Abstract
Background:
Numerous studies showed that vascular endothelial growth factor (VEGF) gene polymorphisms were linked with the regularity of stroke, but the results remained controversial. The aim of this meta-analysis was to determine the associations between VEGF gene polymorphisms and the risk of stroke.
Methods:
A systematic literature search of PubMed, Embase, Wed of Science, The Cochrane Library, Elsevier, China National Knowledge Infrastructure, China Biology Medicine disc, WanFang Data, VIP Database for Chinese Technical Periodicals, and Science paper Online was conducted. Two authors independently assessed trial quality and extracted data. The pooled odds ratio (OR) with 95% confidence interval (CI) was used to assess the strength of associations. Begger funnel plot and Egger test were used to estimate the publication bias of included studies. Heterogeneity assumption was assessed by Cochran Chi-squared-based Q-statistic test and I2 test.
Results:
Thirteen publications including 23 trails with a total of 3794 stroke patients and 3094 control subjects were enrolled. About 3747 cases and 2868 controls for +936C/T, 2134 cases and 1424 controls for −2578C/A, and 2187 cases and 1650 controls for −1154G/A were examined, respectively. The results indicated that VEGF +936C/T (T vs C, OR = 1.19, 95% CI = 1.01–1.40) or −2578C/A (A vs C, OR = 1.13, 95% CI = 1.02–1.27) was positively associated with the risk of stroke, whereas there was no association between −1154G/A (A vs G, OR = 0.99, 95% CI = 0.87–1.11) polymorphism and stroke risk in our study. Among the subgroup analyses on ethnicity, the results showed that VEGF +936C/T was an increased risk of stroke in Asian population (T vs C, OR = 1.21, 95% CI = 1.01–1.44), but not −1154G/A.
Conclusion:
Our findings suggest that VEGF +936C/T and −2578C/A might be related to the risk of stroke, especially in the Asian population, but not −1154G/A.
Keywords: gene polymorphisms, meta-analysis, stroke, vascular endothelial growth factor
1. Introduction
In 2013, stroke was the 2nd most common cause of deaths worldwide, and the 3rd most cause of disability from all diseases.[1] It is well known that genetic and environmental backgrounds play an important role in the pathogenesis of stroke.[2] To the best of our knowledge, there are few studies to investigate the relationship between gene loci and stroke, compared with those to investigate the relationship between environmental factors and stroke. Although genome-wide association studies on stroke have been published, mixed results have been demonstrated.[3,4]
A number of studies have proved that vascular endothelial growth factor (VEGF) is involved in atherosclerosis, angiogenesis, brain edema, and vascular repair after ischemic stroke.[5] The VEGF gene is located on chromosome 6 at location 6p21.3 and comprised of 8 exons and 7 introns.[6] Neural-derived VEGF plays an important part in neurovascular development and vessel patterning.[7] It has documented there were >30 single-nucleotide polymorphisms (SNPs) in the VEGF gene, such as rs3025039, rs1570360, and rs30250202, which have been reported to be associated with the expression of VEGF protein.[8,9] Three of them were found to be involved in the activity of VEGF signaling pathway and investigated the most frequently: +936C/T (rs3025039), −2578C/A (rs699947) and −1154G/A (rs1570360). Recently, a meta-analysis proved that SNPs of VEGF is associated with increased risk of diabetic foot ulcer.[10]
An increasing number of studies have been undertaken to study the relationship between VEGF gene polymorphism and stroke. Although emerging meta-analysis showed that +936C/T may be involved in the risk of stroke, there were some conflicting results.[11] For example, the literature was not comprehensive enough, and not all the articles were consistent with the Hardy–Weinberg equilibrium (HWE). To further examine the role of VEGF in stroke, we performed a meta-analysis to evaluate the association between VEGF gene polymorphisms and stroke risk.
2. Methods
2.1. Search strategy
A systematic literature search of PubMed, Embase, Wed of Science, The Cochrane Library, Elsevier, China National Knowledge Infrastructure, China Biology Medicine disc, WanFang Data, VIP Database for Chinese Technical Periodicals, and Sciencepaper Online were conducted by two investigators independently. The latest data for searching articles were May 1, 2018. Key words used in the research were: “VEGF” or “vascular endothelial growth factor” or “vasculotropin,” “single-nucleotide polymorphism” or “SNP” or “polymorphism” or “mutation” or “genetics” or “variant,” and “stroke” or “cerebral infarction” or “cerebrovascular disorders.”
2.2. Inclusion and exclusion criteria
Studies eligible for inclusion in this meta-analysis needed to meet the following criteria: independently published case-control studies focused on associations between VEGF polymorphism and the risk of stroke; these studies provided genotype or allelic distributions; and genotype or allelic distributions in the control group was in accordance with HWE.
The exclusion criteria for the meta-analysis included: animal studies; there were a large difference in the general data of the subjects, such as age, gender, and there may be a significant bias in the literature. When individual authors published several articles from the same patient population, only the most recent or complete articles were taken into account in the analysis.
2.3. Data extraction
All qualified information were drawn from all the eligible publications. The following data were collected from each study: the 1st author's name, the date of publication, country, ethnicity, sample size, and the genotyping method.
2.4. Quality score assessment
We used the Newcastle Ottawa scale (NOS) to assess the quality of these case–control studies. The NOS ranges from 0 to 9 stars, and more than a score of 7 was taken to be of high quality. Two authors independently evaluated the quality of the included studies and resolved all the differences through discussion.
2.5. Evaluation of statistic association
All the statistical analysis was conducted by Review manager 5.3. We performed the association between +936C/T, −2578C/A, and −1154G/A polymorphisms and the risk of stroke by calculating odds ratio (OR) and 95% confidence interval (CI). The association was estimated with the use of the allelic contrast, the dominant model, the recessive model, and the homozygous contrast. When the P value was >.1 and I2 < 50%, the pooling data was performed by fixed-effects model or random-effects model. Heterogeneity assumption was assessed by Cochran Chi-squared-based Q-statistic test and I2 test. The pooled OR was counted by the method of Mantel–Haenszel, with 95% CI calculated by Woolf method. The potential publication bias was valued by Begg funnel plot and Egger test.[12] The HWE of the genotype distribution of controls was assessed by Pearson Chi-squared test.
2.6. Ethical approval
Ethical approval was not necessary under the ethical committee of Jinan University, since this study was a meta-analysis of previous literature works, which informed consents had been obtained by the previous clinical researchers.
3. Results
3.1. Included studies
Figure 1 displays the process of retrieving eligible studies. Briefly, our sensitive search strategy identified 230 articles. After reviewed and considered the titles and abstracts of all articles, 206 articles were excluded. After systematically reading full texts and calculating HWE value, we excluded another 11 articles. Finally, 13 case–control studies with a total of 3794 patients with stroke and 3094 control subjects were included,[13–25] including 3747 cases and 2868 controls for +936C/T, 2134 cases and 1424 controls for −2578C/A, and 2187 cases and 1650 controls for −1154G/A, respectively. The characteristics of 13 included studies are summarized in Table 1. Table 2 shows the distribution of VEGF genotype and allele between the case group and the control group. The NOS scores demonstrated that all included studies were high quality, which suggested the reliability of our findings (Table 3).
Figure 1.
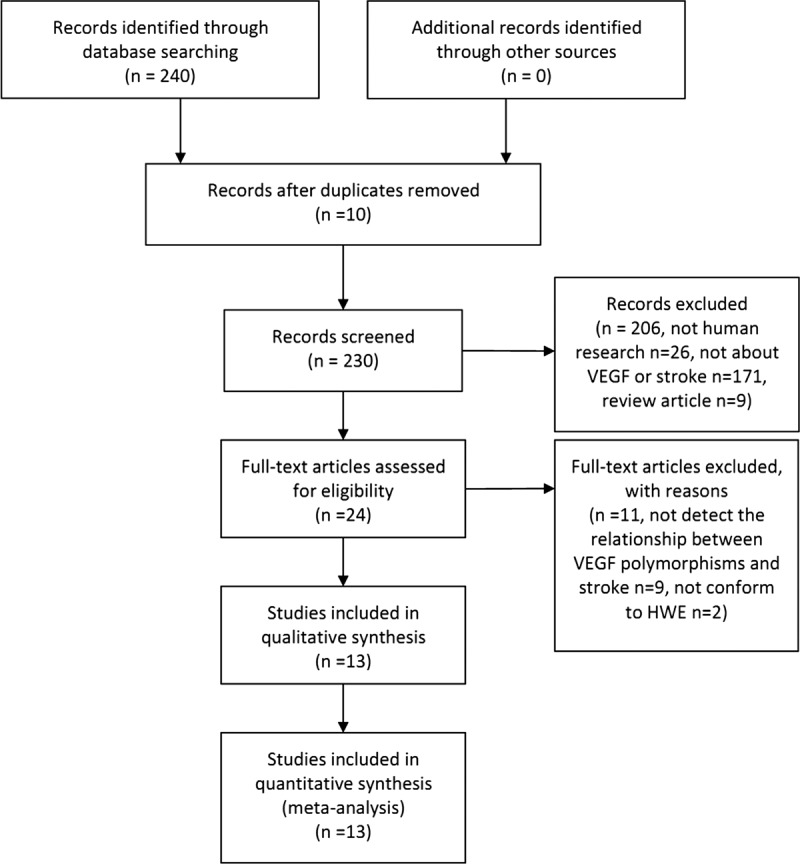
Flow chart of the literature search and selection procedures.
Table 1.
Characteristics of studies included in the meta-analysis.

Table 2.
Distribution of vascular endothelial growth factor genotype and allele among stroke patients and controls in 3 single-nucleotide polymorphisms.

Table 3.
Quality assessment of the included studies.

3.2. Quantitative synthesis
3.2.1. VEGF +936C/T
Table 4 shows the assessment of association between VEGF polymorphisms and stroke risk. The meta-analysis results showed that VEGF +936C/T polymorphism was linked to the risk of stroke under all genetic models. Among the subgroup analyses on race, the results showed that those variants were an increased risk of stroke in Asian population (T vs C: OR = 1.21, 95% CI = 1.01–1.44, P = .03). However, no significant association was found in the Caucasian (T vs C: OR = 0.96, 95% CI = 0.63–1.45, P = .83). Overall, the significant association was discovered between VEGF +936C/T polymorphism and the risk of stroke in allele contrast (OR = 1.19, 95% CI = 1.01–1.40, P = .04, Fig. 2A), dominant model (OR = 1.32, 95% CI = 1.08–1.62, P = .008), recessive model (OR = 1.35, 95% CI = 1.08–1.70, P = .009), and homozygous contrast (OR = 1.41, 95% CI = 1.11–1.79, P = .004).
Table 4.
Main results for the vascular endothelial growth factor polymorphism with the risk of stroke based on OR and 95% CI.

Figure 2.
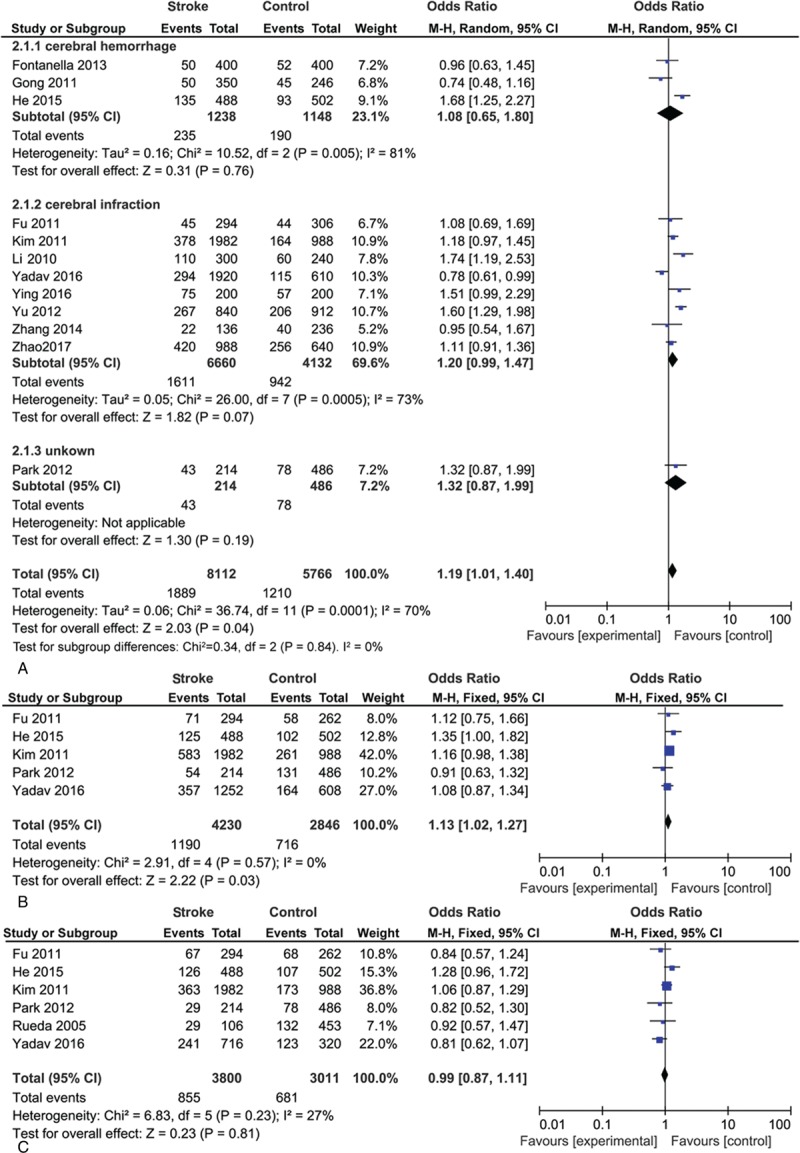
Overall odds ratio (ORs) for the association between vascular endothelial growth factor (VEGF) gene polymorphisms and the risk of stroke under allele contrast. (A) VEGF +936C/T. (B) VEGF −2578C/A. (C) VEGF −1154G/A.
3.2.2. VEGF −2578C/A
Analysis of the correlation of the VEGF susceptibility loci and stroke was discovered (allele contrast, OR = 1.13, 95% CI = 1.02–1.2, P = .03, Fig. 2B; dominant model, OR = 1.15, 95% CI = 1.00–1.32, P = .06; recessive model, OR = 1.27, 95% CI = 0.97–1.26, P = 1.67; and homozygous contrast, OR = 1.33, 95% CI = 0.65–1.01, P = .04).
3.2.3. VEGF −1154G/A
No correlation with stroke risk was found in −1154G/A polymorphism under all genetic models (allele contrast, OR = 0.99, 95% CI = 0.87–1.11, P = .81, Fig. 2C; dominant model, OR = 1.01, 95% CI = 0.87–1.17, P = .90; recessive model, OR = 0.98, 95% CI = 0.76–1.27, P = .13; and homozygous contrast, OR = 0.98, 95% CI = 0.75–1.29, P = .90). Table 4 also shows no difference between Asian and Caucasian.
3.3. Sensitivity analysis
We did a sensitivity analysis by removing each study in turn (Fig. 3). The results indicated no significant differences, suggesting that our results were fairly robust.
Figure 3.
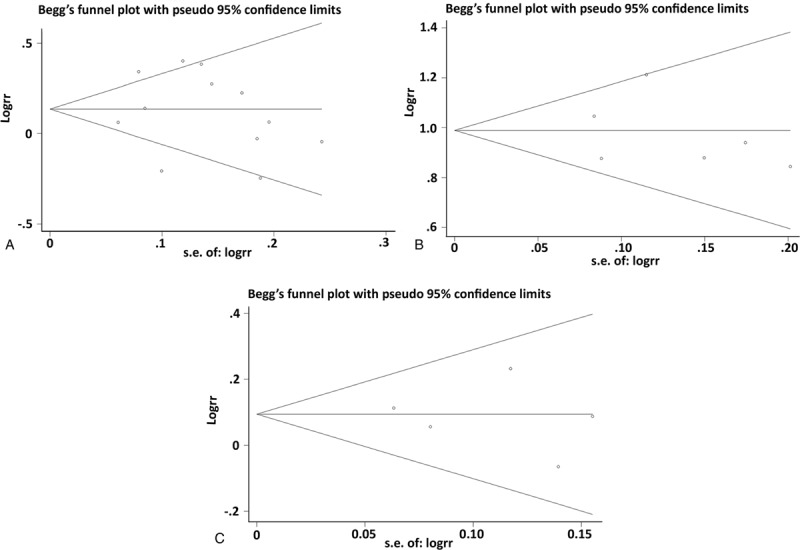
Sensitivity analysis of the summary odds ratio (OR) coefficients on the association between vascular endothelial growth factor (VEGF) gene polymorphisms and the risk of stroke under allele contrast. (A) VEGF +936C/T. (B) VEGF −2578C/A. (C) VEGF −1154G/A.
3.4. Publication bias
Begger funnel plot and Egger test were performed to assess the publication bias of included studies. No obvious evidence of publication bias was indicated by the results (Fig. 4, Table 5).
Figure 4.
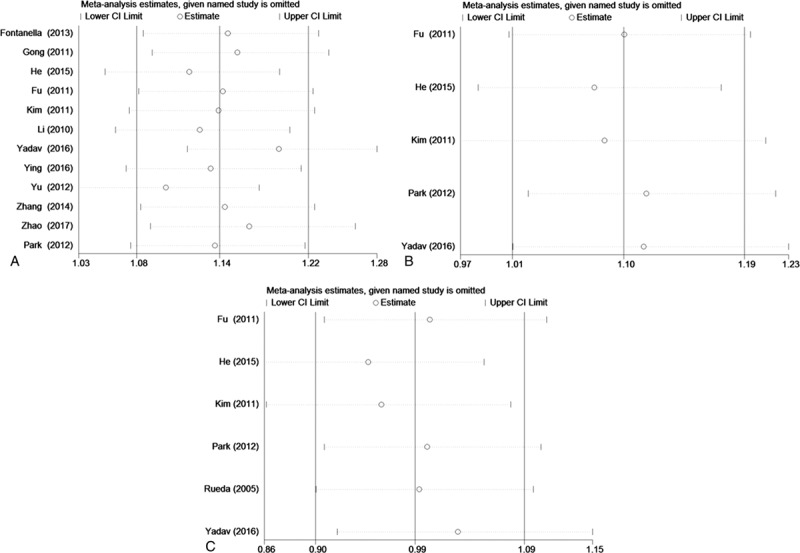
Begger funnel plot in assessing publication bias about vascular endothelial growth factor (VEGF) gene polymorphisms and stroke under allele contrast. (A) VEGF +936C/T. (B) VEGF −2578C/A. (C) VEGF −1154G/A.
Table 5.
Egger linear regression test to measure the funnel plot asymmetric under allele contrast.

4. Discussion
The VEGF, commonly known as vascular permeability factor, belongs to a gene family that contains mainly VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placental growth factor which has been extensively studied and concerned.[26] VEGF can maintain homeostasis of blood vessels and strengthen neoangiogenesis under physiologic or pathologic conditions.[27] It has demonstrated that VEGF, particularly in brain,[28] may mediate the response of increases in permeability and angiogenesis when exposed to hypoxia. They also take a critical role in inflammation, wound healing, as well as in cancer pathology. There are several evidences that VEGF +936C/T, −2578C/A, and −1154G/A SNPs are associated with the progress of several diseases.[18,29] Although genome-wide association studies on stroke have been published, the results remain controversial, which may be linked with sufficient sample size, appropriate control selection, standardized clinical classification, stroke subtypes, and so on.[3,4]
Thus, it is particularly important to explore what role it may play in the pathogenesis of stroke. Loss of vascular integrity is a critical step in ischemic brain tissue, which can promote the proliferation of endothelial cells induce by VEGF. Pericytes release angiopoietin-1 and participate in the formation of tight junctions that bind to the endothelial Tie-2 receptor.[30] VEGF can also counteract the maturation of newborn blood vessels by disrupting pericytes coverage of vessels.[31] On the contrary, VEGF can induce the proliferation of endothelial cells to participate in physiologic and pathologic angiogenesis after cerebral ischemia, and its expression level may increase the likelihood of stroke. In hemorrhagic brain tissue, VEGF may promote the formation of brain edema after subarachnoid hemorrhage[32] and increase the risk of plaque rupture.[33] The content of matrix metalloproteinase is positively correlated with the increasing risk of cerebral hemorrhage in patients with cerebral arteriovenous malformation, which may be linked with upregulated matrix metalloproteinase activity stimulated by the VEGF.[34] Although over expression of VEGF121 and VEGF165 accelerates the growth and breakdown of micro vessels around the tumor,[35] targeting neuropilin-1 or its cytoplasmic domain interactors may reduce VEGF165-induced edema.[36] The new blood vessels can not only improve the blood supply in the infarct area, but also cause bleeding due to the rupture of the new small blood vessels. Therefore, the release of VEGF at the right place and time is particularly crucial. This may explain that subgroup analysis showed that there was no difference between cerebral infarction and hemorrhage groups. It is possible to influence VEGF signaling pathway by using anti-VEGF therapy, especially in the management of neoplasms and ophthalmic diseases.[37] However, how the VEGF signaling pathway affects the pathogenesis of stroke is still unknown.
In the present study, the associations between 3 genetic loci in VEGF and stroke were investigated from 13 studies. We found genetic variations in +936C/T and −2578C/A were associated with the risk of stroke, especially in the Asian population, but not −1154G/A. There are several interpretations of this phenomenon. +936C/T resides in the 3-untranslated region which includes key regulatory elements. It is responsive to hypoxia.[38] +936C/T also associates with VEGF serum levels.[12] Even though the fact that both −2578C/A and −1154G/A are located at the promoter region, −2578C/A is correlated with a decreased VEGF expression.[39] Due to the complexity of angiogenesis mediated by VEGF, combined analysis of various pathways may be more feasible.
However, there were certain limitations in this meta-analysis. First, the number of studies in this meta-analysis was not enough. The risk assessment of +936C/T, −2578C/A, −1154G/A polymorphism, and stroke was based on unadjusted environmental effect estimates. If there are detailed personal data, more accurate analysis can be carried out. Second, 8 of the 13 articles were taken from China. Therefore, the regional distribution of the literature is more concentrated, which the results would have yet to be confirmed. Third, significant heterogeneity in +936C/T was found, which may be caused by different genotyping methods. Finally, +936C/T (OR = 1.19) and −2578C/A (OR = 1.13) showed a relatively small risk. Some researchers have showed that stroke is associated with multiple gene loci,[10,12,40] which may leads to a decrease in the probability of stroke at each gene locus. In addition, Yadav et al[13] had proved that there may be a joint effect between +936C/T and −2578C/A with the risk of stroke. However, we need more high-quality, large sample, multicenter studies to evaluate the relationship between VEGF polymorphism and stroke.
In conclusion, our meta-analysis suggests that VEGF −1154G/A polymorphism has no association with stroke risk, whereas VEGF +936C/T and −2578C/A might be associated with an increased risk of stroke.
Author contributions
All authors discussed the results and commented on the manuscript.
Conceptualization: Bingdong Xu, Rui Zhan, Yusheng Zhang.
Data curation: Bingdong Xu, Rui Zhan.
Formal analysis: Rui Zhan.
Investigation: Hongcheng Mai.
Methodology: Rui Zhan, Hongcheng Mai.
Project administration: Bingdong Xu.
Software: Rui Zhan.
Supervision: Yubin Liang.
Validation: Bingdong Xu.
Visualization: Bingdong Xu.
Writing – original draft: Bingdong Xu, Zhengdong Wu, Peizhi Zhu.
Writing – review & editing: Bingdong Xu, Yubin Liang, Yusheng Zhang.
Footnotes
Abbreviations: CI = confidence interval, HWE = Hardy–Weinberg equilibrium, LDR = ligase detection reaction, MALDI-TOF MS = matrix-assisted laser desorption/ionization time of flight mass spectrometry, NOS = Newcastle Ottawa scale, OR = odds ratio, PCR-RFLP = polymerase chain reaction-restriction fragment length polymorphism, SNPs = single-nucleotide polymorphisms, VEGF = vascular endothelial growth factor.
This work was supported by Guangzhou Science and Technology Program of China (2014Y2-00505, 201508020004), Natural Science Foundation of Guangdong Province (2014A030313384), and National Natural Science Foundation of China (81171084).
The authors have no conflicts of interest to disclose.
References
- [1].Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res 2017;120:439–48. [DOI] [PubMed] [Google Scholar]
- [2].Bevan S, Traylor M, Adib-Samii P, et al. Genetic heritability of ischemic stroke and the contribution of previously reported candidate gene and genomewide associations. Stroke 2012;43:3161–7. [DOI] [PubMed] [Google Scholar]
- [3].Dichgans M, Markus HS. Genetic association studies in stroke: methodological issues and proposed standard criteria. Stroke 2005;36:2027–31. [DOI] [PubMed] [Google Scholar]
- [4].Pruissen DM, Kappelle LJ, Rosendaal FR, et al. Genetic association studies in ischemic stroke: replication failure and prospects. Cerebrovasc Dis 2009;27:290–4. [DOI] [PubMed] [Google Scholar]
- [5].Greenberg DA, Jin K. Vascular endothelial growth factors (VEGFs) and stroke. Cell Mol Life Sci 2013;70:1753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vincenti V, Cassano C, Rocchi M, et al. Assignment of the vascular endothelial growth factor gene to human chromosome 6p21.3. Circulation 1996;93:1493–5. [DOI] [PubMed] [Google Scholar]
- [7].James JM, Gewolb C, Bautch VL. Neurovascular development uses VEGF-A signaling to regulate blood vessel ingression into the neural tube. Development 2009;136:833–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kong SY, Lee HL, Eom HS, et al. Reference intervals for circulating angiogenic cytokines. Clin Chem Lab Med 2008;46:545–50. [DOI] [PubMed] [Google Scholar]
- [9].Al-Habboubi HH, Sater MS, Almawi AW, et al. Contribution of VEGF polymorphisms to variation in VEGF serum levels in a healthy population. Eur Cytokine Netw 2011;22:154–8. [DOI] [PubMed] [Google Scholar]
- [10].Li X. The association between MCP-1, VEGF polymorphisms and their serum levels in patients with diabetic foot ulcer. Medicine 2018;97:e10959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wu T, Qiu S, Wang P, et al. The association between vascular endothelial growth factor gene polymorphisms and stroke: a meta-analysis. Brain Behav 2016;6:e00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yadav BK, Yadav R, Shin B-S. Single-nucleotide polymorphisms in vascular endothelial growth factor gene associated with stroke subtype in LAA and SVO. Int J Gerontol 2016;11:16–21. [Google Scholar]
- [14].Kim OJ, Hong SH, Oh SH, et al. Association between VEGF polymorphisms and homocysteine levels in patients with ischemic stroke and silent brain infarction. Stroke 2011;42:2393–402. [DOI] [PubMed] [Google Scholar]
- [15].Gong ZP, Qiao ND, Gu YX, et al. Polymorphisms of VEGFA gene and susceptibility to hemorrhage risk of brain arteriovenous malformations in a Chinese population. Acta Pharmacol Sin 2011;32:1071–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fontanella M, Gallone S, Panciani PP, et al. Vascular endothelial growth factor gene polymorphisms and intracranial aneurysms. Acta Neurochir (Wien) 2013;155:1511–5. [DOI] [PubMed] [Google Scholar]
- [17].Fu Y, Ni PH, Ma JF, et al. Polymorphisms of human vascular endothelial growth factor gene are associated with acute cerebral infarction in the Chinese population. Eur Neurol 2011;66:47–52. [DOI] [PubMed] [Google Scholar]
- [18].He QS, Yang LF, Wang WB, et al. Vascular endothelial growth factor gene is associated with hypertensive cerebellar hemorrhage and rehabilitative treatment. Genet Mol Res 2015;14:9849–57. [DOI] [PubMed] [Google Scholar]
- [19].Park YS, Jeon YJ, Kim HS, et al. The role of VEGF and KDR polymorphisms in moyamoya disease and collateral revascularization. PLoS One 2012;7:e47158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhao J, Bai Y, Jin L, et al. A functional variant in the 3′-UTR of VEGF predicts the 90-day outcome of ischemic stroke in Chinese patients. PLoS One 2017;12:e0172709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rueda B, Lopeznevot MA, Lopezdiaz MJ, et al. A functional variant of vascular endothelial growth factor is associated with severe ischemic complications in giant cell arteritis. J Rheumatol 2005;32:1737–41. [PubMed] [Google Scholar]
- [22].Li T, liao X, Wen G, et al. Single nucleotide polymorphisms 936C/T, (460T/C, 405G/C in vascular endothelial growth factor gene and their association with cerebral infarction. J Chongqing Med Univ 2010;35:1014–7. [Google Scholar]
- [23].Zhang H, Li G, Su F, et al. Gene polymorphisms and plasma concentration of vascular endothelial growth factor receptors and risk for TIA. Chin J Trauma Disability Med 2014;12:47. [Google Scholar]
- [24].Yu Y, Yu T, Wang M, et al. Association between 936C/T polymorphisms in vascular endothelial growth factor gene with cerebral infarction subtypes in the Han Chinese population. Available at: http://www.paper.edu.cn/releasepaper/content/201211-413. [Google Scholar]
- [25].Ying T, Fang JJ, Tao J, et al. Association of vascular endothelial growth factor single nucleotide polymorphisms with atherosclerotic cerebral infarction (Chinese). Mod Pract Med 2016;28:876–8. [Google Scholar]
- [26].Ferrara N, Gerber HP, Lecouter J. The biology of VEGF and its receptors. Nat Med 2003;9:669–76. [DOI] [PubMed] [Google Scholar]
- [27].Lee S, Chen TT, Barber CL, et al. Autocrine VEGF signaling is required for vascular homeostasis. Cell 2007;130:691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ogunshola OO, Stewart WB, Mihalcik V, et al. VEGF expression correlates with angiogenesis in postnatal developing rat brain. Dev Brain Res 2000;119:139–53. [DOI] [PubMed] [Google Scholar]
- [29].Li X, Lu Y, Wei P. Association between VEGF genetic variants and diabetic foot ulcer in Chinese Han population: a case–control study. Medicine 2018;97:e10672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci 2016;19:771–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hermann DM, Zechariah A. Implications of vascular endothelial growth factor for postischemic neurovascular remodeling. J Cereb Blood Flow Metab 2009;29:1620–43. [DOI] [PubMed] [Google Scholar]
- [32].Liu L, Fujimoto M, Kawakita F, et al. Vascular endothelial growth factor in brain edema formation after subarachnoid hemorrhage. Acta Neurochir Suppl 2016;121:173–7. [DOI] [PubMed] [Google Scholar]
- [33].Camaré C, Pucelle M, Nègre-Salvayre A, et al. Angiogenesis in the atherosclerotic plaque. Redox Biol 2017;12:18–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lee CZ, Xue Z, Zhu Y, et al. Matrix metalloproteinase-9 inhibition attenuates vascular endothelial growth factor-induced intracerebral hemorrhage. Stroke 2007;38:2563–8. [DOI] [PubMed] [Google Scholar]
- [35].Cheng SY, Nagane M, Huang HS, et al. Intracerebral tumor-associated hemorrhage caused by overexpression of the vascular endothelial growth factor isoforms VEGF121 and VEGF165 but not VEGF189. Proc Natl Acad Sci U S A 1997;94:12081–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Fantin A, Lampropoulou A, Senatore V, et al. VEGF165-induced vascular permeability requires NRP1 for ABL-mediated SRC family kinase activation. J Exp Med 2017;214:1049–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Pozarowska D, Pozarowski P. The era of anti-vascular endothelial growth factor (VEGF) drugs in ophthalmology, VEGF and anti-VEGF therapy. Cent Eur J Immunol 2016;41:311–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Liu Y, Cox SR, Morita T, et al. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5′ enhancer. Circ Res 1995;77:638–43. [DOI] [PubMed] [Google Scholar]
- [39].Mohammadi M, Bazrafshani MR, Day PJ, et al. Vascular endothelial growth factor production is regulated by gene polymorphisms. Iran J Immunol 2009;6:119–29. [PubMed] [Google Scholar]
- [40].Qiu S, Wu T, Wang P, et al. The Association between VEGFR gene polymorphisms and stroke: a meta-analysis. PLoS One 2016;11:e0151371. [DOI] [PMC free article] [PubMed] [Google Scholar]


