Abstract
Cell and animal experiments have found that in addition to being a retinol transporter, Stimulated by Retinoic Acid 6 (STRA6) also functions as a surface signaling receptor by which retinol regulates insulin responses. Several studies revealed that the STRA6 gene may contribute to the pathogenesis of type 2 diabetes mellitus (T2DM). Gestational diabetes mellitus (GDM) and T2DM have some risk factors in common. The present study was directed to investigate whether the 3 single nucleotide polymorphism (SNPs) (rs11633768, rs351219, and rs736118) of STRA6 correlate with the development of GDM in Chinese pregnant women. We also aimed to estimate the relationship between SNPs with fasting blood glucose level, 1-hour and 2-hour blood glucose levels after 75 g oral glucose intake, fasting insulin and insulin resistance levels to better study the relationship between STRA6 and glucose metabolism.
Case–control studies were conducted to compare the GDM and control groups. A total of 334 cases and 367 controls were recruited. Three tagSNPs of STRA6, rs11633768, rs351219, and rs736118, were selected. A chi-square test, logistic regression, and linear regression were used to estimate the relationship between SNPs with GDM risk and oral glucose tolerance test (OGTT), fasting insulin and homeostasis model assessment of insulin resistance (HOMA-IR) levels. Regression analyses were all adjusted by maternal age, pre-pregnancy BMI, and weekly BMI growth. The Bonferroni correction was applied for multiple comparisons.
After adjusting the maternal age, pre-pregnancy BMI and weekly BMI growth, STRA6 rs736118 was associated with fasting insulin level (Beta = −1.468, P = .036), and the association between rs736118 and HOMA-IR was of borderline significance (Beta = −0.290, P = .093) under the dominance model.
This study found that there is a significant association between STRA6 polymorphism and GDM.
Keywords: gestational diabetes mellitus, single nucleotide polymorphisms, stimulated by retinoic acid 6
1. Introduction
Gestational diabetes mellitus (GDM) is defined as glucose intolerance first detected during pregnancy.[1] The prevalence of GDM has been increasing in recent decades, moving from 1.7% to 11.6% among populations.[2] During pregnancy, insulin resistance is enhanced physiologically and can be further strengthened by some factors such as obesity, leading to a high risk of GDM.[3–5]
Retinol-binding protein 4 (RBP4) has been implicated as a driver of insulin resistance in rodents and humans.[6] RBP4, an adipocytokine, is mainly synthesized by hepatocytes and adipose tissues. An increase in serum RBP4 levels can induce hepatic expression of phosphoenolpyruvate carboxykinase, a gluconeogenic enzyme, to increase gluconeogenesis, and impair insulin signaling in muscles through decreasing the expression of phosphoinositide-3 kinase.[7] Further studies on the mechanism by which RBP4 can lead to insulin resistance showed that Stimulated by Retinoic Acid 6 (STRA6) played an important role. STRA6 is a membrane protein acting as a receptor for retinol-RBP4 complex (holo-RBP) to remove the retinol from the complex and transport it across the cell membrane.[8] Berry and colleagues found that the binding of holo-RBP to STRA6 results in recruitment of Janus Kinases 2 (JAK2) that, in turn, catalyze phosphorylation of a tyrosine residue in the cytosolic domain of STRA6, leading to recruitment and activation of the transcription factor Signal Transducers and Activators of Transcription 5 (STAT5).[9] The expression levels of cytokine signaling 3 (SOCS3) and peroxisome proliferator-activated receptor γ (PPARγ), 2 endogenous STAT target genes, can be upregulated by holo-RBP. PPARγ is a key regulator of adipose lipid storage. SOCS3 is a potent inhibitor of insulin receptor-mediated signaling. Treatment of adipocytes with holo-RBP inhibited insulin-induced activation of insulin receptor and insulin-induced mobilization of the glucose transporter GluT4 to plasma membranes. Berry et al found that holo-RBP induces the expression of STAT target genes and inhibits insulin signaling only in STRA6-expressing tissues in Vivo. These abovementioned findings establish that, in addition to being a retinol transporter, STRA6 also functions as a surface signaling receptor by which retinol regulates insulin responses.[9]
Many studies have focused on the association between RBP4 and GDM. A meta-analysis showed that high serum RBP4 levels represented a risk factor for GDM. The GDM diagnostic criteria affected the strength of association between RBP4 level and GDM risk. Adopting a higher threshold of oral glucose tolerance test (OGTT) would result in a larger difference of serum RBP4 level between GDM women and controls.[10]To date, several genetic variants that affect RBP4 expression levels (e.g., rs3758539 and rs12265684) have been investigated for their potential association with the risk of GDM.[11–13] However, to date, no studies have focused on the association between GDM and polymorphisms in STRA6, the only identified high-affinity receptor for RBP4. Nair et al found that the STRA6 rs974456 T allele, rs736118 A allele, and rs4886578 A allele were associated with a lower risk of type 2 diabetes mellitus (T2DM) in a south Indian population.[14] Huang et al analyzed the association of STRA6 rs974456, rs736118, rs4886578 and rs17173617 with T2DM in southern Han Chinese and verified the results of Nair et al on rs974456 and rs736118.[15] STRA6 may not only be associated with T2DM but also may be associated with the risk of GDM. The present study investigated whether the 3 SNPs (rs11633768, rs351219, and rs736118) of STRA6 correlate with the development of GDM in Chinese pregnant women. We also aimed to estimate the relationship between SNPs with fasting blood glucose level, 1-hour and 2-hour blood glucose levels after 75 g oral glucose intake, fasting insulin and insulin resistance levels to better study the relationship between STRA6 and glucose metabolism.
2. Methods
2.1. Ethics statement
The study protocol was reviewed and approved by the Central-South University's Ethical and Confidentiality Committee. All participants provided written informed consent. The authors assert that all procedures/methods were carried out in accordance with the approved guidelines.
2.2. Study design
The research population and most parts of the statistical methods of this study were consistent with one of our previous articles,[16] therefore, the same content was not repeated here. Briefly, this was a case–control study which enrolled pregnant women with GDM and pregnant women with normal glucose tolerance who visited prenatal clinics regularly and underwent OGTT from 24 to 28 weeks. The boundaries of OGTT were 5.1 mmol/L, 10.0 mmol/L, and 8.5 mmol/L for fasting glucose and 1 and 2 hours after 75 g oral glucose intake. When 1 or more OGTT indicators reached or exceeded the abovementioned boundaries, the pregnant woman was diagnosed with GDM. The following information was collected on the OGTT morning: maternal age, gestational age, parity, height, weight, fasting insulin levels, systolic blood pressure, and diastolic blood pressure. Homeostasis model assessment of insulin resistance (HOMA-IR) = fasting insulin (mIU/L)∗fasting blood glucose (mmol/L)/22.5. Weekly body mass index (BMI) growth = (BMI on the OGTT morning–pre-pregnancy BMI)/gestational age (weeks). A chi-square test, logistic regression, and linear regression were used to estimate the relationship between SNPs with GDM risk and OGTT, fasting insulin and HOMA-IR levels. Regression analyses were all adjusted by maternal age, pre-pregnancy BMI and weekly BMI growth. Three SNPs were included in the analysis; therefore, α was equal to 0.017 (0.017 = 0.05/3). The alleles, minor allele frequency (MAF) and SNPs covered by tagSNP are shown in Table 1. The primers for each SNP are shown in Table 2.
Table 1.
The information of selected SNPs.

Table 2.
Primers of the selected SNPs.

3. Results
3.1. Demographic and clinical characteristics
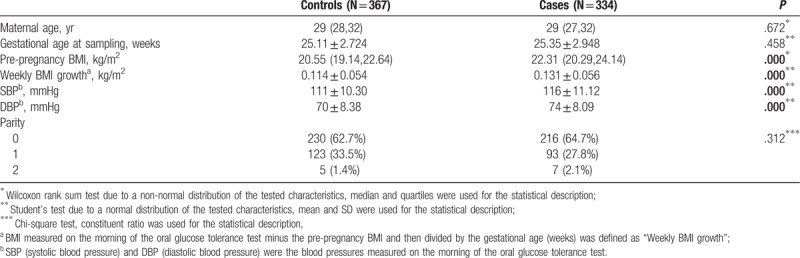
A total of 334 cases and 367 controls were analyzed. The clinical characteristics of cases and controls are summarized in Table 3. Compared with the control group, the case group had higher pre-pregnancy BMI (P <.001), larger weekly BMI growth (P <.001), higher systolic blood pressure (P <.001) and higher diastolic blood pressure (P <.001).
Table 3.
Demographic and clinical characteristics of the study subjects.
3.2. Test for Hardy–Weinberg equilibrium and LD analysis
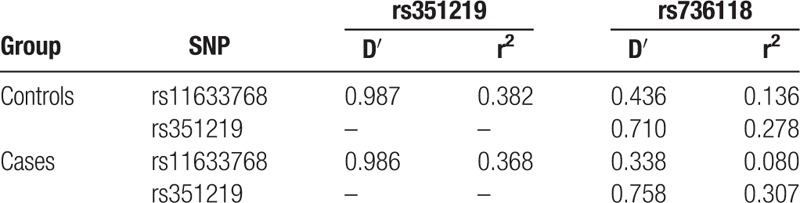
The SNP genotyping detection rate was 99.5%. For all SNPs, the Hardy–Weinberg equilibrium (HWE) was observed in the control group (Table 1). Pairwise linkage disequilibrium parameters (D’ and r2) were estimated for STRA6 rs11633768, rs351219, and rs736118 (Table 4).
Table 4.
Pair-wise linkage disequilibrium analyses of STRA6 rs11633768, rs351219 and rs736118.
3.3. Association between alleles and genotypes with GDM
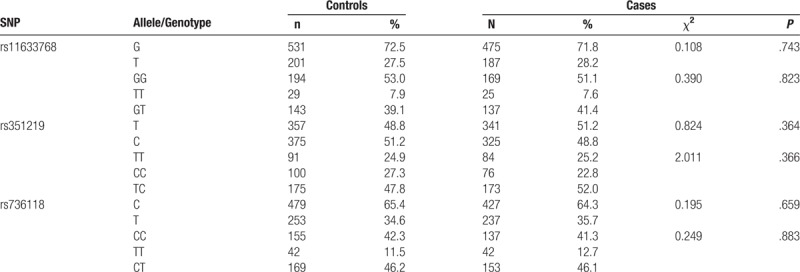
As shown in Table 5, no significant differences in the alleles and genotypes of STRA6 rs11633768, rs351219, and rs736118 were observed between cases and controls.
Table 5.
The distribution of alleles and genotypes of STRA6 rs11633768, rs351219 and rs736118.
3.4. Association between genetic models with GDM
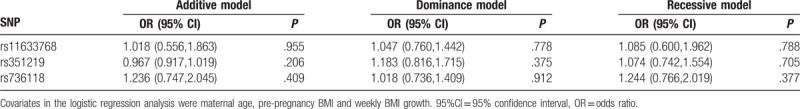
As shown in Table 6, after adjusting the maternal age, pre-pregnancy BMI and weekly BMI growth, the results of the logistic regression analysis revealed that comparing cases with controls, STRA6 rs11633768, rs351219, and rs736118 were not associated with GDM, regardless of additive model, dominant model, or recessive model comparisons.
Table 6.
Correlation analysis after the adjustment in 3 genetic models.
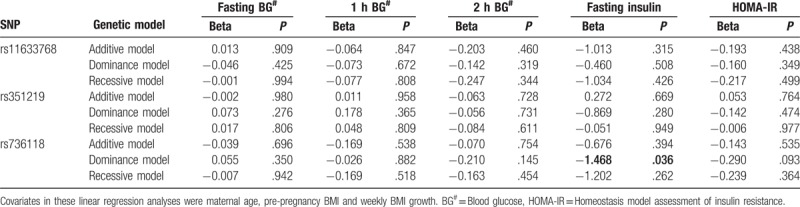
3.5. Association analysis of genetic variants in STRA6 with OGTT, fasting insulin and HOMA-IR levels
In addition to fasting blood glucose level, OGTT 1-hour blood glucose level and 2-hour blood glucose level, which constitute the diagnostic criteria for GDM, fasting insulin level and HOMA-IR are also important indicators for evaluating the level of glucose metabolism. To better study the relationship between STRA6 and the level of glucose metabolism, we analyzed the relationship between fasting insulin level and other continuous indicators with STRA6 rs11633768, rs351219, and rs736118. As shown in Table 7, after adjusting the maternal age, pre-pregnancy BMI, and weekly BMI growth, the results of the linear regression analysis revealed that under the dominance model, STRA6 rs736118 was associated with fasting insulin level (Beta = −1.468, P = .036), and the association between rs736118 and HOMA-IR was of borderline significance (Beta = −0.290, P = .093). However, after correction for multiple testing, the association did not remain statistically significant. We inferred that the STRA6 rs736118 T allele might protect Chinese pregnant women from GDM. No significant results were observed in the association analysis of rs11633768, rs351219 with OGTT, fasting insulin, and HOMA-IR.
Table 7.
The association of genetic variants in STRA6 with OGTT, fasting insulin and HOMA-IR levels.
4. Discussion
STRA6 was identified as a specific membrane receptor for RBP in 2007 by Kawaguchi and colleagues. STRA6 binds to RBP with high affinity and has robust vitamin A uptake activity from the vitamin A–RBP complex. It is widely expressed in embryonic development and in the adult brain, spleen, kidney, female genital tract, and testis (and at lower quantities in heart and lung).[8] Consistent with the expression of STRA6 and the diverse functions of vitamin A in embryonic development, mutations in STRA6 can cause a broad spectrum of malformations, including anophthalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary dysplasia, lung hypoplasia, and mental retardation.[17,18] In addition to being a retinol transporter, STRA6 also functions as a surface signaling receptor by which retinol regulates insulin responses. Binding of holo-RBP to STRA6 induces STRA6 phosphorylation, leading to the recruitment and activation of JAK2 and STAT5, then resulting in the upregulation of expression of STAT target genes, including suppressor of SOCS3, which inhibits cytokine signaling mediated by the JAK/STAT pathway, and PPARγ, which controls adipocyte lipid homeostasis.[9] Berry and colleagues found the mutations of STRA6 would disrupt the above signaling cascade. STRA6 variants lacking the SH2 domain-binding motif (Y643F, T644 M, ΔC) inhibited holo-RBP–induced STRA6/STAT5 phosphorylation and exerted dominant negative activities of the holo-RBP response of both SOCS3 and PPARγ.[9] Berry and colleagues generated and analyzed Stra6-null mice. They confirmed that ablation of STRA6 effectively protected mice from RBP-induced suppression of insulin signaling.[19]
In the present study, we analyzed the association between STRA6 rs11633768, rs351219, and rs736118 with GDM risk. We found STRA6 rs736118 was associated with fasting insulin level and inferred that the rs736118 T allele might protect Chinese pregnant women from GDM. The STRA6 gene is located on Chromosome 15q24.1. STRA6 rs736118 presents in exon 17. For the SNP of rs736118, the C→T variant will cause amino acid change from methionine to isoleucine. In addition, this SNP is located at the cytosolic C terminus of the protein, containing a docking site for the transcription factors STATs.[18] As mentioned above, SOCS3, an endogenous STAT target gene, is a potent inhibitor of insulin receptor-mediated signaling. To date, this study is the first study concerning the association between STRA6 variants and GDM risk. STRA6 rs736118 has been studied twice in the T2DM region. Nair et al. analyzed 2002 unrelated South Indian individuals (1002 cases with T2DM and 1000 normoglycemic control subjects) and found the STRA6 rs736118 T allele was associated with a lower risk of T2DM; after adjustment for maternal age, sex, and BMI, the association still existed.[14] Huang et al analyzed 571 T2DM patients and 632 normal control subjects and found the allele T of SNP rs736118 on STRA6 was significantly associated with a lower risk of T2DM, after adjustment for sex, BMI, and triglycerides.[15] To have better comparability with the previous results, we also adjusted BMI, including pre-pregnancy BMI and weekly BMI growth. In fact, doing so may have underestimated the impact of STRA6 on GDM risk. The STAT target gene PPARγ is a key regulator of adipose lipid storage.[20] Treatment of adipocytes with holo-RBP increased triglyceride accumulation by the cells and did so in a STRA6-dependent manner.[9] This evidence hinted that STRA6 may associate with BMI through its function in long-term lipid metabolism regulation. The association between BMI and GDM risk is obvious.[4,5] We inferred that BMI is one of the pathophysiological links between STRA6 and GDM. If BMI was not adjusted, STRA6 rs736118 was still associated with fasting insulin level (Beta = −1.533, P = .041), and the Beta value was greater than the value after adjustment. The results of HOMA-IR (Beta = −0.306) followed the same pattern.
To the best of our knowledge, this is the first study that has evaluated the relationship between genetic variants of STRA6 and GDM risk. However, the study has certain limitations. First, genetic susceptibility analysis only provides some hints about the association; the results need to be validated at other omics levels, such as proteomics. Second, the results need to be confirmed in other races and larger samples.
Author contributions
Conceptualization: Shimin Hu, Junxia Yan, Xun Li.
Data curation: Shimin Hu, Yiping You, Guilian Yang, Xin Liao.
Formal analysis: Xun Li, Xin Liao.
Funding acquisition: Hongzhuan Tan.
Investigation: Shimin Hu, Yiping You, Guilian Yang.
Methodology: Shimin Hu, Junxia Yan, Xun Li, Hongzhuan Tan.
Project administration: Yiping You, Guilian Yang, Hui Zhou.
Writing – original draft: Shimin Hu.
Writing – review & editing: Shimin Hu, Junxia Yan, Hui Zhou, Hongzhuan Tan.
Footnotes
Abbreviations: GDM = gestational diabetes mellitus, holo-RBP = retinol-RBP4 complex, HOMA-IR = homeostasis model assessment of insulin resistance, JAK2 = Janus Kinases 2, OGTT = oral glucose tolerance test, PPARγ = peroxisome proliferator-activated receptor γ, RBP4 = retinol binding protein 4, SNP = single nucleotide polymorphism, SOCS3 = cytokine signaling 3, STAT5 = Activators of Transcription 5, STRA6 = Stimulated by Retinoic Acid 6, T2DM = type 2 diabetes mellitus.
This work was supported by the National Natural Science Foundation of China (81373088 and 81773535) and the Central South University Postdoctoral Launch Foundation (175870). The funding source had no role in the study design, data collection, data analysis, data interpretation, or writing of this manuscript.
The authors have no conflicts of interest to disclose.
References
- [1].Buchanan TA, Xiang AH. Gestational diabetes mellitus. J Clin Invest 2005;115:485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Schneider S, Bock C, Wetzel M, et al. The prevalence of gestational diabetes in advanced economies. J Perinat Med 2012;40:511–20. [DOI] [PubMed] [Google Scholar]
- [3].Xiang AH, Takayanagi M, Black MH, et al. Longitudinal changes in insulin sensitivity and beta cell function between women with and without a history of gestational diabetes mellitus. Diabetologia 2013;56:2753–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Stupin JH, Arabin B. Overweight and obesity before, during and after pregnancy: part 1: pathophysiology, molecular biology and epigenetic consequences. Geburtshilfe Frauenheilkunde 2014;74:639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Athukorala C, Rumbold AR, Willson KJ, et al. The risk of adverse pregnancy outcomes in women who are overweight or obese. BMC Pregnancy Childbirth 2010;10:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gliniak CM, Brown JM, Noy N, et al. Receptor STRA6 regulates diurnal insulin responses. J Biol Chem 2017;292:15080–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yang Q, Graham TE, Mody N, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 2005;436:356–62. [DOI] [PubMed] [Google Scholar]
- [8].Kawaguchi R, Yu J, Honda J, et al. A membrane receptor for retinol binding protein mediates cellular uptake of vitamin A. Science 2007;315:820–5. [DOI] [PubMed] [Google Scholar]
- [9].Berry DC, Jin H, Majumdar A, et al. Signaling by vitamin A and retinol-binding protein regulates gene expression to inhibit insulin responses. Proc Natl Acad Sci U S A 2011;108:4340–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hu S, Liu Q, Huang X, et al. Serum level and polymorphisms of retinol-binding protein-4 and risk for gestational diabetes mellitus: a meta-analysis. BMC Pregnancy Childbirth 2016;16:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hiraoka M, Urschitz J, Sultan O, et al. A polymorphism in the retinol binding protein 4 gene is not associated with gestational diabetes mellitus in several different ethnic groups. Hawaii Med J 2011;70:164–7. [PMC free article] [PubMed] [Google Scholar]
- [12].Ping F, Xiang HD, Li M, et al. Effects of variation in retinol binding protein 4 gene and adipose specific expression of gestational diabetes in Beijing, China. Diabetes Res Clin Pract 2012;97:283–9. [DOI] [PubMed] [Google Scholar]
- [13].Saucedo R, Zarate A, Basurto L, et al. RBP4 gene variants are associated with insulin resistance in women with previous gestational diabetes. Dis Markers 2014;2014:269208–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nair AK, Sugunan D, Kumar H, et al. Case-control analysis of SNPs in GLUT4, RBP4 and STRA6: association of SNPs in STRA6 with type 2 diabetes in a South Indian population. PLoS One 2010;5:e11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Huang HW, Liang BY, Li YX. Association of polymorphisms in STRA6 and RARRES2 genes with type 2 diabetes in Southern Han Chinese. BioMed Res Int 2016;2016:6589793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hu S, Ma SJ, Li X, et al. Relationships of SLC2A4, RBP4, PCK1 and PI3K gene polymorphisms with gestational diabetes mellitus in a Chinese population. Biomed Res Int 2019;2019:7398063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pasutto F, Sticht H, Hammersen G, et al. Mutations in STRA6 cause a broad spectrum of malformations including anophthalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary dysplasia, lung hypoplasia, and mental retardation. Am J Hum Genet 2007;80:550–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].White T, Lu T, Metlapally R, et al. Identification of STRA6 and SKI sequence variants in patients with anophthalmia/microphthalmia. Mol Vis 2008;14:2458–65. [PMC free article] [PubMed] [Google Scholar]
- [19].Berry DC, Jacobs H, Marwarha G, et al. The STRA6 receptor is essential for retinol-binding protein-induced insulin resistance but not for maintaining vitamin A homeostasis in tissues other than the eye. J Biol Chem 2013;288:24528–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature 2000;405:421–4. [DOI] [PubMed] [Google Scholar]


