomized placebo‐controlled trial. Expert Opinion on Pharmacotherapy 2010;11(4):493‐8. [DOI: 10.1517/14656560903535898] [DOI] [PubMed] [Google Scholar]
- Predel HG, Koll R, Pabst H, Dieter R, Gallacchi G, Giannetti B, et al. Diclofenac patch for topical treatment of acute impact injuries: a randomised, double blind, placebo controlled, multicentre study. British Journal of Sports Medicine 2004;38(3):318‐23. [DOI: 10.1136/bjsm.2003.005017] [DOI] [PMC free article] [PubMed] [Google Scholar]
Predel 2012 {published data only}
- Predel HG, Hamelsky S, Gold M, Giannetti B. Efficacy and safety of diclofenac diethylamine 2.32% gel in acute ankle sprain. Medicine and Science in Sports and Exercise 2012;44(9):1629‐36. [DOI: 10.1249/MSS.0b013e318257ed41] [DOI] [PubMed] [Google Scholar]
Predel 2013a {published data only}
- Predel HG, Giannetti B, Seigfried B, Novellini R, Menke G. A randomized, double‐blind, placebo‐controlled multicentre study to evaluate the efficacy and safety of diclofenac 4% spray gel in the treatment of acute uncomplicated ankle sprain. Journal of International Medical Research 2013;41(4):1187‐202. [DOI: 10.1177/0300060513487639] [DOI] [PubMed] [Google Scholar]
Predel 2013b {published data only}
- Predel HG, Giannetti B, Pabst H, Schaefer A, Hug AM, Burnett I. Efficacy and safety of diclofenac diethylamine 1.16% gel in acute neck pain: a randomized, double‐blind, placebo‐controlled study. BMC Musculoskeletal Disorders 2013;14:250. [DOI: 10.1186/1471-2474-14-250] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramesh 1983 {published data only}
- Ramesh N, Steuber U. Ibuprofen in a cream vehicle for accidental and sport injuries [Dolgit creme bei unfall‐ und sportverletzungen]. Therapiewoche 1983;33:4563‐70. [Google Scholar]
Rowbotham 2003 {published data only}
- Rowbotham M, Galer B, Block J, Backonja M. Flector Tissugel: efficacy and safety in minor sport injuries. A vs controlled clinical trial. Journal of Sports Traumatology 2003;20:IS15‐IS20. [Google Scholar]
Russell 1991 {published data only}
- Russell AL. Piroxicam 0.5% topical gel compared to placebo in the treatment of acute soft tissue injuries: a double‐blind study comparing efficacy and safety. Clinical and Investigative Medicine 1991;14(1):35‐43. [PubMed] [Google Scholar]
Saillant 1998 {published data only}
- Lionberger DR, Joussellin E, Yanchick J, Magelli M, Lanzarotti A. Pooled analysis of clinical trial data evaluating the safety and effectiveness of diclofenac epolamine topical patch 1.3% for the treatment of acute ankle sprain. Open Access Journal of Sports Medicine 2011;2:75‐84. [DOI: 10.2147/OAJSM.S17048] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saillant G. Study comparing the efficacy and tolerance of Flector Tissugel® to that of a placebo in the treatment of benign ankle sprains [Étude comparant l’efficacité et la tolérance de FlectorTissugel® à celles d’un placebo dans le traitement des entorses bénignes de la cheville]. Medicine du Sport 1998;72:1‐5. [Google Scholar]
Sanguinetti 1989 {published data only}
- Sanguinetti C. Treatment of soft tissue injury with BPAA gel. Results of an Italian multicenter study vs. placebo [Trattemento con BPAA gel dei traumi dei tessuti moli]. Clinica Terapeutica 1989;130(5):255‐258. [PubMed] [Google Scholar]
Sinniger 1981 {published data only}
- Sinniger M, Blanchard P. Controlled clinical trial with Fentiazac cream in sport microtraumatology. Journal of International Medical Research 1981;9(4):300‐2. [DOI] [PubMed] [Google Scholar]
Spacca 2005 {published data only}
- Spacca G, Cacchio A, Forg?cs A, Monteforte P, Rovetta G. Analgesic efficacy of a lecithin‐vehiculated diclofenac epolamine gel in shoulder periarthritis and lateral epicondylitis: a placebo‐controlled, multicenter, randomized, double‐blind clinical trial. Drugs under Experimental and Clinical Research 2005;31(4):147‐54. [PubMed] [Google Scholar]
Sugioka 1984 {published data only}
- Sugioka Y. Multicenter clinical evaluation of piroxicam gel vs. indomethacin gel in the treatment of non‐traumatic diseases of tendon or muscle. Japanese Pharmacology and Therapeutics 1984;12:139‐53. [Google Scholar]
Thorling 1990 {published data only}
- Thorling J, Linden B, Berg R, Sandahl A. A double blind comparison of naproxen gel and placebo in the treatment of soft tissue injuries. Current Medical Research and Opinion 1990;12:242‐8. [DOI] [PubMed] [Google Scholar]
Tonutti 1994 {published data only}
- Tonutti A. The use of ketoprofen gel 5% (orudis gel) in traumatology: controlled double‐blind study vs etofenamate [Utilizzazione del ketoprofene gel 5% (orudis gel) nella pratica traumatologica: studio in doppio cieco controllato verso etofenamato]. Ortopedia e Traumatologia Oggi 1994;14(3):119‐25. [Google Scholar]
Vecchiet 1991 {published data only}
- Vecchiet L, Colozzi A. Effects of meclofenamic acid in the treatment of lesions deriving from minor traumatology. Clinical Journal of Pain 1991;7 Suppl 1:S54‐9. [PubMed] [Google Scholar]
Whitefield 2002 {published data only}
- Whitefield M, O'Kane CJA, Anderson S. Comparative efficacy of a proprietary topical ibuprofen gel and oral ibuprofen in acute soft tissue injuries: a randomised, double blind study. Journal of Clinical Pharmacy and Therapeutics 2002;27(6):409‐17. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ambrus 1987 {published data only}
- Ambrus P, Böhmer D. Mobilat ointment in acute sprains [Mobilat Salbe bei akuten Distorsionen]. Fortschritte der Medizin 1987;105(13):259‐62. [PubMed] [Google Scholar]
Anon 1993 {unpublished data only}
- Anon. Comparative clinical efficacy of Oruvail, piroxicam and diclofenac gels in soft tissue injury. Unpublished.
Ascherl 1982 {published data only}
- Ascherl R, Schlemmer H, Blumel G, Lechner F. The effectiveness of etofenamate in minor sports injuries of the knee and ankle joint. A double blind study [Die Wirksamkeit der Etofenamat in kleineren Sportverletzungen am Knie und Sprunggelenk. Eine Doppelblind‐Studie]. Fortschritte der Medizin 1982;100(37):1729‐34. [PubMed] [Google Scholar]
Bagliani 1976 {published data only}
- Bagliani A, Montalbetti L. Topical treatment of thrombophlebitis with feprazone and benzydamine. Controlled clinical study [Il trattamento topico di tromboflebite con feprazone e benzidamina. Studio clinico controllato]. Minerva Medica 1976;67(14):880‐4. [PubMed] [Google Scholar]
Baracchi 1982 {published data only}
- Baracchi G, Messina Denaro S, Piscini S. Experience of the topical use of isobutylfenylproprionic acid (Ibuprofen) in traumatic inflammation. A double‐blind comparison with placebo [Esperienza sull'impiego topico dell'acido isobutil‐fenil‐propionico (ibuprofen) nella flogosi traumatica. Confronto a doppia cecita con placebo]. Gazzetta Medica Italiana 1982;141(12):691‐4. [Google Scholar]
Böhmer 1995 {published data only}
- Böhmer D, Ambrus P. Treatment of muscular injuries with diclofenac‐diethylammonium emugel [Behandlung von muskelverletzungen mit diclofenac‐diethylammonium emugel]. Sportverletzung Sportschaden 1995;9:94‐5. [DOI] [PubMed] [Google Scholar]
Burnham 1998 {published data only}
- Burnham R, Gregg R, Healy P, Steadward R. The effectiveness of topical diclofenac for lateral epicondylitis. Clinical Journal of Sport Medicine 1998;8(2):78‐81. [DOI] [PubMed] [Google Scholar]
Cesarone 2008 {published data only}
- Cesarone MR, Belcaro G, Pellegrini L, Ledda A, Vinciguerra G, Ricci A, et al. Treatment of ankle sprain in patients with vascular diseases of the lower limbs. Minerva Cardioangiologica 2008;56 (5 Suppl):39‐46. [PubMed] [Google Scholar]
Coulibaly 2009 {published data only}
- Coulibaly SK, Courau S, Staiger C. The sprained ankle: comparative study of a comfreyroot extract ointment versus a diclofenac gel [Entorses de la cheville: étude comparative entre un extrait à base de racines de consoude et le diclofénac]. Phytothérpie 2009;7:147‐9. [DOI: 10.1007/s10298-009-0383-x] [DOI] [Google Scholar]
Diebschlag 1985 {published data only}
- Diebschlag W. Benzydamine cream in post‐traumatic oedema. International Journal of Tissue Reactions 1985;7(3):219‐23. [PubMed] [Google Scholar]
Diebschlag 1986 {published data only}
- Diebschlag W. Diclofenac in blunt traumatic ankle joint swelling. Volumetric monitoring in a placebo controlled double blind trial [Diclofenac in stumpfen traumatischen Sprunggelenk Schwellung. Volumetrische Überwachung in einer Placebo‐kontrollierten Doppelblind‐Studie]. Fortschritte der Medizin 1986;104(21):437‐40. [PubMed] [Google Scholar]
Diebschlag 1992 {published data only}
- Diebschlag W, Nocker W, Lehmacher W. Treatment of acute sprains of the ankle joint. A comparison of the effectiveness and tolerance of two gel preparations containing indomethacin [Die Behandlung der akuten Verstauchungen des Sprunggelenks. Ein Vergleich der Wirksamkeit und Verträglichkeit von zwei Gel‐Zubereitungen mit Indometacin]. Fortschritte der Medizin 1992;110(6):64‐72. [PubMed] [Google Scholar]
Fantato 1971 {published data only}
- Fantato S, Gregorio M. Clinical evaluation of topical benzydamine in traumatology. Arzneimittel‐Forschung 1971;21(10):1530‐5. [PubMed] [Google Scholar]
Galer 2000 {published data only}
- Galer BS, Rowbotham M, Perander J, Devers A, Friedman E. Topical diclofenac patch relieves minor sports injury pain: results of a multicenter controlled clinical trial. Journal of Pain and Symptom Management 2000;19(4):287‐94. [DOI] [PubMed] [Google Scholar]
Hallmeier 1986 {published data only}
- Hallmeier B, Michelbach B. Etofenamate under tape bandages ‐ a controlled study [Etofenamat unter tape‐verbänden]. Medizinische Welt 1986;37(43):1344‐8. [Google Scholar]
Hallmeier 1988 {published data only}
- Hallmeier B. Efficacy and tolerance of etofenamate and diclofenac in acute sports injuries [Wirksamkeit und Verträglichkeit von Etofenamat und Diclofenac bei akuten Sportverletzungen]. Rheuma 1988;8:183‐6. [Google Scholar]
Kaneko 1999 {published data only}
- Kaneko M, Shimojo H, Saito H, Onuma Y, Yamashita K. Clinical evaluation of felbinac patch (SELSPOT) on post‐traumatic disease: clinical comparative study versus commercially available patch. Japanese Pharmacology and Therapeutics 1999;27:75‐85. [Google Scholar]
Kockelbergh 1985b {published data only}
- Kockelbergh M, Verspeelt P, Caloine R, Dermaux F. Local anti‐inflammatory treatment with a ketoprofen gel: current clinical findings [Traitement anti‐inflammatoire local par un gel de kétoprofène: données cliniques récentes]. Journal Belge de Medecine Physique et de Rehabilitation 1985;8(4):205‐13 (study 2). [PubMed] [Google Scholar]
Kuwabara 2013 {published data only}
- Kuwabara Y, Hamamoto H, Hikake S, Miwa Y. A randomized, multi‐center, double‐blind, placebo‐controlled phase II/III trial to evaluate the eftopical patch in the treatment of pain. Journal of Pain. 2013; Vol. 14 (4 Suppl 1):S73.
Lee 1991 {published data only}
- Lee EH, Lee PY, Ngai AT, Chiu EH. Treatment of acute soft tissue trauma with a topical non‐steroidal anti‐inflammatory drug (biphenylacetic acid 3% gel). Singapore Medical Journal 1991;32(4):238‐41. [PubMed] [Google Scholar]
Link 1996 {published data only}
- Link R, Balint G, Pavlik G, Otto J, Krause W. Topical treatment of soft tissue rheumatism and athletic injuries. Effectiveness and tolerance of a new ketoprofen gel [Topische Behandlung von Weichteil‐Rheumatismus und Sportverletzungen. Wirksamkeit und Verträglichkeit eines neuen Ketoprofen Gel]. Fortschritte der Medizin 1996;114(25):311‐4. [PubMed] [Google Scholar]
May 2007 {published data only}
- May JJ, Lovell G, Hopkins WG. Effectiveness of 1% diclofenac gel in the treatment of wrist extensor tenosynovitis in long distance kayakers. Journal of Science and Medicine in Sport 2007;10(1):59‐65. [DOI] [PubMed] [Google Scholar]
Oakland 1993 {published data only}
- Oakland C, Rapier C. A comparison of the efficacy of the topical NSAID felbinac and ultrasound in the treatment of acute ankle injuries. British Journal of Clinical Research 1993;4:89‐96. [Google Scholar]
Odaglia 1987 {unpublished data only}
- Odaglia G, Sereni G. Sports minor traumatology: results of a double‐blind controlled clinical study ketoprofen (fastum gel 2.5%) versus placebo. Menarini unpublished data.
Picardi 1993 {published data only}
- Picardi E, Iasio R. Efficacy of percutaneous anti‐inflammatory drugs (NSAIDs) in swimmers and waterpolo players [Efficacia dei farmaci antinfiammatori (FANS) per via percutanea in atleti di nuoto e pallanuoto]. Clinica Terapeutica 1993;143(6):507‐9. [PubMed] [Google Scholar]
Taboada 1992 {published data only}
- Taboada A. Controlled trial of piroxicam gel associated with ultrasound in acute disturbances of the locomotive system [Experienca controlada con gel de piroxicam asociado a ultrasonidos en afecciones agudas del aparato locomotor]. Prensa Medica Argentina 1992;79(10):630‐2. [Google Scholar]
Vanderstraeten 1990 {published data only}
- Vanderstraeten G, Schuermans P. Study on the effect of etofenamate 10% cream in comparison with an oral NSAID in strains and sprains due to sports injuries. Acta Belgica Medica Physica 1990;13(3):139‐41. [PubMed] [Google Scholar]
Vinciguerra 2008 {published data only}
- Vinciguerra G, Belcaro G, Cesarone MR, Errichi BM, Renzo A, Errichi S, et al. Management of uncomplicated ankle sprains with topical or oral ketoprofen treatment. A registry study. Minerva Cardioangiologica 2008;56 (5 Suppl):47‐53. [PubMed] [Google Scholar]
Von Klug 1977 {published data only}
- Klug H. Experience with a locally applied anti‐inflammatory drug [Erfahrungen mit einem local anwendbaren antirheumatikum]. Arzneimittel‐Forschung/Drug Research 1977;27:1350‐4. [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT00351104 {published data only}
- Endo Pharmaceuticals (Sponsors). A randomized, double‐blind, placebo‐controlled, parallel group phase III study of the efficacy, tolerability and safety of ketoprofen topical patch, 20% (KTP) in the treatment of pain associated with grade 1 or grade 2 ankle sprain or strain. clinicaltrials.gov/ct2/show/NCT00351104 (accessed 3 February 2015) 2008. [CTG: NCT00351104]
NCT00352625 {unpublished data only}
- Endo Pharmaceuticals (Sponsors). A randomized, double‐blind, placebo‐controlled, parallel group phase III study of the efficacy, tolerability and safety of ketoprofen topical patch, 20% (KTP) in the treatment of pain associated with tendonitis or bursitis of the shoulder, elbow or knee. clinicaltrials.gov/ct2/show/NCT00352625 (accessed 3 February 2015) 2008. [CTG: NCT00352625]
NCT00426985 {unpublished data only}
- Endo Pharmaceuticals (Sponsors). A randomized, double‐blind, placebo‐controlled, parallel group phase iii study of the efficacy, tolerability and safety of ketoprofen topical patch, 20% (KTP) in the treatment of pain associated with tendonitis or bursitis of the shoulder, elbow or knee. clinicaltrials.gov/ct2/show/NCT00426985 (accessed 3 February 2015) 2008. [CTG: NCT00426985]
NCT00640705 {unpublished data only}
- Cerimon Pharmaceuticals (Sponsors). A randomized, double‐blind, placebo‐controlled study of the efficacy and safety of a diclofenac sodium patch for the topical treatment of pain due to mild to moderate ankle sprain. clinicaltrials.gov/ct2/show/NCT00640705 (accessed 3 February 2015) 2008. [CTG: NCT00640705]
NCT00640939 {unpublished data only}
- Cerimon Pharmaceuticals (Sponsors). A randomized, double‐blind, placebo‐controlled study of the efficacy and safety of a diclofenac sodium patch for the topical treatment of pain due to mild to moderate tendonitis or bursitis. clinicaltrials.gov/ct2/show/NCT00640939 (accessed 3 February 2015) 2008. [CTG: NCT00640939]
NCT00680472 {unpublished data only}
- Hisamitsu Pharmaceutical Co, Inc (Sponsors). A randomized, multicenter, double‐blind, placebo‐controlled, two‐week study to assess the efficacy and safety of HKT‐500 in subjects with acute shoulder pain. clinicaltrials.gov/ct2/show/NCT00680472 (accessed 3 February 2015) 2015. [CTG: NCT00680472]
NCT00680784 {unpublished data only}
- Hisamitsu Pharmaceutical Co, Inc (Sponsors). A randomized, multicenter, double‐blind, placebo‐controlled study to evaluate the efficacy and safety of HKT‐500 in the treatment of pain associated with Grade I or Grade II ankle sprain. clinicaltrials.gov/ct2/show/NCT00680784 (accessed 3 February 2015) 2015. [CTG: NCT00680784]
NCT00765700 {unpublished data only}
- Ekman E, Skrepnik S, Jones M, Lawson K, Schupp J. Efficacy and safety of ketoprofen 10% cream in acute soft tissue injuries (phase 3 study tdlp‐110‐001). Proceedings of the 13th World Congress on Pain 2010 Aug 29 – Sept 2; Montreal, Canada.
- Imprimis Pharmaceuticals, Inc (Sponsors). A randomized, multicenter, double‐blind, placebo‐controlled, parallel‐group, phase 3 study to assess the efficacy and safety of Ketotransdel™ (Ketoprofen topical cream 10%) in the treatment of pain associated with mild to moderate acute soft tissue injury. clinicaltrials.gov/ct2/show/NCT00765700 (accessed 3 February 2015) 2013. [CTG: NCT00765700]
NCT00869063 {unpublished data only}
- Cerimon Pharmaceuticals (Sponsors). A randomized, double‐blind, placebo‐controlled study of the efficacy and safety of a diclofenac sodium patch for the topical treatment of acute pain due to mild to moderate wrist sprain, strain or contusion. clinicaltrials.gov/ct2/show/NCT00869063 (accessed 3 February 2015) 2010. [CTG: NCT00869063]
NCT00869180 {unpublished data only}
- Cerimon Pharmaceuticals (Sponsors). A randomized, double‐blind, placebo‐controlled study of the efficacy and safety of a diclofenac sodium patch for the topical treatment of acute pain due to mild to moderate ankle sprain. clinicaltrials.gov/ct2/show/NCT00869180 (accessed 3 February 2015) 2010. [CTG: NCT00869180]
NCT00931866 {unpublished data only}
- Cerimon Pharmaceuticals (Sponsors). A randomized, double‐blind, placebo‐controlled study of the efficacy and safety of a diclofenac sodium patch for the topical treatment of acute pain due to mild to moderate soft tissue injuries. clinicaltrials.gov/ct2/show/NCT00931866 (accessed 3 February 2015) 2010. [CTG: NCT00931866]
NCT01874626 {unpublished data only}
- Strategic Science & Technologies, LLC (Sponsors). A randomized, double‐blind, placebo‐controlled, multi‐dose, pivotal study to determine the efficacy and safety of SST 0225, a topical ibuprofen cream, in the treatment of pain associated with acute ankle sprain. clinicaltrials.gov/ct2/show/NCT01874626 (accessed 3 February 2015) 2014. [CTG: NCT01874626]
NCT01957215 {unpublished data only}
- GlaxoSmithKline (Sponsors). A clinical study to assess the efficacy of pain relief of topical indomethacin patch over placebo in ankle sprain patients. clinicaltrials.gov/ct2/show/NCT01957215 (accessed 3 February 2015) 2015. [CTG: NCT01957215]
NCT02324270 {unpublished data only}
- Actavis Inc (Sponsors). Randomized, double‐blind, multiple‐center, placebo‐controlled study comparing the safety and efficacy of generic diclofenac epolamine to Flector® patch in the treatment of acute pain due to minor ankle sprain. clinicaltrials.gov/ct2/show/NCT02324270 (accessed 3 February 2015) 2014. [CTG: NCT02324270]
Sarzi‐Puttini 2014 {published data only}
- Sarzi‐Puttini P, Atzeni F, Damiani C, Casale R, Barbagallo M, Cazzola M. A double‐blind, randomised, parallel group, active controlled, multicentre study to assess the therapeutic non‐inferiority of SKP‐021, a 0.3% ketoprofen patch, versus diclofenac sodium patch in patients with acute inflammatory musculoskeletal injuries. Proceeding of the Annual European Congress of Rheumatology of the European League Against Rheumatism, EULAR 2014 June 11‐14; Paris, France. 2014:73.
References to ongoing studies
NCT01945034 {unpublished data only}
- Pfizer (Sponsors). Placebo‐controlled, double‐blind evaluation of the efficacy and safety of ibuprofen 5% topical gel for the treatment of ankle sprain. clinicaltrials.gov/ct2/show/NCT01945034 (accessed 3 February 2015) 2014. [CTG: NCT01945034]
NCT02100670 {unpublished data only}
- GlaxoSmithKline (Sponsors). A clinical study to assess the efficacy and onset of pain relief of topical MFC51123 diclofenac‐menthol gel versus controls in ankle sprain. clinicaltrials.gov/ct2/show/NCT02100670 (accessed 3 February 2015) 2014. [ClinicalTirals.gov: NCT02100670]
NCT02290821 {unpublished data only}
- Novartis (Sponsors). A randomized, double‐blind, placebo‐controlled, parallel group study to evaluate the efficacy and safety of diclofenac sodium topical gel (DSG) 1% applied four times daily in subjects with acute blunt soft tissue injuries/contusions of the limbs. clinicaltrials.gov/ct2/show/NCT02290821 (accessed 3 February 2015) 2015. [CTG: NCT02290821]
Additional references
Anon 2005
- Anon. Topical analgesics: a review of reviews and a bit of perspective. www.jr2.ox.ac.uk/Bandolier/Extraforbando/Topextra3.pdf 2005.
Cook 1995
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ 1995;310(6977):452‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Counsell 1994
- Counsell CE, Clarke MJ, Slattery J, Sandercock PA. The miracle of DICE therapy for acute stroke: fact or fictional product of subgroup analysis?. BMJ 1994;309(6970):1677‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dechartres 2013
- Dechartres A, Trinquart L, Boutron I, Ravaud P. Influence of trial sample size on treatment effect estimates: meta‐epidemiological study. BMJ 2013;346:f2304. [DOI: 10.1136/bmj.f2304] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2012a
- Derry S, Moore RA. Topical capsaicin (low concentration) for chronic neuropathic pain in adults. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD010111] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2012b
- Derry S, Moore RA, Rabbie R. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD007400.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2013
- Derry S, Sven‐Rice A, Cole P, Tan T, Moore RA. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD007393.pub3] [DOI] [PubMed] [Google Scholar]
Derry 2014
- Derry S, Matthews PR, Wiffen PJ, Moore RA. Salicylate‐containing rubefacients for acute and chronic musculoskeletal pain in adults. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD007403.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Evans 1995
- Evans JM, McMahon AD, McGilchrist MM, White G, Murray FE, McDevitt DG, et al. Topical non‐steroidal anti‐inflammatory drugs and admission to hospital for upper gastrointestinal bleeding and perforation: a record linkage case‐control study. BMJ 1995;311(6996):22‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
FitzGerald 2001
- FitzGerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase‐2. New England Journal of Medicine 2001;345(6):433‐42. [DOI] [PubMed] [Google Scholar]
Haroutiunian 2010
- Haroutiunian S, Drennan DA, Lipman AG. Topical NSAID therapy for musculoskeletal pain. Pain Medicine 2010;11(4):535‐49. [DOI: 10.1111/j.1526-4637.2010.00809.x] [DOI] [PubMed] [Google Scholar]
Hawkey 1999
- Hawkey CJ. Cox‐2 inhibitors. Lancet 1999;353(9149):307‐14. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jadad 1996a
- Jadad AR, Carroll D, Moore A, McQuay H. Developing a database of published reports of randomised clinical trials in pain research. Pain 1996;66(2‐3):239‐46. [DOI] [PubMed] [Google Scholar]
Jadad 1996b
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
L'Abbé 1987
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107(2):224‐33. [DOI] [PubMed] [Google Scholar]
Makris 2010
- Makris UE, Kohler MJ, Fraenkel L. Adverse effects of topical nonsteroidal antiinflammatory drugs in older adults with osteoarthritis: a systematic literature review. Journal of Rheumatology 2010;37(6):1236‐43. [DOI: 10.3899/jrheum.090935] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mason 2004a
- Mason L, Moore RA, Edwards JE, Derry S, McQuay HJ. Topical NSAIDs for acute pain: a meta‐analysis. BMC Family Practice 2004;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mason 2004b
- Mason L, Moore RA, Edwards JE, Derry S, McQuay HJ. Topical NSAIDs for chronic musculoskeletal pain: systematic review and meta‐analysis. BMC Musculoskeletal Disorders 2004;5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
McPherson 2013
- McPherson ML, Cimino NM. Topical NSAID formulations. Pain Medicine 2013;14 Suppl 1:S35‐9. [DOI: 10.1111/pme.12288] [DOI] [PubMed] [Google Scholar]
MHRA 2009
- Medicines and Healthcare Products Regulatory Agency. Topical ketoprofen: reminder on risk of photo‐sensitivity reactions. Drug Safety Update 2009 June; Vol. 2, issue 11:6.
Moore 1998a
- Moore RA, Tramèr MR, Carroll D, Wiffen PJ, McQuay HJ. Quantitative systematic review of topically applied non‐steroidal anti‐inflammatory drugs. BMJ 1998;316(7128):333‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 1998b
- Moore RA, Gavaghan D, Tramèr MR, Collins SL, McQuay HJ. Size is everything ‐ large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209‐16. [DOI] [PubMed] [Google Scholar]
Moore 2003
- Moore RA, Edwards J, Barden J, McQuay HJ. Bandolier's Little Book of Pain. Oxford: Oxford University Press, 2003. [ISBN: 0‐19‐263247‐7] [Google Scholar]
Moore 2006
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2006:15‐23. [ISBN: 978‐0‐931092‐69‐5] [Google Scholar]
Moore 2008a
- Moore RA, Derry S, McQuay HJ. Topical agents in the treatment of rheumatic pain. Rheumatic Diseases Clinics of North America 2008;34(2):415‐32. [DOI] [PubMed] [Google Scholar]
Moore 2008b
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐24. [ISBN: 978‐0‐931092‐69‐5] [Google Scholar]
Moore 2013
- Moore RA, Straube S, Aldington D. Pain measures and cut‐offs ‐ 'no worse than mild pain' as a simple, universal outcome. Anaesthesia 2013;68(4):400‐12. [DOI: 10.1111/anae.12148] [DOI] [PubMed] [Google Scholar]
Morris 1995
- Morris JA, Gardner MJ. Calculating confidence intervals for relative risk, odds ratios and standardised ratios and rates. In: Gardner MJ, Altman DG editor(s). Statistics with Confidence ‐ Confidence Intervals and Statistical Guidelines. London: British Medical Journal, 1995:50‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2008
- National Institute for Health and Care Excellence. Osteoarthritis. The care and management of osteoarthritis in adults, 2008. www.nice.org.uk/CG059fullguideline.
Nüesch 2010
- Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Tschannen B, Altman DG, et al. Small study effects in meta‐analyses of osteoarthritis trials: meta‐epidemiological study. BMJ 2010;341:c3515. [DOI: 10.1136/bmj.c3515] [DOI] [PMC free article] [PubMed] [Google Scholar]
PACT 2014
- Prescribing and Primary Care team, Health and Social Care Information Centre. Prescrption Cost Analysis, England 2013. Health and Social Care Information Centre. The NHS Information Centre, 2014:298‐301. [ISBN: 978‐1‐78386‐089‐0]
Quane 1998
- Quane PA, Graham GG, Zeigler JB. Pharmacology of benzydamine. Inflammopharmacology 1998;6(2):95‐107. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roth 2012
- Roth SH, Fuller P. Pooled safety analysis of diclofenac sodium topical solution 1.5% (w/w) in the treatment of osteoarthritis in patients aged 75 years or older. Clinical Interventions in Aging 2012;7:127‐37. [DOI: 10.2147/CIA.S30884] [DOI] [PMC free article] [PubMed] [Google Scholar]
Simon 2009
- Simon LS, Grierson LM, Naseer Z, Bookman AA, Zev Shainhouse J. Efficacy and safety of topical diclofenac containing dimethyl sulfoxide (DMSO) compared with those of topical placebo, DMSO vehicle and oral diclofenac for knee osteoarthritis. Pain 2009;143(3):238‐45. [DOI: 10.1016/j.pain.2009.03.008] [DOI] [PubMed] [Google Scholar]
Tramer 1997
- Tramer MR, Reynolds DJ, Moore RA, McQuay HJ. Impact of covert duplicate publication on meta‐analysis: a case study. BMJ 1997;315(7109):635‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tugwell 2004
- Tugwell PS, Wells GA, Shainhouse JZ. Equivalence study of a topical diclofenac solution (pennsaid) compared with oral diclofenac in symptomatic treatment of osteoarthritis of the knee: a randomized controlled trial. Journal of Rheumatology 2004;31(10):2002‐12. [PubMed] [Google Scholar]
Zimmerman 1995
- Zimmerman J, Siguencia J, Tsvang E. Upper gastrointestinal hemorrhage associated with cutaneous application of diclofenac gel. American Journal of Gastroenterology 1995;90(11):2032‐4. [PUBMED: 7485017] [PubMed] [Google Scholar]
References to other published versions of this review
Massey 2010
- Massey T, Derry S, Moore RA, McQuay HJ. Topical NSAIDs for acute pain in adults. Cochrane Database of Systematic Reviews 2010, Issue 6. [DOI: 10.1002/14651858.CD007402.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


