Abstract
Verticipyrone has recently been isolated from the culture broth of Verticillium sp. and shown to inhibit NADH fumarate reductase, as well as NADH oxidoreductase (complex I) of the mitochondrial electron transport chain. In order to assess the structural elements in verticipyrone essential for complex I inhibitor, 15 structural analogues were prepared and analyzed for their effects on mitochondrial NADH oxidoreductase and NADH oxidase activities. Also measured were the abilities of several of the analogues to inhibit respiration as judged by a shift to glycolysis, and to inhibit the growth of several mammalian cell lines. The nature of the pyrone ring was shown to be important to potency of inhibition, as was the length and nature of substituents in the side chain of the analogues.
Keywords: Verticipyrone analogues, Mitochondrial complex I, NADH-ubiquinone oxidoreductase, NADH oxidase
1. Introduction
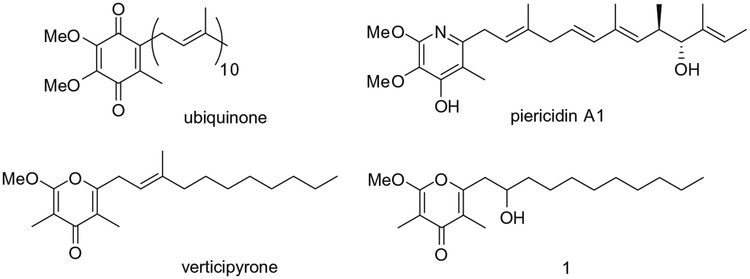
Recently, verticipyrone (Fig. 1) was isolated from the culture broth of the fungus Verticillium sp. FKI-1083 as an inhibitor of NADH fumarate reductase and complex I (NADH oxidoreductase) of the mitochondrial electron transport chain.1 The fully substituted γ-pyrone core and the unsaturated side chain of verticipyrone exhibit structural similarities both with ubiquinone as well as piericidin, the most potent inhibitor of complex I (Fig. 1). Omura and co-workers reported the total synthesis of verticipyrone and analogues which inhibited NADH fumarate reductase from Ascariis suum with IC50 values in the subnanomolar range.2
Figure 1.
Structures of ubiquinone (coenzyme Q10), piericidin A1, verticipyrone and previously synthesized analogue 1.
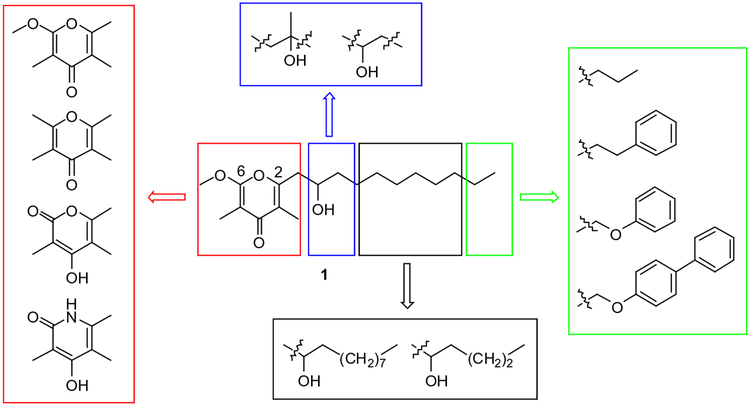
Given the interesting biological profile of verticipyrone and its analogues,2 a variety of new verticipyrone analogues were envisioned and prepared to investigate the structural elements of the natural product responsible for its effects on mitochondrial electron transport. The structures of the new analogues were designed to differ from verticipyrone analogue 1 in several respects, and included modification of the pyrone moiety and aliphatic side chain (Fig. 2).
Figure 2.
Structural modifications of verticipyrone, shown by individual structural domains.
The analogues were studied to define their effects on the mitochondrial electron transport chain. Specific effects investigated included (i) complex I function, as measured by NADH-ubiquinone oxidoreductase, (ii) function of complexes I, III and IV, as measured by NADH oxidase, (iii) evaluation of the consequences of inhibition of respiration as measured by a shift to glycolysis, and (iv) evaluation of the cytotoxicities of key compounds in several mammalian cell lines.
The verticipyrone analogues exhibited a range of potencies as inhibitors of the mitochondrial respiratory chain. Modification of the 6-methoxy group of the γ-pyrone ring with a methyl group did not drastically affect the complex I inhibitory potency. However, alterations of the γ-pyrone moiety to a c-hydroxy-α-pyrone reduced the inhibitory potency dramatically. The position and stereochemistry of the hydroxyl group on the side chain did not significantly affect the potency of the prepared analogues, but introduction of a biphenyl ether moiety led to more potent analogues than those containing alkyl chains. Reported herein is the preparation, biochemical and biological characterization of these interesting new mitochondrial respiratory chain inhibitors. In addition to providing compounds of utility for probing mitochondrial complex function, the findings bear relevance to the design of exogenous complex I substrates.
2. Results and discussion
2.1. Chemistry
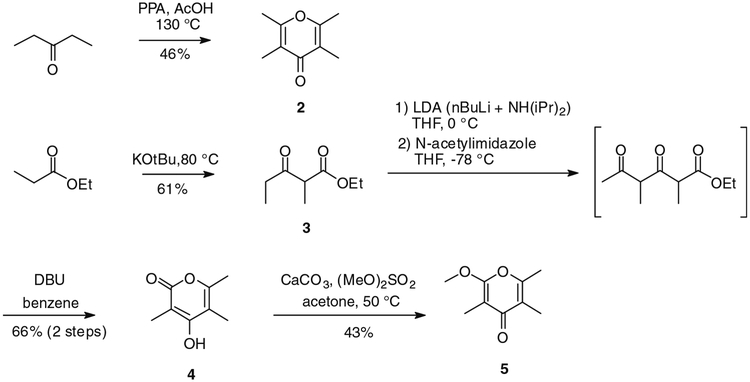
The targeted pyrones were prepared first. Tetramethyl-γ-pyrone (2) was prepared in 46% yield following a literature procedure by treating 3-pentanone with polyphosphoric acid and acetic acid (Scheme 1).3 Known β-ketoester 3 was obtained by intermolecular Claisen reaction of ethyl propionate in the presence of KOtBu.4 By using an approach similar to that used for the reported synthesis of iromycin,5 3 was first acetylated using LDA and N-acetylimidazole, affording the unstable intermediate diketo ester; treatment with DBU afforded the expected γ-hydroxy-α-pyrone 4 via cyclization of the intermediate (Scheme 1). This scheme was shorter and more convenient operationally than a published route,2 although proceeding in lower yield. The preparation of the corresponding α methoxy-γ-pyrone (5) by selective O-methylation of 4 was accomplished by using dimethyl sulfate. According to the method of Hosokawa et al., treatment of 4 with dimethyl sulfate in presence of a large excess of CaCO3 in acetone gave desired methylated pyrone 5 after four days of reaction at 50 °C.6 This compound was separated from its isomeric γ-methoxy-α-pyrone by chromatography on a silica gel column.
Scheme 1.
Synthesis of pyrones 2, 4 and 5.
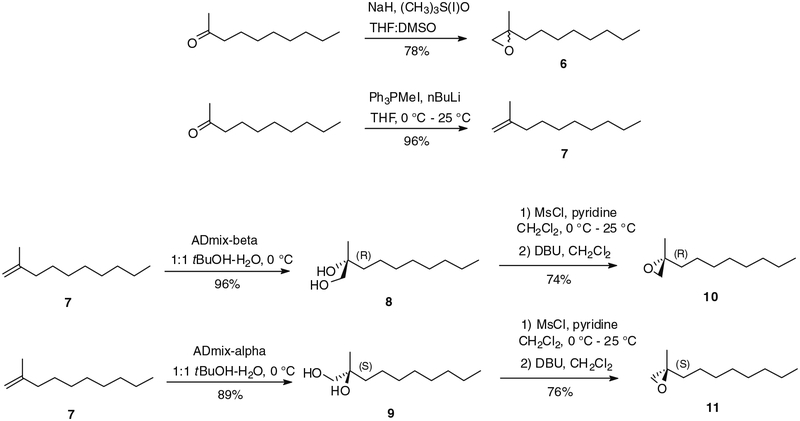
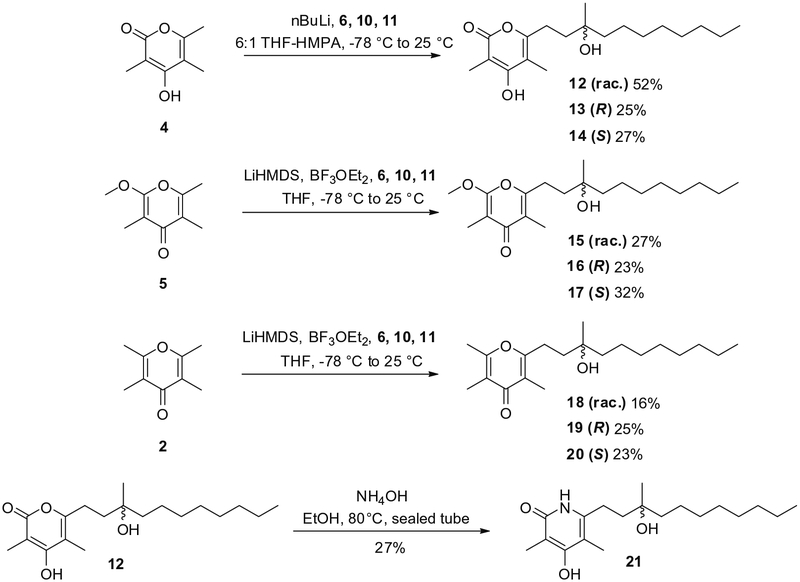
With pyrones 2, 4 and 5 in hand, the synthetic approach envisioned to obtain the tertiary alcohol analogues involved regioselective opening of suitable racemic, (R) and (S) epoxides by anions derived from the pyrones. In support of this strategy, Zhang et al. have recently reported regioselective alkylation of hydroxypyrones by using nBuLi in a mixture of THF and HMPA.7 This report also established the feasibility of epoxide opening by this method. Further, Shimamura et al. found that the C2 methyl group of methoxypyrone 5 can be regioselectively deprotonated by using either LiHMDS or LDA.2 It was thought that tetramethyl pyrone 2 should exhibit the same reactivity, presuming the use of stoichiometric amounts of base to avoid the formation of disubstituted secondary products. According to this strategy, the racemic epoxide 6 was first prepared by treatment of 2-decanone with trimethylsulfoxonium iodide and NaH in DMSO (Scheme 2) following a literature procedure.8 The 2-decanone also provided access to the corresponding 2-methyl-1-decene (7) in almost quantitative yield by means of a Wittig reaction using triphenylmethylphosphonium iodide and nBuLi (Scheme 2).9 Stereoselective Sharpless dihydroxylation of 7 by the use of ADmix β and α afforded the expected (R) and (S) diols 8 and 9, respectively.10 The selective activation of the primary alcohols was achieved by conversion to the respective mesylates,11 the latter of which were treated with 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU)12 to give the corresponding (R) and (S)-epoxides 10 and 11 in ~75% yields (Scheme 2).
Scheme 2.
Preparation of epoxides 6, 10 and 11.
The regioselective opening of epoxides 6, 10 and 11 by c-hydro-xy-α-pyrone 4 using conditions reported by Zhang et al. led to the expected coupling products (Scheme 3).7 Compounds 12, 13 and 14 were obtained in this fashion, albeit in modest yields (25–52%), probably due to the low reactivity of the epoxides, as well as the need for careful purification of the formed product mixtures. However, opening of the epoxides was observed to be regioselective; the undesired isomers were not formed. Similar coupling reactions with methoxypyrone 5 proved to be more difficult. In fact, after formation of the carbanion of 5 by treatment with LiHMDS or LDA in THF at −78 °C, no coupling was observed following addition of the epoxides. The addition of known epoxide activating agents such as LiCl or LiOTf also produced disappointing results. Finally, extensive investigation revealed that the addition of BF3 Et2O, associated with the use of LiHMDS as a base, permitted the formation of coupling products 15, 16 and 17 in low (23–32%) yields (Scheme 3). The same reaction conditions were applied successfully to tetramethylpyrone 2 by using stoichiometric amounts of LiHMDS (Scheme 3). After purification, the expected coupling products 18, 19 and 20 were obtained in low (16–25%) yields as well.
Scheme 3.
Synthesis of pyrone analogues 12–20 and pyridone analogue 21.
Another synthetic challenge involved the preparation of a γ-hydroxy-α-pyridone derivative of coupling product 12. Initially, it was thought that the synthesis of suitably protected γ-hydroxy-α-pyri-done as well as γ-methoxy-α-pyridone would provide a route to fully elaborated pyridone analogues via coupling with epoxides and pyridone deprotection. This synthetic approach was abandoned after many unsuccessful coupling attempts. It was then decided to synthesize the pyridone moiety after alkyl epoxide coupling of the γ-hydroxy-α-pyrone 4. Accordingly, γ-hydroxy-α-pyridone derivative 21 was then prepared by treatment of 12 with NH4OH in a sealed tube (Scheme 3). However, after purification by preparative thin layer chromatography, the compound proved to be rather unstable. Therefore, freshly purified pyridone 21 was utilized immediately for biochemical and biological assays, as well as for characterization by MALDI mass spectrometry and 1H NMR spectroscopy.
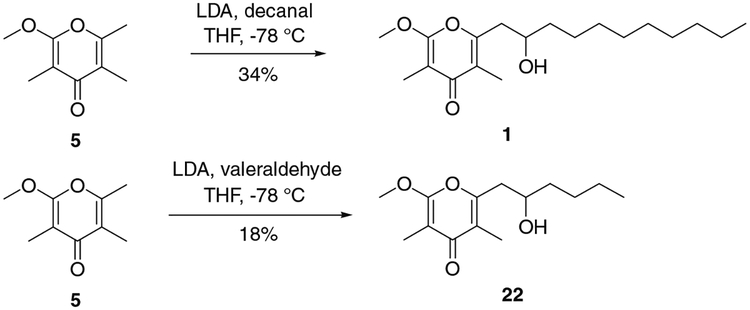
The strong inhibitory activity of analogue 1 toward NADH fumarate reductase prompted the preparation of this compound and structural analogues to permit their more detailed study. Treatment of methoxypyrone 5 with decanal in presence of LDA gave the expected product 1 in 34% yield (Scheme 4). Study of the influence of side chain length was enabled by preparing a similar compound with a shorter side chain. Accordingly, the coupling conditions used with decanal were then applied successfully with valeraldehyde, affording desired analogue 22, albeit in low (18%) yield (Scheme 4).
Scheme 4.
Synthesis of 1 and analogue 22.
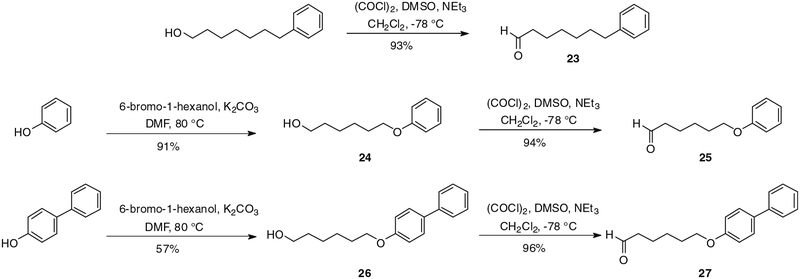
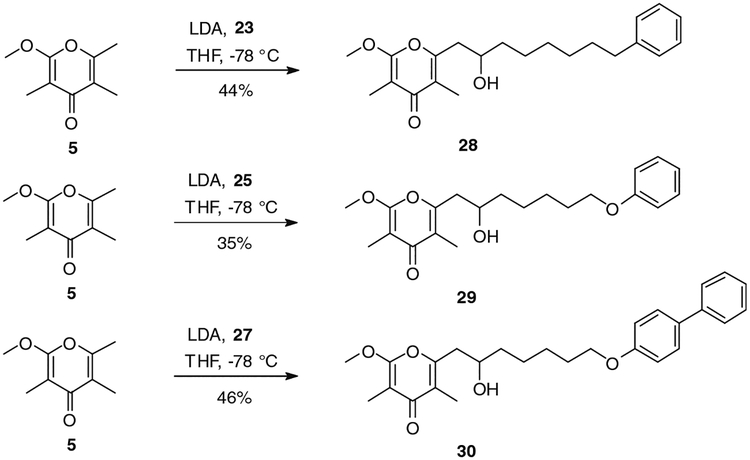
Another class of analogues was prepared keeping the nature of the pyrone ring (α-methoxy-γ-pyrone) as well as the position of the hydroxyl group on the side chain unchanged. The structural modification of these compounds involved the end of the side chain with the incorporation of phenyl, phenoxy and 4-phenylphenoxy groups. Access to the first envisioned analogue was achieved by Swern oxidation of commercially available 7-phenylheptanol, leading almost quantitatively to corresponding aldehyde 23 (Scheme 5), followed by coupling to 5 using the conditions described above (cf. Schemes 4 and 6). Coupling product 28 was obtained in 44% yield after purification (Scheme 6). 6-Phenoxy-1-hexanol (24) was produced in good yield, following the literature procedure involving a nucleophilic substitution of 6-bromo-1-hexanol by phenol in presence of K2CO3 (Scheme 5).13 6-Phenoxyhexanol was converted to unstable aldehyde 25 in 94% yield (Scheme 5). The latter was condensed with 5, affording verticipyrone analogue 29 in 35% yield (Scheme 6). In the same manner, alcohol 26 was obtained in 57% yield from 4-phenylphenol and oxidized to corresponding aldehyde 27 in 96% yield (Scheme 5). The coupling reaction afforded verticipyrone analogue 30 in 46% yield (Scheme 6).
Scheme 5.
Synthesis of intermediate aldehydes 23, 25 and 27.
Scheme 6.
Synthesis of verticipyrone analogues 28, 29 and 30.
2.2. Inhibition of NADH oxidase and NADH-ubiquinone oxidoreductase
The NADH oxidase and NADH-ubiquinone oxidoreductase activities of compounds 1, 12–22, 28, 29 and 30 were measured. NADH oxidase activity reflects electron transfer in the respiratory chain from complex I to IV; electrons are first transported from complex I (NADH: ubiquinone oxidoreductase) to complex III (ubiquinol: cytochrome c oxidoreductase) by coenzyme Q10 and then from complex III to complex IV (cytochrome c oxidase) by the peripheral membrane protein cytochrome c. Nine of the 15 assayed pyrone analogues (compounds 1, 15–20, 28 and 30), were found to be potent inhibitors of the integrated electron transfer chain (NADH oxidase) activity in beef heart submitochondrial particles (SMPs) with IC50 values less than 75 nM (Table 1). Compound 30 was found to be the most potent inhibitor with an IC50 value of 35.9 nM. Compound 22 did not inhibit mitochondrial respiratory chain function, undoubtedly reflecting its very short alkyl side chain. These results support the conclusion that the aliphatic chain plays an important role in the interaction of the verticipyrone analogues with complex I and possibly other components of the respiratory chain. In addition, the potent inhibitory activity of analogue 30 demonstrates that the phenylphenoxy group promotes particularly effective interaction with the respiratory chain. The α-methoxy-γ-pyrones (1, 15–17, 28, 29 and 30) as well as the trimethylpyrone analogues (18–20) showed minimal differences as inhibitors of mitochondrial respiratory chain function (Table 1).
Table 1.
Inhibitory activities toward mitochondrial complex Ia of the described verticipyrone analogues
| Compound | IC50b | |
|---|---|---|
| NADH oxidase (nM) | NADH-ubiquinone (Q1) oxidoreductase (nM) | |
| 1 | 64.9 ± 1.7 | 230.9 ± 5.7 |
| 12 | 13,590 ± 500 | 16,410 ± 240 |
| 13 | 14,740 ± 460 | 19,020 ± 450 |
| 14 | 10,670 ± 690 | 13,300 ± 1050 |
| 15 | 52.6 ± 1.0 | 151.1 ± 4.6 |
| 16 | 45.9 ± 1.7 | 133.1 ± 3.2 |
| 17 | 43.2 ± 2.2 | 126.2 ± 2.2 |
| 18 | 55.0 ± 1.7 | 180.2 ± 8.8 |
| 19 | 50.8 ± 2.3 | 145.4 ± 5.9 |
| 20 | 47.5 ± 3.4 | 135.3 ± 4.6 |
| 21 | 4290 ± 130 | 1250 ± 96 |
| 22 | >20,000 | >25,000 |
| 28 | 72.5 ± 1.2 | 257.1 ± 13.1 |
| 29 | 218.9 ± 8.5 | 595 ± 35.8 |
| 30 | 35.9 ± 1.1 | 101.6 ± 4.0 |
| Rotenone | 4.36 ± 0.07 | Nt |
Performed using bovine heart submitochondrial particles (SMP).
The molar concentration required for half-maximal inhibition of the control. Values are mean ± SD of three independent experiments. NADH-ubiquinone oxidoreductase was determined with ubiquinone 1 (Q1) as the ubiquinone substrate. Control activity was approximately 0.75–0.9 μmol min−1 mg−1 for NADH oxidase and 0.65–0.75 lmol min−1 mg−1 for NADH-ubiquinone oxidoreductase. Nt, not determined.
In three cases (12 vs 13 and 14; 15 vs 16 and 17; 18 vs 19 and 20), the inhibitory properties of compounds racemic at the carbon atom bearing the tertiary OH group were compared with those of each of the enantiomers. As is clear from Table 1, no dramatic differences were noted, although there was a possible tendency for the racemates to be slightly less potent than the individual enantiomers. Perhaps more significantly, for those compounds with reasonable inhibitory potency, the two enantiomers had quite similar potencies, indicating a lack of effect of stereochemistry at the tertiary OH center.
Interestingly, analogue 21 had more than 10-fold greater potency than 12 as an inhibitor of NADH-ubiquinone oxidoreductase, suggesting that the interactions of 21 with the mitochondrial respiratory complex may differ from those of the other analogues in some respects. Inhibition of complex I was measured directly by measuring NADH-ubiquinone oxidoreductase activity in presence of a lipid-soluble exogenous ubiquinone-1 (Q1) through complex I, as well as complex III inhibitor antimycin A and complex IV inhibitor cyanide. It is interesting that for those compounds exhibiting reasonably potent inhibitory activity, IC50 values for the NADH oxidase activity were lower with respect to those of the NADH-ubiquinone oxidoreductase activity (Table 1). Such relative extents of inhibitory potencies have been noted previously for other complex I inhibitors.1,14,15 The substrate concentration was higher in Q1-dependent NADH oxidation than in the integrated NADH oxidase assay (which contained only ~0.1–0.2 Mm Q1016,17), consistent with the interpretation that these inhibitors compete with ubiquinone for binding to complex I.
2.3. Induction of lactate production
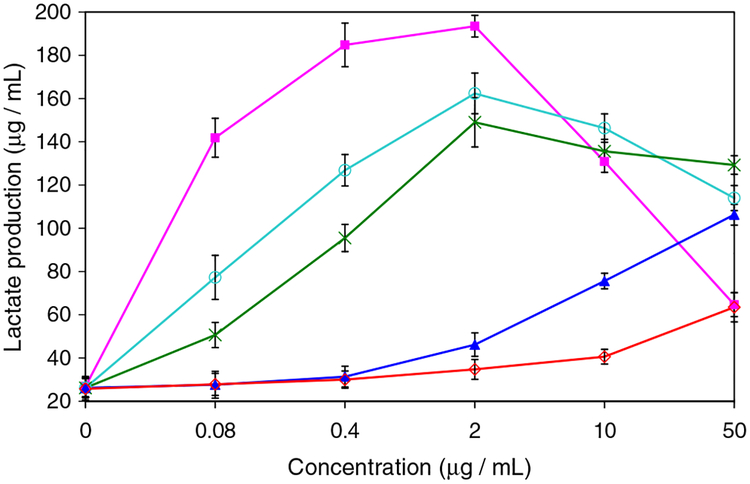
More than 70 years ago, Warburg observed that cancer cells frequently exhibit increased glycolysis and depend significantly on this metabolic pathway for the generation of ATP to meet their energy needs.18 The compromised ability of cancer cells to generate ATP through oxidative phosphorylation forces the cells to increase glycolysis to maintain their energy supply, thus rendering cancer cells more highly dependent on this metabolic pathway for survival.19 Since glycolysis in the absence of mitochondrial function yields two ATP and two lactate molecules per glucose catabolized, it follows that the incremental lactate production arising from inhibition of respiration is proportional to the extent of inhibition of mitochondrial ATP production, assuming that the Pasteur effect20 compensates for respiratory inhibition in proportion to the extent of inhibition. We have used this principle (the Pasteur effect) to evaluate known mitochondrial toxins, such as the Δlacacetogenins,14 as well as the verticipyrones that are the focus of this study. While the basal lactate levels reflect glycolytic ATP, the difference in the lactate concentrations (Δ-lactate) measured in presence (mitochondrial inhibition) and absence (basal condition) of antimycin A represent an amount of energy which correlates well with that produced by the mitochondria prior to the respiratory chain inhibition. Figure 3 shows lactate production by semi-confluent MCF-7 cells exposed to varying concentrations of several verticipyrone analogues for 3 h. Lactate production, which is low under basal conditions, was strongly stimulated after incubation with some of the verticipyrone analogues (1, 17, and 30) and weakly stimulated by others (14, 22). In order to compare these compounds, the concentration required to obtain half maximal lactate production (IC50) was calculated (Table 2). The potency of the tested compounds in inducing a shift to glycolysis was in reasonable agreement with the trend for mitochondrial complex I inhibition (cf. Tables 1 and 2).
Figure 3.
Lactate production by MCF-7 cells exposed to compound 1 ( ), 14 (
), 14 ( ), 17 (
), 17 ( ), 22 (
), 22 ( ), or 30 (
), or 30 ( ) for 3 h. Data shown are from a typical experiment, repeated at least three times, and each point represents the mean ± SD of three determinations.
) for 3 h. Data shown are from a typical experiment, repeated at least three times, and each point represents the mean ± SD of three determinations.
Table 2.
Lactate production by MCF-7 human breast cancer cells incubated for 3 h in the presence of some of the verticipyrone analogues
| Compound (μM) | IC50a (μM) | Incremental lactate production (μM) |
|---|---|---|
| 1 | 0.90 | 1365 |
| 12 | 4.9 | 1175 |
| 13 | 8.6 | 692 |
| 14 | 16.6 | 891 |
| 15 | 0.38 | 1745 |
| 16 | 0.30 | 2229 |
| 17 | 0.18 | 1852 |
| 18 | 0.94 | 1099 |
| 22 | >20 | 419 |
| 28 | 0.88 | 1135 |
| 30 | 0.34 | 1513 |
| Antimycin A | <0.15 | 1520 |
IC50 values were calculated as the dose which produced a 50% inhibition of the maximum lactate production measured.
2.4. Cytotoxicity toward mammalian cell lines
Table 3 shows the IC50 values for several verticipyrone analogues against cultured human and mouse cell lines. The cytotoxicity of the γ-hydroxy-α-pyrone analogues (12–14) was minimal (IC50 >31 μM) in all tested cell lines, in parallel with their lack of significant inhibition of mitochondrial complex I. On the other hand, the cytotoxicity of the α-methoxy-γ-pyrones (1, 16, 17, 28 and 30) were in the low micromolar range. Interestingly, trim-ethylpyrone 18 exhibited no cytotoxicity against the cultured cells. In contrast with the effects of the verticipyrone analogues on lactate production, the data in Table 3 reflect less correlation between the cytotoxicity data and the effects of individual compounds on mitochondrial complex I. This may reflect factors such as differences in cellular uptake and metabolism for the individual compounds, especially where the analogues (e.g., for 18) have unexpectedly low cytotoxic potency. Alternatively, it may result from the action of some of the analogues at cellular loci in addition to mitochondrial complex I (e.g., for 16 and 17).
Table 3.
Inhibition values (IC50, lM) of several verticipyrone analogues determined by MTT assay using human and mouse cell linesa
| Compound | MCF-7 | MCF-10A | CRL-2365 | CRL-2366 | A549 | 3LL |
|---|---|---|---|---|---|---|
| 1 | 23.2 ± 0.6 | 9.9 ± 0.3 | 29.9 ± 0.6 | >31 | 20.7 ± 0.6 | 22.2 ± 5 |
| 12 | >31 | >31 | >31 | >31 | >31 | >31 |
| 13 | >31 | >31 | >31 | >31 | >31 | >31 |
| 14 | >31 | >31 | >31 | >31 | >31 | >31 |
| 16 | 3.3 ± 0.3 | 5.3 ± 0.3 | 3.3 ± 2.4 | 2.7 ± 0.3 | 5.3 ± 0.1 | 0.39 ± 0.07 |
| 17 | 3.3 ± 0.3 | 5.6 ± 0.3 | 2.4 ± 0.3 | 3.3 ± 1.5 | 4.1 ± 0.3 | 0.34 ± 0.05 |
| 18 | >31 | >31 | >31 | >31 | >31 | >31 |
| 28 | >27 | 15.1 ± 0.8 | >27 | >27 | >27 | 17.3 ± 1.7 |
| 30 | 17.4 ± 0.9 | 9.2 ± 0.2 | 12.4 ± 0.9 | 19.5 ± 0.7 | 17.0 ± 0.9 | 13.5 ± 0.2 |
Cell Type: MCF-7 (breast carcinoma); MCF-10A (‘normal’ human breast); CRL-2365 (brain glioblastoma); CRL-2366 (DNA repair deficient brain glioblastoma); A549 (lung carcinoma); 3LL (mouse Lewis lung carcinoma). DMSO was used as vehicle in the testing.
3. Conclusions
The preparation and study of several analogues of verticipyrone was accomplished during the course of this study. The biochemical studies of analogues 12–21 showed that the nature of the pyrone ring could be very important to the potency of mitochondrial complex I inhibition, but the hydroxyl group at the C3 position of the side chain was not. The α-methoxy-γ-pyrones (1, 15–17, 28, 29 and 30) and trimethyl-γ-pyrones (18–20) showed limited differences in their inhibitory potency toward mitochondrial complex I. In comparison, the cytotoxicity of the α-methoxy-γ-pyrones (1, 16, 17, 28 and 30) tended to exhibit somewhat greater cytotoxicity toward cultured mammalian cells than trimethyl-γ-pyrone 18. Verticipyrone analogue 22 showed no inhibitory activity compared to 1, underscoring the importance of the length and lipophilic nature of the side chain. While the present compounds were designed as inhibitors of complex I function, the findings regarding optimal side chain structure for complex I binding may guide efforts to identify exogenous complex I substrates of potential utility for augmenting the function of the mitochondrial electron transport chain.
4. Experimental
4.1. Chemistry
Chemicals and solvents were of reagent grade and were used without further purification. Anhydrous grade solvents were purchased from VWR. All reactions involving air or moisture sensitive reagents or intermediates were performed under an argon atmosphere. Flash chromatography was carried out using Silicycle 200–400 mesh silica gel. Analytical TLC was carried out using0.25 mm EM Silica Gel 60 F250 plates that were visualized by irradiation (254 nm) or by staining with p-anisaldehyde stain. 1H and 13C NMR spectra were obtained using Gemini 300, Inova 400, Inova 500 and VNMRS 800 MHz Varian instruments. Chemical shifts were reported in parts per million (ppm,δ) referenced to the residual 1H resonance of the solvent (CDCl, 7.26 ppm). 13C spectra were referenced to the residual 13C resonance of the solvent (CDCl3, 77 ppm). Splitting patterns were designated as follow: s, singlet; br, broad; d, doublet; dd, doublet of doublets; t, triplet; q, quartet; m, multiplet. High resolution mass spectra were obtained at the Ohio State University Mass Spectrometry Facility.
4.1.1. 2,3,5,6-Tetramethyl-4-pyrone (2)2
Compound 2 was prepared according to the method of Letsinger and Jamison.3 To a mixture of 195 g of polyphosphoric acid and 120 mL of acetic acid was added 4.90 mL (4.00 g, 46.4 mmol) of 3-pentanone. The resulting solution was heated at 130 °C with vigorous stirring for 8 h. The resulting dark black solution was cooled to 0 °C and 500 mL of water was added. The mixture was extracted with portions of ethyl ether. The combined organic phase was washed with 10% aq KOH, dried over anhydrous MgSO4 and concentrated under diminished pressure to afford a crude residue. The residue was purified by chromatography on a silica gel column (40 × 10 cm). Elution with chloroform gave 2 as a yellow solid: yield 3.25 g (46%); silica gel TLC Rf 0.63 (10:1 chloroform/methanol); 1H NMR (CDCl3) δ 1.86 (s, 6H) and 2.18 (s, 6H); 13C NMR (CDCl3) δ 9.8, 17.5, 118.5, 159.9 and 179.2.
4.1.2. Ethyl 2-methyl-3-oxo-pentanoate (3)2
Compound 3 was prepared according to the method of Yoshizawa et al.4 A solution containing 3.56 g (34.8 mmol) of ethyl propionate and 2.75 g (24.5 mmol) of powdered KOtBu was heated at 80 °C for 3 h. The reaction mixture was cooled and neutralized with 1 N HCl. The mixture was extracted with portions of ethyl ether. The combined organic phase was dried over anhydrous MgSO4, filtered and concentrated under diminished pressure to afford a crude oil. The residue was purified by chromatography on a silica gel column (20 × 2 cm). Elution with 7:1 hexanes/ethyl acetate gave 3 as a colorless oil: yield 2.40 g (61%); silica gel TLC Rf0.62 (2:1 hexanes/ethyl acetate); 1H NMR (CDCl3) δ 0.98 (t, 3H, J = 7.2 Hz), 1.18 (t, 3H, J = 7.2 Hz,), 1.25 (d, 3H, J = 7.2 Hz), 2.47 (m, 2H), 3.45 (q, 1H, J = 14.1, 7.2 Hz) and 4.09 (q, 2H, J = 13.8,7.2 Hz).
4.1.3. 4-Hydroxy-3,5,6-trimethyl-2-pyrone (4)2
To a solution containing 5.75 mL (4.13 g, 40.8 mmol) of iPr2NH in 80 mL of THF at 0 °C was added dropwise 25.0 mL (40.6 mmol) of nBuLi (1.6 M in hexane). The reaction mixture was stirred at 0 °C for 30 min. A solution containing 2.47 g (15.6 mmol) of 3 in 5 mL of THF was then added dropwise. The reaction mixture was stirred at 0 °C for 1 h and then cooled to −78 °C. A solution of2.60 g (23.4 mmol) of N-acetylimidazole in 30 mL of THF was then added dropwise. The reaction mixture was stirred at −78 °C for 2 h, quenched with satd aq NH4Cl and extracted with portions of ethyl acetate. The combined organic phase was washed with water and brine, dried over anhydrous MgSO4, filtered and concentrated under diminished pressure to afford the crude diketoester (3.00 g) which was used immediately for the next step due to its instability.
To a solution containing 3.00 g (14.9 mmol) of diketoester in 50 mL of benzene was added 2.70 mL (17.9 mmol) of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU). The reaction mixture was heated at reflux for 10 h. After cooling to room temperature, the mixture was poured into 30 mL of cooled (0 °C) satd aq NaHCO3. The aqueous layer was washed with portions of Et2O. The aqueous layer was then acidified to pH 2 with 12 N aqueous HCl. The mixture was extracted with portions of Et2O. The combined organic extract was dried over anhydrous MgSO4, filtered and concentrated under diminished pressure to afford a crude residue. The residue was purified by chromatography on a silica gel column (10 × 2 cm). Elution with chloroform gave 4 as a colorless solid: yield 1.35 g (57% over two steps); silica gel TLC Rf 0.21 (10:1 chloroform/methanol); 1H NMR (CDCl3) δ 1.96 (s, 3H), 1.99 (s, 3H) and 2.21 (s, 3H); 13C NMR (CDCl3) δ 8.6, 10.0, 17.1, 98.4, 108.1, 166.4, 155.2 and 167.2.
4.1.4. 2-Methoxy-3,5,6-trimethyl-4-pyrone (5)2
Compound 5 was prepared according to the method of Hosokawa et al.6 To a solution containing 365 mg (2.37 mmol) of 4 and1.20 g (12.0 mmol) of CaCO3 in 15 mL of acetone was added0.27 mL (2.81 mmol) of (MeO)2SO2. The reaction mixture was heated to 50 °C and stirred for 4 days, cooled to room temperature and filtered. Excess solvent was removed under diminished pressure to afford a crude residue. The residue was purified by chromatography on a silica gel column (15 × 3 cm). Elution with chloroform gave 5 as a white solid: yield 172 mg (43%); silica gel TLC Rf 0.39 (10:1 chloroform/methanol); 1H NMR (CDCl3) δ 1.82 (s, 3H), 1.90 (s, 3H), 2.24 (s, 3H) and 3.92 (s, 3H); 13C NMR (CDCl3) δ 6.8, 10.0, 16.8, 55.3, 99.4, 118.4, 154.8, 162.1 and 180.8.
4.1.5. (rac) 1-Epoxy-2-methyldecane (6)
To a suspension of 485 mg (12.1 mmol) of NaH (60%) in a mixture of 4 mL of THF and 3 mL of DMSO was added dropwise a solution containing 2.66 g (12.1 mmol) of trimethylsulfoxonium iodide in 35 mL of DMSO while keeping the reaction temperature between 6 °C and 10 °C. The reaction mixture was stirred at 6 °C for 15 min and a solution containing 2.01 g (1.65 g, 10.5 mmol) of 2-decanone in 5 mL of DMSO was added dropwise over a period of 5 min. The reaction mixture was stirred at room temperature overnight and 20 mL of hexane and 30 mL of semi-satd aq NH4Cl were added successively. The reaction mixture was extracted with portions of hexanes. The combined organic phase was dried over anhydrous MgSO4, filtered and concentrated under diminished pressure to afford a crude oil. The residue was purified by chromatography on a silica gel column (15 × 2 cm). Elution with 12:1 hexanes/ethyl ether gave 6 as a colorless oil: yield 1.41 g (78%); silica gel TLC Rf 0.52 (8:1 hexanes/ethyl ether); ; 1H NMR (CDCl3) δ 0.87 (t, 3H, J = 7.1 Hz), 1.27 (m, 10H), 1.29 (s, 3H),1.37 (m, 2H), 1.46 (m, 1H), 1.58 (m, 1H) and 2.57 (dd, 2H, J = 13,4.5 Hz); 13C NMR (CDCl3) δ 14.3, 21.1, 22.9, 25.5, 29.4, 29.7, 29.9,32.1, 36.9, 54.1 and 57.3.
4.1.6. 2-Methyl-dec-1-ene (7)
Compound 7 was prepared according the method of Farhat et al.21 To a solution containing 3.60 g (8.96 mmol) of methyltriphenylphosphonium iodide in 30 mL of THF at 0 °C was added dropwise 5.60 mL (8.96 mmol) of nBuLi (1.6 M in hexane). The reaction mixture was stirred at room temperature for 1 h, cooled to 0 °C and a solution containing 1.00 mL (824 mg, 5.28 mmol) of 2-decanone in 5 mL of THF was added dropwise. The reaction mixture was stirred at 0 °C for 15 min and then at room temperature for 8 h. The solution was diluted with 50 mL of hexanes and filtered through a pad of silica gel which was washed with 100 mL of 1:1 hexanes/ethyl ether. The filtrate was concentrated carefully under diminished pressure to afford a crude oil. The residue was purified by chromatography on a silica gel column (15 × 2 cm). Elution with 20:1 hexanes/ethyl ether gave 7 as a colorless oil: yield 700 mg (86%); silica gel TLC Rf 0.89 (8:1 hexanes/ethyl ether); 1HNMR (CDCl3) δ 0.90 (t, 3H, J = 7.1 Hz), 1.30 (m, 10H), 1.44 (m, 2H), 1.72 (s, 3H), 2.01 (t, 2H, J = 7.5 Hz) and 4.69 (d, 2H, J = 10.5 Hz); 13C NMR (CDCl3) δ 13.1, 22.2, 22.7, 27.6, 29.3, 29.4,29.5, 31.9, 37.8, 103.4 and 146.2.
4.1.7. (R)-2-Hydroxy-2-methyldecanol (8)
A 250-mL three necked round-bottomed flask equipped with a mechanical stirrer was charged with 13.0 mL of a 1:1 mixture of tBuOH/H2O and 1.80 g of ADmix-β. The resulting mixture was stirred vigorously at room temperature for 30 min and then cooled to 0 °C. A precipitate appeared and 200 mg (1.30 mmol) of 7 was added in one portion. The resulting mixture was stirred vigorously at 0 °C for 6 h and 1.93 g (15.3 mmol) of sodium sulfide was added. The reaction mixture was allowed to warm to room temperature and was stirred for 1 h. Twenty milliliters of CH2Cl2 and 40 mL of water were then added successively and the reaction mixture was extracted with portions of CH2Cl2. The combined organic phase was dried over anhydrous MgSO4, filtered and concentrated under diminished pressure to afford a crude oil. The residue was purified by chromatography on a silica gel column (8 × 2 cm). Step gradient elution with 4:1 hexanes/ethyl acetate→2:1 hexanes/ethyl acetate gave 8 as a colorless oil: yield 235 mg (96%); ; (c 0.48, CHCl3); silica gel TLC Rf 0.24 (6:3 hexanes/ethyl acetate); 1H NMR (CDCl3) δ 0.88 (t, 3H, J = 7 Hz), 1.16 (s, 3H), 1.29 (m, 12H), 1.49 (m, 2H), 2.05–2.38 (2 br s, 2H) and 3.43 (dd, 2H, J = 20, 11 Hz); 13C NMR (CDCl3) δ 14.3, 22.8, 23.4, 23.9, 29.5, 29.8,30.4, 32.1, 38.9, 70.0 and 73.2; mass spectrum (ESI), m/z 211.1666 (M+Na)+ (C11H24O2Na requires m/z 211.1674).
4.1.8. (S)-2-Hydroxy-2-methyldecanol (9)
A 250-mL three necked round-bottomed flask equipped with a mechanical stirrer was charged with 13.0 mL of 1:1 tBuOH/H2O and 1.80 g of ADmix-α. The resulting mixture was stirred vigorously at room temperature for 30 min and then cooled to 0 °C. A precipitate appeared and 200 mg (1.30 mmol) of 7 was added in one portion. The resulting mixture was stirred vigorously at 0 °C for 6 h and 1.93 g (15.3 mmol) of sodium sulfide was added. The reaction mixture was allowed to warm to room temperature and was stirred for 1 h. Twenty milliliters of CH2Cl2 and 40 mL of water were then added successively and the reaction mixture was extracted with portions of CH2Cl2. The combined organic phase was dried over anhydrous MgSO4, filtered and concentrated under diminished pressure to afford a crude oil. The residue was purified by chromatography on a silica gel column (8 × 2 cm). Step gradient elution with 4:1 hexanes/ethyl acetate→2:1 hexanes/ethyl acetate gave 9 as a colorless oil: yield 217 mg (89%); ; silica gel TLC Rf 0.24 (6:3 hexanes/ethyl acetate); 1H NMR (CDCl3) δ 0.88 (t, 3H, J = 7 Hz), 1.16 (s, 3H), 1.29 (m, 12H), 1.49 (m, 2H), 2.05–2.38 (2 br s, 2H) and 3.43 (dd, 2H, J = 20.0,11.2 Hz); 13C NMR (CDCl3) δ 14.3, 22.8, 23.4, 23.9, 29.5, 29.8,30.4, 32.1, 38.9, 70.0 and 73.2; mass spectrum (ESI), m/z 211.1673 (M+Na)+ (C11H24O2Na requires m/z 211.1674).
4.1.9. (R)-1-Epoxy-2-methyldecane (10)
To a solution containing 127 mg (0.67 mmol) of 8 in 1.50 mL of CH2Cl2 at 0 °C was added 136 μL (133 mg, 1.69 mmol) of pyridine. The reaction mixture was stirred at 0 °C for 5 min and 80.0 lL(1.15 mmol) of mesyl chloride (MsCl) was added dropwise. The reaction mixture was stirred at room temperature for 1 h, quenched with satd aq NaHCO3, and extracted with portions of CH2Cl2. The combined organic phase was washed with brine, dried over anhydrous MgSO4, filtered and partially concentrated to a volume of ~3 mL under diminished pressure.
To the solution containing the crude mesylate was added at room temperature 1.31 mL (8.76 mmol) of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU). The reaction mixture was stirred for 10 h, diluted with 7 mL of CH2Cl2, quenched with satd aq NH4Cl and extracted with portions of CH2Cl2. The combined organic phase was washed with brine, dried over anhydrous MgSO4, filtered and concentrated under diminished pressure to afford a crude oil. The residue was purified by chromatography on a silica gel column (5 × 1 cm). Elution with 17:1 hexanes/ethyl ether gave epoxide 10 as a colorless oil: yield 85.0 mg (74% over two steps); silica gel TLC Rf 0.52 (8:1 hexanes/ethyl ether); ; 1H NMR (CDCl3) δ 0.87 (t, 3H, J = 7 Hz), 1.27 (m, 10H), 1.29 (s, 3H),1.37 (m, 2H), 1.46 (m, 1H), 1.58 (m, 1H) and 2.57 (dd, 2H, J = 13.1,4.5 Hz); 13C NMR (CDCl3) δ 14.3, 21.1, 22.9, 25.5, 29.4, 29.7, 29.9,32.1, 36.9, 54.1 and 57.3; mass spectrum (MALDI-TOF), m/z 171.2(M)+ (theoretical m/z 171.3).
4.1.10. (S)-1-Epoxy-2-methyldecane (11)
To a solution containing 145 mg (0.77 mmol) of 9 in 2 mL of CH2Cl2 at 0 °C was added 155 lL (151 mg, 1.69 mmol) of pyridine. The reaction mixture was stirred at 0 °C for 5 min and 90.0 μL(1.17 mmol) of mesyl chloride (MsCl) was added dropwise. The reaction mixture was stirred at room temperature for 1 h, quenched with satd aq NaHCO3, and extracted with portions of CH2Cl2. The combined organic phase was washed with brine, dried over anhydrous MgSO4, filtered and partially concentrated to a volume of ~3 mL under diminished pressure.
To the solution containing the crude mesylate was added at room temperature 1.50 mL (10.0 mmol) of DBU. The reaction mixture was stirred for 10 h, diluted with 10 mL of CH2Cl2, quenched with satd aq NH4Cl and extracted with portions of CH2Cl2. The combined organic phase was washed with brine, dried over anhydrous MgSO4, filtered and concentrated under diminished pressure to afford a crude oil. The residue was purified by chromatography on a silica gel column (5 × 1 cm). Elution with 17:1 hexanes/ethylether gave epoxide 11 as a colorless oil: yield 100 mg (76% over two steps); silica gel TLC Rf 0.52 (8:1 hexanes/ethyl ether); ; 1H NMR (CDCl3) δ 0.87 (t, 3H, J = 7 Hz), 1.27 (m, 10H), 1.29 (s, 3H), 1.37 (m, 2H), 1.46 (m, 1H), 1.58 (m, 1H) and 2.57 (dd, 2H, J = 13.1, 4.5 Hz); 13C NMR (CDCl3) δ 14.3, 21.1,22.9, 25.5, 29.4, 29.7, 29.9, 32.1, 36.9, 54.1 and 57.3; mass spectrum, m/z 170.9 (M)+ (theoretical m/z 171.3).
4.1.11. (rac)-6-[3-Hydroxy-3-methylundecyl]-3,5-dimethyl-4-hydroxypyran-2-one (12)
To a solution containing 41.0 mg (0.266 mmol) of 4 in 1 mL of 6:1 THF/HMPA at −78 °C was added 382 μL (0.612 mmol) of nBuLi (1.6 M in hexanes). The reaction mixture was stirred at 78 °C for 1 h and 90.0 mg (0.532 mmol) of neat racemic epoxide 6 was added. The reaction mixture was stirred at 78 °C for 1 h and was then allowed to warm slowly to room temperature and stirred overnight. The reaction mixture was quenched with 1 N HCl and extracted with portions of EtOAc. The combined organic phase was washed with brine, dried over anhydrous MgSO4, filtered and concentrated under diminished pressure to afford a crude oil. The residue was purified by chromatography on a silica gel column (6 × 1.5 cm). Step gradient elution with 3:1 hexanes/ethyl acetate→1:1 hexanes/ethyl acetate gave 12 as a colorless oil: yield 45.0 mg (52%); silica gel TLC Rf 0.28 (9:1 chloroform/methanol);; 1H NMR (CDCl3) δ 0.86 (t, 3H, J = 6.5 Hz), 1.18 (s, 3H), 1.20–1.31 (m, 12H), 1.46 (m, 2H), 1.73 (m, 2H), 1.75 (m, 2H), 1.96 (s, 3H), 1.97 (s, 3H) and 2.58 (m, 2H); 13C NMR (CDCl3) δ 8.7, 9.9, 14.3, 22.9, 24.2, 25.7, 26.7, 29.5, 29.8,30.4, 32.1, 39.0, 42.4, 72.7, 98.4, 105.0, 159.5, 165.0 and 166.3; mass spectrum (ESI), m/z 347.2196 (M+Na)+ (C19H32O4Na requires m/z 347.2198).
4.1.12. (R)-6-[3-Hydroxy-3-methylundecyl]-3,5-dimethyl-4-hydroxypyran-2-one (13)
To a solution containing 30.0 mg (0.194 mmol) of 4 in 1 mL of a 6:1 mixture of THF/HMPA at −78 °C was added 279 μL (0.448 mmol) of nBuLi (1.6 M in hexanes). The reaction mixture was stirred at −78 °C for 1 h and a solution containing 66.0 mg (0.388 mmol) of (R)-epoxide 10 in 0.5 mL of THF was added. The reaction mixture was stirred at −78 °C for 1 h and was then allowed to warm slowly to room temperature and stirred overnight. The reaction was quenched with 1 M HCl and extracted with portions of EtOAc. The combined organic phase was washed with brine, dried over anhydrous MgSO4, filtered and concentrated under diminished pressure to afford a crude oil. The residue was purified by chromatography on a silica gel column (6 × 1 cm). Elution with 3:1 hexanes/ethyl acetate→1:1 hexanes/ethyl acetate gave 13 as a colorless oil: yield 16.0 mg (25%); silica gel TLC Rf 0.28 (9:1 chloroform/methanol); 1H NMR (CDCl3) δ 0.86 (t, 3H, J = 6.5 Hz), 1.18 (s, 3H), 1.20–1.31 (m, 12H),1.46 (m, 2H), 1.73 (m, 2H), 1.75 (m, 2H), 1.96 (s, 3H), 1.97 (s, 3H) and 2.58 (m, 2H); 13C NMR (CDCl3) δ 8.7, 9.9, 14.3, 22.9, 24.2,25.7, 26.7, 29.5, 29.8, 30.4, 32.1, 39.0, 42.4, 72.7, 98.4, 105.0, 159.5, 165.0 and 166.3; mass spectrum (ESI), m/z 347.2186 (M+Na)+ (C19H32O4Na requires m/z 347.2198).
4.1.13. (S)-6-[3-Hydroxy-3-methylundecyl]-3,5-dimethyl-4-hydroxypyran-2-one (14)
To a solution containing 30 mg (0.194 mmol) of 4 in 1 mL of a 6:1 mixture of THF/HMPA at −78 °C was added 279 μL (0.448 mmol) of nBuLi (1.6 M in hexanes). The reaction mixture was stirred at −78 °C for 1 h and a solution containing 66.0 mg (0.388 mmol) of (S)-epoxide 11 in 0.5 mL of THF was added. The reaction mixture was stirred at −78 °C for 1 h and was then allowed to warm slowly to room temperature and stirred overnight. The reaction was quenched with 1 M HCl and extracted with portions of EtOAc. The combined organic phase was washed with brine, dried over anhydrous MgSO4, filtered and concentrated under diminished pressure to afford a crude oil. The residue was purified by chromatography on a silica gel column (6 × 1 cm). Elution with 3:1 hexanes/ethyl acetate→1:1 hexane/ethyl acetate gave 14 as a colorless oil: yield 17.0 mg (27%); silica gel TLC Rf 0.28 (9:1 chloroform/methanol); ; 1H NMR (CDCl3) δ 0.86 (t, 3H, J = 6.5 Hz), 1.18 (s, 3H), 1.20–1.31 (m, 12H),1.46 (m, 2H), 1.73 (m, 2H), 1.75 (m, 2H), 1.96 (s, 3H), 1.97 (s, 3H) and 2.58 (m, 2H); 13C NMR (CDCl3) δ 8.7, 9.9, 14.3, 22.9, 24.2,25.7, 26.7, 29.5, 30.4, 32.1, 39.0, 42.4, 72.7, 98.4, 105.0, 159.5, 165.0 and 166.3; mass spectrum (ESI), m/z 347.2200 (M+Na)+ (C19H32O4Na requires m/z 347.2198).
4.1.14. (rac)-2-[3-Hydroxy-3-methylundecyl]-6-methoxy-3,5-dimethyl-4H-pyran-4-one (15)
To a solution containing 11.1 mg (0.065 mmol) of 5 in 0.40 mL of THF at −78 °C was added dropwise 131 μL (0.131 mmol) of 1 M LiHMDS solution. The resulting mixture was stirred at −78 °C for 1 h and 23.0 mg (0.131 mmol) of neat racemic epoxide 6 was added followed by 18.0 μL (0.131 mmol) of BF3 Et2O. The reaction mixture was stirred at −78 °C for 1 h and was then allowed to warm slowly to room temperature and stirred overnight. The reaction mixture was quenched with satd aq NH4Cl and extracted with portions of EtOAc. The combined organic phase was washed with brine, dried over anhydrous MgSO4, filtered and concentrated under diminished pressure to afford a crude oil. The residue was purified by chromatography on a silica gel column (4 × 1 cm). Elution with 30:1 chloroform/methanol gave 15 as a colorless oil: yield 6.02 mg (27%); silica gel TLC Rf 0.42 (9:1 chloroform/methanol); ; 1H NMR (CDCl3) δ 0.87 (t, 3H, J = 7 Hz), 1.23 (s, 3H), 1.26–1.37 (m, 12H), 1.49 (m, 2H),1.75 (m, 2H), 1.84 (s, 3H), 1.94 (s, 3H), 2.66 (m, 2H) and 3.94 (s, 3H); 13C NMR (CDCl3) δ 6.9, 9.8, 14.1, 22.6, 24.0, 25.6, 26.8, 29.2,29.6, 30.1, 31.9, 38.4, 42.0, 55.3, 72.1, 99.4, 118.0, 158.5, 162.1 and 181.0; mass spectrum (ESI), m/z 361.2356 (M+Na)+ (C20H34O4Na requires m/z 361.2355).
4.1.15. (R)-2-[3-Hydroxy-3-methylundecyl]-6-methoxy-3,5-dimethyl-4H-pyran-4-one (16)
To a solution containing 35.1 mg (0.208 mmol) of 5 in 1 mL of THF at −78 °C was added dropwise 416 μL (0.416 mmol) of 1 M LiHMDS solution. The reaction mixture was stirred at −78 °C for 1 h and a solution containing 50.0 mg (0.416 mmol) of (R)-epoxide 10 in 0.5 mL of THF was added followed by 52.0 lL(0.416 mmol) of BF3 Et2O. The reaction mixture was stirred at −78 °C for 1 h and was then allowed to warm slowly to room temperature and stirred overnight. The reaction mixture was quenched with satd aq NH4Cl and extracted with portions of EtOAc. The combined organic phase was washed with brine, dried over anhydrous MgSO4, filtered and concentrated under diminished pressure to afford a crude oil. The residue was purified by chromatography on a silica gel column (5 × 1.5 cm). Elution with 30:1 chloroform/methanol gave 16 as a colorless oil: yield 10.2 mg (23%); silica gel TLC Rf 0.42 (9:1 chloroform/methanol); ; 1H NMR (CDCl3) δ 0.87 (t, 3H, J = 7 Hz), 1.23 (s, 3H), 1.26–1.37 (m, 12H), 1.49 (m, 2H), 1.75 (m, 2H), 1.84 (s, 3H), 1.94 (s, 3H), 2.66 (m, 2H) and 3.94 (s, 3H); 13C NMR (CDCl3) δ 6.9, 9.8, 14.1, 22.6, 24.0, 25.6, 26.8,29.2, 29.6, 30.1, 31.9, 38.4, 42.0, 55.3, 72.1, 99.4, 118.0, 158.5, 162.1 and 181.0; mass spectrum (ESI), m/z 361.2353 (M+Na)+ (C20H34O4Na requires m/z 361.2355).
4.1.16. (S)-2-[3-Hydroxy-3-methylundecyl]-6-methoxy-3,5-dimethyl-4H-pyran-4-one (17)
To a solution containing 25.1 mg (0.148 mmol) of 5 in 1 mL of THF at −78 °C was added dropwise 300 μL (0.298 mmol) of 1 M LiHMDS solution. The reaction mixture was stirred at 78 °C for 1 h and a solution containing 36.0 mg (0.298 mmol) of (S)-epoxide 11 in 0.5 mL of THF was added, followed by 40.0 μL(0.298 mmol) of BF3 Et2O. The reaction mixture was stirred at −78 °C for 1 h and was then allowed to warm slowly to room temperature and stirred overnight. The reaction mixture was quenched with satd aq NH4Cl and extracted with portions of EtOAc. The combined organic phase was washed with brine, dried over anhydrous MgSO4, filtered and concentrated under diminished pressure to afford a crude oil. The residue was purified by chromatography on a silica gel column (5 × 1 cm). Elution with 30:1 chloroform/methanol gave 17 as a colorless oil: yield 16.3 g (32%); silica gel TLC Rf 0.42 (9:1 chloroform/methanol); ; 1H NMR (CDCl3) δ 0.87 (t, 3H, J = 7.1 Hz), 1.23 (s, 3H), 1.26–1.37 (m, 12H), 1.49 (m, 2H), 1.75 (m, 2H), 1.84 (s, 3H), 1.94 (s, 3H), 2.66 (m, 2H) and 3.94 (s, 3H); 13C NMR (CDCl3) δ 6.9, 9.8, 14.1, 22.6, 24.0, 25.6, 26.8,29.2, 29.6, 30.1, 31.9, 38.4, 42.0, 55.3, 72.1, 99.4, 118.0, 158.5, 162.1 and 181.0; mass spectrum (ESI), m/z 361.2357 (M+Na)+ (C20H34O4Na requires m/z 361.2355).
4.1.17. (rac)-2-[3-Hydroxy-3-methylundecyl]-3,5,6-trimethyl-4H-pyran-4-one (18)
To a solution containing 30.6 mg (0.197 mmol) of 2 in 1 mL of THF at −78 °C was added dropwise 406 μL (0.394 mmol) of 1 M LiHMDS solution. The reaction mixture was stirred at −78 °C for 1 h and 34.0 mg (0.394 mmol) of neat racemic epoxide 6 was added followed by 53.0 μL (0.394 mmol) of BF3 Et2O. The reaction mixture was stirred at −78 °C for 1 h and was then allowed to warm slowly to room temperature and stirred overnight. The reaction mixture was quenched with satd aq NH4Cl and extracted with portions of EtOAc. The combined organic phase was washed with brine, dried over anhydrous MgSO4, filtered and concentrated under diminished pressure to afford a crude oil. The residue was purified by chromatography on a silica gel column (7 × 2 cm). Elution with 40:1 chloroform/methanol gave 18 as a colorless oil: yield 10.7 mg (16%); silica gel TLC Rf 0.51 (9:1 chloroform/methanol);; 1H NMR (CDCl3) δ 0.88 (t, 3H, J = 6.5 Hz), 1.21 (s, 3H), 1.23–1.35 (m, 12H), 1.49 (m, 2H), 1.74 (m, 2H), 1.93 (s, 3H), 1.95 (s, 3H), 2.26 (s, 3H) and 2.64 (m, 2H); 13C NMR (CDCl3) δ 9.9, 10.2, 14.3, 17.9, 22.9, 24.2, 26.4, 26.9,29.5, 29.8, 30.4, 32.1, 38.9, 42.3, 72.4, 118.5, 119.0, 160.4, 164.0 and 179.8; mass spectrum (ESI), m/z 345.2402 (M+Na)+ (C20H34O3Na requires m/z 345.2406).
4.1.18. (R)-2-[3-Hydroxy-3-methylundecyl]-3,5,6-trimethyl-4H-pyran-4-one (19)
To a solution containing 17.4 mg (0.104 mmol) of 2 in 0.5 mL of THF at −78 °C was added dropwise 208 μL (0.208 mmol) of 1 M LiHMDS solution. The reaction mixture was stirred at −78 °C for 1 h and a solution containing 25.0 mg (0.208 mmol) of (R)-epoxide 10 in 0.5 mL of THF was added followed by26.0 lL (0.208 mmol) of BF3 Et2O. The reaction mixture was stir red at −78 °C for 1 h and was then allowed to warm slowly to room temperature and stirred overnight. The reaction mixture was quenched with satd aq NH4Cl and extracted with portions of EtOAc. The combined organic phase was washed with brine, dried over anhydrous MgSO4, filtered and concentrated under diminished pressure to afford a crude residue. The residue was purified by chromatography on a silica gel column (5 × 1.5 cm). Elution with 40:1 chloroform/methanol gave 19 as a colorless oil: yield 8.3 mg (25%); silica gel TLC Rf 0.51 (9:1 chloroform/methanol); ; 1H NMR (CDCl3) δ 0.88 (t, 3H, J = 6.5 Hz), 1.21 (s, 3H), 1.23–1.35 (m, 12H), 1.49 (m, 2H),1.74 (m, 2H), 1.93 (s, 3H), 1.95 (s, 3H), 2.26 (s, 3H) and 2.64 (m, 2H); 13C NMR (CDCl3) δ 9.9, 10.2, 14.3, 17.9, 22.9, 24.2,26.4, 26.9, 29.5, 29.8, 30.4, 32.1, 38.9, 42.3, 72.4, 118.5, 119.0, 160.4, 164.0 and 179.8; mass spectrum (ESI), m/z 345.2402 (M+Na)+ (C20H34O3Na requires m/z 345.2406).
4.1.19. (S)-2-[3-Hydroxy-3-methylundecyl]-3,5,6-trimethyl-4H-pyran-4-one (20)
To a solution containing 12.5 mg (0.074 mmol) of 2 in 0.5 mL of THF at −78 °C was added dropwise 150 μL (0.150 mmol) of 1 M LiHMDS solution. The reaction mixture was stirred at −78 °C for 1 h and a solution containing 18.0 mg (0.150 mmol) of (S)-epoxide 11 in 0.5 mL of THF was added followed by 20.0 μL (0.150 mmol) of BF3 Et2O. The reaction mixture was stirred at −78 °C for 1 h and was then allowed to warm slowly to room temperature and stirred overnight. The reaction mixture was quenched with satd aq NH4Cl and extracted with portions of ethyl acetate. The combined organic phase was washed with brine, dried over anhydrous MgSO4, filtered and concentrated under diminished pressure to afford a crude residue. The residue was purified by chromatography on a silica gel column (5 × 1 cm). Elution with 30:1 chloroform/methanol gave 20 as a colorless oil: yield 5.5 mg (23%); silica gel TLC Rf0.51 (9:1 chloroform/methanol); ; 1H NMR (CDCl3) δ 0.88 (t, 3H, J = 6.5 Hz), 1.21 (s, 3H), 1.23–1.35 (m, 12H), 1.49 (m, 2H), 1.74 (m, 2H), 1.93 (s, 3H), 1.95 (s, 3H), 2.26 (s, 3H) and 2.64 (m, 2H); 13C NMR (CDCl3) δ 9.9, 10.2, 14.3,17.9,22.9, 24.2, 26.4, 26.9, 29.5, 29.8, 30.4, 32.1, 38.9, 42.3, 72.4, 118.5, 119.0, 160.4, 164.0 and 179.8; mass spectrum (ESI), m/z 345.2399 (M+Na)+ (C20H34O3Na requires m/z 345.2406).
4.1.20. (rac)-6-[3-Hydroxy-3-methylundecyl]-3,5-dimethyl-4-hydroxypyridin-2-one (21)
To a solution containing 23.4 mg (0.071 mmol) of 12 in 0.5 mL of EtOH was added 4 mL of NH4OH. The reaction mixture was heated to 70 °C in a sealed tube for 4 days, cooled to 0 °C, neutralized to pH 7 with 1 N HCl and extracted with portions of ethyl acetate. The combined organic phase was dried over anhydrous MgSO4, filtered and concentrated under diminished pressure to afford a crude oil. The residue was purified by preparative thin layer chromatography. Elution with 9:1 chloroform/methanol gave 21 as a yellow oil: yield 6.21 mg (27%); silica gel TLC Rf 0.23 (9:1 chloroform/methanol); ; 1H NMR (CDCl3) δ 0.86 (t, 3H, J = 6.5 Hz), 1.12–1.42 (m, 12H), 1.23 (s, 3H), 1.58 (s, 3H),1.75 (m, 2H), 1.87 (s, 3H), 2.11 (m, 2H), 2.58 (m, 2H), 3.62 (br s, 1H) and 8.7 (br s, 1H); mass spectrum (ESI), m/z 346.2353 (M+Na)+ (C19H33NO3Na requires m/z 346.2358).
4.1.21. 2-(2-Hydroxyundecyl)-6-methoxy-3,5-dimethyl-4H-pyran-4-one (1)2
To a solution containing 23.1 mg (0.136 mmol) of 5 in 2 mL of THF at −78 °C was added dropwise 258 μL (0.204 mmol) of 0.79 M LDA solution in THF. The reaction mixture was stirred at −78 °C for 1 h and a solution containing 256 μL (212 mg, 1.36 mmol) of decanal in 1 mL of THF was added. The reaction mixture was stirred at −78 °C for 3 h, quenched with satd aq NH4Cl and extracted with portions of ethyl acetate. The combined organic phase was washed with brine, dried over anhydrous MgSO4, filtered and concentrated under diminished pressure to afford a crude oil. The residue was purified by chromatography on a silica gel column (5 × 1 cm). Elution with 30:1 chloroform/methanol gave 1 as a colorless oil: yield 14.9 mg (34%); silica gel TLC Rf0.46 (9:1 chloroform/methanol); 1H NMR (CDCl3) δ 0.83 (t, 3H, J = 6.8 Hz), 1.26–1.37 (m, 14H), 1.40 (m, 2H), 1.77 (s, 3H), 1.92 (s, 3H), 2.70 (m, 2H), 3.95 (s, 3H) and 3.99 (m, 1H); 13C NMR (CDCl3) δ 6.7, 10.2, 14.1, 22.6, 25.7, 29.3, 29.5, 29.6, 31.8, 37.4, 39.2, 55.3,69.8, 99.3, 119.8, 156.3, 162.3 and 180.9.
4.1.22. 2-(2-Hydroxyhexyl)-6-methoxy-3,5-dimethyl-4H-pyran-4-one (22)
To a solution containing 23.2 mg (0.136 mmol) of 5 in 2 mL of THF at −78 °C was added dropwise 258 μL (0.204 mmol) of 0.79 M LDA solution in THF. The reaction mixture was stirred at −78 °C for 1 h and a solution containing 145 μL (117 mg, 1.36 mmol) of valeraldehyde in 1 mL of THF was added. The reaction mixture was stirred at −78 °C for 3 h, quenched with satd aq NH4Cl and extracted with portions of ethyl acetate. The combined organic phase was washed with brine, dried over anhydrous MgSO4, filtered and concentrated under diminished pressure to afford a crude oil. The residue was purified by chromatography on a silica gel column (5 × 1 cm). Elution with 50:1 chloroform/methanol gave 22 as a colorless oil: yield 7.21 mg (18%); silica gel TLC Rf0.43 (9:1 chloroform/methanol); 1H NMR (CDCl3) δ 0.89 (t, 3H, J = 7.1 Hz), 1.36 (m, 2H), 1.53 (m, 2H), 1.67 (m, 2H), 1.79 (s, 3H),1.93 (s, 3H), 2.51 (br s, 1H), 2.73 (m, 2H), 3.95 (s, 3H) and 3.99 (m, 1H); 13C NMR (CDCl3) d 6.8, 10.2, 14.1, 22.6, 27.8, 33.8, 37.1,39.1, 69.9, 99.4, 119.9, 155.9, 162.3 and 180.9; mass spectrum (ESI), m/z 277.1414 (M+Na)+ (C14H22O4Na requires m/z 277.1416).
4.1.23. 7-Phenylheptanal (23)
To a solution containing 257 μL (381 mg, 3.00 mmol) of oxalyl chloride in 7 mL of CH2Cl2 at −78 °C was added dropwise 287 μL (316 mg, 4.00 mmol) of DMSO. The reaction mixture was stirred at −78 °C for 30 min and a solution containing 300 μL (288 mg, 1.50 mmol) of 7-phenylheptanol in 2 mL of CH2Cl2 was added dropwise. The reaction mixture was stirred at −78 °C for 1 h and2.09 mL (1.51 g, 15.0 mmol) of NEt3 was added dropwise. The reaction mixture was stirred at −78 °C for 45 min at which time it was allowed to warm to room temperature, quenched with satd aq NH4Cl and extracted with portions of CH2Cl2. The combined organic phase was washed with brine, dried over anhydrous MgSO4, filtered and concentrated under diminished pressure to afford a crude oil. The residue was purified by chromatography on a silica gel column (10 × 1 cm). Elution with 7:1 hexanes/ethyl acetate gave aldehyde 23 as a yellow oil: yield 267 mg (93%); silica gel TLC Rf 0.62 (4:1 hexanes/ethyl acetate); 1H NMR (CDCl3) δ 1.37 (m, 4H), 1.64 (m, 4H), 2.42 (m, 2H), 2.62 (t, 2H, J = 7.6 Hz), 7.18 (m, 3H), 7.29 (m, 2H) and 9.76 (t, 1H, J = 1.6 Hz); 13C NMR (CDCl3) δ 22.0, 29.0, 31.3, 35.9, 43.8, 125.7, 128.3, 128.4, 128.5, 142.6 and 202.8.
4.1.24. 6-Phenoxy-1-hexanol (24)
Compound 24 was prepared according the method of Suzuki et al.13 To a solution containing 312 mg (3.31 mmol) of phenol and 216 lL (300 mg, 1.65 mmol) of 6-bromo-1-hexanol in 7 mL of DMF was added 462 mg (3.34 mmol) of K2CO3. The reaction mixture was heated to 80 °C and stirred for 2 h. The reaction mixture was diluted in ethyl acetate and washed with water and brine. The combined organic phase was dried over anhydrous MgSO4, filtered and concentrated under diminished pressure to afford a crude colorless solid. The residue was purified by chromatography on a silica gel column (12 × 3 cm). Elution with 3:1 hexanes/ethyl acetate gave alcohol 24 as a colorless solid: yield 293 mg (91%); silica gel TLC Rf 0.15 (4:1 hexanes/ethyl acetate); 1H NMR (CDCl3) d1.38–1.75 (m, 6H), 1.85 (m, 2H), 2.80 (br s, 1H), 3.61 (t, 2H, J = 6.8 Hz), 3.94 (t, 2H, J = 6.4 Hz), 6.91 (m, 3H) and 7.29 (m, 2H); 13C NMR (CDCl3) δ 25.6, 29.3, 32.7, 33.9, 62.4, 67.9, 114.4, 114.5, 120.6, 129.3, 129.5 and 159.0; mass spectrum (ESI), m/z 217.1201 (M+Na)+ (C12H18O2Na requires m/z 217.1204).
4.1.25. 7-Phenoxy-1-hexanal (25)
To a solution containing 133 μL (197 mg, 1.54 mmol) of oxalyl chloride in 5 mL of CH2Cl2 at −78 °C was added dropwise 149 μL (164 mg, 2.08 mmol) of DMSO. The reaction mixture was stirred at −78 °C for 30 min and a solution containing 151 mg (0.77 mmol) of 24 in 2 mL of CH2Cl2 was added dropwise. The reaction mixture was stirred at −78 °C for 1 h and 1.07 mL (777 mg, 7.70 mmol) of NEt3 was added dropwise. The reaction mixture was stirred at −78 °C for 45 min at which time it was allowed to warm to room temperature, quenched with satd aq NH4Cl and extracted with portions of CH2Cl2. The combined organic phase was washed with brine, dried over anhydrous MgSO4, filtered and concentrated under diminished pressure to afford a crude oil. The residue was purified by chromatography on a silica gel column (10 × 2 cm). Elution with 6:1 hexanes/ethyl acetate gave aldehyde 25 as a yellow oil: yield 142 mg (94%); silica gel TLC Rf 0.57 (4:1 hexanes/ethyl acetate); 1H NMR (CDCl3) δ 1.45–1.89 (m, 6H), 2.47 (m, 2H), 3.86 (m, 2H), 6.90 (m, 3H), 7.27 (m, 2H) and 9.77 (t, 1H, J = 1.6 Hz); 13C NMR (CDCl3) δ 25.7, 29.1, 33.4, 43.8, 67.4, 114.4, 114.5, 120.5, 129.3, 129.5, 158.9 and 202.8.
4.1.26. 6-[(4-Phenyl)-phenoxy]-1-hexanol (26)
To a solution containing 563 mg (3.31 mmol) of 4-phenylphenol and 216 μL (300 mg, 1.65 mmol) of 6-bromo-1-hexanol in 10 mL of DMF was added 462 mg (3.34 mmol) of K2CO3. The reaction mixture was heated to 80 °C and stirred for 2 h. The reaction mixture was diluted in ethyl acetate and washed with water and brine. The combined organic phase was dried over anhydrous MgSO4, filtered and concentrated under diminished pressure to afford a crude colorless solid. The residue was purified by chromatography on a silica gel column (12 × 2 cm). Step gradient elution with 6:1 hexanes/ethyl acetate→2:1 hexanes/ethyl acetate gave alcohol 26 as a colorless solid: yield 258 mg (57%); silica gel TLC Rf 0.15 (4:1 hexanes/ethyl acetate); 1H NMR (CDCl3) δ 1.42–1.57 (m, 4H),1.62 (m, 2H), 1.83 (m, 2H), 3.67 (t, 2H, J = 6.4 Hz), 4.01 (t, 2H, J = 6.4 Hz), 6.96 (m, 2H), 7.30 (m, 1H), 7.43 (t, 2H, J = 7.2 Hz) and7.55 (m, 4H); 13C NMR (CDCl3) d 25.6, 25.9, 29.3, 32.7, 62.9, 67.9, 114.7, 126.8, 128.0, 128.2, 128.6, 133.6, 140.8 and 158.6; mass spectrum (ESI), m/z 293.1515 (M+Na)+ (C18H22O2Na requires m/z 293.1517).
4.1.27. 6-[(4-Phenyl)phenoxy]-1-hexanal (27)
To a solution containing 154 μL (228 mg, 1.80 mmol) of oxalyl chloride in 5 mL of CH2Cl2 at −78 °C was added dropwise 174 μL (191 mg, 2.43 mmol) of DMSO. The reaction mixture was stirred at −78 °C for 30 min and a solution containing 244 mg (0.90 mmol) of 26 in 5 mL of CH2Cl2 was added dropwise. The reaction mixture was stirred at −78 °C for 1 h and 1.31 mL (9.00 mmol) of NEt3 was added dropwise. The reaction mixture was stirred at −78 °C for 45 min at which time it was allowed to warm to room temperature, quenched with satd aq NH4Cl and extracted with portions of CH2Cl2. The combined organic phase was washed with brine, dried over anhydrous MgSO4, filtered and concentrated under diminished pressure to afford a crude colorless solid. The residue was purified by chromatography on a silica gel column (10 × 1 cm). Elution with 7:1 hexanes/ethyl acetate gave aldehyde 27 as a colorless solid: yield 235 mg (96%); silica gel TLC Rf 0.48 (4:1 hexanes/ethyl acetate); 1H NMR (CDCl3) δ 1.55 (m, 2H), 1.73 (m, 2H), 1.84 (m, 2H), 2.48 (m, 2H), 4.01 (t, 2H, J = 6.4 Hz), 6.96 (m, 2H), 7.31 (m, 1H), 7.42 (t, 2H, J = 7.2 Hz), 7.55 (m, 4H) and 9.79 (t, 1H, J = 1.6 Hz); 13C NMR (CDCl3) δ 21.8, 25.7, 29.1, 43.8,67.6, 114.7, 126.8, 128.0, 128.2, 128.6, 133.7, 140.8, 158.7 and 202.5.
4.1.28. 2-(2-Hydroxy-8-phenyloctyl)-6-methoxy-3,5-dimethyl-4H-pyran-4-one (28)
To a solution containing 20.3 mg (0.119 mmol) of 5 in 2 mL of THF at −78 °C was added dropwise 226 μL (0.178 mmol) of 0.79 M LDA solution in THF. The reaction mixture was stirred at −78 °C for 1 h and a solution containing 114 mg (0.595 mmol) of 23 in 1 mL of THF was added. The reaction mixture was stirred at −78 °C for 3 h, quenched with satd aq NH4Cl and extracted with portions of ethyl acetate. The combined organic phase was washed with brine, dried over anhydrous MgSO4, filtered and concentrated under diminished pressure to afford a crude oil. The residue was purified by chromatography on a silica gel column (7 2 cm). Elution with 50:1 chloroform/methanol gave 28 as a colorless oil: yield 19.3 mg (44%); silica gel TLC Rf 0.44 (9:1 chloroform/methanol); 1H NMR (CDCl3) δ 1.35 (m, 5H), 1.53 (m, 3H), 1.61 (m, 2H),1.78 (s, 3H), 1.92 (s, 3H), 2.60 (m, 2H), 2.70 (m, 2H), 3.95 (s, 3H),3.98 (m, 1H), 7.16 (m, 3H) and 7.28 (m, 2H); 13C NMR (CDCl3) δ 6.7, 10.3, 25.6, 29.4, 31.4, 35.9, 37.4, 39.2, 55.3, 69.8, 99.3, 119.8, 128.3, 128.4, 128.5, 142.7, 156.0, 162.3 and 180.9; mass spectrum (ESI), m/z 381.2045 (M+Na)+ (C22H30O4Na requires m/z 381.2042).
4.1.29. 2-(2-Hydroxy-7-phenoxyheptyl)-6-methoxy-3,5-dimethyl-4H-pyran-4-one (29)
To a solution containing 23.2 mg (0.135 mmol) of 5 in 2 mL of THF at −78 °C was added dropwise 253 μL (0.200 mmol) of 0.79 M LDA solution in THF. The reaction mixture was stirred at −78 °C for 1 h and a solution containing 261 mg (1.35 mmol) of 25 in 1 mL of THF was added. The reaction mixture was stirred at −78°C for 3 h, quenched with satd aq NH4Cl and extracted with portions of ethyl acetate. The combined organic phase was washed with brine, dried over anhydrous MgSO4, filtered and concentrated under diminished pressure to afford a crude oil. The residue was purified by chromatography on a silica gel column (8 × 2 cm). Elution with 50:1 chloroform/methanol gave 29 as a colorless oil: yield 17.8 mg (35%); silica gel TLC Rf 0.46 (9:1 chloroform/methanol); 1H NMR (CDCl3) δ 1.56–1.63 (m, 6H), 1.78 (s, 3H), 1.84 (m, 2H), 1.93 (s, 3H), 2.73 (m, 2H), 3.95 (s, 3H), 3.96 (m, 2H), 3.98 (m, 1H), 6.89 (m, 3H) and 7.27 (m, 2H); 13C NMR (CDCl3) δ 6.7,10.3, 29.2, 37.2, 39.3, 55.8, 67.6, 68.6, 99.3, 114.4, 119.9, 120.6, 129.5, 155.9, 159.3, 162.3 and 180.9; mass spectrum (ESI), m/z 383.1837 (M+Na)+ (C21H28O5Na requires m/z 383.1834).
4.1.30. 2-[2-Hydroxy-7-(4-phenyl)phenoxyheptyl]-6-methoxy-3,5-dimethyl-4H-pyran-4-one (30)
To a solution containing 20.4 mg (0.119 mmol) of 5 in 2 mL of THF at −78 °C was added dropwise 226 μL (0.178 mmol) of 0.79 M LDA solution in THF. The reaction mixture was stirred at −78 °C for 1 h and a solution containing 159 mg (0.595 mmol) of 27 in 2 mL of THF was added. The reaction mixture was stirred at −78 °C for 3 h, quenched with satd aq NH4Cl and extracted with portions of ethyl acetate. The combined organic phase was washed with brine, dried over anhydrous MgSO4, filtered and concentrated under diminished pressure to afford a crude colorless solid. The residue was purified by chromatography on a silica gel column (8 × 2 cm). Elution with 50:1 chloroform/methanol gave 30 as an oil: yield 23.8 mg (46%); silica gel TLC Rf 0.44 (9:1 chloroform/methanol); 1H NMR (CDCl3) δ 1.44–1.67 (m, 6H), 1.78 (s, 3H), 1.83 (m, 2H), 1.93 (s, 3H), 2.71 (m, 2H), 3.95 (s, 3H), 3.97 (m, 1H), 4.01 (t, 2H, J = 6.4 Hz), 6.96 (m, 2H), 7.29 (m, 1H), 7.41 (t, 2H, J = 7.2 Hz) and 7.53 (m, 4H); 13C NMR (CDCl3) δ 6.7, 10.3, 25.6, 26.1, 29.2,37.3, 39.3, 55.3, 67.8, 69.7, 99.3, 114.7, 119.8, 126.8, 128.0, 128.2, 128.6, 133.6, 140.8, 156.0, 158.6, 162.3 and 180.9; mass spectrum (ESI), m/z 459.2151 (M+Na)+ (C27H32O5Na requires m/z 459.2147).
4.2. Biochemical and biological evaluation
4.2.1. Lactate assay
Using an adaptation of the method of Everse,22 mitochondrial toxins were assessed using MCF-7 human breast carcinoma cells. Cells were plated at 2 × 105 cells per well in tissue culture treated 12-well plates and incubated for 24 h (resulting in about 50% cell confluence). The wells were rinsed with a pre-warmed solution of Krebs-Ringer buffer (Sigma) and the compounds were then added at the appropriate concentrations in 0.5 mL of buffer and incubated for 3 h in comparison with controls containing the same amount of buffer. The supernatant was recovered and the variation in absorbance (△OD) was measured at 363 nm. Lactate concentration (μg/mL) was calculated from the following formula: [lactate] = [(△OD363) (10f)(90)]/9.1 where f is the dilution factor used to bring the lactate concentration within the measurable range of the lactate titration curve, 90 represents the molecular weight of lactic acid and 9.1 is the extinction coefficient (mM−1 cm−1) of the reaction at 363 nm. IC50 values were calculated as the dose which produced a 50% inhibition of the maximum lactate production measured.
4.2.2. Cell culture and MTT assay
MCF-7, A-549, and 3LL cells were cultured using RPMI-1640 medium, whereas CRL-2365 and CRL-2366 were maintained in DMEM medium, all supplemented with 10% fetal bovine serum, penicillin G (100 U/mL), and streptomycin (100 μg/mL). MCF-10A cells were cultured in Mammary Epithelial Growth Medium (MEGM, Lonza) according to the manufacturer’s protocol.
In vitro inhibition of human cancer cell growth was assessed using the standard MTT assay, essentially as describe.23
4.2.3. Mitochondrial complex I activity
Beef heart mitochondria were obtained by a large-scale procedure.24 Inverted submitochondrial particles (SMP) were prepared by the method of Matsuno-Yagi and Hatefi,25 and stored in a buffer containing 0.25 M sucrose and 10 mM Tris–HCl, pH 7.4, at −80 °C. Inhibitory effects of verticipyrone analogues on bovine heart mitochondrial complex I (NADH oxidase and NADH: ubiquinone oxidoreductase) were evaluated by modification of a method described previously.26 Stock solutions (2 mg/mL in ethanol) of verticipyrone analogues were prepared and kept in the dark at −80 °C. Maximal ethanol concentration never exceeded 2% and had no influence on the control enzymatic activity. Beef heart SMP were diluted to0.5 mg/mL, and treated with 300 lV NADH to activate complexI.27 The enzymatic activities were assayed at 30 °C and monitored spectrophotometrically with a Molecular Devices SPECTRA Max-M5 (340 nm, ε 6.22 mM−1 cm−1). NADH oxidase activity was determined in a reaction medium (2.5 mL) containing 50 mM Hepes, pH7.5, containing 5 mM MgCl2. The final amount of mitochondrial protein was 30 μg. The reaction was initiated by adding 50 μM NADH after the pre-equilibration of SMP with inhibitor for 5 min. The initial rates were calculated from the linear portion of the traces. The inhibition of NADH-Q1 oxidoreductase activity was also determined under the same experimental conditions except that the reaction medium (2.5 mL) contained 0.25 M sucrose, 1 mM MgCl2, 2 μM antimycin A, 2 mM KCN, 50 μM ubiquinone Q1 and 50 mM phosphate buffer, pH 7.4.
IC50 values were taken as the final compound concentrations in the assay cuvette that yielded 50% inhibition of the enzymatic activity. When ubiquinone-1 (Q1) was used as a substrate, the rates in the presence of 1 μM rotenone were subtracted (nonphysiological electron transfer) (~15%).28 Verticipyrone analogues needed to reach a greater concentration to achieve full inhibition, a slower decay of the complex I and NADH oxidase activities beyond the IC50. Maximal inhibition of NADH oxidation was not complete, even at a relatively high concentration of inhibitor (data not shown).
Acknowledgment
KST was supported by NIH Research Grant R25GM071798, awarded by the National Institute of General Medical Sciences.
References and notes
- 1.Ui H; Shiomi K; Suzuki H; Hatano H; Morimoto H; Yamaguchi Y; Masuma R; Sunazuka T; Shimamura H; Sakamoto K; Kita K; Miyoshi H; Tomoda H; Omura SJ Antibiot. 2006, 59, 785. [DOI] [PubMed] [Google Scholar]
- 2.Shimamura H; Sunazuka T; Izuhara T; Hirose T; Shiomi K; Omura S Org. Lett 2007, 9, 65. [DOI] [PubMed] [Google Scholar]
- 3.Letsinger RL; Jamison JD J. Am. Chem. Soc 1961, 83, 193. [Google Scholar]
- 4.Yoshizawa K; Toyota S; Toda F Tetrahedron Lett. 2001, 42, 7983. [Google Scholar]
- 5.Surup F; Wagner O; vonFrieling J; Schleicher M; Oess S; Muller P; Grond SJ Org. Chem 2007, 72, 5085. [DOI] [PubMed] [Google Scholar]
- 6.Hosokawa S; Yokota K; Imamura K; Suzuki Y; Kawarasaki M; Tatsuta K Tetrahedron Lett. 2006, 47, 5415. [Google Scholar]
- 7.Zhang X; McLaughlin M; Lizeth R; Munoz P; Hsung RP; Wang J; Swidorski J Synthesis 2007, 749. [Google Scholar]
- 8.Mischitz M; Kroutil W; Wandel U; Faber K Tetrahedron: Asymmetry 1995, 6, 1261. [Google Scholar]
- 9.Ishmuratov GY; Yakovleva MP; Galyautdinova AV; Faifer LV; Kharisov RY; Zorin VV; Tolstikov GA Chem. Nat. Compd 2001, 37, 486. [Google Scholar]
- 10.Sharpless KB; Amberg W; Bennani YL; Crispino GA; Hartung J; Jeong K-S; Kwong H-L; Morikawa K; Wang Z-M; Xu D; Zhang X-LJ Org. Chem 1992, 57, 2768. [Google Scholar]
- 11.Morimoto Y; Nishikawa Y; Takaishi MJ Am. Chem. Soc 2005, 127, 5806. [DOI] [PubMed] [Google Scholar]
- 12.Bondar D; Liu J; Muller T; Paquette LA Org. Lett 2005, 7, 1813. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki T; Nagano Y; Kouketsu A; Matsuura A; Maruyama S; Kurotaki M; Nakagawa H; Miyata NJ Med. Chem 2005, 48, 1019. [DOI] [PubMed] [Google Scholar]
- 14.Chapuis JC; Khdour O; Cai X; Lu J; Hecht SM Bioorg. Med. Chem 2008, 17, 2204. [DOI] [PubMed] [Google Scholar]
- 15.Gallardo T; Saez J; Granados H; Tormo JR; Velez ID; Brun N; Torres B; Cortes DJ Nat. Prod 1998, 61, 1001. [DOI] [PubMed] [Google Scholar]
- 16.Ernster L; Lee I-Y; Norling B; Persson B Eur. J. Biochem 1969, 9, 299. [DOI] [PubMed] [Google Scholar]
- 17.Lass A; Agarwal S; Sohal RS J. Biol. Chem 1997, 272, 19199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warburg O; Wind F; Negalein EJ J. Physiol 1927, 8, 519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zu XL; Guppy M Biochem. Biophys. Res. Commun 2004, 313, 459. [DOI] [PubMed] [Google Scholar]
- 20.Pasteur LC R. Acad. Sci 1861, 52, 1260. [Google Scholar]
- 21.Farhat S; Zouev I; Marek I Tetrahedron 2004, 60, 1329. [Google Scholar]
- 22.Everse J Methods Enzymol. 1975, 9, 41. [DOI] [PubMed] [Google Scholar]
- 23.Mosmann TJ Immunol. Methods 1983, 65, 55. [DOI] [PubMed] [Google Scholar]
- 24.Smith AL Methods Enzymol. 1967, 10, 81. [Google Scholar]
- 25.Matsuno-Yagi A; Hatefi YJ Biol. Chem 1985, 260, 14424. [PubMed] [Google Scholar]
- 26.Hamada T; Ichimaru N; Abe M; Fujita D; Kenmochi A; Nishioka T; Zwicker K; Brandt U; Miyoshi H Biochemistry 2004, 43, 3651. [DOI] [PubMed] [Google Scholar]
- 27.Esposti MD; Ghelli A; Crimi M; Estornell E; Fato R; Lenaz G Biochem. Biophys. Res. Commun 1993, 190, 1090. [DOI] [PubMed] [Google Scholar]
- 28.Esposti MD; Ghelli A; Ratta M; Cortes D; Estornell E Biochem. J 1994, 301, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]