Abstract
With the increase in the elderly population, we are witnessing an increase in the rate of patients with underlying diseases and those under treatment with antithrombotic drugs.
In this study, we compared the treatment outcomes of endoscopic submucosal dissection (ESD) and other parameters in the following 3 groups: super-elderly, elderly, and nonelderly.
Compared with the other groups, the super-elderly group showed a significantly higher incidence of underlying diseases and the rate of antithrombotic treatment (P < .05). However, we observed no significant difference in the rate of curative resection or incidence of complications among the 3 groups. ESD is a relatively safe technique when performed on super-elderly patients. However, we have identified some cases in the super-elderly group, for which ESD was selected as a minimally invasive treatment for lesions that did not meet the inclusion criteria for open surgery as well as for which follow-up observations were selected rather than additional surgery for noncurative resections.
Further investigations concerning ESD are required, focusing on aspects such as indications, additional surgery, and informed consent of the patient or family, particularly when ESD is performed for super-elderly patients.
Keywords: antithrombotic drug, early gastric cancer, elderly patient, endoscopic submucosal dissection
1. Introduction
According to the Japanese Ministry of Health, Labour and Welfare in 2015, the number of individuals aged over 65 years is expected to reach 36.57 million and reach a peak in 2042, at 38.78 million individuals. The proportion of elderly individuals aged over 75 years in the entire population is expected to exceed 25% by 2055.[1] Furthermore, the increased number of elderly in society has led to an increase in the occurrence of various underlying diseases as well as the rate of oral antithrombotic therapy.[2]
Nowadays, endoscopic submucosal dissection (ESD) has become a useful minimally invasive treatment for elderly patients with early-stage gastric cancer,[3–5] because it is less invasive than open surgical procedures and is highly advantageous in terms of organ preservation.[6,7] Recently, some patients in the expanded indications group, that is, very elderly patients (age over 80 years) who are taking anticoagulation drug, are treated by ESD. However, there are few discussions on this topic, such as the occurrence of procedure-related adverse events when performing ESD in elderly patients.[8–11]
In the present study, we retrospectively evaluated the therapeutic outcomes of ESD for elderly patients to clarify their benefit and harm.
2. Patients and methods
2.1. Patients
Among 501 lesions from 452 patients (mean age: 71.9 ± 9.5 years; male-to-female ratio: 328:124) who underwent ESD at our hospital between November 2012 and November 2016, those aged over 80 years constituted group A (107 lesions among 94 patients with a mean age of 83.9 ± 3.9 years and a male-to-female ratio of 65:29), those aged 65 to 79 years constituted group B (293 lesions among 266 patients with a mean age of 72.3 ± 4.2 years and a male-to-female ratio of 190:76), and those aged less than 65 years constituted group C (101 lesions among 92 patients with a mean age of 58.1 ± 6.2 years and a male-to-female ratio of 73:19).
2.2. ESD procedure
The GIF-Q260J (Olympus Medical Systems Corp, Tokyo, Japan) endoscope was primarily used. Devices used included the insulation-tipped diathermic knife (IT knife) 2 (Olympus Medical Systems Corp, Tokyo, Japan) and dual knife (Olympus Medical Systems Corp,). Totally, 20 mL of physiological saline with 0.8 mg of indigo carmine was used as the local injection solution.
The indications for endoscopic resection and postendoscopic resection evaluation were determined in accordance with the Japanese Classification of Gastric Carcinoma in 2016 (ver. 3).[12] Lesions that met absolute indications were defined as differentiated cancer diagnosed as macroscopic intramucosal carcinoma (cT1a) measuring less than 2 cm and lesions limited to UL (–), regardless of the macroscopic type. Lesions that met expanded indications were defined as
UL (–) cT1a differentiated carcinomas greater than 2 cm in diameter,
UL (+) cT1a differentiated carcinomas less than 3 cm in diameter, and
UL (–) cT1a undifferentiated carcinomas less than 2 cm in diameter.
Lesions exceeding the expanded indication were considered as the ones that did not meet the inclusion criteria for endoscopic treatment. Furthermore, curative resection was determined based on all the following criteria being met: the tumor is resected en bloc, is <2 cm in diameter, and is a differentiated type of cancer with a depth of pT1a, HM0, VM0, ly (–), and v (–). Curative resection for lesions that met the expanded indications is determined when the tumor is resected en bloc and the resected specimen is
-
(1)
UL (–) pT1a differentiated carcinoma of ≥2 cm,
-
(2)
UL (+) pT1a differentiated carcinoma of <3 cm,
-
(3)
UL (–) pT1a undifferentiated carcinoma of <2 cm, or
-
(4)
differentiated-type with pT1b (SM1) invasion (less than 500 μm from the muscularis mucosae) of <3 cm and HM0, VM0, ly (–), and v (–).
When one of the conditions in the absolute and expanded indications for curative resection is not met, it is defined as noncurative resection.
A proton pump inhibitor was administered to all patients on the day of ESD, and use was regularly continued for at least 56 days after ESD. Second-look endoscopy was not performed after ESD without post-ESD bleeding. Antithrombotic drug treatment was managed according to the JGES guidelines in 2014.[13]
2.3. Statistical analysis
The present study was performed with the approval of the Ethical Review Board of Tokyo Medical University Hospital (No. 2017-045). The 3 groups were compared in terms of the underlying disease, the presence or absence of oral antithrombotic therapy, therapeutic outcomes, the presence or absence of procedural accidents, and the treatment plan following noncurative resection. SPSS (version 22, Chicago, IL) was used for all statistical analyses. Analysis was performed using analysis of variance, and P < .05 was considered to indicate a significant difference.
3. Results
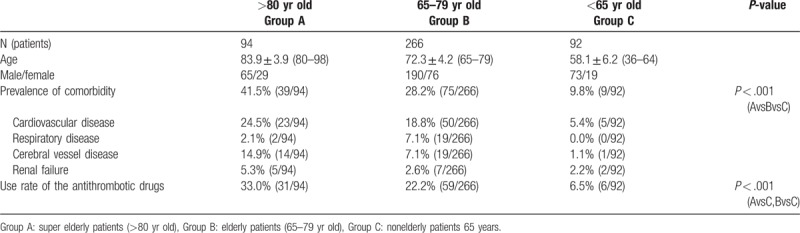
Upon comparing groups A, B, and C, the prevalence of underlying diseases, including heart disease, lung disease, kidney disease, and cerebrovascular disease, were 41.5% (39/94), 28.2% (39/94), and 9.8% (9/92), respectively, indicating a significantly higher prevalence in groups A and B than in group C. The rates of administering oral antithrombotic therapy were 33.0% (31/94), 22.2% (59/266), and 6.5% (6/92) in groups A, B, and C, respectively, and this rate was found to significantly increase with age (Table 1).
Table 1.
Patient's characteristics.
3.1. ESD treatment outcomes
There were 73, 236, and 83 lesions corresponding to the indications in the guidelines; 27, 51, and 14 lesions that met the expanded indications, and 7, 6, and 4 lesions in groups A, B, and C, respectively that did not meet the inclusion criteria for ESD. On comparing the pathological diagnosis of the resected specimens in each of the 3 groups in terms of differentiation, depth, presence or absence of ulceration, lymphatic invasion, and vascular invasion, no significant difference was observed (Table 2) (Fig. 1).
Table 2.
Categories of lesion.
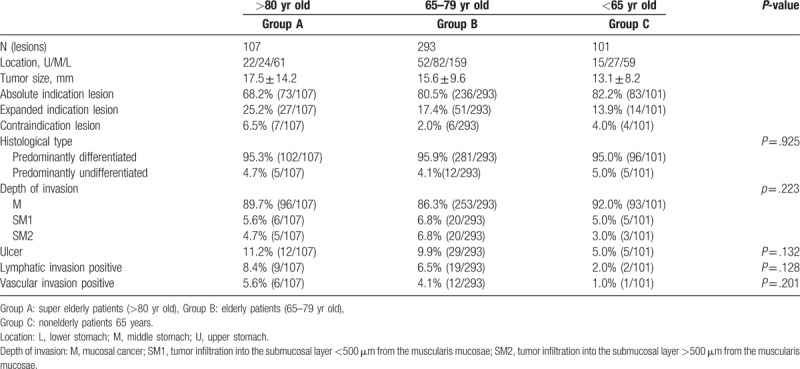
Figure 1.
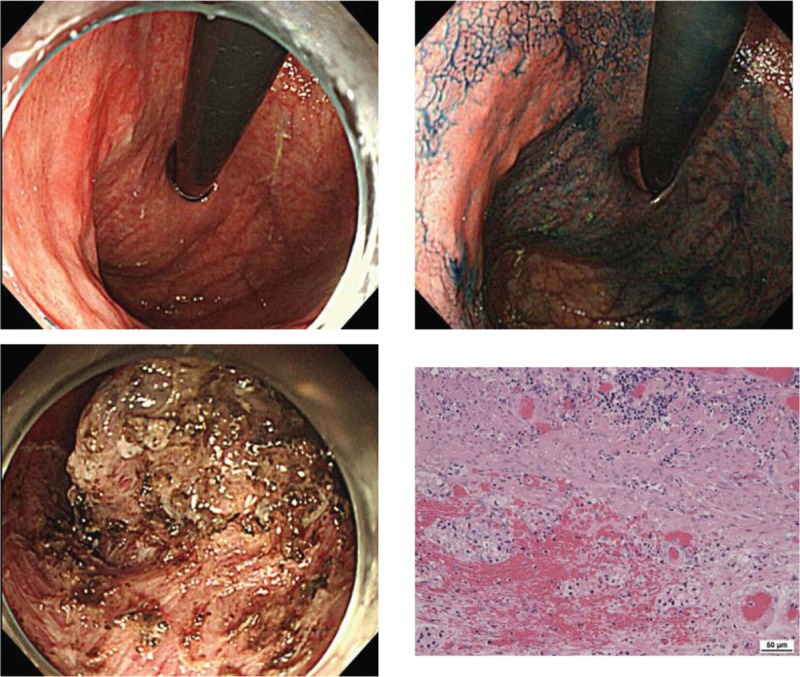
A 91-year-old man presenting a 0-IIa lesion measuring 25 mm in the posterior wall of the upper gastric body, with por2 > sig (preoperative biopsy). Although the lesion did not meet the inclusion criteria, en bloc resection was performed by ESD as per the wishes of the patient's family. Pathological findings included por2 > sig, 0-IIa, 18 × 18 mm, pT1b2 (SM2 ≧800 μm), UL (−), ly (+), v (+), HM0, and VM1. The procedure was deemed a noncurative resection. Upon performing additional surgery, the subject developed postgastrectomy syndrome 1 month after surgery, which led to the gradual deterioration of his nutritional status due to impaired food intake. Seven months after surgery, the subject went into septic shock caused by a urinary tract infection and passed away. ESD = endoscopic submucosal dissection.
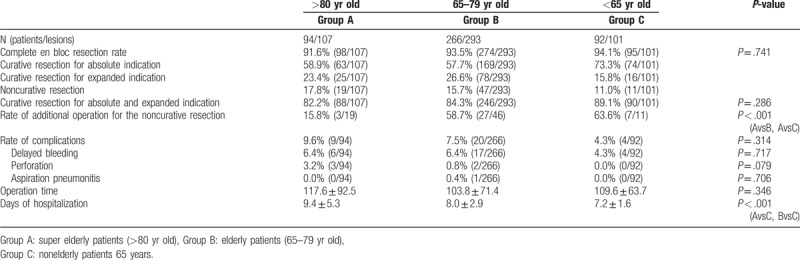
On comparing groups A, B, and C, there was no significant difference observed in the en bloc resection rate (96.3% [103/107], 98.0% [287/293], and 94.1% [95/101]), complete en bloc resection rate (91.6% [98/107], 93.5% [274/293], and 94.1% [95/101]), and curative resection rate (82.2% [88/107], 84.3% [246/293], and 89.1% [90/101]), respectively. On comparing the duration of endoscopic treatment between the 3 groups, there was no significant difference found. Furthermore, the incidence of procedural accidents of bleeding, perforation, and aspiration pneumonitis between groups A, B, and C was 9.6% (9/94), 7.5% (20/266), and 4.3% (4/92), respectively, with no significant difference observed. On the other hand, the rate of additional surgery performed for noncurative lesions was 15.8% (3/19), 58.7% (27/46), and 63.6% (7/11) in groups A, B, and C, respectively, indicating a significantly lower rate in group A than in groups B and C. Moreover, the length of the hospital stay was significantly shorter in group C than in groups A and B (Table 3).
Table 3.
Treatment outcomes.
3.2. Correlation with antithrombotic drugs
Oral antithrombotic therapy was used in 96 of the 452 patients, accounting for 21.2% overall (mean age: 76.5 ± 7.3, male-to-female ratio of 73:23, and oral antithrombotic therapy with a single agent-to-multiple agents ratio of 73/23 patients). Compared to the group that did not receive oral antithrombotic therapy, the rate of after-bleeding was significantly higher (group with oral antithrombotic therapy vs the group without oral antithrombotic therapy: 10.4% >4.8%, P = .038).
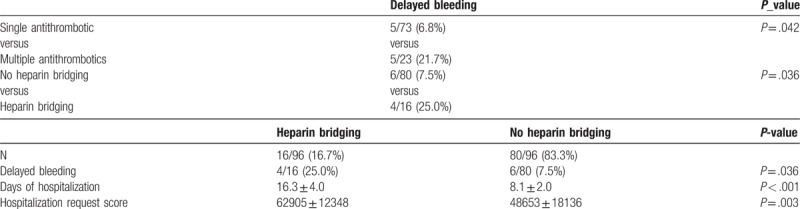
The rate of after-bleeding in patients receiving oral antithrombotic therapy reached 10.4% (11/96) and was significantly higher for patients receiving multiple agent oral therapies (P = .042) and patients with heparinization (P = .036). Compared to patients without heparinization, those with heparinization had significantly longer hospital stays (16.3 ± 4.0 vs 8.1 ± 2.0 days; P < .001) than those who accumulated significantly higher medical care fee points during hospitalization (62905 ± 12348 points vs 48653 ± 18136 points (P = .003). There were no cases of thrombotic procedural accidents in the group with heparinization or the group without (Tables 4 and 5).
Table 4.
Characteristics of 96 patients receiving antithrombotic therapy.
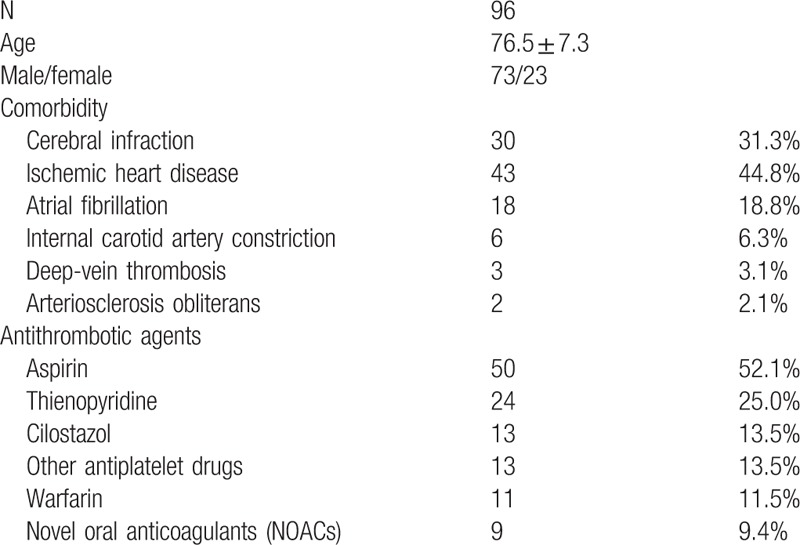
Table 5.
Treatment outcome of 96 patients receiving antithrombotic therapy.
3.3. Noncurative cases
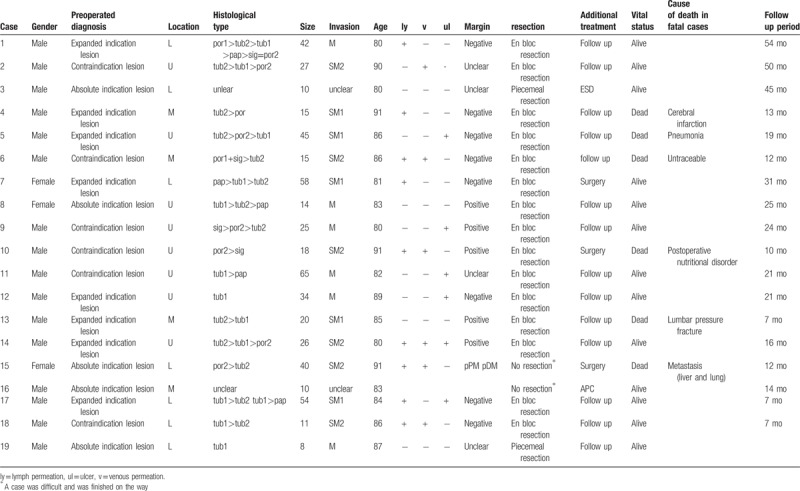
Among the patients in group A, the 19 cases with noncurative resections are presented in Table 6. Of these 19 patients, 3 patients underwent an additional surgical resection, 2 patients underwent additional endoscopic treatment by ESD or argon plasma coagulation, and 15 patients underwent follow-up observation. Follow-up observation at our hospital for noncurative resection cases involves examination for localized recurrence by endoscopy or CT at 6 months to 1 year following ESD. Five patients passed away while undergoing follow-up, including 3 patients who died from other causes after ESD; 1 patient who had multiple metastasis following ESD, and 1 patient who died due to poor nutritional status after additional surgery.
Table 6.
Noncurative resection in over 80 yr patients.
4. Discussion
Endoscopic mucosal resection was developed in the 1980s and led to the widespread popularity of endoscopic treatment for early-stage gastric cancer.[14] Subsequently, in the 1990s, the development of the IT knife and the advent of various devices led to the rapid popularization of ESD in Japan.[15,16] Advantages of ESD include that it enables en bloc resection even of extensive lesions and accurate pathological evaluation.[17–22] Furthermore, the aging of the population is associated with an increased number of cases in which the selection of minimally invasive surgery is recommended for elderly patients from various perspectives, including postoperative quality of life (QOL) and procedural accidents.[23–25] Compared to open surgical procedures, ESD is minimally invasive, and has thus been established at various institutions as a highly effective endoscopic treatment for elderly patients. Reports of ESD in elderly patients are associated with various controversies, and many points remain unclear regarding safety, postoperative ADL (activities of daily life), and the approach for lesions that do not meet the inclusion criteria. In the present study, we analyzed the characteristics of the therapeutic outcomes of ESD in elderly patients and examined the indications of ESD for elderly patients.
The prevalence of underlying disease, and the rate of oral antithrombotic therapy were both significantly higher in elderly patients; however, there was no significant difference between the 3 groups in terms of the curative resection rate, treatment duration, and the incidence of procedural accidents. Thus, ESD was considered to be performed relatively safer for elderly patients. The reason that the hospital stay was significantly longer in groups A and B is thought to be attributed to the fact that careful follow-up observation was required following the endoscopic treatment for elderly patients with procedural accidents, and with low PS. Furthermore, in patients aged younger than 65 years, the rate of oral antithrombotic therapy was low, and there were few patients with heparinization, which was thought to have resulted in shorter hospital stays.
Patients receiving oral antithrombotic therapy are considered to be at high risk of developing thrombosis upon drug cessation; thus, heparinization was administered in accordance with the guidelines.[26] However, in recent years, the risk of hemorrhage in heparinization has gradually become clear.[27,28] Furthermore, disadvantages arise in routine clinical practice (eg, complications at hospital admission, and longer hospital stays). With the increased incidence of underlying diseases, there are many elderly patients who undergo oral antithrombotic therapy for the prevention of cerebrovascular and cardiovascular disease. In addition to the increased risk of late bleeding, since there is a negative medical economic effect (eg, the length of the hospital stay and cost of medical care), we believe that the continuation of antithrombotic therapy and the need for heparinization should be examined from various perspectives.
Group A included some patients for whom ESD was selected as minimally invasive treatment rather than open surgery for lesions that do not meet the inclusion criteria, as well as some patients who underwent follow-up observation without additional surgery for noncurative resection. In the present study, while the safety of ESD was suggested, a few procedural events occurred, including bleeding and perforation.
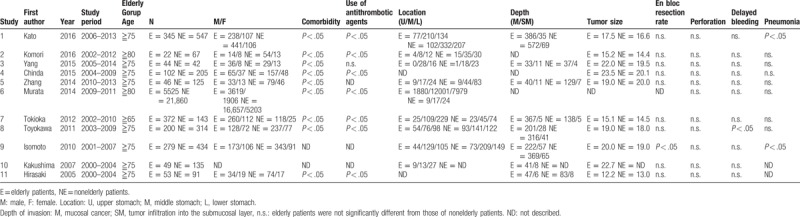
Until January 2018, the ESD study that compared with an elderly person and the nonelderly person was 11 cases in total (Table 7).[23,29–38] Those studies reported the en bloc resection rates and complication. Most of these studies describe that there is not significant difference in en bloc resection rate and complication between the 2 groups similar to the present study.
Table 7.
Characteristics of study for elderly patient's ESD.
Among limitations, in elderly patients, some procedures become fatal because the patients have considerably reduced residual function of various organs. Thus, more careful consideration for the treatment and management of the patient's general condition is required. It has been reported that additional surgery for noncurative resection can help to improve the survival rate.[38,39] However, as seen in the case presented above, there are some patients who undergo an additional resection for noncurative lesions by ESD, which consequently leads to the deterioration of their nutritional status due to impaired food intake caused by postgastrectomy syndrome; therefore, in cases of elderly patients, judgment can be difficult. While ESD is advantageous since it enables the removal of cancer, the burden of minimally invasive surgery cannot be ignored, and follow-up observation can also be considered an option. Additional surgery for elderly patients remains controversial and while there are no established clear determination criteria. Therefore, in addition to age, some experts consider that ADL, PS (performance statue), and the prognostic nutritional index (Onodera's PNI) could serve as factors to determine the treatment plan.[40–41,5] Elderly patients are at high risk of dying from other diseases, and the treatment should be carefully determined, taking ADL and nutritional status into consideration. In the present study, on comparing the pathological diagnosis in terms of differentiation, depth, presence or absence of ulceration, lymphatic invasion, and vascular invasion, no significant difference was observed. On the other hand, there are reports that pathological risk factors are very important. By early stomach cancer treatment study group, they have established a risk-scoring system, termed the “eCura system,” for the risk stratification of lymph node metastasis in patients who have received noncurative ESD for early gastric cancer.[42] The eCura system seems to be useful for selection of a treatment policy after ESD for elderly people.
In our hospital, treatment decisions for elderly patients are made with regard to ADL, PS, age, and comorbidities. We believe that prior to surgery, the patient concerned and his or her family members should be fully informed of the significance of treatment, as well as possible procedural accidents. Furthermore, informed consent should be obtained.
There are several limitations for this study. First, it was a retrospective study and performed at a single center, which may introduce bias into the results of the study. Second, the number of patients in the super-elderly patients is small. Third, technical problems by ESD operators may have a considerable impact on complications. Fourth, we did not compare survival rates in super-elderly patients between those who underwent ESD and those who did not. However, even considering these limitations, the results of this study are clinically meaningful.
In conclusion, ESD appears to be safely performed, even in elderly patients. In contrast, when performing ESD although further examination is needed with regards to the indications, criteria for determining whether or not to perform additional surgery in the future.
Acknowledgments
We are indebted to Professor J. Patrick Barron, Chairman of the Department of International Medical Communications of Tokyo Medical University for their editorial review of the English manuscript.
Author contributions
All authors have read and approved the submitted version of the paper.
Conceptualization: Hayato Yamaguchi, Masakatsu Fukuzawa, Takashi Kawai.
Data collection: Hayato Yamaguchi.
Data curation: Hayato Yamaguchi, Masakatsu Fukuzawa.
Endoscopic procedures:Hayato Yamaguchi, Taisuke Matsumoto , Maya Suguro, Kumiko Uchida, Yohei Koyama, Akira Madarame , Takashi Morise, Yuki Aoki, Akihiko Sugimoto, Yoshiya Yamauchi, Shin Kono, Yuichiro Tsuji , Kenji Yagi, Masakatsu Fukuzawa, Takashi Kawai.
Formal analysis: Hayato Yamaguchi, Takashi Kawai.
Investigation: Taisuke Matsumoto, Maya Suguro, Kumiko Uchida, Yohei Koyama, Akira Madarame, Takashi Morise, Yuki Aoki, Akihiko Sugimoto, Yoshiya Yamauchi, Shin Kono, Yuichiro Tsuji, Kenji Yagi.
Manuscript supervisors: Masakatsu Fukuzawa, Takashi Kawai, Takao Itoi
Methodology: Taisuke Matsumoto, Maya Suguro, Kumiko Uchida, Yohei Koyama, Akira Madarame, Takashi Morise, Yuki Aoki, Akihiko Sugimoto, Yoshiya Yamauchi, Shin Kono, Yuichiro Tsuji, Kenji Yagi.
Project administration: Masakatsu Fukuzawa, Takashi Kawai, Takao Itoi.
Study design, data analysis, and script preparation: Hayato Yamaguchi, Takashi Kawai, Takao Itoi.
Supervision: Masakatsu Fukuzawa, Takashi Kawai, Takao Itoi.
Writing – original draft: Hayato Yamaguchi.
Writing – review and editing: Hayato Yamaguchi, Masakatsu Fukuzawa, Takashi Kawai, Takao Itoi.
Footnotes
Abbreviations: ADL = activities of daily life, CT = computed tomography, ESD = endoscopic submucosal dissection, ly = lymph permeation, PS = performance statue, UL = ulcer, v = venous permeation.
All authors disclose no financial relationships relevant to this publication.
Institutional review board statement: This study was approved by our institutional review board (Tokyo Medical University No. 2017-045).
Informed consent was obtained from all the patients.
All authors have no financial relationships with a commercial entity producing health-care related products and services relevant to this article.
The authors have no conflicts of interest to disclose.
References
- [1]. Ministry of Health, Labour and Welfare in Japan: Welfare Labor White Paper 2016 (Journal of Japanese Society of Internal Medicine) :4-9, 2016. (In Japanese) [Google Scholar]
- [2].Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;30:1849–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lee JY, Choi IJ, Cho SJ, et al. Endoscopic submucosal dissection for metachronous tumor in the remnant stomach after distal gastrectomy. Surg Endosc 2009;24:1360–6. [DOI] [PubMed] [Google Scholar]
- [4].Takenaka R, Kawahara Y, Okada H, et al. Endoscopic submucosal dissection for cancers of the remnant stomach after distal gastrectomy. Gastrointest Endosc 2008;67:359–63. [DOI] [PubMed] [Google Scholar]
- [5].Park CH, Lee H, Kim DW, et al. Clinical safety of endoscopic submucosal dissection compared with surgery in elderly patients with early gastric cancer: a propensity-matched analysis. Gastrointest Endosc 2014;80:599–609. [DOI] [PubMed] [Google Scholar]
- [6].Akasaka T, Nishida T, Tsutsui S, et al. Short-term outcomes of endoscopic submucosal dissection (ESD) for early gastric neoplasm: multicenter survey by osaka university ESD study group. Dig Endosc 2011;23:73–7. [DOI] [PubMed] [Google Scholar]
- [7].Abe N, Gotoda T, Hirasawa T, et al. Multicenter study of the long-term outcomes of endoscopic submucosal dissection for early gastric cancer in patients 80 years of age or older. Gastric Cancer 2012;15:70–5. [DOI] [PubMed] [Google Scholar]
- [8].Oda I, Gotoda T, Hamanaka H, et al. Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications from a large consecutive series. Dig Endosc 2005;17:54–8. [Google Scholar]
- [9].Watanabe K, Ogata S, Kawazoe S, et al. Clinical outcomes of EMR for gastric tumors: historical pilot evaluation between endoscopic submucosal dissection and conventional mucosal resection. Gastrointest Endosc 2006;63:776–82. [DOI] [PubMed] [Google Scholar]
- [10].Oka S, Tanaka S, Kaneko I, et al. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastroint Endosc 2006;64:877–83. [DOI] [PubMed] [Google Scholar]
- [11].Takizawa K, Oda I, Gotoda T, et al. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection – an analysis of risk factors. Endoscopy 2008;40:179–83. [DOI] [PubMed] [Google Scholar]
- [12].Ono H, Yao K, Fujishiro M, et al. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig Endosc 2016;28:3–15. [DOI] [PubMed] [Google Scholar]
- [13].Fujimoto K, Fujishiro M, Kato M, et al. Guidelines for gastroenterological endoscopy in patients undergoing antithrombotic treatment. Dig Endosc 2014;26:1–4. [DOI] [PubMed] [Google Scholar]
- [14].Tanabe S, Koizumi W, Mitomi H, et al. Clinical outcome of endoscopic aspiration mucosectomy for early stage gastric cancer. Gastrointest Endosc 2002;56:708–13. [DOI] [PubMed] [Google Scholar]
- [15].Ono H, Kondo H, Gotoda T, et al. Endoscopic mucosal resection for treatment of early gastric cancer. Gut 2001;48:225–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ono H, Hasuike N, Inui T, et al. Usefulness of a novel electrosurgical knife, the insulation-tipped diathermic knife-2, for endoscopic submucosal dissection of early gastric cancer. Gastric Cancer 2008;11:47–52. [DOI] [PubMed] [Google Scholar]
- [17].Imagawa A, Okada H, Kawahara Y, et al. Endoscopic submucosal dissection for early gastric cancer: results and degrees of technical difficulty as well as success. Endoscopy 2006;8:987–90. [DOI] [PubMed] [Google Scholar]
- [18].Oda I, Saito D, Tada M, et al. A multicenter retrospective study of endoscopic resection for early gastric cancer. Gastric Cancer 2006;9:262–70. [DOI] [PubMed] [Google Scholar]
- [19].Park JC, Lee SK, Seo JH, et al. Predictive factors for local recurrence after endoscopic resection for early gastric cancer: long-term clinical outcome in a single-center experience. Surg Endosc 2010;24:2842–9. [DOI] [PubMed] [Google Scholar]
- [20].Park YM, Cho E, Kang HY, et al. The effectiveness and safety of endoscopic submucosal dissection compared with endoscopic mucosal resection for early gastric cancer: a systematic review and metaanalysis. Surg Endosc 2011;25:2666–77. [DOI] [PubMed] [Google Scholar]
- [21].Lee S, Oh ST, Lee H, et al. Associated risk factors for psychological distress in patients with gastric epithelial neoplasm undergoing endoscopic submucosal dissection. Medicine (Baltimore) 2018;97:e13912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bang CS, Choi JH, Lee JJ, et al. Endoscopic submucosal dissection of papillary adenocarcinoma of stomach; protocol for a systematic review and meta-analysis. Medicine (Baltimore) 2018;97:e13905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Endo S, Dousei T, Yoshikawa Y, et al. Prognosis of gastric carcinoma patients aged 85 years or older who underwent surgery or who received best supportive care only. Int J Clin Oncol 2013;18:1014–9. [DOI] [PubMed] [Google Scholar]
- [24].Isomoto H, Ohnita K, Yamaguchi N, et al. Clinical outcomes of endoscopic submucosal dissection in elderly patients with early gastric cancer:. Eur J Gastroenterol Hepatol 2010;22:311–7. [DOI] [PubMed] [Google Scholar]
- [25].Kakushima N, Fujishiro M, Kodashima S, et al. Technical feasibility of endoscopic submucosal dissection for gastric neoplasms in the elderly Japanese population. J Gastroenterol Hepatol 2007;22:311–4. [DOI] [PubMed] [Google Scholar]
- [26].Fujimoto K, Fujishiro M, Kato M, et al. Guidelines for gastroenterological endoscopy in patients undergoing antithrombotic treatment; Japan Gastroenterological Endoscopy Society. Dig Endosc 2014;26:1–4. [DOI] [PubMed] [Google Scholar]
- [27].Katsuhiro Mabe, Mototsugu Kato, Koji Oba, et al. The Sapporo Consensus Study Group. A prospective, multicenter survey on the validity of shorter periendoscopic cessation of antithrombotic agents in Japan. J Gastroenterol 2017;52:50–60. [DOI] [PubMed] [Google Scholar]
- [28].Ono S, Fujishiro M, Niimi K. Technical feasibility of endoscopic submucosal dissection for early gastric cancer in patients taking anti-coagulants or anti-platelet agents. Dig Liver Dis 2009;41:725–8. [DOI] [PubMed] [Google Scholar]
- [29].Kato M, Michida T, Kusakabe A, et al. Safety and short-term outcomes of endoscopic submucosal dissection for early gastric cancer in elderly patients. Endosc Int Open 2009;4:E521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Komori K, Nakamura K, Ihara E, et al. Endoscopic submucosal dissection is feasible for very elderly patients with early gastric cancer: comparison of short-term and long-term outcomes in very elderly and non-elderly patients. Fukuoka Igaku Zasshi (J Jpn Soc Intern Med) 2016;107:72–81. [PubMed] [Google Scholar]
- [31].Yang TC, Hou MC, Chen PH, et al. Clinical outcomes and complications of endoscopic submucosal dissection for superficial gastric neoplasms in the elderly. Medicine (Baltimore) 2015;94:1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chinda D, Sasaki Y, Tatsuta T, et al. Perioperative complications of endoscopic submucosal dissection for early gastric cancer in elderly Japanese patients 75 years of age or older. Intern Med 2015;54:267–72. [DOI] [PubMed] [Google Scholar]
- [33].Zhang Y, Huang L, Li L, et al. Endoscopic submucosal dissection for early gastric neoplasms in elderly patients. J Laparoendosc Adv Surg Tech A 2014;24:391–8. [DOI] [PubMed] [Google Scholar]
- [34].Murata A, Muramatsu K, Ichimiya Y, et al. Endoscopic submucosal dissection for gastric cancer in elderly Japanese patients: an observational study of financial costs of treatment based on a national administrative database. J Dig Dis 2014;15:62–70. [DOI] [PubMed] [Google Scholar]
- [35].Tokioka S, Umegaki E, Murano M, et al. Utility and problems of endoscopic submucosal dissection for early gastric cancer in elderly patients. J Gastroenterol Hepatol 2012;3:63–9. [DOI] [PubMed] [Google Scholar]
- [36].Toyokawa T, Fujita I, Morikawa T, et al. Clinical outcomes of ESD for early gastric neoplasms in elderly patients. Eur J Clin Invest 2011;41:474–8. [DOI] [PubMed] [Google Scholar]
- [37].Isomoto H, Ohnita K, Yamaguchi N, et al. Clinical outcomes of endoscopic submucosal dissection in elderly patients with early gastric cancer. Eur J Gastroenterol Hepatol 2010;22:311–7. [DOI] [PubMed] [Google Scholar]
- [38].Hirasaki S, Tanimizu M, Nasu J, et al. Treatment of elderly patients with early gastric cancer by endoscopic submucosal dissection using an insulated-tip diathermic knife. Intern Med 2005;44:1033–8. [DOI] [PubMed] [Google Scholar]
- [39].Kusano C, Iwasaki M, Kaltenbach T, et al. Should elderly patients undergo additional surgery after non-curative endoscopic resection for early gastric cancer? Long-term comparative outcomes. Am J Gastroenterol 2011;106:1064–9. [DOI] [PubMed] [Google Scholar]
- [40].Jiang N, Deng JY, Ding XW, et al. Prognostic nutritional index predicts postoperative complications and long-term outcomes of gastric cancer. World J Gastroenterol 2014;20:10537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hayashi T, Yoshikawa T, Aoyama T, et al. Severity of complications after gastrectomy in elderly patients with gastric cancer. World Surg 2012;36:2139–45. [DOI] [PubMed] [Google Scholar]
- [42].Hatta W, Gotoda T, Oyama T, et al. Is the eCura system useful for selecting patients who require radical surgery after noncurative endoscopic submucosal dissection for early gastric cancer? A comparative study. Gastric Cancer 2018;21:481–9. [DOI] [PubMed] [Google Scholar]


