Abstract
Background:
Restrictive red blood cell transfusion strategy is implemented to minimize risk following allogeneic blood transfusion in adult cardiac surgery. However, it is still unclear if it can be applied to pediatric cardiac patients. The purpose of this systematic review and meta-analysis was to determine the effect of postoperative restrictive transfusion thresholds on clinical outcomes based on up-to-date results of randomized controlled trials (RCTs) and observational studies in pediatric cardiac surgery.
Method:
We searched for RCTs and observational studies in the following databases: the Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE, and ClinicalTrials.gov from their inception to October 26, 2017. We also searched reference lists of published guidelines, reviews, and relevant articles, as well as conference proceedings. No language restrictions were applied and no observational study met the inclusion criteria.
Results:
Four RCTs on cardiac surgery involving 454 patients were included. There were no differences in the pooled fixed effects of intensive care unit (ICU) stay between the liberal and restrictive transfusion thresholds (standardized mean difference SMD, 0.007; 95% confidence interval CI, −0.18–0.19; P = .94). There were also no differences in the length of hospital stay (SMD, −0.062; 95% CI, −0.28−0.15; P = .57), ventilation duration (SMD, −0.015; 95% CI, −0.25–0.22; P = .90), mean arterial lactate level (SMD, 0.071; 95% CI, −0.22–0.36; P = .63), and mortality (risk ratio, 0.49; 95% CI, 0.13–1.94; P = .31). There was no inter-trial heterogeneity for any pooled analysis. Publication bias was tested using Egger, Begg, or the trim-and-fill test, and the results indicated no significant publication bias.
Conclusion:
Evidence from RCTs in pediatric cardiac surgery, though limited, showed non-inferiority of restrictive thresholds over liberal thresholds in length of ICU stay and other outcomes following red blood cell transfusion. Further high-quality RCTs are necessary to confirm the findings.
Keywords: cardiac surgery, pediatric, postoperative, transfusion trigger/threshold
1. Introduction
Cardiac surgery is associated with frequent allogeneic blood transfusions, at a reported rate of approximately 10% to 90%.[1,2] The pathophysiological rationale for perioperative red blood cell (RBC) transfusion is that anemia is an independent risk factor for postoperative morbidity and mortality in cardiac patients.[3] However, comparison of transfused and non-transfused patients after cardiac surgery showed that transfusions were associated with significant morbidity and mortality, including increased incidences of renal failure; respiratory, cardiac, and neurologic complications; and infections.[4,5] A recent meta-analysis revealed that findings favoring restrictive strategy were largely based on observational studies, but randomized controlled trials (RCTs) on cardiac surgery refuted the findings.[6] All the studies were conducted in adult populations. The situation in the pediatric population was unclear due to less evidence supported by high-quality studies. It is more difficult to conduct comparative studies in pediatric cardiac patients than in adult cardiac patients because of small case volume and significant diagnostic and management heterogeneity, and so far, there are only a few relevant studies in the literature.
We systematically reviewed and critically assessed results of RCTs and observational studies that compared postoperative restrictive and liberal RBC transfusion strategies in pediatric cardiac surgery to explore evidence in support of restrictive transfusion strategy in clinical settings.
2. Methods
We drafted a predefined review protocol and registered with the PROSPERO international prospective register of systematic reviews (http://www.crd.york.ac.uk/PROSPERO; registration number: CRD42017078087). We followed the PRISMA guidelines for analyses.[7] Ethical approval was waived since this was a meta-analysis of published articles.
2.1. Study identification
Relevant databases such as Cochrane Central Register of Controlled Trials, PubMed, EMBASE, and ClinicalTrials.gov were searched from their inception to October 26, 2017 for articles and trials. No search restrictions were applied to publication status, date, or language. Reference lists of relevant reviews, original articles, and available online conference proceedings, as well as all included trials were extensively and thoroughly searched.
2.2. Eligibility criteria
The following 4 inclusion criteria had to be met in this systematic review:
-
1.
study design: RCT or observational study;
-
2.
patient population: cardiac surgery patients aged 18 years and below;
-
3.
intervention: patients postoperatively managed with 2 different RBC transfusion strategies. The patients in the restrictive transfusion group received RBCs at lower hemoglobin concentrations or hematocrit levels than those in the liberal transfusion group; and
-
4.
outcomes: intensive care unit (ICU) length of stay, hospital length of stay, ventilation duration, and mortality, as well as biomarkers including mean arterial lactate level. Studies were excluded if they reported preoperative or intraoperative transfusion, no transfusion threshold, or none of the a priori outcomes.
2.3. Study selection and data collection
Two reviewers independently screened query results to select relevant studies that met the inclusion criteria. The same 2 reviewers independently extracted relevant data and outcomes, including study design, methodology, transfusion thresholds, patient characteristics, and outcomes. Disagreements were resolved by consensus.
2.4. Risk of bias assessment
The methodological quality of individual studies was assessed independently by the 2 reviewers using the Cochrane Collaboration tool for assessing risk of bias as described in the Cochrane Handbook for Systematic Reviews of Interventions.[8] The following categories were assessed in each study: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other potential sources of bias. Performance in these categories and the overall judgment of the risk of bias for each entry were described as follows: “low,” “unclear” (indicating unclear or unknown risk of bias), and “high” risk of bias.
2.5. Data synthesis
The pooled standardized mean difference (SMD) with 95% confidence interval (CI) was used to estimate effect on continuous outcomes. Mean ± standard deviation (SD) was considered as following normal distribution whether it was mentioned in the original studies or not. Data that did not follow a normal distribution and were presented as medians ± interquartile ranges/ranges were converted to means ± SDs according to previously published methods.[9] Data from all the studies were combined to estimate the pooled risk ratio (RR) and associated 95% CI for binary outcomes. Pooled RRs and mean differences were estimated using the fixed-effects model to estimate variances. The heterogeneity between studies was tested using a weighted inverse variance chi-square test and quantified using the I2 test. I2 is greater than 50% was considered substantial heterogeneity. As for the chi-square test, a P value less than .10 was used to indicate the presence of statistically significant heterogeneity. All statistical analyses were performed using Stata version 15.0 software (Stata Corporation, College Station, TX). We considered P values less than .05 to be statistically significant for pooled results. Due to the limited number of included studies, we did not examine funnel plots for evidence of publication bias but only used Egger, Begg, or the trim-and-fill test.
3. Results
3.1. Characteristics of included and excluded studies
The literature search found 1316 studies. Of these, 1299 were excluded from further assessment at the stage of title and abstract screening, and 17 studies underwent full-text screening (Fig. 1). The rest of the studies were excluded for the following reasons: threshold omission,[10–15] unavailability of full data,[16] inclusion of preoperative transfusion,[17–19] inclusion of multi-interventions related to transfusion,[20] and irrelevant study purpose.[21,22]Table 1 shows the patient demographics, biomarker status, and clinical outcomes. All 4 included studies were RCTs. There were significant variations in patient age and transfusion threshold. One study included both cyanotic and acyanotic patients,[23] while the others included either cyanotic or acyanotic patients others.[24–26]
Figure 1.
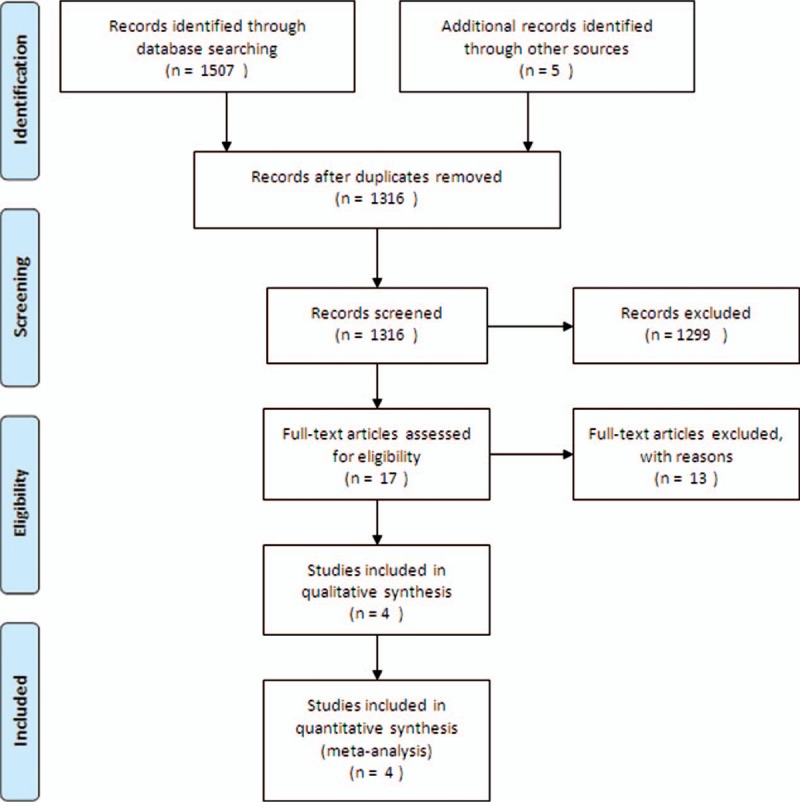
Flow diagram showing the study selection steps of the meta-analysis.
Table 1.
Patient population, biomarker, and clinical outcomes of included studies.

3.2. Quality assessment and risk of bias
The quality of the included studies were assessed using the Cochrane Collaboration risk of bias tool.[8] All the studies had 3 or less “unclear” or “high risk” items according to Cochrane and were considered of acceptable quality. One study claimed to be open-labeled and was therefore “high risk” in allocation concealment.[24] It did not blind participants/personnel, and it was also unclear if a blinded outcome assessment was performed. Two other studies by the same researchers did not mention allocation concealment or blinding of participants/personnel/outcome assessment.[23,26] The fourth study[27] was a secondary subgroup study of the primary study,[27] and it did not blind patients or care-givers (Table 2).
Table 2.
Study quality assessment using Cochrane collaboration risk of bias tool.

3.3. Quantitative analysis
Due to the limited number of studies, we did not perform subgroup analysis based on cyanotic status, procedure types, or age. In the study that included cyanotic and acyanotic patients, both patient groups were pooled as 1 single group.[23]
3.4. ICU stay
All 4 studies reported this parameter. The pooled SMD showed no statistically significant effect on ICU stay (SMD, 0.007; 95% CI, −0.18–0.19; P = .94; test for heterogeneity P = .82; I2 = 0%) (Fig. 2).
Figure 2.
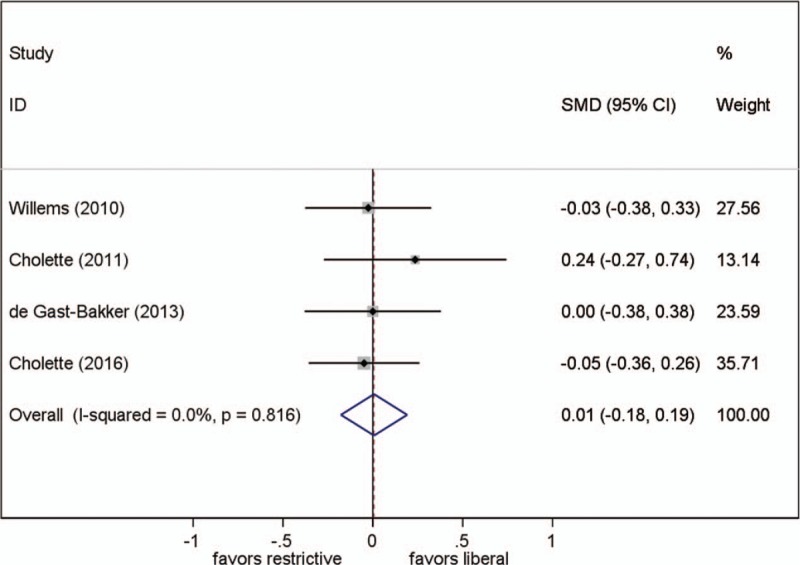
Effect of RBC transfusion thresholds on length of intensive care unit stay. SMD = standardized mean difference.
3.5. Length of hospital stay
Three studies reported this parameter. One study reported this parameter to be 1 day shorter in the restrictive group than in the liberal group,[24] while the other 2 reported no difference between the groups. Different transfusion strategies had no statistically significant effect on the length of hospital stay (SMD, −0.062; 95% CI, −0.28–0.15; P = .57; test for heterogeneity P = .51; I2 = 0%) (Fig. 3).
Figure 3.
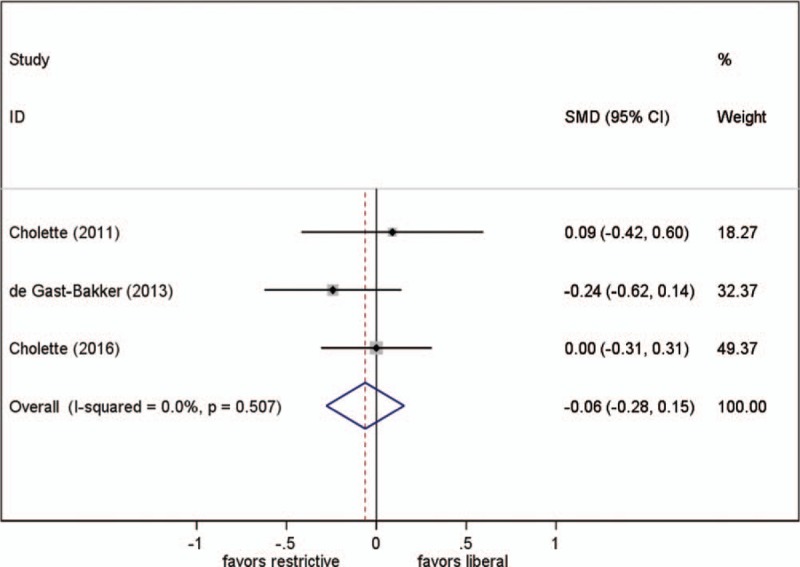
Effect of RBC transfusion thresholds on length of hospital stay. SMD = standardized mean difference.
3.6. Ventilation duration
Three studies reported this parameter. Different transfusion strategies had no statistically significant effect on ventilation duration (SMD, −0.015; 95% CI, −0.25–0.22; P = .90; test for heterogeneity P = .40; I2 = 0%) (Fig. 4).
Figure 4.
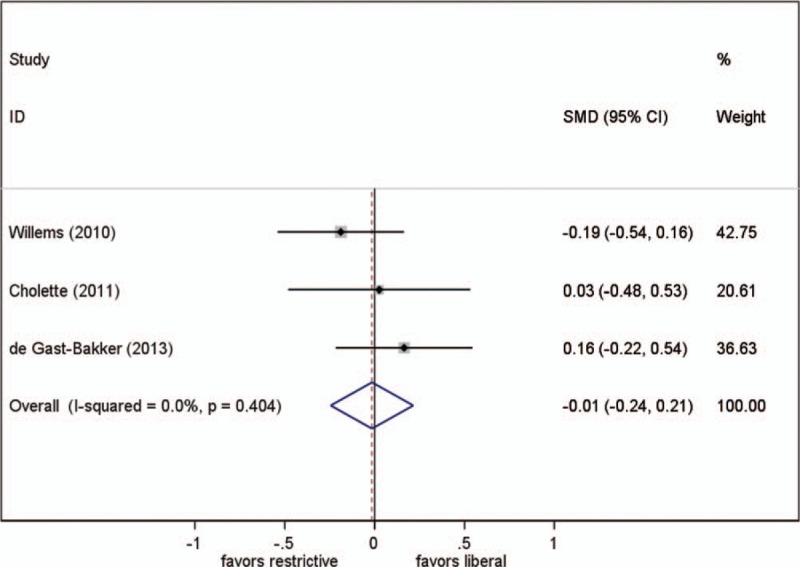
Effect of RBC transfusion thresholds on ventilator duration. SMD = standardized mean difference.
3.7. Mean arterial lactate level
Three studies reported this parameter, but we could only obtain original data from 2 of them. Different transfusion strategies had no statistically significant effect on the mean arterial lactate level (SMD, 0.071; 95% CI, −0.22–0.36; P = .63; test for heterogeneity P = .74; I2 = 0%) (Fig. 5).
Figure 5.
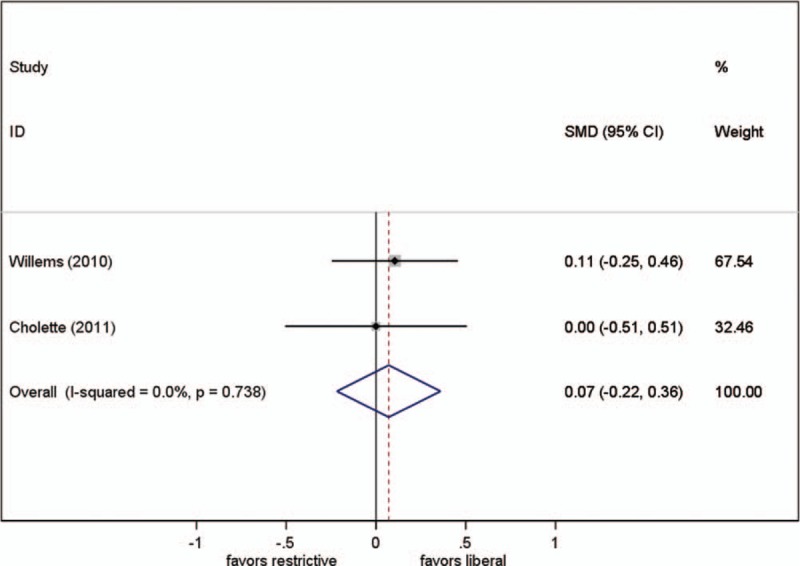
Effect of RBC transfusion thresholds on mean lactate. SMD = standardized mean difference.
3.8. Mortality
Three studies reported this parameter. Different transfusion strategies had no statistically significant effect on mortality when data was pooled from the 3 trials (RR, 0.49; 95% CI, 0.13–1.94; P = .31; test for heterogeneity P = .63; I2 = 0%) (Fig. 6).
Figure 6.
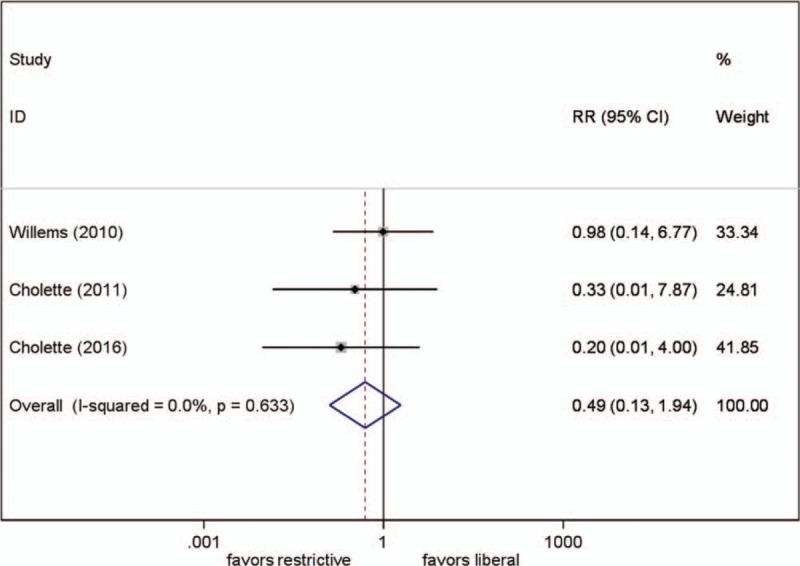
Effect of RBC transfusion thresholds on mortality. RR = relative risk.
We did not pool analyzed frequencies of transfusion[23,24,26] or volumes of transfusion[23–25] because of variety and incompleteness of data. Nevertheless, all the studies showed a decreased transfusion need in the restrictive group.
Other parameters including surrogate measures of oxygen delivery, specifically the arteriovenous and arteriocerebral oxygen content differences, were reported in only 2 studies and were not pooled due to scarcity of data.
Publication bias was tested using Egger, Begg, or trim-and-fill tests. Egger test (P = .03) for ICU stay differed from Begg test (P = .08), but the trim-and-fill test showed no statistical difference. Only Egger test showed no statistical difference in the other 4 parameters (P = .8).
4. Discussion
Although traditional narrative reviews have been published, this review is the first exclusive meta-analysis on postoperative RBC transfusion in pediatric cardiac surgery. In this systematic review and meta-analysis of studies on thresholds in RBC transfusion after pediatric cardiac surgery, restrictive transfusion strategy resulted in less frequency of RBC transfusion with noninferior biochemical and clinical outcomes than liberal transfusion strategy.
In adults, restrictive strategy can result in significant cost savings[28] and decreased incidence of adverse events secondary to transfusion.[29] The rationale for restrictive transfusion strategy has previously been extensively discussed.[30,31] Anemia often translates to increased risk of acute kidney injury and increased incidence of early and late mortality,[32,33] and the association between mortality/morbidity and increased transfusion has been confirmed by several studies.[34–36] A recent meta-analysis showed that findings favoring restrictive strategy were largely based on observational studies, but RCTs in cardiac surgery refute these findings.[6] Furthermore, although studies have shown that RBC transfusions following cardiac surgery in pediatric populations are associated with worse clinical outcomes, the studies were mainly observational studies, which makes their findings less credible.[37–41] In congenital cardiac patients, the practice of RBC transfusion greatly varies depending on the diagnosis/morphologic characteristics, procedure, and institution. Because of heterogeneity and small case volume, studies on surgical blood management in pediatric cardiac surgery are scarce compared to similar studies in adult cardiac surgery.
Unlike in adults, age and weight varied significantly in pediatric patients, with a tendency for increased transfusion demand in younger patients undergoing congenital heart surgery due to their comparatively higher susceptibility to anemic insult.[42] There were also wide intra-study and inter-study age varieties in the 4 original studies included. Furthermore, there is a wide range of diseases and procedures in this subspecialty, and patients can be categorized as cyanotic or acyanotic after surgery. These 2 patient groups are considered to have different hemoglobin needs after surgery.[43,44] One study included both cyanotic and acyanotic patients, while the other studies included only 1 of the patient groups. The mixture of patient groups may have made the conclusion less definitive. In considering the complexity of patient conditions, 1 study only included acyanotic patients older in age than neonates.[24] There were no patients in the Risk Adjustment for Congenital Heart Surgery (RACHS-1) category 4 and the mean RACHS-1 score was lower than that in the other studies.[45] As the differences between the 2 transfusion strategies have been regarded as most prominent in critically ill patients, this may explain the finding of a shorter length of hospital stay in the restrictive transfusion group than in the liberal transfusion group in the de Gast-Bakker study,[24] while no difference was found in the other studies. The 4 studies also used different thresholds to inform a transfusion decision, which was due to the cyanotic status of individual patients and inter-institutional protocol variances. It is difficult to draw a conclusion on the optimal RBC transfusion threshold since the data included is limited and heterogeneous.
Critically ill patients are the most susceptible patients after cardiac surgery. As earlier mentioned, there were no RACHS-1 category 4 patients in 1 study,[24] and there were only few patients with the most complex defects and highest risk in the other studies. Future studies should focus on this subgroup of pediatric patients and involve more neonates with higher RACHS-1 scores to find out if the 2 transfusion strategies lead to different outcomes.
A recent study[46] on cerebral oxygen metabolism before and after major surgery including congenital cardiac repair in infants raised the question of the impact of transfusion threshold on cerebral fractional tissue oxygen extraction and hence, neurodevelopment outcome. According to the study, as no previous studies had investigated cerebral metabolism and neurodevelopment, the non-inferiority of restrictive transfusion strategy should be interpreted with caution. It also stated that further studies were warranted.
Our review has strengths which include methods to minimize bias such as comprehensive literature search and duplicate data extraction. It also has limitations, particularly the limited data in this area of study. As expected, the number of studies that met the inclusion criteria was small and the studies were generally of low quality and had small sample sizes. Even though statistical heterogeneity in our meta-analysis was low, the significant variance in patient characteristics such as lesion type, weight, and age is concerning. To make our study more homogenous, we considered postoperative studies only, but this further reduced the number of studies included. The outcomes analyzed in the original studies were not always consistent and data output was not universal, which made the statistical results less conclusive. In addition, cerebral metabolism and neurodevelopment were omitted in all the included studies. Therefore, more multicenter clinical RCTs are necessary to confirm the results and define a clear threshold for RBC transfusion in pediatric cardiac patients.
5. Conclusion
This systematic review and meta-analysis showed that restrictive RBC transfusion strategy is at least as safe as liberal RBC transfusion strategy. The studies included were of reasonable quality even though they were limited in number. The optimal RBC transfusion thresholds are yet to be defined.
Author contributions
XD conceived the study. YW, XD carried out the literature search. PH, JL, XD and YX wrote the manuscript. XD and JQ performed the statistical analysis. XD, YX and GY revised and finalized the manuscript. All authors have reviewed the final manuscript and approved it.
Conceptualization: Xicheng Deng, Yefeng Wang.
Data curation: Xicheng Deng.
Investigation: Xicheng Deng, Yefeng Wang.
Writing – original draft: Xicheng Deng, Peng Huang.
Writing – review & editing: Xicheng Deng, Yefeng Wang, Jun Qiu, Peng Huang, Jinwen Luo, Yunbin Xiao, Guangxian Yang.
Footnotes
Abbreviations: CI = confidence interval, ICU = intensive care unit, RACHS-1 = risk adjustment for congenital heart surgery, RBC = red blood cell, RCTs = randomized controlled trials, RR = risk ratio, SD = standard deviation, SMD = standardized mean difference.
The study was supported by the following funds: Health and Family Planning Commission Research foundation of Hunan Province (No. B2013-106), Institutional fund of Hunan Children's Hospital.
The authors have no conflicts of interest to disclose.
References
- [1].Ferraris VA, Ferraris SP, Saha SP, et al. Society of Thoracic Surgeons Blood Conservation Guideline Task Force. Perioperative blood transfusion and blood conservation in cardiac surgery: the Society of Thoracic Surgeons and The Society of Cardiovascular Anesthesiologists clinical practice guideline. Ann Thorac Surg 2007;83(5 Suppl):S27–86. [DOI] [PubMed] [Google Scholar]
- [2].Bennett-Guerrero E, Zhao Y, O’Brien SM, et al. Variation in use of blood transfusion in coronary artery bypass graft surgery. JAMA 2010;304:1568–75. [DOI] [PubMed] [Google Scholar]
- [3].Carson JL, Duff A, Poses RM, et al. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet 1996;348:1055–60. [DOI] [PubMed] [Google Scholar]
- [4].Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Criti Care Med 2008;36:2667–74. [DOI] [PubMed] [Google Scholar]
- [5].Leal-Noval SR, Rincon-Ferrari MD, Garcia-Curiel A, et al. Transfusion of blood components and postoperative infection in patients undergoing cardiac surgery. Chest 2001;119:1461–8. [DOI] [PubMed] [Google Scholar]
- [6].Patel NN, Avlonitis VS, Jones HE, et al. Indications for red blood cell transfusion in cardiac surgery: a systematic review and meta-analysis. Lancet Haematol 2015;2:e543–53. [DOI] [PubMed] [Google Scholar]
- [7].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Khan Z, Natarajan G, Sallaam S, et al. Association between anemia and packed cell transfusion and outcomes of ventricular septal defect and atrioventricular canal repair in children. Pediatr Cardiol 2014;35:471–8. [DOI] [PubMed] [Google Scholar]
- [11].Dasgupta R, Parsons A, McClelland S, et al. Association of haematocrit and red blood cell transfusion with outcomes in infants with shunt-dependent pulmonary blood flow and univentricular physiology. Blood Transfus 2015;13:417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kipps AK, Wypij D, Thiagarajan RR, et al. Blood transfusion is associated with prolonged duration of mechanical ventilation in infants undergoing reparative cardiac surgery. Pediatr Crit Care Med 2011;12:52–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mazine A, Rached-D’Astous S, Ducruet T, et al. Blood transfusions after pediatric cardiac operations: a North American multicenter prospective study. Ann Thorac Surg 2015;100:671–7. [DOI] [PubMed] [Google Scholar]
- [14].Whitney G, Daves S, Hughes A, et al. Implementation of a transfusion algorithm to reduce blood product utilization in pediatric cardiac surgery. Paediatr Anaesth 2013;23:639–46. [DOI] [PubMed] [Google Scholar]
- [15].Howard-Quijano K, Schwarzenberger JC, Scovotti JC, et al. Increased red blood cell transfusions are associated with worsening outcomes in pediatric heart transplant patients. Anesth Analg 2013;116:1295–308. [DOI] [PubMed] [Google Scholar]
- [16].Chkhaidze M, Metreveli I, Tsintsadze A. Comparison of two RBC transfusion strategies in pediatric cardiac surgery patients. Eur J Anaesth (serial on the Internet) 2014;31Available from: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/777/CN-01023777/frame.html. Accessed February 13, 2019. [Google Scholar]
- [17].Redlin M, Boettcher W, Kukucka M, et al. Blood transfusion during versus after cardiopulmonary bypass is associated with postoperative morbidity in neonates undergoing cardiac surgery. Perfusion (United Kingdom) 2014;29:327–32. [DOI] [PubMed] [Google Scholar]
- [18].Gunaydin S, McCusker K, Vijay V. Perioperative blood conservation strategies in pediatric patients undergoing open-heart surgery: impact of non-autologous blood transfusion and surface-coated extracorporeal circuits. Perfusion 2011;26:199–205. [DOI] [PubMed] [Google Scholar]
- [19].Willems A, Van Lerberghe C, Gonsette K, et al. The indication for perioperative red blood cell transfusions is a predictive risk factor for severe postoperative morbidity and mortality in children undergoing cardiac surgery. Eur J Cardio-Thorac Surg 2014;45:1050–7. [DOI] [PubMed] [Google Scholar]
- [20].Kwak JG, Park MK, Lee JK, et al. Multiple approaches to minimize transfusions for pediatric patients in open-heart surgery. Cardiology 2016;37:44–9. [DOI] [PubMed] [Google Scholar]
- [21].Mulaj M, Faraoni D, Willems A, et al. Predictive factors for red blood cell transfusion in children undergoing noncomplex cardiac surgery. Ann Thorac Surg 2014;98:662–7. [DOI] [PubMed] [Google Scholar]
- [22].Machovec KA, Smigla G, Ames WA, et al. Reduction in blood transfusion in a cohort of infants having cardiac surgery with cardiopulmonary bypass after instituting a goal-directed transfusion policy. Perfusion 2016;31:598–603. [DOI] [PubMed] [Google Scholar]
- [23].Cholette JM, Swartz MF, Rubenstein J, et al. Outcomes using a conservative versus liberal red blood cell transfusion strategy in infants requiring cardiac operation. Ann Thorac Surg 2017;103:206–14. [DOI] [PubMed] [Google Scholar]
- [24].De Gast-Bakker DH, De Wilde RBP, Hazekamp MG, et al. Safety and effects of two red blood cell transfusion strategies in pediatric cardiac surgery patients: A randomized controlled trial. Intensive Care Med 2013;39:2011–9. [DOI] [PubMed] [Google Scholar]
- [25].Willems A, Harrington K, Lacroix J, et al. Comparison of two red-cell transfusion strategies after pediatric cardiac surgery: a subgroup analysis. Crit Care Med 2010;38:649–56. [DOI] [PubMed] [Google Scholar]
- [26].Cholette JM, Rubenstein JS, Alfieris GM, et al. Children with single-ventricle physiology do not benefit from higher hemoglobin levels post cavopulmonary connection: results of a prospective, randomized, controlled trial of a restrictive versus liberal red-cell transfusion strategy. Pediatr Crit Care Med 2011;12:39–45. [DOI] [PubMed] [Google Scholar]
- [27].Lacroix J, Hebert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med 2007;356:1609–19. [DOI] [PubMed] [Google Scholar]
- [28].Shander A, Hofmann A, Ozawa S, et al. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion 2010;50:753–65. [DOI] [PubMed] [Google Scholar]
- [29].Murphy WG. Disease transmission by blood products: past, present and future. Pathophysiol Haemost Thromb 2002;32suppl 1:1–4. [DOI] [PubMed] [Google Scholar]
- [30].Napolitano LM, Kurek S, Luchette FA, et al. Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. Crit Care Med 2009;37:3124–57. [DOI] [PubMed] [Google Scholar]
- [31].Task F, Ferraris VA, Brown JR, et al. Society of Thoracic Surgeons Blood Conservation Guideline. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg 2011;91:944–82. [DOI] [PubMed] [Google Scholar]
- [32].van Straten AH, Hamad MA, van Zundert AJ, et al. Preoperative hemoglobin level as a predictor of survival after coronary artery bypass grafting: a comparison with the matched general population. Circulation 2009;120:118–25. [DOI] [PubMed] [Google Scholar]
- [33].Klein AA, Collier TJ, Brar MS, et al. The incidence and importance of anaemia in patients undergoing cardiac surgery in the UK - the first Association of Cardiothoracic Anaesthetists national audit. Anaesthesia 2016;71:627–35. [DOI] [PubMed] [Google Scholar]
- [34].Ranucci M, Pazzaglia A, Bianchini C, et al. Body size, gender, and transfusions as determinants of outcome after coronary operations. Ann Thorac Surg 2008;85:481–6. [DOI] [PubMed] [Google Scholar]
- [35].Murphy GJ, Reeves BC, Rogers CA, et al. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation 2007;116:2544–52. [DOI] [PubMed] [Google Scholar]
- [36].Habib RH, Zacharias A, Schwann TA, et al. Role of hemodilutional anemia and transfusion during cardiopulmonary bypass in renal injury after coronary revascularization: implications on operative outcome. Crit Care Med 2005;33:1749–56. [DOI] [PubMed] [Google Scholar]
- [37].Du Pont-Thibodeau G, Harrington K, Lacroix J. Anemia and red blood cell transfusion in critically ill cardiac patients. Ann Intensive Care 2014;4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Costello JM, Graham DA, Morrow DF, et al. Risk factors for surgical site infection after cardiac surgery in children. Ann Thorac Surg 2010;89:1833–41. discussion 41-2. [DOI] [PubMed] [Google Scholar]
- [39].Kneyber MC, Grotenhuis F, Berger RF, et al. Transfusion of leukocyte-depleted RBCs is independently associated with increased morbidity after pediatric cardiac surgery. Pediatr Crit Care Med 2013;14:298–305. [DOI] [PubMed] [Google Scholar]
- [40].Salvin JW, Scheurer MA, Laussen PC, et al. Blood transfusion after pediatric cardiac surgery is associated with prolonged hospital stay. Ann Thorac Surg 2011;91:204–10. [DOI] [PubMed] [Google Scholar]
- [41].Szekely A, Cserep Z, Sapi E, et al. Risks and predictors of blood transfusion in pediatric patients undergoing open heart operations. Ann Thorac Surg 2009;87:187–97. [DOI] [PubMed] [Google Scholar]
- [42].Parker RI. Transfusion in critically ill children: indications, risks, and challenges. Crit Care Med 2014;42:675–90. [DOI] [PubMed] [Google Scholar]
- [43].Demaret P, Tucci M, Ducruet T, et al. Red blood cell transfusion in critically ill children (CME). Transfusion 2014;54:365–75. quiz 64. [DOI] [PubMed] [Google Scholar]
- [44].Tremblay-Roy JS, Poirier N, Ducruet T, et al. Red blood cell transfusion in the postoperative care of pediatric cardiac surgery: survey on stated practice. Pediatr Cardiol 2016;37:1266–73. [DOI] [PubMed] [Google Scholar]
- [45].Jenkins KJ, Gauvreau K. Center-specific differences in mortality: preliminary analyses using the Risk Adjustment in Congenital Heart Surgery (RACHS-1) method. J Thorac Cardiovasc Surg 2002;124:97–104. [DOI] [PubMed] [Google Scholar]
- [46].Neunhoeffer F, Hofbeck M, Schuhmann MU, et al. Cerebral oxygen metabolism before and after RBC transfusion in infants following major surgical procedures. Pediatr Crit Care Med 2018;19:318–27. [DOI] [PubMed] [Google Scholar]


