Abstract
To evaluate the clinical efficacy and safety of adenomyomectomy using “H” type incision combined with Mirena (LNG-IUS) in the treatment of adenomyosis.
A total of 57 women with adenomyosis who underwent adenomyomectomy using “H” type incision combined with LNG-IUS were selected. Visual analog scale (VAS), menstrual flow, uterine volume, serum CA125 levels and hemoglobin amounts were compared before and after the surgery. Meanwhile, postoperative pregnancy, adverse reactions, and recurrence were observed.
VAS score, menstrual flow, uterine volume, and serum CA125 levels in 53 patients were significantly reduced after surgery (P < . 001). Moreover, statistical significances were obtained for VAS score at 13 and 6 months, menstrual flow at 1, 3, 6, 12, and 24 months, uterine volume at 1, 3, 6, 12, 24, and 36 months and CA125 levels at 1 and 3 months (P < .05). Of the 5 patients with fertility requirements, 1 became pregnant after IVF-ET, progressed to preterm, and delivered healthy twins. Among all related adverse reactions, amenorrhea was the most common (n = 20, 37.7%). There were no cases of LNG-IUS removal, ectopia, expulsion, and incarceration, except in 2 patients due to pregnancy, 1 due to uterine bleeding, and 1 due to Mirena perforation from incision of the uterine fundus. All patients showed no relapse.
Adenomyomectomy using “H” type incision combined with Mirena constitutes a novel and effective conservative surgical procedure for adenomyosis treatment.
Keywords: adenomyomectomy using “H” type incision, adenomyosis, conservative surgery, Mirena
1. Introduction
Adenomyomectomy using “H” type incision combined with Mirena is a novel and effective conservative surgical procedure for adenomyosis treatment and has low postoperative recurrence rate. Adenomyosis is a common benign disorder, which usually occurs in reproductive-aged women between 30 and 45 years old, with the main symptoms of secondary progressive dysmenorrhea, menorrhagia, chronic pelvic pain, or infertility.[1] The incidence rate of adenomyosis, generally defined on the basis of hysterectomy specimens, is extremely variable (ranging between 10% and 66%) mainly because of the lack of widely accepted criteria for histopathological diagnosis.[2] The several available medical and surgical options are used for treating adenomyosis. Generally used medical approach to adenomyosis disease include gonadotropin-releasing hormone agonists (GnRH-a), levonorgestrel-releasing intrauterine device (LNG-IUD), oral contraceptive combined pill, progestogens, and danazol.[3–5] The principal limit of these medical options is that they induce regression but not eradication of the pathology, with symptom recurrence after drug discontinuation. Adenomyosis can be radially cured by traditional hysterectomy. However, conservative surgery, as an optional and effective treatment tool, has been constantly developed for the treatment of patients with mild and moderate adenomyosis after ineffective conservative treatment, especially young individuals with fertility requirements. It is currently possible to perform treatment for patients with severe adenomyosis strongly desiring uterus retention. Since the first description of traditional adenomyomectomy by Hyam[6] in 1952, various adenomyomectomy protocols have been used clinically, including the ‘transverse H incision technique’ of Fujishita et al[7] in 2004, “asymmetric dissection” of Nishida et al[8] in 2010, and ‘triple-flap’ method of Osada et al[9] in 2011. However, the above methods are associated with risk of uterine rupture during pregnancy[10] and a certain recurrence rate. The best method of surgery is yet to be seen. Therefore, efficient, safe and feasible surgical methods for adenomyomectomy are urgently needed, as well as measures that could effectively prevent recurrence in the long run for conservative surgery in adenomyosis treatment.
Levonorgestrel-initiated intrauterine system (LNG-IUS, Mirena) is a T-shaped intrauterine device with a filament tail, which constantly releases progesterone to the uterus within the 5 years of validity, achieving efficient contraception.[11,12] Its noncontraceptive benefits from adenomyosis have been extensively studied in recent years.[13–16] Our current study performed a retrospective analysis for more than 5 years of 318 patients with mild and moderate adenomyosis treated with LNG-IUS. We found that LNG-IUS significantly reduced menstrual flow, relieved the symptoms of dysmenorrhea and reduced uterine volume, constituting a very effective nonsurgical method for the treatment of mild and moderate adenomyosis. In patients with mild, moderate, and severe adenomyosis who underwent ineffective conservative treatment, we used a novel adenomyomectomy using “H” type incision to resect lesions extensively. Long-term postoperative adjuvant therapy was performed in combination with intraoperative LNG-IUS. Moreover, the clinical efficacy and safety of this method were evaluated.
2. Materials and methods
2.1. Study subjects
A total of 57 patients with adenomyosis confirmed by postoperative pathology who underwent adenomyomectomy using “H” type incision combined with intraoperative LNG-IUS in the Second Hospital of Hebei Medical University, Shijiazhuang, China, were selected between February 2012 and May 2015, which was approved by the Ethical Committee of the Second Hospital of Hebei Medical University, Shijiazhuang. The date of issue and registration number are July 5, 2012 and 2012 L-7 respectively. All the subjects signed informed consent prior to their participation. Inclusion criteria were: mild and moderate cases who underwent ineffective or failed conservative treatment (oral contraceptives, GnRH-a, and Mirena, etc.) due to severe dysmenorrhea or menorrhagia; severe adenomyosis (gynecological examination showing uterus>12 weeks size, transvaginal sonography (TVS) indicating a maximum uterine diameter exceeding 8 cm, increase in anterior or posterior uterine wall thickness above 5 cm, and adenomyotic nodule diameter >5 cm); fertility requirements or strong desire to retain the uterus; no surgical and LNG-IUS insertion contraindications. Patient ages ranged from 29 to 46 years, averaging 37.8 ± 4.4 years. All the patients were married except one. Average gravidity was 2.8 ± 1.6 times. A total of 53 cases had given birth, and 1 had reproductive desire. Three cases were nulligravid, including 2 with a successful history of in vitro fertilization and embryo transfer (IVF-ET) after administration of GnRH-a. One case had a history of embryonic arrest after natural pregnancy. A total of 15 cases had a history of abdominal operations (e.g., endometriosis, uterine fibroids, ovarian cysts, and cesarean section). Thirty-six cases were combined with anemia, with mean hemoglobin (Hb) levels of 86.4 ± 14.3 g/L, of which 5 underwent blood transfusion due to preoperative Hb < 70 g/L. There were 52 cases combined with abnormal serum CA125 levels.
2.2. Study methods
2.2.1. Surgical methods
All the 57 patients underwent laparotomy (Fig. 1), with 6U pituitrin injected into the uterine wall (Fig. 1E). Then, the uterine wall was vertically incised to the uterine cavity from the middle of the 2 cornua uteri of the uterine fundus; the incision was extended to the anterior and posterior walls until the cervix (Fig. 1A and F). If combined with hysteromyoma, the latter should be removed. The operation was guided by the left index finger in the uterine cavity (Fig. 1B). There were 4 regions, including the front, rear, left, and right regions. Adenomyomectomy using “H” type incision can be used to resect adenomyotic lesions, 0.5 cm to the submucosal layer inwardly and about 0.5 cm to the strata subserosum outwardly. The remaining tissues of the uterine wall were soft (Fig. 1C and G). Meanwhile, the endometrium was scraped and collected. Absorbable 3-0 line suture was used to intermittently stitch the endometrium in order to close the uterine cavity (Fig. 1C and G); absorbable 0 line suture was used to perform plication for the seromuscular layer of the anterior and posterior walls to give shape to the uterus (Fig. 1D and H). Pelvic adhesion, ovarian cysts, and ovarian teratoma should be treated if found. In case of fertility requirement, hydrotubation or fallopian tube shaping operation was performed as well. Uterine incision was covered with an Interceed pad or implanted 6 mL medical chitosan to avoid adhesion. The patients were changed to the lithotomy position; after vulva, vagina and cervix disinfection, a LNG-IUS was implanted.
Figure 1.
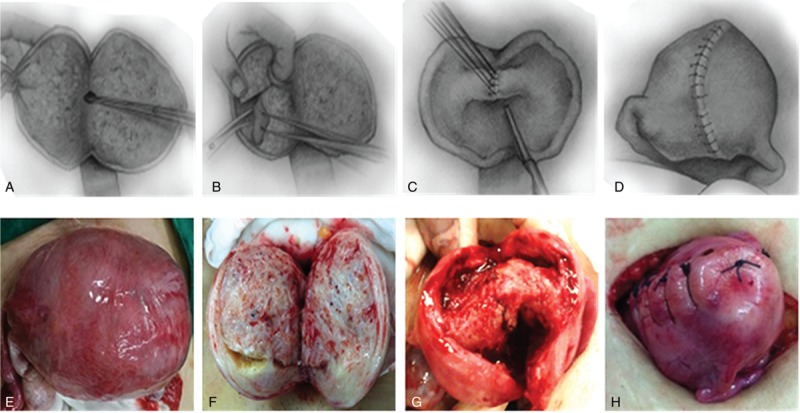
Surgical sketch[9] and operative methods of adenomyomectomy using “H” type incision.
2.2.2. Outcome measures and follow-up
All patients were followed-up in our gynecological clinic at postoperative 1, 3, 6, 12, 24, and 36 months, respectively, and follow-up indicators included the following:
-
1.
Dysmenorrhea:
Dysmenorrhea severity was evaluated before and after surgery, based on a visual analog scale (VAS): 0 point, no pain; 1–3 points, mild pain; 4–6 points, moderate pain; 7–9 points, severe pain; 10 points, exquisite pain. Complete remission referred to complete disappearance of dysmenorrhea after surgery.
-
2.
Menstrual flow:
This study referred to the improved VAS method of Osada et al.[9] Preoperative menstrual flow was considered to be 10 points for all patients, and changes of postoperative menstrual flow were evaluated with 10 points as standard. 0 point reflected amenorrhea.
-
3.
Levels of serum CA125 and Hb:
Venous blood was extracted before and after surgery from all patients. CA125 >35 IU/mL was considered to be positive, while Hb < 110 g/L was considered to reflect anemia. Hemoglobin levels were assessed only at postoperative 1 and 3 months.
-
4.
Uterine volume:
Uterine volume was calculated based on the formula of elliptical volume (V = 0.5236abc), where a, b, and c are diameters of the 3 uterine sections determined by TVS, respectively.
-
5.
Postoperative pregnancy:
Pregnancy criteria were evaluated after 12-month follow-up in patients with fertility requirements, including: significant improvement of symptoms; uterine morphology is basically normal by hysteroscopy; uterine size and morphology is basically normal by TVS or magnetic resonance imaging (MRI). The intrauterine device was removed if all above criteria were met. Patients with one time normal menstruation after removal of the intrauterine device tried to conceive, or received assisted reproductive technology (ART) based on a comprehensive evaluation of age, intraoperative conditions and surgical complications; the pregnancy outcome was then observed.
-
6.
Incidence rate of adverse reactions and recurrence rate:
Follow-up was performed regularly and surgery-related complications (ischemic necrosis of the uterus, hematoma of uterine incision, infection, and rupture, etc.) and LNG-IUS-induced adverse reactions (e.g., irregular vaginal bleeding, amenorrhea, breast tenderness, ovarian cysts, hot flashes, night sweats, LNG-IUS removal, incarceration, ectopia, and perforation) were recorded.
2.3. Statistical method
Data analysis was performed with the SPSS 13.0 statistical software. Continuous data are x ± s, and repeated measure analysis of variance (ANOVA) was used for comparison. P < .05 was considered statistically significant.
3. Results
3.1. Surgery and complications
The 57 cases underwent laparotomy successfully. The mean surgical time was 116.9 ± 20.7 minutes, for an average intraoperative blood loss of 110.7 ± 53.5 mL. Two cases underwent intraoperative blood transfusion, 30 underwent adenomyomectomy combined with intraoperative LNG-IUS alone, 1 was combined with ovarian teratoma, 7 were combined with pelvic adhesions, 9 were combined with endometriosis ovarian ectopic disease, 16 were combined with uterine fibroid, and 2 underwent salpingoplasty. There were 4 cases with no intraoperative LNG-IUS insertion due to giant uterine volume (about the size of 5-month pregnancy) and uterine cavity depth > 9 cm after the operation. These 4 cases were treated with GnRH-a or LNG-IUS insertion post-treatment.
3.2. Postoperative dysmenorrhea, menstrual flow, uterine volume, and serum CA125 and Hb levels
Of the 57 patients who were followed-up, 4 were lost to follow-up. Complete remission rates for dysmenorrhea in the remaining 53 cases were 54.2% (26/48), 72.1% (31/43), 80.0% (40/50), 83.8% (31/37), 87.5% (21/24), and 88.9% (16/18), respectively, at various time points postoperation (Fig. 2). As shown in Table 1, VAS score, menstrual flow, uterine volume, and serum CA125 levels at various follow-up time points after the operation were decreased significantly compared with preoperative values (P < .001), with VAS score (1, 3, and 6 months), menstrual flow (1, 3, 6, 12, and 24 months), uterine volume (1, 3, 6, 12, 24, and 36 months), and serum CA125 levels (1 and 3 months) showing statistically significant differences (P < .05). Dysmenorrhea in almost all patients disappeared, with menstrual volume significantly decreased; uterine volume was gradually reduced to the normal level, while serum CA125 returned to normal at postoperative 3 months and remained in the normal range. However, 1 case showed continued CA125 level increase, to an abnormal level after reduction to a normal level at postoperative 30 months; this was combined with a gradual increase of uterine volume, but not reaching the preoperative value. Moreover, dysmenorrhea and menstrual flow showed no significant changes. In the 36 cases combined with anemia before surgery, hemoglobin levels at postoperative 1 and 3 months were increased significantly (P < .001), basically reaching normal levels.
Figure 2.
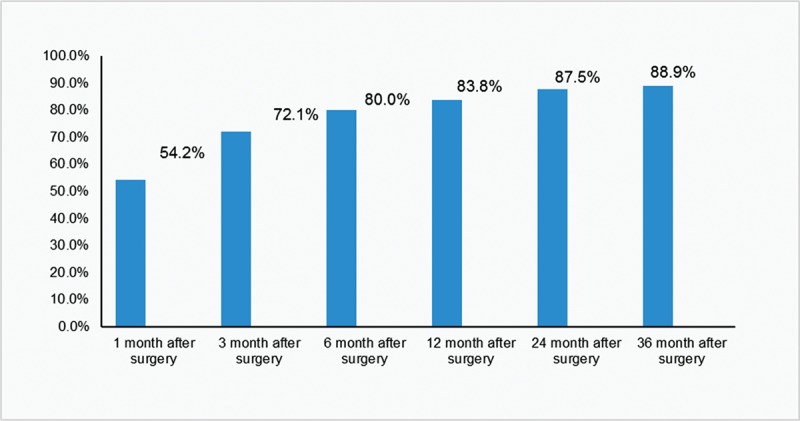
Complete remission rate of dysmenorrhea after surgery.
Table 1.
Changes of postoperative VAS score, menstrual flow, uterine volume, and serum CA125 and Hb levels.

3.3. Pregnancy
In this study, there were 5 cases desire a future pregnancy; 1 case was unmarried and would not reproduce, 2 patients did not require removal of the intrauterine device 12 months postsurgery, and 1 case had the intrauterine device removed, and postoperative uterine cavity, morphology, and size were evaluated by hysteroscopy and TVS (Fig. 3). The latter patient underwent adjuvant pregnancy in the reproductive department. In addition, 1 case was pregnant at postoperative 36 months by IVF-ET with twins, and underwent cesarean section at 34+3 weeks. This patient successfully delivered 2 healthy babies, and was combined with hypertension and placenta previa during the pregnancy period. However, there are no severe complications such as placenta implantation, placenta adhesions, and uterine rupture.
Figure 3.
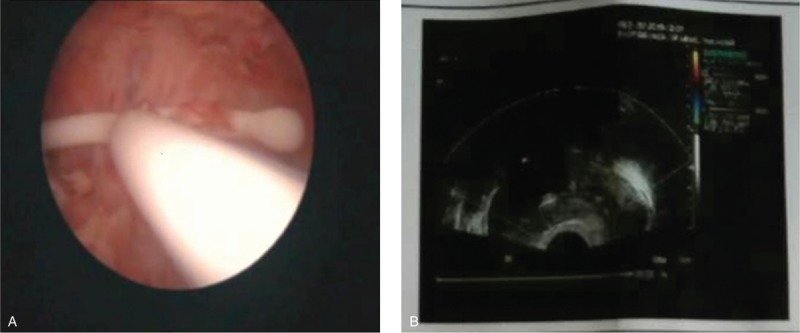
(A) Laparoscopy was performed 12 months after surgery; (B) TVS was performed through vagina 12 months after surgery. TVS = transvaginal sonography.
3.4. Incidence of adverse reactions and recurrence rate
Adverse reactions found during follow-up included amenorrhea (20 cases, 37.7%), ovarian cysts alone (12 cases or 22.6%. which were all naturally absorbed within 3 months), abnormal uterine bleeding (10 cases or 18.9%, which disappeared at postoperative 6–12 months), breast pain (9 cases, 17.0%), weight gain >2 kg (8 cases, 15.1%), increased vaginal secretions (6 cases, 11.3%), lower abdominal pain and soreness of waist (6 cases, 11.3%), massive hemorrhage of the uterus (1 case, 1.9%), and Mirena perforation from incision of the uterine fundus (1 case, 1.9%). In all follow-up patients, except for 2 individuals desiring pregnancy, 1 case with massive uterine hemorrhage and 1 patient with Mirena perforation from incision of the uterine fundus who removed the intrauterine device, the remaining cases showed no ectopia, expulsion and internalization. Moreover, all patients had no relapse after the surgery.
4. Discussion
Adenomyosis is a benign uterine disorder characterized by the presence of heterotopic endometrial glands and stroma in the myometrium and reactive fibrosis of the surrounding smooth muscles cells of the myometrium.[17] On the basis of myometrial invasion extension, it can be either diffuse or focal. In the diffuse type, endometrial glands and/or stroma are extensively intermingled with myometrial muscle fibers with an increase in uterine volume; focal adenomyosis is generally a single nodular aggregate located in the myometrium, which may have a histologic spectrum from mostly (“adenomyoma”) solid to mostly cystic (“adenomyotic cyst”).[18,19] The several available medical and surgical options are used for treating adenomyosis. For patients who are refractory or unsuitable to long-term medical treatment or those with focal adenomyoma or severe adenomyosis, the traditional hysterectomy remains the main therapeutic strategy in the long term. However, considering the relevant technical progress of conservative surgical techniques in recent years, and the expansion of lesion removal, the short-term efficacy of conservative surgical techniques is significant in improving adenomyosis related dysmenorrhea, abnormal uterine bleeding, and infertility[20]; in addition, such surgeries somewhat reduce recurrence with less complications.[21] However, adenomyosis typically involves the myometrium in an irregular and massive way, characterized by lesions with unclear borders, so much so that complete excision of adenomyotic tissue is not possible, with obligate loss of healthy myometrium,[22] it is plausible that residual small lesions may continue to grow and relapse.[23,24] Wang et al.[25] compared and analyzed the recurrence rate of patients with symptomatic adenomyoma after surgical–medical treatment and surgery alone, relapse were 28.1% and 49%, respectively.[25] Compared with old classical reduction surgery, Fujishita's innovative H incision technique may be considered a useful method for an easy surgical approach and satisfactory removal of adenomyotic lesions, the major complication or pregnancy or subjective relief of pain may be better.[7] But the best method of surgery is yet to be seen, therefore, long-term efficacy of simple conservative surgery in the treatment of adenomyosis should be supported by further evidence.
Studies have demonstrated that LNG-IUS downregulates endometrial estrogen and progesterone receptors by constantly releasing effective progesterone to the uterine cavity. This results in loss of sensitivity of the endometrium to circulatory estradiol, generating a strong proliferative antagonism in the endometrium.[26] The device's strong local effects on the endometrium benefit women with various benign gynaecological conditions such as menorrhagia, dysmenorrhea, leiomyomata, adenomyosis, and endometriosis. There is also evidence to support its role in endometrial protection during postmenopausal estrogen replacement therapy, and in the treatment endometrial hyperplasia.[27] Its noncontraceptive benefits from adenomyosis have been extensively studied in recent years. Multiple studies demonstrated that dysmenorrhea score, menstrual flow, uterine volume, and serum CA125 levels are significantly improved in patients with adenomyosis after LNG-IUS implantation.[13,15,16,28] But Park et al[14] found no significant change in postoperative uterine volume compared with the preoperative value after LNG-IUS in the treatment of large and symptomatic adenomyosis, with an expulsion rate of intrauterine device of up to 37.5%. It is therefore obvious that LNG-IUS can be effectively used in the treatment of mild and moderate adenomyosis; however, for patients with severe adenomyosis having a large uterus, such treatment is limited.
Therefore, our study applied a novel open adenomyomectomy with “H” type incision to vertically cut the uterus to the uterine cavity. The endometrium and serosa were used as signs, digital palpation of the uterus is necessary to delineate the involved areas, limiting the excision of healthy myometrium, and adenomyotic lesions were divided into 4 quadrants and removed satisfactorily to the maximum extent possible. One month after the surgery, symptoms in all patients were improved; the complete remission rate of dysmenorrhea reached 54.2%, and dysmenorrhea was alleviated significantly or resolved. Moreover, menstrual flow, uterine volume and serum CA125 levels were also decreased significantly. As important factors reflecting surgical efficacy, these indicators were significantly improved 1 month postsurgery, suggesting that the novel adenomyomectomy using “H” type incision had rapid, definite and significant effects in the treatment of adenomyosis. In a systematic review, Younes and Tulandi[29] evaluated 27 studies; the results showed that excision of adenomyosis is effective for symptom control such as menorrhagia and dysmenorrhea and most probably for adenomyosis-related infertility. In addition, appositional suture was performed to maintain the integrity of the uterine cavity; the “Double-folding” method was used to reconstruct the uterine wall, preserving at least 1–1.5 cm of myometrial thickness; thus multiple layers of interrupted sutures are preferred for good repair and better obstetrical outcome.[30] Fallopian tube functions were retained. These are necessary conditions for preserving the reproductive function by conservative surgery for adenomyosis.[9] Using our standard after a myomectomy, we recommend a waiting time of at least 12 months between surgery and trial to conceive. After removing the intrauterine device, it is necessary to comprehensive evaluate at postoperative 12 months. The factors to be taken into consideration in order to choose natural pregnancy or ART are patient age, intraoperative conditions and surgical complications. Indeed, in our study, 2 cases restored menstruation 1 month after removal of the intrauterine device; 1 of them (33 years) became pregnant with twins using IVF-ET at postoperative 36 months and successfully delivered. In addition, the patient had normal pregnancy without a risk of uterine rupture. Saremi et al[31] also used a novel technique of wedge resection of adenomyomatic uterus, there were 2 cases of uterine rupture at 37 and 32 weeks. The result possibly showed that the novel adenomyomectomy using “H” type incision was effective and safe for adenomyosis-related infertility to a certain extent. However, the sample size of this study was small, and only 2 patients met pregnancy requirements; therefore, fertility outcome with pregnancy rate and safety should be further assessed in long term, large sample size and prospective clinical studies.
Complete excision can be difficult because adenomyosis generally lacks a cleavage plane; residual small lesions may continue to relapse. According to a systematic review of Younes et al,[29] depending on the duration of follow-up, recurrence rates after excision differ from no recurrence to almost a half of the patients. In 2009, study of Wang et al[25] showed that adjuvant application of drug (GnRH-a) after adenomyomectomy in the treatment of adenomyosis can effectively reduce postoperative recurrence, but with a recurrence rate after drug discontinuation of up to 28.1%. Meanwhile, systemic medical treatment resulted in some side effects. In our study, 5-year (long-term) adjuvant therapy was performed, with the novel adenomyomectomy using “H” type incision combined with intraoperative LNG-IUS. There were no statistically significant differences in VAS score for dysmenorrhea at postoperative 6–36 months, menstrual flow at postoperative 24–36 months, and serum CA125 levels at postoperative 3–36 months. This may be explained by the fact that after the operation dysmenorrhea almost disappeared, menstrual flow was reduced significantly, and even amenorrhea occurred, while serum CA125 levels returned to normal in all patients. Nevertheless, with increasing follow-up time, uterine volume was reduced gradually, with statistical significance. Except for 2 cases to meet fertility requirements, 1 patient with massive uterine hemorrhage, and 1 case of pyosalpinx who removed the intrauterine device due to Mirena perforation from incision of the uterine fundus, the remaining patients did not remove the intrauterine device, and showed no ectopia, expulsion, and incarceration. Thus, the patients were all satisfied. Despite adverse reactions such as amenorrhea and breast pain in some patients, the latter tolerated well and were willing to continue implanting the intrauterine device. Thus, LNG-IUS, as adjuvant therapy after adenomyomectomy, can significantly inhibit the development of residual small adenomyotic lesions leading to ectopia, effectively reducing recurrence.
In our study, the preoperative uterus looked like 5 month pregnancy in a 35-year-old patient (V = 615.73 cm3). The latter patient suffered from massive hemorrhage of the uterus 22 months after the surgery, and hysteroscopy found that uterine morphology and size were basically normal; the endometrial was eutopic, with endometrial blood vessels in the uterine cavity of the lower uterus exposed and overt active bleeding. Evidence-based interpretation cannot be given because there was no relevant literature report. The reason of this complication might be that high efficient progesterone released by LNG-IUS caused excessive atrophy and thinness of the endometrium, and the exposure of endometrial blood vessels led to massive hemorrhage. Removal of the intrauterine device and hemostasis by intrauterine compression of the balloon were performed before switching to oral administration of combined oral contraceptives (COCs, drospirenone and ethinyl-estradiol) for 3 courses, followed by LNG-IUS implantation. To date, the patient condition was stable. In another case, the uterus before the surgery resembled an over 4 month pregnancy (V = 532.66 cm3), with serum CA125 levels of 657.1 IU/mL. Twenty months post-surgery, the patient had abdominal pain with high fever; laparoscopy showed pyosalpinx on the left side and Mirena perforation from incision of the uterine fundus. This might have been caused by loose suture of the serosal layer and infection due to Mirena perforation. Both fallopian tubes were removed, and uterine incision was repaired. Meanwhile, COCs (drospirenone and ethinyl-estradiol) were orally administered after removal of the intrauterine device. To the above 2 extraordinary cases, we used COCs for short-term adjuvant therapy and hemostasis after removal of LNG-IUS. The rationale for using COCs in adenomyosis was related to the induced decidualization and subsequent atrophy of the endometrium, reducing pain and abnormal uterine bleeding.[32] There was another case with serum CA125 levels ascending to abnormal amounts 30 months after the surgery and gradual increase in uterine volume. Although serum CA125 cannot completely reflect the severity of adenomyosis, it constitutes an effective biomarker for diagnosing adenomyosis and monitoring treatment efficacy as well as recurrence.[33] Serum CA125 increase in the latter patient may be explained by the weakened inhibitory effects of LNG-IUS after 30 months on the residual ectopic endometrium, which resulted in increased endometrial activity. Since there were no significant symptom changes in this patient, we recommended continuous observation and LNG-IUS replace if necessary.
In summary, for patients who are refractory or unsuitable to long-term medical treatment or ineffective conservative treatment or infertility association with adenomyosis or those with focal adenomyoma or severe adenomyosis, or strongly desiring to retain the uterus, the novel adenomyomectomy using “H” type incision combined with LNG-IUS can remove adenomyotic lesions to the maximum extent possible, significantly improve symptoms, markedly decrease the uterine volume, reduce CA125 levels and improve anemia. In addition, the new technique inhibits the development of residual lesions in the long term while effectively reducing recurrence. Therefore, it may be considered a useful method for an effective surgical approach for the treatment of adenomyosis. However, based on the above 2 cases, the following should be considered for adenomyosis patients with giant uterine volume. Three courses of GnRH-a may be administered preoperatively to reduce the uterine volume and decrease surgical difficulty. Mirena may be implanted 2–3 months after the surgery when the morphology of the uterine cavity is basically recovered and the incision healed, to avoid Mirena perforation form the uterine incision. Meticulous suture without leaving any dead space should be performed for reconstructing uterine wall. Improvement of pregnancy and safety evaluation should be further assessed in large sample size prospective clinical studies in the long term. Overall, these findings suggest that adenomyosis may be normally treated in the near future by conservative surgery combined with LNG-IUS, in patients desiring to retain the uterus.
Author contributions
Bin Shi and Yanfei Gao carried out the studies, participated in collecting data, and drafted the manuscript. Yanfei Gao, Shuzhi Shan and Xin Zhao performed the statistical analysis and participated in its design. Bin Shi, Jing Jiang and Dongxiao Li helped to draft the manuscript. All authors read and approved the final manuscript.
Conceptualization: Bin Shi.
Investigation: Yanfei Gao, Shuzhi Shan, Xin Zhao, Jing Jiang, Dongxiao Li.
Methodology: Yanfei Gao, Shuzhi Shan, Xin Zhao, Jing Jiang, Dongxiao Li.
Writing – original draft: Yanfei Gao.
Footnotes
Abbreviations: ANOVA = analysis of variance, ART = assisted reproductive technology, COCs = combined oral contraceptives, GnRH-a = gonadotrophin-releasing hormone agonists, Hb = hemoglobin, IVF-ET = in vitro fertilization and embryo transfer, LNG-IUS = levonorgestrel-releasing intrauterine device, MRI = magnetic resonance imaging, TVS = transvaginal sonography, VAS = visual analog scale.
The authors have no conflicts of interest to disclose.
References
- [1].Botsis D, Kassanos D, Antoniou G, et al. Adenomyoma and leiomyoma: differential diagnosis with transvaginal sonography. J Clin Ultrasound 1998;26:21–5. [DOI] [PubMed] [Google Scholar]
- [2].Vercellini P, Vigano P, Somigliana E, et al. Adenomyosis: epidemiological factors. Best Pract Res Clin Obstetr Gynaecol 2006;20:465–77. [DOI] [PubMed] [Google Scholar]
- [3].Nelson JR, Corson SL. Long-term management of adenomyosis with a gonadotropin-releasing hormone agonist: a case report. Fertil Steril 1993;59:441–3. [DOI] [PubMed] [Google Scholar]
- [4].Fedele L, Bianchi S, Raffaelli R, et al. Treatment of adenomyosis-associated menorrhagia with a levonorgestrel-releasing intrauterine device. Fertil Steril 1997;68:426–9. [DOI] [PubMed] [Google Scholar]
- [5].Cucinella G, Granese R, Calagna G, et al. Oral contraceptives in the prevention of endometrioma recurrence: does the different progestins used make a difference? Arch Gynecol Obstet 2013;288:821–7. [DOI] [PubMed] [Google Scholar]
- [6].Hyams LL. Adenomyosis; its conservative surgical treatment (hysteroplasty) in young women. N Y State J Med 1952;52:2778–84. [PubMed] [Google Scholar]
- [7].Fujishita A, Masuzaki H, Khan KN, et al. Modified reduction surgery for adenomyosis. A preliminary report of the transverse H incision technique. Gynecol Obstet Invest 2004;57:132–8. [DOI] [PubMed] [Google Scholar]
- [8].Nishida M, Takano K, Arai Y, et al. Conservative surgical management for diffuse uterine adenomyosis. Fertil Steril 2010;94:715–9. [DOI] [PubMed] [Google Scholar]
- [9].Osada H, Silber S, Kakinuma T, et al. Surgical procedure to conserve the uterus for future pregnancy in patients suffering from massive adenomyosis. Reprod Biomed Online 2011;22:94–9. [DOI] [PubMed] [Google Scholar]
- [10].Wada S, Kudo M, Minakami H. Spontaneous uterine rupture of a twin pregnancy after a laparoscopic adenomyomectomy: a case report. J Minimally Inv Gynecol 2006;13:166–8. [DOI] [PubMed] [Google Scholar]
- [11].Rose S, Chaudhari A, Peterson CM. Mirena (Levonorgestrel intrauterine system): a successful novel drug delivery option in contraception. Adv Drug Deliv Rev 2009;61:808–12. [DOI] [PubMed] [Google Scholar]
- [12].Bednarek PH, Jensen JT. Safety, efficacy and patient acceptability of the contraceptive and non-contraceptive uses of the LNG-IUS. Int J Womens Health 2010;1:45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sheng J, Zhang WY, Zhang JP, et al. The LNG-IUS study on adenomyosis: a 3-year follow-up study on the efficacy and side effects of the use of levonorgestrel intrauterine system for the treatment of dysmenorrhea associated with adenomyosis. Contraception 2009;79:189–93. [DOI] [PubMed] [Google Scholar]
- [14].Park DS, Kim ML, Song T, et al. Clinical experiences of the levonorgestrel-releasing intrauterine system in patients with large symptomatic adenomyosis. Taiwanese J Obstetr Gynecol 2015;54:412–5. [DOI] [PubMed] [Google Scholar]
- [15].Bragheto AM, Caserta N, Bahamondes L, et al. Effectiveness of the levonorgestrel-releasing intrauterine system in the treatment of adenomyosis diagnosed and monitored by magnetic resonance imaging. Contraception 2007;76:195–9. [DOI] [PubMed] [Google Scholar]
- [16].Cho S, Nam H, Kim D, et al. Clinical effects of the levonorgestrel-releasing intrauterine device in patients with adenomyosis. Am J Obstet Gynecol 2008;198:373–7. [DOI] [PubMed] [Google Scholar]
- [17].Harada T, Khine YM, Kaponis A, et al. The impact of adenomyosis on women's fertility. Obstet Gynecol Surv 2016;71:557–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Van den Bosch T, Dueholm M, Leone FP, et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: a consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet Gynecol 2015;46:284–98. [DOI] [PubMed] [Google Scholar]
- [19].Levgur M, Abadi MA, Tucker A. Adenomyosis: symptoms, histology, and pregnancy terminations. Obstet Gynecol 2000;95:688–91. [DOI] [PubMed] [Google Scholar]
- [20].Koo YJ, Im KS, Kwon YS. Conservative surgical treatment combined with GnRH agonist in symptomatic uterine adenomyosis. Pakistan J Med Sci 2011;27:365–70. [Google Scholar]
- [21].Grimbizis GF, Mikos T, Tarlatzis B. Uterus-sparing operative treatment for adenomyosis. Fertil Steril 2014;101:472–87. [DOI] [PubMed] [Google Scholar]
- [22].Di Spiezio Sardo A, Calagna G, Santangelo F, et al. The role of hysteroscopy in the diagnosis and treatment of adenomyosis. Biomed Res Int 2017;2017:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Maheshwari A, Gurunath S, Fatima F, et al. Adenomyosis and subfertility: a systematic review of prevalence, diagnosis, treatment and fertility outcomes. Hum Reprod Update 2012;18:374–92. [DOI] [PubMed] [Google Scholar]
- [24].Louis LS, Saso S, Chatterjee J, et al. Adenomyosis and infertility. Reprod Biomed Online 2012;24:586. [DOI] [PubMed] [Google Scholar]
- [25].Wang PH, Liu WM, Fuh JL, et al. Comparison of surgery alone and combined surgical-medical treatment in the management of symptomatic uterine adenomyoma. Fertil Steril 2009;92:876–85. [DOI] [PubMed] [Google Scholar]
- [26].Zhang L, Weng L. Clinical study on women with amenorrhea after levonorgestrel intrauterine system. Zhonghua Fu Chan Ke Za Zhi 2001;36:675–7. [PubMed] [Google Scholar]
- [27].Fraser IS. Non-contraceptive health benefits of intrauterine hormonal systems. Contraception 2010;82:396–403. [DOI] [PubMed] [Google Scholar]
- [28].Cho S, Nam A, Kim H, et al. Clinical effects of the levonorgestrel-releasing intrauterine device in patients with adenomyosis. Am J Obstet Gynecol 2008;198:373.e1–7. [DOI] [PubMed] [Google Scholar]
- [29].Younes G, Tulandi T. Conservative surgery for adenomyosis and results: a systematic review. J Minim Invasive Gynecol 2018;25:265–76. [DOI] [PubMed] [Google Scholar]
- [30].Fedele L, Bianchi S, Zanconato G, et al. Conservative treatment of diffuse uterine leiomyomatosis. Fertil Steril 2004;82:450–3. [DOI] [PubMed] [Google Scholar]
- [31].Saremi A, Bahrami H, Salehian P, et al. Treatment of adenomyomectomy in women with severe uterine adenomyosis using a novel technique. Reprod Biomed Online 2014;28:753–60. [DOI] [PubMed] [Google Scholar]
- [32].Schindler AE. Non-contraceptive use of hormonal contraceptives. Gynecol Endocrinol 2008;24:235–6. [DOI] [PubMed] [Google Scholar]
- [33].Huang X, Huang Q, Chen S, et al. Efficacy of laparoscopic adenomyomectomy using double-flap method for diffuse uterine adenomyosis. BMC Women's Health 2015;15:24. [DOI] [PMC free article] [PubMed] [Google Scholar]


