Supplemental Digital Content is available in the text
Keywords: chronic obstructive pulmonary disease (COPD), lung cancer, poor prognosis, postoperative complications
Abstract
Background:
Nowadays, there is growing recognition that chronic obstructive pulmonary disease (COPD) may have influence on lung cancer. However, coexisted COPD related to prognosis of lung cancer is still elusive. We conducted this meta-analysis to examine the association between COPD and 5-year overall survival (OS) and postoperative pulmonary complications of patients with lung cancer.
Methods:
A comprehensive computer-based online search was conducted using PubMed, Embase, Medline, and the Cochrane Library for articles published before September 30, 2017. We identified 29 eligible studies, which included 70,111 patients in the related literature.
Results:
Twenty-two of the 29 studies provided hazard ratio for OS (1.18, 95% confidence interval: 1.11–1.25; P < .001), it suggested that the presence of COPD indicated poor survival for the patients with lung cancer. In subgroup analysis, the relationship between COPD and OS occurrence remained statistically prominent in the subgroups stratified by study designs, COPD diagnosis timing, lung cancer surgery, cancer stage, and origins of patients. The presence of COPD increased the risk of bronchopleural fistula, pneumonia, prolonged air leakage, and prolonged mechanical ventilation.
Conclusions:
The present meta-analysis suggested that coexisting COPD is associated with poor survival outcomes in patients with lung cancer and higher rates of postoperative pulmonary complications.
1. Introduction
Lung cancer accounts for 12% of all cancers diagnosed worldwide, and becomes the most common malignancy. There are approximately over 1 million people died of lung cancer each year.[1] Despite the advances in diagnosis and mechanistic understanding of the pathophysiology of lung cancer, there has been little improvement in 5-year survival rates.[2,3] Furthermore, 50% to 70% of patients diagnosed with lung cancer have evidence of chronic obstructive pulmonary disease (COPD).[4] COPD is a chronic inflammatory disease featured by progressive destruction of lung tissues and airway obstruction. It is the most common pulmonary comorbidities and the third leading cause of death globally.[5] The recent studies found that the presence of COPD increased the risk of lung cancer and patients with coexistence of COPD were more susceptible to lung cancer.[6,7] In addition, pulmonary complications after lung cancer surgery are the major causes of morbidity for patients with COPD.[8]
Smoking habits could explain the association between COPD and lung cancer. The studies have found that COPD is an independent risk factor for lung cancer.[5,9] Recent studies have explored the clinical features and prognoses of patients with lung cancer, and the results suggested that COPD could induce the poor prognosis of lung cancer. However, the details of the relationship between COPD and prognosis of lung cancer are still elusive.
In the present study, we performed a meta-analysis to assess the impact of COPD on the overall survival (OS) of lung cancer patients and determine the incidence of various types of postoperative complications in these patients undergoing curative surgery in the presence or absence of COPD. The understanding of the relationship between COPD and prognosis of lung cancer will help physicians to develop the proper treatment of the lung cancer.
2. Materials and methods
2.1. Ethics statement
As all analyses were based on previously published studies, no ethical approval or patient consent was required.
2.2. Search strategy
A comprehensive literature search on the retrieved publications (the last search was done on September 30, 2017) was performed independently by 2 authors (Lin and Lu) associated with this current study. No language limitation was imposed during the retrieval. The primary sources for the literature search were the following electronic databases: PubMed, Embase, Medline, and the Cochrane Library. The search was limited by using the following search terms: (COPD OR chronic obstructive pulmonary disease) AND (lung OR pulmonary) AND (cancer OR neoplasms OR carcinoma OR tumor) AND (prognosis OR prognostic OR outcome OR survival). The title and abstract of each identified study were scanned to exclude any irrelevant publications. The remaining articles were reviewed to determine whether they contained information on the topic of interest. We also supplemented this search by examining the reference lists of all of the retrieved publications and by identifying additional relevant articles.
2.3. Eligibility criteria
We formulated the following inclusion and exclusion criteria to determine the eligible studies included in our meta-analysis.
Inclusion criteria:
-
(1)
studies compared patients with lung cancer with and without COPD;
-
(2)
lung cancer was the study's primary disease;
-
(3)
the target outcomes were survival related and (or) postoperative pulmonary complications rate;
-
(4)
the hazard ratio (HR) or odds ratio (OR) with corresponding 95% confidence interval (CI) was validly reported in original literature or generated by sufficient data;
-
(5)
studies where the total number of enrolled patients, as well as those with COPD, were both reported.
Exclusion criteria:
-
(1)
studies had no control patients;
-
(2)
studies reported postsurgical or in-hospital mortality only.
Letters, comments, review articles, conference proceedings, case-reports, and unpublished data were excluded. We included more recent articles with the largest sample sizes to avoid overlapping patient data in duplicate publications.
2.4. Data extraction
Data from the included studies were extracted and summarized independently by the 2 authors mentioned earlier (Lin and Lu). The extracted data primarily included (Table 1): first author, publication year, country, study design, sample sizes included the number of total patients and patients with COPD, cancer stage, whether to be treated by lung cancer surgery and COPD diagnosis timing. The target statistical data were 5-year OS and (or) postsurgical resection pulmonary complications rate in COPD group and non-COPD group, respectively.
Table 1.
Characteristics of included studies in the meta-analysis.

2.5. Assessment of study quality
The quality assessment of studies included in this article was undertaken by authors Lin and Lu. Newcastle–Ottawa scale (NOS) was employed to assist with the above-mentioned quality assessment.[10] Three perspectives including selection, comparability, and exposure were considered for a semiquantitative estimation. The “star system” with a maximum of 9 stars was used as the assessment tool. After grading all of the included studies, we regarded 8 to 9 stars as good quality; 6 to 7 stars as medium quality; and lower than 6 stars as poor quality.
2.6. Statistical analysis
We finally applied HR with 95% CI as the appropriate summarized statistics of the primary endpoint, OS. OR with 95% CI served as the summarized statistics for the association between COPD and risk of pulmonary complications. Remarkably, for OS, an HR >1 indicated the poorer survival for the COPD group. Meanwhile, a significant relationship between COPD and increased pulmonary complications risk could be proved when the pooled OR >1.
2.7. Heterogeneity, publication bias, and sensitivity test
Heterogeneity across studies was tested by using I2 statistic, which is a quantitative measure of inconsistency, with the suggested thresholds of 25% to 50% for low, 50% to 70% for moderate and >75% for high heterogeneity, respectively. If the I2 statistic was >50%, a random-effects model (DerSimonian and Laird) was used. Otherwise, fixed-effects models (inverse-variance method) were employed. Pooled effects were calculated and the P-values less than .05 were considered as statistically significant.[11]
For additional analysis, we performed a sensitivity analysis to further evaluate the stability of the summarized estimates. We removed the study which was identified to be associated with the increased heterogeneity and repeated a meta-analysis of the remaining studies for adjustments. The robustness of our meta-analysis would be confirmed if no substantial variation was identified between the adjusted estimates and primary estimates.[11]
Funnel plots, Egger and Begg tests,[12] and the Duval and Tweedie[13] “trim and fill” method were used for the evaluation of publication bias.
The present study employed Stata 12.0 software (StataCorp, College Station, Texas) to perform the aggregate data meta-analyses and evaluate the heterogeneity of the included studies.
3. Results
3.1. Study selection
Figure 1 summarizes the details of the electronic databases searching results. In brief, a total of 7433 studies were retrieved, 1350 (18.2%) studies were removed as duplicates. After title and abstract evaluation, 55 (0.74%) remained for the full-text review. Of these, 26 (0.35%) studies were additionally excluded for the following reasons: 6 (0.36%) were all COPD patients, no control patients; 2 (0.027%) had no relation to lung cancer; 2 (0.027%) not mainly focused on COPD; 3 (0.040%) reported lung cancer-specific survival only; 3 (0.040%) diagnosed COPD unclearly; 4 (0.054%) reported insufficient data; 2 (0.027%) from the same research team using the same database; 1 (0.013%) was case report; 1 (0.013%) was letter; 2 (0.027%) were reviews. Thus, 29 (0.39%) studies[9,14–41] (involving 70,111 patients) were deemed eligible after a comprehensive literature search. Of these 29 studies, 3 articles[9,16,22] presented data for both OS and postoperative pulmonary complications rate, 19 articles[15,17–20,23,24,26,28–30,32–37,39,40] reported OS only, 7 articles[14,21,25,27,31,38,41] reported the postoperative pulmonary complications rate only. Figure 1 shows the process involved in the assessment of the studies and in accordance to the process highlighted in the figure the studies were identified, those of them that fulfilled the conditions detailed earlier were included and those that did not were excluded.
Figure 1.

Flow diagram of studies identified, included, and excluded.
3.2. The quality of included studies
We identified that the average NOS score of these studies was 8 (range, 7–9), suggesting a generally good quality level (Table 1). The core “NOS” items and complete details are outlined in the Appendix Table 1 (see Table, Supplemental Digital Content 1, which illustrates the quality level of studies included in this article).
3.3. Meta-analysis of the association between COPD and lung cancer OS
Twenty-two of the 29 studies provided HR for OS.[9,15–20,22–24,26,28–30,32–37,39,40] On the basis of quantitative integrations of eligible statistics from all the included studies, the pooled HR was 1.18 (95% CI: 1.11–1.25) with heterogeneity across studies (I2 = 68.1%, P < .001), suggesting that COPD patients had increased risk of poorer OS compared with the non-COPD counterparts (P < .001) (Fig. 2).
Figure 2.
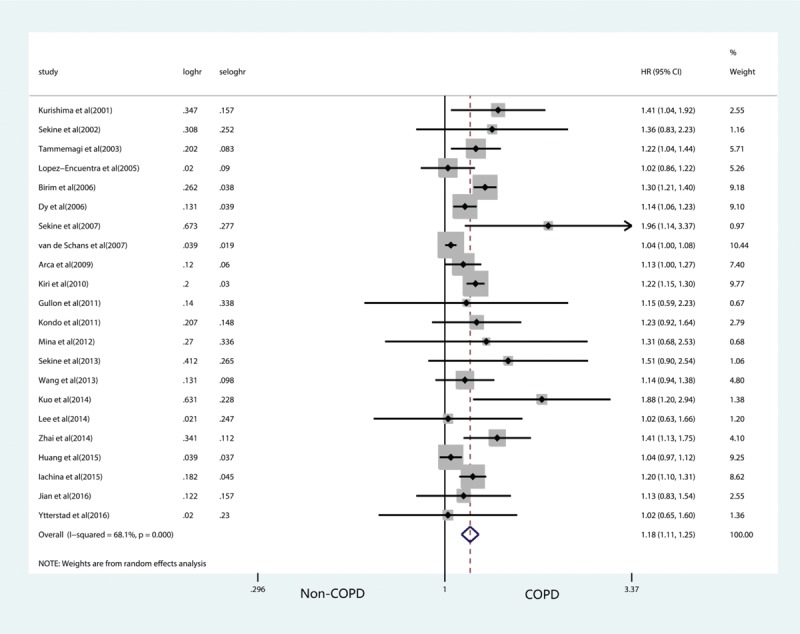
Forest plots of the impacts of COPD on lung cancer overall survival. COPD = chronic obstructive pulmonary disease.
3.4. Meta-analysis of the association between COPD and postoperative pulmonary complications
Ten studies[9,14,16,21,22,25,27,31,38,41] were included to assess the association between COPD and risk of pulmonary complications, as bronchopleural fistula (BPF), pneumonia, prolonged air leakage, prolonged mechanical ventilation, and empyema in patients undergoing lung cancer surgery. Eight studies[9,14,16,21,22,25,27,31] reported a frequency of BPF; 5[9,16,22,38,41] reported pneumonia; 3[16,38,41] reported prolonged air leakage; 3[9,16,22] reported prolonged mechanical ventilation; 3[9,22,38] reported empyema.
According to the level of heterogeneity (BPF: I2 = 20.1%; pneumonia: I2 = 0.0%; prolonged mechanical ventilation: I2 = 2.9%; empyema: I2 = 0.0%), 4 studies (BPF group; pneumonia group; prolonged mechanical ventilation group; empyema group) used fixed-effects models, whereas the remaining 1 (prolonged air leakage: I2 = 51.6%) used a random-effects model.
For BPF, the overall OR was 1.84 (95% CI: 1.30–2.60; P = .001; Fig. 3A), indicating that the incidence of BPF in patients with lung cancer and COPD was higher than that in patients with lung cancer who did not have COPD. For pneumonia, the overall summarized OR was 3.62 (95% CI: 2.60–5.03; P < .001; Fig. 3B), that demonstrated COPD was significantly associated with the risk of pneumonia after lung cancer surgery. COPD could also significantly predispose to prolonged air leakage and prolonged mechanical ventilation formation in patients undergoing lung cancer surgery, the pooled OR was 2.77 (95% CI: 1.30–5.90; P = .008; Fig. 3C) and 2.60 (95% CI: 1.52–4.43; P < .001; Fig. 3D), respectively. However, the pooled OR for empyema revealed that there was no significant difference in the incidence of empyema between lung cancer with or without COPD (1.45, 95% CI: 0.69–3.03; P = .330; Fig. 3E).
Figure 3.

Overall analysis for the association between COPD and risk of pulmonary complications in patients undergoing lung cancer surgery. (A) BPF; (B) pneumonia; (C) prolonged air leakage; (D) prolonged mechanical ventilation; (E) empyema. BPF = bronchopleural fistula, COPD = chronic obstructive pulmonary disease.
3.5. Subgroup analysis of the impacts of COPD on lung cancer OS
To further evaluate the impact of COPD on lung cancer OS, we classified all cases into 5 subgroups according to study designs, COPD diagnosis timing, lung cancer surgery, cancer stage, and origins of patients.
The results of subgroup analysis confirmed that the OS of lung cancer in COPD group was worse (each group's P value was less than .05). In the subgroups stratified by study designs, 14 published retrospective HR outcomes.[9,15–17,19,22,24,26,29,30,32–35] The integrated HR of these 14 studies was 1.25 (95% CI: 1.19–1.31; I2 = 9.6%, P < .001), indicating that COPD was significantly associated with poorer OS. The summarized estimates integrating the clinical data of prospective studies from 8 articles[18,20,23,28,36,37,39,40] also suggested that poorer OS in patients with COPD (HR: 1.09, 95% CI: 1.03–1.14; I2 = 43.8%, P = .001). For the timing of COPD diagnosis, 12 studies diagnosed COPD after lung cancer diagnosis[9,15–19,26,29,30,34,35,40] (HR: 1.24, 95% CI: 1.19–1.29; I2 = 0.0%, P < .001), 2 studies did not report the diagnosis timing[22,33] (HR: 1.91, 95% CI: 1.35–2.70; I2 = 0.0%, P < .001), 8 studies diagnosed COPD diagnosis before the time of lung cancer diagnosis[20,23,24,28,32,36,37,39] (HR: 1.10, 95% CI: 1.05–1.15; I2 = 47.1%, P < .001). Subgroup analyzed according to other variables, such as the origins of patients, cancer stage, and whether to be treated by lung cancer surgery, unanimously indicated that COPD patients had increased risks of the poorer OS compared with the non-COPD counterparts. Their results are summarized in Table 2.
Table 2.
Meta-analysis of the association between COPD and lung cancer overall survival.

3.6. Sensitivity analysis of the impacts of COPD on lung cancer OS
We evaluated the effect of each study on the pooled results by excluding single study sequentially; the forest plot derived from sensitivity analysis was shown in Figure 4. None of the individual HR for OS statistics was out of the estimated ranges by visual inspection, and no substantial variation interfere the primary summarized HR was observed, indicating that the data were not overly influenced by any studies. Therefore, the leave-one-out approach and further adjustments of heterogeneity were no longer necessary. The strong rationality and reliability of our meta-analysis of the OS were thus confirmed.
Figure 4.

Sensitivity analysis of the impacts of COPD on lung cancer overall survival. COPD = chronic obstructive pulmonary disease.
3.7. Publication bias
Visual inspection of the funnel plot indicated certain asymmetry for the association between COPD and lung cancer OS (Fig. 5A). However, no evidence for significant publication bias was detected by visually inspecting the symmetry of the funnel plot conducted through the trim and fill method (7 studies trimmed; Fig. 5B). Before adjustment, the HR = 0.151 (95% CI: 0.089–0.213; P < .001), and the adjusted value HR = 0.110 (95% CI: 0.049–0.171; P < .001), suggesting that the change of the summary results after trim and fill was not obvious. Furthermore, the Begg and Egger test did not show significant evidence of publication bias (Begg test, P = .756; Egger test, P = .105). We did not examine the publication bias for the meta-analysis for the association of COPD and postoperative pulmonary complications, due to insufficient studies available to render a valid statistical test.
Figure 5.

Publication bias for the association between COPD and lung cancer overall survival. (A) Funnel plot; (B) Funnel plot by using trim and fill method. COPD = chronic obstructive pulmonary disease.
4. Discussion
The main results of our meta-analysis of 29 eligible studies with 70,111 patients suggested that coexisting COPD is associated with poor survival outcomes and higher rates of postoperative pulmonary complications in patients with lung cancer. To our knowledge, this is the latest meta-analysis focused on the prognostic value of concomitant COPD on lung cancer survival.
In addition, a subgroup analysis was performed to explore the heterogeneity in 5 variables: study design, COPD diagnosis timing, lung cancer surgery, origins of patients, and staging of lung cancer. By grouping the studies according to the timing of COPD diagnosis, we found the I2 was lowered from 68.1% to 0.0%, 47.1%, 0.0% in 3 subgroups, respectively. With subgroup analysis of the present study, the change of heterogeneity was also significant. Therefore, the timing of COPD diagnosis and study design may have accounted for the majority of heterogeneity.
Lung cancer and COPD share a common environmental risk factor in cigarette smoke exposure. Interestingly, COPD which is characterized by chronic inflammation of the lower airway is also a major independent risk factor for lung carcinoma among the long-term smokers. The evidence suggested that COPD related to poor prognosis in patients with lung cancer was biologically plausible. COPD is characterized by local (pulmonary) and chronic systemic inflammation, which may result in repeating injury and repair, stimulating cell turnover, potential genetic errors, and ultimately inducing lung cancer.[42,43] Increased oxidative stress in COPD induced DNA damage and carcinogenesis DNA repair capacity, which is associated with poorer survival in patients with lung cancer.[44,45] Furthermore, COPD is associated with abnormal apoptosis, cell cycle regulation,[46] and epigenetic alterations,[47,48] which is a critical mechanism implicated in the prognosis of lung cancer.[49]
The mechanism of the correlation between prognosis and the immunologic status of the patients is also worth to be explored. A new study[50] reported that COPD was associated with an increased risk of the lung cancer and an aberrant microbiota of the lung. IL-17C mediated the recruitment of tumor-associated neutrophils and lung tumor growth. In addition, Dr Zhu et al.[51] aimed at identifying candidate biomarker predicting lung cancer risk among patients with chronic respiratory diseases. They concluded that inflammatory conditions could lead to increased myocyte enhancer factor 2D (MEF2D) expression, and contribute to the development of lung cancer by influencing cancer microenvironment and cell bio-behaviors. MEF2D might be a potential biomarker to predict the risk of lung cancer among patients with chronic respiratory diseases.
The present results suggested that the presence of COPD was associated with poorer OS than for patients without by analyzing 29 eligible studies including 70,111 patients. The presence of COPD also increased the risk of BPF, pneumonia, prolonged air leakage, and prolonged mechanical ventilation.
However, we need to promote more analysis on the following limitations. One was that unadjusted estimates and more accurate results would come from adjustments for other confounders such as cardiovascular comorbidity, smoking status preresection and postresection, and the criteria for excluding patients from resection. Another is that present study did not mention the different degrees of COPD severity for prognostic value of lung cancer because of the scarcity of available data from these literatures.
5. Conclusions
The results of our meta-analysis for the first time demonstrated that coexisting COPD was related to the poor survival outcomes and higher rates of postoperative pulmonary complications in patients with lung cancer. Patients with lung cancer need special attention when they are diagnosed with COPD. However, further clinical trials are needed to verify and modify the relationship between severity degree, the timing of COPD and prognosis of lung cancer in the future.
Acknowledgment
The authors thank Dr Chaiyakiat Eamratsameekool for proofreading of the manuscript.
Author contributions
Conceptualization: Hefeng Lin and Yunlong Lu, Junqiang Fan.
Data curation: Hefeng Lin, Yunlong Lu, Ke Meng.
Formal analysis: Hefeng Lin and Ke Meng.
Funding acquisition: Junqiang Fan.
Investigation: Hefeng Lin and Liya Lin.
Methodology: Hefeng Lin, Junqiang Fan.
Project administration: Junqiang Fan.
Resources: Hefeng Lin, Junqiang Fan.
Supervision: Junqiang Fan.
Validation: Hefeng Lin, Junqiang Fan.
Visualization: Hefeng Lin.
Writing – original draft: Hefeng Lin.
Writing – review and editing: Yunlong Lu, Liya Lin, Hefeng Lin, Junqiang Fan.
Supplementary Material
Footnotes
Abbreviations: BPF = bronchopleural fistula, CI = confidence interval, COPD = chronic obstructive pulmonary disease, HR = hazard ratio, IL-17C= interleukin-17C, MEF2D= myocyte enhancer factor 2D, NOS = Newcastle–Ottawa scale, OR = odds ratio, OS = overall survival.
The work has not been published previously (except in the form of an abstract or as part of a published lecture or academic thesis or as an electronic preprint).
The study is supported by the Natural Science Foundation of Zhejiang Province (LY15H160034), China.
The authors declare that they have no conflict of interest.
Supplemental Digital Content is available for this article.
References
- [1].Goldstraw P, Ball D, Jett JR, et al. Non-small-cell lung cancer. Lancet 2011;378:1727–40. [DOI] [PubMed] [Google Scholar]
- [2].Luchtenborg M, Jakobsen E, Krasnik M, et al. The effect of comorbidity on stage-specific survival in resected non-small cell lung cancer patients. Eur J Cancer 2012;48:3386–95. [DOI] [PubMed] [Google Scholar]
- [3].Sen F, Tambas M, Ozkaya K, et al. Concomitant etoposide and cisplatin provided improved survival compared with docetaxel and cisplatin in patients with locally advanced non-small cell lung cancer treated with chemoradiotherapy. Medicine (Baltimore) 2016;95:e4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Read WL, Tierney RM, Page NC, et al. Differential prognostic impact of comorbidity. J Clin Oncol 2004;22:3099–103. [DOI] [PubMed] [Google Scholar]
- [5].Rennard SI, Drummond MB. Early chronic obstructive pulmonary disease: definition, assessment, and prevention. Lancet 2015;385:1778–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sin DD, Man SF. Impact of cancers and cardiovascular diseases in chronic obstructive pulmonary disease. Curr Opin Pulm Med 2008;14:115–21. [DOI] [PubMed] [Google Scholar]
- [7].Jian ZH, Lung CC, Huang JY, et al. The coexistence of common pulmonary diseases on the histologic type of lung cancer in both genders in Taiwan: a STROBE-compliant article. Medicine (Baltimore) 2014;93:e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Licker MJ, Widikker I, Robert J, et al. Operative mortality and respiratory complications after lung resection for cancer: impact of chronic obstructive pulmonary disease and time trends. Ann Thorac Surg 2006;81:1830–7. [DOI] [PubMed] [Google Scholar]
- [9].Sekine Y, Suzuki H, Yamada Y, et al. Severity of chronic obstructive pulmonary disease and its relationship to lung cancer prognosis after surgical resection. Thorac Cardiovasc Surg 2013;61:124–30. [DOI] [PubMed] [Google Scholar]
- [10].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [11].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [13].Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. [DOI] [PubMed] [Google Scholar]
- [14].Algar FJ, Alvarez A, Aranda JL, et al. Prediction of early bronchopleural fistula after pneumonectomy: a multivariate analysis. Ann Thorac Surg 2001;72:1662–7. [DOI] [PubMed] [Google Scholar]
- [15].Kurishima K, Satoh H, Ishikawa H, et al. Lung cancer patients with chronic obstructive pulmonary disease. Oncol Rep 2001;8:63–5. [PubMed] [Google Scholar]
- [16].Sekine Y, Behnia M, Fujisawa T. Impact of COPD on pulmonary complications and on long-term survival of patients undergoing surgery for NSCLC. Lung Cancer 2002;37:95–101. [DOI] [PubMed] [Google Scholar]
- [17].Tammemagi CM, Neslund-Dudas C, Simoff M, et al. Impact of comorbidity on lung cancer survival. Int J Cancer 2003;103:792–802. [DOI] [PubMed] [Google Scholar]
- [18].Lopezencuentra A, Astudillo J, Cerezal J, et al. Prognostic value of chronic obstructive pulmonary disease in 2994 cases of lung cancer. Eur J Cardio-Thorac Sur 2005;27:8–13. [DOI] [PubMed] [Google Scholar]
- [19].Birim O, Kappetein AP, Waleboer M, et al. Long-term survival after non-small cell lung cancer surgery: development and validation of a prognostic model with a preoperative and postoperative mode. J Thorac Cardiovasc Surg 2006;132:491–8. [DOI] [PubMed] [Google Scholar]
- [20].Dy SM, Sharkey P, Herbert R, et al. Comorbid illnesses and health care utilization among medicare beneficiaries with lung cancer. Crit Rev Oncol Hematol 2006;59:218–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yena S, Doddoli C, Doumbia S, et al. Bronchial fistula postpneumonectomy: predictive factors. Ann Chir 2006;131:22–6. [DOI] [PubMed] [Google Scholar]
- [22].Sekine Y, Yamada Y, Chiyo M, et al. Association of chronic obstructive pulmonary disease and tumor recurrence in patients with stage IA lung cancer after complete resection. Ann Thorac Surg 2007;84:946–50. [DOI] [PubMed] [Google Scholar]
- [23].van de Schans SA, Janssen-Heijnen ML, Biesma B, et al. COPD in cancer patients: higher prevalence in the elderly, a different treatment strategy in case of primary tumours above the diaphragm, and a worse overall survival in the elderly patient. Eur J Cancer 2007;43:2194–202. [DOI] [PubMed] [Google Scholar]
- [24].Abal Arca J, Parente Lamelas I, Almazan Ortega R, et al. Lung cancer and COPD: a common combination. Arch Bronconeumol 2009;45:502–7. [DOI] [PubMed] [Google Scholar]
- [25].Panagopoulos ND, Apostolakis E, Koletsis E, et al. Low incidence of bronchopleural fistula after pneumonectomy for lung cancer. Interact Cardiovasc Thorac Surg 2009;9:571–5. [DOI] [PubMed] [Google Scholar]
- [26].Kiri VA, Soriano J, Visick G, et al. Recent trends in lung cancer and its association with COPD: an analysis using the UK GP Research Database. Prim Care Respir J 2010;19:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lindner M, Hapfelmeier A, Morresi-Hauf A, et al. Bronchial stump coverage and postpneumonectomy bronchopleural fistula. Asian Cardiovasc Thorac Ann 2010;18:443–9. [DOI] [PubMed] [Google Scholar]
- [28].Gullon JA, Suarez I, Medina A, et al. Role of emphysema and airway obstruction in prognosis of lung cancer. Lung Cancer 2011;71:182–5. [DOI] [PubMed] [Google Scholar]
- [29].Kondo R, Yoshida K, Eguchi T, et al. Clinical features of lung cancer in smokers with light and mild chronic obstructive pulmonary disease: a retrospective analysis of Japanese surgical cases. Eur J Cardiothorac Surg 2011;40:1439–43. [DOI] [PubMed] [Google Scholar]
- [30].Mina N, Soubani AO, Cote ML, et al. The relationship between chronic obstructive pulmonary disease and lung cancer in African American patients. Clin Lung Cancer 2012;13:149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hu XF, Duan L, Jiang GN, et al. A clinical risk model for the evaluation of bronchopleural fistula in non-small cell lung cancer after pneumonectomy. Ann Thorac Surg 2013;96:419–24. [DOI] [PubMed] [Google Scholar]
- [32].Wang HM, Liao ZX, Komaki R, et al. Improved survival outcomes with the incidental use of beta-blockers among patients with non-small-cell lung cancer treated with definitive radiation therapy. Ann Oncol 2013;24:1312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kuo CH, Wu CY, Lee KY, et al. Chronic obstructive pulmonary disease in stage I non-small cell lung cancer that underwent anatomic resection: the role of a recurrence promoter. COPD 2014;11:407–13. [DOI] [PubMed] [Google Scholar]
- [34].Lee SJ, Lee J, Park YS, et al. Impact of chronic obstructive pulmonary disease on the mortality of patients with non-small-cell lung cancer. J Thorac Oncol 2014;9:812–7. [DOI] [PubMed] [Google Scholar]
- [35].Zhai R, Yu X, Shafer A, et al. The impact of coexisting COPD on survival of patients with early-stage non-small cell lung cancer undergoing surgical resection. Chest 2014;145:346–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Huang JY, Jian ZH, Ndi Nfor O, et al. The impact of coexisting asthma, chronic obstructive pulmonary disease and tuberculosis on survival in patients with lung squamous cell carcinoma. PLoS One 2015;10:e0133367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Iachina M, Jakobsen E, Moller H, et al. The effect of different comorbidities on survival of non-small cells lung cancer patients. Lung 2015;193:291–7. [DOI] [PubMed] [Google Scholar]
- [38].Yoshida Y, Kage H, Murakawa T, et al. Worse prognosis for stage IA lung cancer patients with smoking history and more severe chronic obstructive pulmonary disease. Ann Thorac Cardiovasc Surg 2015;21:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jian ZH, Huang JY, Nfor ON, et al. Pre-existing pulmonary diseases and survival in patients with stage-dependent lung adenocarcinoma: a STROBE-compliant article. Medicine (Baltimore) 2016;95:e2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ytterstad E, Moe PC, Hjalmarsen A. COPD in primary lung cancer patients: prevalence and mortality. Int J Chron Obstruct Pulmon Dis 2016;11:625–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Takegahara K, Usuda J, Inoue T, et al. Preoperative management using inhalation therapy for pulmonary complications in lung cancer patients with chronic obstructive pulmonary disease. Gen Thorac Cardiovasc Sur 2017;65:388–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lee G, Walser TC, Dubinett SM. Chronic inflammation, chronic obstructive pulmonary disease, and lung cancer. Curr Opin Pulm Med 2009;15:303–7. [DOI] [PubMed] [Google Scholar]
- [43].O’Callaghan DS, O’Donnell D, O’Connell F, et al. The role of inflammation in the pathogenesis of non-small cell lung cancer. J Thorac Oncol 2010;5:2024–36. [DOI] [PubMed] [Google Scholar]
- [44].Bosken CH. An analysis of DNA repair as a determinant of survival in patients with non-small-cell lung cancer. CancerSpectr Knowl Environ 2002;94:1091–9. [DOI] [PubMed] [Google Scholar]
- [45].Anderson GP, Bozinovski S. Acquired somatic mutations in the molecular pathogenesis of COPD. Trends Pharmacol Sci 2003;24:71–6. [DOI] [PubMed] [Google Scholar]
- [46].Demedts IK, Demoor T, Bracke KR, et al. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir Res 2006;7:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Liloglou T, Bediaga NG, Brown BR, et al. Epigenetic biomarkers in lung cancer. Cancer Lett 2014;342:200–12. [DOI] [PubMed] [Google Scholar]
- [48].Qiu W, Baccarelli A, Carey VJ, et al. Variable DNA methylation is associated with chronic obstructive pulmonary disease and lung function. Am J Respir Crit Care Med 2012;185:373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Singhal S, Vachani A, Antin-Ozerkis D, et al. Prognostic implications of cell cycle, apoptosis, and angiogenesis biomarkers in non-small cell lung cancer: a review. Clin Cancer Res 2005;11:3974–86. [DOI] [PubMed] [Google Scholar]
- [50].Jungnickel C, Schmidt LH, Bittigkoffer L, et al. IL-17C mediates the recruitment of tumor-associated neutrophils and lung tumor growth. Oncogene 2017;36:4182–90. [DOI] [PubMed] [Google Scholar]
- [51].Zhu HX, Shi L, Zhang Y, et al. Myocyte enhancer factor 2D provides a cross-talk between chronic inflammation and lung cancer. J Transl Med 2017;15:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


