Abstract
The relevance of LP(a), Hcy, and D-D in ischemic cerebrovascular disease remains undefined. This study aimed to assess the associations of plasma LP(a), Hcy and D-D levels with the subtype of ischemic cerebrovascular disease.
Patients with ischemic cerebrovascular disease admitted to the Taixing People's Hospital were retrospectively enrolled from November 2017 to July 2018. Immunoturbidimetry was used to assess 119 LAA, 107 SAO, and 112 TIA patients for plasma LP(a), Hcy, and D-D levels.
Plasma LP(a), Hcy, and D-D levels in the large artery atherosclerosis (LAA) group were significantly lower than those of the transient ischemic attack (TIA) group (all P < .05). LP(a), Hcy, and D-D levels were significantly reduced in the SAO group compared with the TIA group (both P < .05). The LAA and SAO groups showed comparable values for all the above parameters (P > .05).
LP(a), Hcy, and D-D levels differ according to the subtype of ischemic cerebrovascular disease.
Keywords: D-D, homocysteine, ischemic stroke, lipoprotein(a)
1. Introduction
Ischemic cerebrovascular disease is a common ailment that seriously threatens the physical and mental health of elderly people, accounting for 75% to 85% of all stroke cases.[1] In China, 2.5 million new stroke cases are diagnosed yearly, with a total of 7.5 million stroke survivors.[2] The high morbidity, mortality and disability rates of this devastating disease impose a heavy burden on the patients, families, and the society at large.[3]
Ischemic cerebrovascular disease is a general term used for an ailment that involves a variety of pathological and risk factors, which can result in different clinical types, severity, and clinical outcomes.[4] Transient ischemic attack (TIA), large artery atherosclerosis (LAA), and small artery occlusion (SAO) cerebral infarction in the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification are the most common subtypes.[5–7] According to the above classification, TIA represents a short duration dysfunction in a specific brain region; LAA cases show paraclinical brain imaging findings of either significant (>50%) stenosis or occlusion of large arteries or their major branches, which is likely associated with atherosclerosis; SAO, or lacunar stroke, is characterized by one of the traditional clinical lacunar syndromes with HR-MRI findings unremarkable or showing a relevant lesion with a diameter below 1.5 cm.
In recent years, many studies have shown that Hcy is closely related to the occurrence and development of stroke; in addition, high Hcy levels in serum is one of the independent risk factors for stroke.[8,9] Meanwhile, D-D, a specific degradation product formed after hydrolysis of cross-linked fibrin protease, is a promising biomarker for the identification of venous thromboembolism in stroke cases.[10] Another factor implicated in stroke is LP(a), a low density lipoprotein strongly inducing thrombosis.[11] Despite the available wealth of knowledge regarding the associations of LP(a), Hcy and D-D with stroke in China and other countries,[8–11] few studies have assessed the associations of these factors with stroke subtype. Therefore, the purpose of the current study was to assess the associations of plasma LP(a), Hcy, and D-D levels with ischemic cerebrovascular disease subtypes, including TIA, LAA, and SAO.
2. Methods
2.1. Patients
Patients with ischemic cerebrovascular disease (TIA, LAA, and SAO) admitted to the Taixing People's Hospital were retrospectively enrolled from November 2017 to July 2018. The diagnosis of TIA was based on the updated Chinese expert consensus on transient ischemic attack in 2011.[12] LAA and SAO were diagnosed according to the revised diagnostic criteria of the 4th National Conference on Cerebrovascular Diseases.[13] Cerebral infarction cases were classified according to the TOAST criteria.[7]
Inclusion criteria were:
-
1.
acute onset, with disease course less than 14 days;
-
2.
CT/MR diagnosis of acute cerebral infarction, with no history of cerebrovascular disease or trauma within 6 months.
Exclusion criteria were:
-
1.
use within 1 month of drugs which may affect plasma Hcy, including antiepileptic drugs (carbamazepine, phenytoin), folic acid, folic acid antagonists (methotrexate), vitamin B6, vitamin B12;
-
2.
history of infection, such as respiratory and urinary system infections, within 2 weeks;
-
3.
comorbidity of autoimmune diseases, hematological diseases, or malignant tumors;
-
4.
serious heart, liver and/or kidney diseases;
-
5.
use of other drugs, such as aspirin, clopidogrel, anticoagulant drugs, immunosuppressants, anti-inflammatory drugs, and hormones, within 1 month before admission;
-
6.
diagnosis of cardioembolism (one of the TOAST types).
The study had approval from the ethics committee of the Taixing People's Hospital (No. TPH201707).
2.2. Clinical data collection
History of hypertension, diabetes mellitus, smoking and drinking, blood biochemistry and coagulation function data, and cranial CT and/or magnetic resonance imaging (MRI), ultrasound cardiogram, electrocardiogram, carotid artery ultrasound, and transcranial Doppler (TCD) findings were collected.
2.3. Blood sample collection and laboratory examination methods
The subjects were examined at 7:30 am, and 3 to 4 ml of fasting venous blood was collected; high protein or high fat diet was prohibited before blood collection. After 2 hours at room temperature, blood samples were centrifuged for 10 minutes at 2000 r/minutes for plasma preparation. Then, the plasma levels of LP(a), Hcy, and D-D in the TIA, LAA, and SAO groups were measured by immunoturbidimetry within 2 hours.
Hcy and LP(a) were assessed on a biochemical analyzer (Beckmann Kurt AU5800) with specific kits (Ningbo Meikang Biotechnology Co., Ltd). Hcy amounts ≥ 15 μmol/L were considered to be elevated. LP(a) levels ≥300 mg/L were considered to be elevated.
D-D was quantitated on an ACL-TOP-700 automated coagulation analyzer with a specific kit (Instrumentation Laboratory, USA), and amounts ≥500 mg/L were considered to be elevated.
2.4. Statistical analysis
SPSS23.0 (SPSS, USA) was used for all statistical analyses. Continuous variables with normal distribution were analyzed by one-way analysis of variance (ANOVA) and post hoc test. Continuous variables with skewed distribution were assessed by the Kruskal–Wallis test. Classification variables were expressed as frequency and percentage, and compared by the Chi-Square test. P < .05 was considered statistically significant.
3. Results
3.1. Patient baseline characteristics
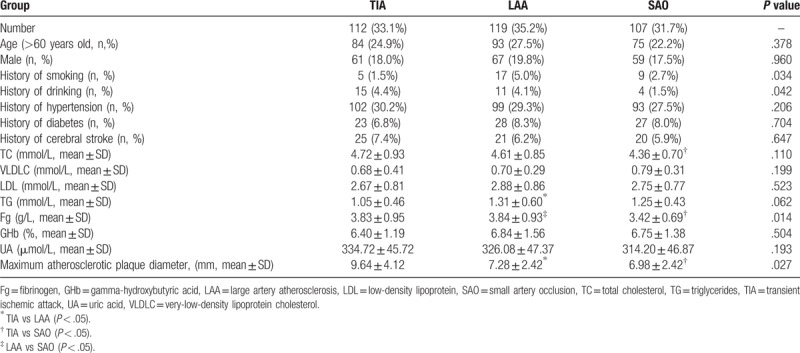
A total of 338 patients with cerebral infarction were assessed. There were 112 (33.1%) TIA cases, of whom 61 (18.0%) and 84 (24.9%) were male and older than 60 years, respectively; there were 119 (35.2%) LAA cases, with 67 (19.8%) males, and 93 (27.5%) individuals older than 60; the remaining 107 (31.7%) cases had SAO, and included 59 (17.5%) males and 75(22.2%) patients above 60 years old. There were no significant differences in age and gender distributions. Among the baseline characteristics assessed, only a few showed differences among the 3 groups (Table 1). For example, TG levels were markedly elevated in the LAA group, while maximum atherosclerotic plaque diameter was significantly reduced, compared with the TIA group; meanwhile, more patients had a history of TC, and Fg levels and maximum atherosclerotic plaque diameter were significantly reduced in the SAO group compared with the TIA group. Although the LAA and SAO groups had similar values for all parameters, Fg was markedly reduced in the latter group (Table 1).
Table 1.
Basic characteristics for different types of ischemic cerebrovascular disease in 338 patients (n, %).
3.2. Plasma levels of Hcy, D-D, and LP(a) in various subtypes of stroke
Plasma Hcy levels were 17.05 ± 5.36, 14.39 ± 5.22, and 13.54 ± 3.72 in the TIA, LAA, and SAO groups, respectively (P = .000) (Table 2 and Fig. 1A). In group pair analyses, Hcy levels in the TIA group were significantly higher than those of the LAA group (P = .002), and Hcy levels in the SAO group were significantly lower than those of the TIA group (P = .000). No significant difference was found between the LAA and SAO groups (P = .337).
Table 2.
Levels of 3 plasma biomarkers in different types of ischemic cerebrovascular disease.

Figure 1.
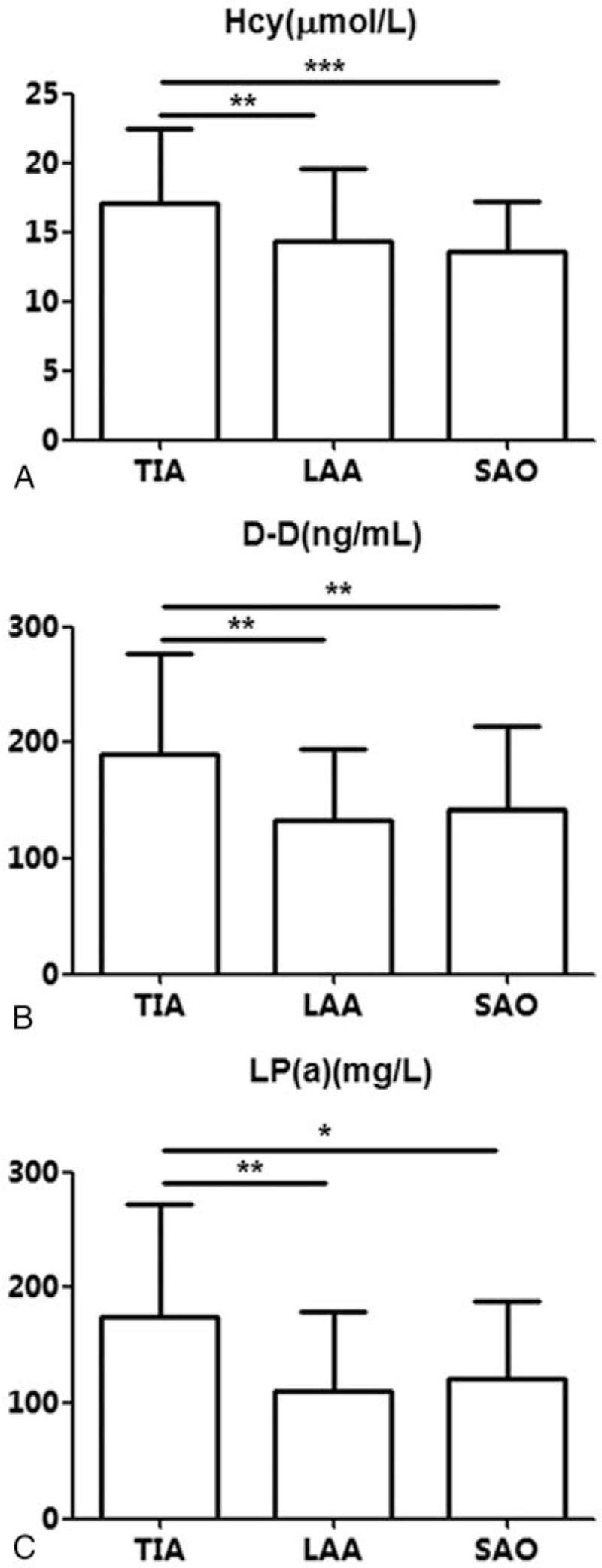
Plasma levels of homocysteine, D-dimer and lipoprotein (a) in various subtypes of stroke. A. homocysteine (Hcy); B. D-dimer (D-D); C. lipoprotein (a) [LP(a)]. ∗P < .05.
Plasma D-D levels were 189.66 ± 86.90, 132.50 ± 61.29, and 142.19 ± 71.80 in the TIA, LAA, and SAO groups, respectively, indicating statistically significant differences (P = .001) (Table 2 and Fig. 1B). Group pair analyses showed that plasma D-D levels were significantly lower in the LAA (P = .001) and SAO (P = .003) groups compared with the TIA group; there was no significant difference in plasma D-D levels between the LAA and SAO groups (P = .565).
Plasma LP(a) levels were 174.61 ± 98.11, 109.76 ± 69.14, and 120.52 ± 68.44 in the TIA, LAA, and SAO groups, respectively (P = .007), indicating statistically significant differences (Table 2 and Fig. 1C). Group pair analyses showed that plasma LP(a) levels were significantly lower in the LAA (P = .003) and SAO (P = .016) groups compared with the TIA group; there was no significant difference in plasma LP(a) levels between the LAA and SAO groups (P = .624).
4. Discussion
The current study demonstrated that LP(a), Hcy, and D-D levels differed in various ischemic cerebrovascular disease subtypes, with TIA clearly demarcated from the LAA and SAO subtypes.
Plasma LP(a), Hcy, and D-D levels are associated with arteriosclerosis and cerebrovascular diseases;[8–11] however, their correlations with ischemic cerebrovascular disease subtypes have been seldom assessed. Therefore, this study evaluated plasma LP(a), Hcy, and D-D levels in 338 patients with ischemic cerebrovascular disease in order to further understand the roles of these factors in different ischemic cerebrovascular disease types.
As shown above, plasma D-D levels in the TIA group were significantly higher than those of the LAA and SAO groups, in agreement with previous findings.[14] A significant increase of D-D in TIA patients also implies an enhanced activity of fibrinolytic enzyme or intravascular microthrombosis, indicating a pre-thrombotic state.[15,16] Detection of D-D level is of great significance for the assessment of thrombosis and thrombolytic therapy.
In addition, plasma Hcy levels in the TIA group were significantly higher than those of the LAA group and SAO group, indicating that Hcy might play a more important role in the occurrence and development of TIA. Previous studies suggested that microembolism might be important in the pathogenesis of TIA, and mainly results from unstable atherosclerotic plaque shedding in atherosclerosis.[17,18] Free radicals produced by Hcy oxidation are highly toxic to vascular endothelial cells, which could promote the oxidation of LDL and increase the synthesis of oxidized low density lipoprotein (OxLDL).[19]This may induce inflammation, accelerating atherosclerosis progression, and plaque instability.[20] In a word, hyperhomocysteinemia is closely related to occurrence of TIA.
Next, we found that LP(a) levels in TIA group were significantly higher than those of LAA and SAO groups. It is known that intima thickening of the internal carotid artery induced by atherosclerotic plaques is prone to ulcer formation, which promotes the adhesion of thrombin, fibrinogen, and platelets, leading to thrombosis.[21] The shed small blood clots form embolus in the brain, causing TIA.[22] Meanwhile, it was proposed that LP(a) increase is not associated with all stroke cases.[23] We believe that the determination of LP(a) levels in patients with TIA has important clinical significance for early intervention therapies and prevention of irreversible cerebral ischemia.
Smoking is an independent risk factor for ischemic stroke, increasing fibrin activation and altering thrombus configuration in patients with aorta atherosclerotic stroke.[24] In addition, cases of intracranial vasospasm caused by smoking have been reported.[25] As shown in Table 1, smoking rates in the LAA and SAO groups were higher than that of the TIA group, confirming a potential role for smoking in LAA and SAO. Meanwhile, we found that the rate of patients with drinking history was higher in TIA patients compared with the other subtypes. The role of drinking in stroke is well documented. For example, the association of alcohol intake with the incidence of ischemic stroke shows a J-shaped curve, with moderate drinkers at lowest risk, while abstainers and heavy drinkers have the highest risk.[26] Furthermore, the rate of hypercholesterolemia in TIA patients was relatively high compared with the other subtypes, corroborating the notion that it is closely related to TIA.[27] TG is another parameter that was increased in patients with TIA (Table 1). Meanwhile, fibrinogen levels differed in various subtypes of cerebral infarction. The highest fibrinogen levels were found in the LAA group. Elevated fibrinogen levels not only increase blood viscosity, but also are closely related to thrombosis, with the “bridging” effect of fibrinogen promoting erythrocyte aggregation.[28] These findings clearly demonstrated that various factors play different roles in the different subtypes of stroke.
The limitations of this study should be mentioned. First it had a retrospective nature, with inherent limitations, including potential selection bias. In addition, it was performed in a single center with a relatively small patient size. Finally, we did not provide mechanistic explanations of the differences observed. Therefore, additional studies addressing these issues should be performed to confirm our findings.
Overall, TIA patients had metabolic disorder of blood lipids, abnormal blood coagulation function, and hyperhomocysteinemia, indicating that LP(a), Hcy, and D-D may play a more important role in the pathogenesis of TIA compared with the other stroke subtypes. For people with high levels of plasma LP(a), Hcy, and D-D, they may be guided to better maintain their health based on their actual situations, such as quitting smoking, staying physically active, controlling weight, adjusting blood lipids, blood pressure, blood sugar, and so on. Medical intervention may be given when necessary, thereby helping to prevent TIA. For patients with TIA, and with high levels of plasma LP(a), Hcy, and D-D, especially, more attention should be paid to the monitoring of above 3 indicators and should be treated with aspirin, statins, vitamin B12, and so on. Early management of these high-risk factors would delay and prevent the onset of TIA. These findings have certain clinical significance in preventing and/or delaying stroke occurrence.
Author contributions
Conceptualization: Deqin Geng.
Investigation: Yong Tang.
Methodology: Yong Tang.
Writing – original draft: Yong Tang.
Writing – review & editing: Deqin Geng.
Footnotes
Abbreviations: D-D = D-dimer, Fg = fibrinogen, GHb = gamma-hydroxybutyric acid, Hcy = homocysteine, LAA = large artery atherosclerosis, LDL = low-density lipoprotein, LP(a) = lipoprotein (a), SAO = small artery occlusion, TC = total cholesterol, TG = triglycerides, TIA = transient ischemic attack, UA = uric acid, VLDLC = very-low-density lipoprotein cholesterol.
The authors have no conflicts of interest to disclose.
References
- [1].Musuka TD, Wilton SB, Traboulsi M, et al. Diagnosis and management of acute ischemic stroke: speed is critical. CMAJ 2015;187:887–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wu X, Zhu B, Fu L, et al. Prevalence, incidence, and mortality of stroke in the Chinese island populations: a systematic review. PLoS One 2013;8:e78629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Feigin VL, Krishnamurthi RV, Parmar P, et al. Update on the Global Burden of Ischemic and Hemorrhagic Stroke in 1990–2013: the GBD 2013 study. Neuroepidemiology 2015;45:161–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Soler EP, Ruiz VC. Epidemiology and risk factors of cerebral ischemia and ischemic heart diseases: similarities and differences. Curr Cardiol Rev 2010;6:138–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tan S, Zhang L, Chen X, et al. Comparison of the Chinese ischemic stroke subclassification and Trial of Org 10172 in acute stroke treatment systems in minor stroke. BMC Neurol 2016;16:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].de Paiva Bezerra R, de Miranda Alves MA, Conforto AB, et al. Etiological classification of stroke in patients with Chagas disease using TOAST, causative classification system TOAST, and ASCOD phenotyping. J Stroke Cerebrovasc Dis 2017;26:2864–9. [DOI] [PubMed] [Google Scholar]
- [7].Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- [8].Wu GH, Kong FZ, Dong XF, et al. Association between hyperhomocysteinemia and stroke with atherosclerosis and small artery occlusion depends on homocysteine metabolism-related vitamin levels in Chinese patients with normal renal function. Metab Brain Dis 2017;32:859–65. [DOI] [PubMed] [Google Scholar]
- [9].Anniwaer J, Liu MZ, Xue KD, et al. Homocysteine might increase the risk of recurrence in patients presenting with primary cerebral infarction. Int J Neurosci 2018;1–6. [DOI] [PubMed] [Google Scholar]
- [10].Zhang D, Li F, Du X, et al. Diagnostic accuracy of biomarker D-dimer in patients after stroke suspected from venous thromboembolism: a diagnostic meta-analysis. Clin Biochem 2018. [DOI] [PubMed] [Google Scholar]
- [11].Milionis HJ, Winder AF, Mikhailidis DP. Lipoprotein (a) and stroke. J Clin Pathol 2000;53:487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Liu LP, Xu AD, Wong LK, et al. Chinese consensus statement on the evaluation and intervention of collateral circulation for ischemic stroke. CNS Neurosci Ther 2014;20:202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang G, Cheng X, Zhang X. Use of various CT imaging methods for diagnosis of acute ischemic cerebrovascular disease. Neural Regen Res 2013;8:655–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yuan W, Shi ZH. The relationship between plasma D-dimer levels and outcome of Chinese acute ischemic stroke patients in different stroke subtypes. J Neural Transm (Vienna) 2014;121:409–13. [DOI] [PubMed] [Google Scholar]
- [15].Zhen LI, Cai L, Zhi WU. Effects of clopidogrel combined with aspirin on fibrinogen,D dimer and C reactive protein in patients with transient ischemic attack. Chin J Diff Compl Cases 2017. [Google Scholar]
- [16].Zhao ZQ, Lan HU, Cai MH. Prospective study on the relationship between D-dimer, fibrinogen and risk stratification of patients with transient ischemic attack. Chin J Arterioscler 2015. [Google Scholar]
- [17].Huang S, Yin L, Xu Y, et al. The homocysteine associated variant rs548987 of SLC17A3 confers susceptibility to ischemic stroke in Chinese population. J Neurol Sci 2016;370:78–81. [DOI] [PubMed] [Google Scholar]
- [18].Hankey GJ, Ford AH, Yi Q, et al. Effect of B vitamins and lowering homocysteine on cognitive impairment in patients with previous stroke or transient ischemic attack: a prespecified secondary analysis of a randomized, placebo-controlled trial and meta-analysis. Stroke 2013;44:2232–9. [DOI] [PubMed] [Google Scholar]
- [19].Singh RB, Mengi SA, Xu YJ, et al. Pathogenesis of atherosclerosis: a multifactorial process. Exp Clin Cardiol 2002;7:40–53. [PMC free article] [PubMed] [Google Scholar]
- [20].Gibson MS, Domingues N, Vieira OV. Lipid and non-lipid factors affecting macrophage dysfunction and inflammation in atherosclerosis. Front Physiol 2018;9:654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Badimon L, Padro T, Vilahur G. Atherosclerosis, platelets and thrombosis in acute ischaemic heart disease. Eur Heart J Acute Cardiovasc Care 2012;1:60–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tutwiler V, Peshkova AD, Andrianova IA, et al. Contraction of blood clots is impaired in acute ischemic stroke. Arterioscler Thromb Vasc Biol 2017;37:271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Siarnik P, Carnicka Z, Krivosikova Z, et al. Association of lipoprotein subfractions with endothelial function and arterial stiffness in acute ischemic stroke. Scand J Clin Lab Invest 2017;77:36–9. [DOI] [PubMed] [Google Scholar]
- [24].Krajcoviechova A, Wohlfahrt P, Mayer O, Jr, et al. Tobacco smoking strongly modifies the association of prothrombin G20210A with undetermined stroke: consecutive survivors and population-based controls. Atherosclerosis 2015;240:446–52. [DOI] [PubMed] [Google Scholar]
- [25].Siddiqui M, Noon MJ, Mehboob N, et al. Smokeless tobacco use and ischemic stroke: a cross-sectional study. Ann Glob Health 2016;82:768–9. [DOI] [PubMed] [Google Scholar]
- [26].Tang L, Xu T, Li H, et al. Hypertension, alcohol drinking and stroke incidence: a population-based prospective cohort study among inner Mongolians in China. J Hypertens 2014;32:1091–6. discussion 1096. [DOI] [PubMed] [Google Scholar]
- [27].Schulz UG, Rothwell PM. Differences in vascular risk factors between etiological subtypes of ischemic stroke: importance of population-based studies. Stroke 2003;34:2050–9. [DOI] [PubMed] [Google Scholar]
- [28].Ariens RA. Elevated fibrinogen causes thrombosis. Blood 2011;117:4687–8. [DOI] [PubMed] [Google Scholar]


