Abstract
To investigate the features and prognosis of the elderly patients with pancreatic neuroendocrine tumor (pNET).
The patients diagnosed with pNETs between 2004 and 2014 were identified from the Surveillance Epidemiology and End Results database. The ethical approval was waived because the present study was analysis of the data from Surveillance Epidemiology and End Results database.
A total of 4608 patients with “one primary only” histologically pNETs were confirmed and 653 were older than 75 years. Cancer-specific survival (CSS) and overall survival (OS) were examined. The elderly patients (≥75 years) have disadvantage in CSS and OS compared with younger cohort. Multivariate logistic regression revealed that the elderly patients have increased poorly differentiated composition, and decreased proportion of Black patients, receipt of surgery, married status, and number of removed lymph node. Multivariate Cox regression analysis demonstrated worse differentiation. Patients of T3–4 and M1 stage were associated with poor CSS, while patients of being female, tumor locating at pancreatic body/tail, receipt of surgery, and being married were associated with better CSS in the elderly patients. Meanwhile, patients with higher histological grade and M1 stage have poor OS, while patients with the characteristics of female, being married, tumor location at pancreatic body/tail and tumor surgery have better OS. Distant metastatic elderly patients underwent primary site surgery had better CSS and OS than the patients without surgery.
The elderly patients have increased possibility of poorly differentiated tumor, and decreased proportion of Black patients, surgery of primary site, number of removed lymph node and married status. Worse differentiation and tumor metastasis were independent risk factors for both CSS and OS, while primary tumor located in body/tail of pancreas, female patients, surgery of tumor primary site, and being married were protective factors.
Keywords: elderly cohort, pancreatic neuroendocrine tumor
1. Introduction
The neuroendocrine tumors (NETs) originate from neuroendocrine cells and may occur in many organs, including lung, gastrointestinal tract, and pancreas. Among them, gastroenteropancreatic NETs account for 65–75% of the whole body NETs.[1] Pancreatic NETs (pNETs) comprise approximately half of the gastroenteropancreatic NETs, and account for approximately <3% of all pancreatic malignancies.[2] As a rare pancreatic neoplasm with an annual incidence of 0.19/100,000–0.32/100,000,[3–5] the incidence of pNET has been rising in the United States over the past several decades.[6] A previous study exhibited that most patients with pNETs possessed the characteristics of indolent physiological behavior and longer survival time than those with other pancreatic malignancies.[7] The 5-year overall survival (OS) rates of patients with NETs were >90% both in China and the United States, and the 5-year disease-free survival rates were also similar in the Chinese and American patients: 63.6% vs. 66.9%.[7] However, pNETs are highly heterogenous tumors and may develop aggressive invasion or fatal metastasis.[8,9]
In the recent decades, owing to an increase in the aging population, the incidence of malignancies among the elderly is increasing.[10–12]
The characteristics of the elderly patients suffering from malignancies are different from those of other patients.[11,13,14] Numerous studies across various malignancies have shown that advanced age is associated with decreased referral to specialists, increased delivery of suboptimal therapy, and increased patient refusal of therapy.[11,13,14] Thereby, the effect of the treatment in the elderly is probably worse than that in the young patients. To this day, the characteristics of the elderly cohort suffering from pNETs are poorly defined. In order to analyze the characteristics and the more effective way of treatment for elderly patients suffering from pNETs, we collected and studied the data from the Surveillance Epidemiology and End Results (SEER) database.
2. Methods
2.1. Database and patient cohort
The SEER database was searched for patients with histologically confirmed pNET from 2004 to 2014, using the SEER∗Stat software version 8.3.5 (accession number: 15076-Nov2016). We defined pNET to include the following International Classification of Diseases for Oncology third edition (ICD-O3) codes: 8150/3, 8151/3, 8152/3, 8153/3, 8155/3, 8156/3, 8240/2, 8240/3, 8241/3, 8242/3, 8243/3, 8246/2, 8246/3, and 8249/3. We included all the pancreatic anatomical sites (C25.0–C25.9) in our study. The patients who had 2 or more malignancies were excluded. An elderly person was defined as age ≥75 years based on the definition by the WHO.[15]
2.2. Patient data collection and outcome measurement
Variables regarding gender, race, primary site of tumor, histological grade, T stage, N stage, M stage, primary site surgery (yes or no), marital status, survival months, and cause-specific death classification were collected.
The primary outcome measure was cancer-specific survival (CSS). CSS was defined as the time elapsed from the diagnosis to death attributable to the pNET. The secondary outcome measure was OS, which was defined as the duration from the diagnosis to death from any cause. The information on systemic treatment was not provided by the SEER database.
2.3. Statistical analyses
Pearson chi-squared test and multivariate logistic regression were utilized to compare the differences in the demographic and tumor characteristics between the younger (<75) and elderly patients. Multivariate Cox proportional hazards model was used to identify the factors which were independently associated with CSS and OS. The Kaplan–Meier analysis with the log-rank test was used to describe the CSS and OS. P < 0.05 was considered as statistically significant. All statistical analyses were performed using the IBM SPSS Statistics 22.0 (IBM, Armonk, NY).
3. Result
3.1. Clinicopathological features of the patients
A total of 6034 patients with histologically confirmed pNETs were extracted from the SEER database, and those with 2 or more malignancies were excluded. In all, 4608 patients with 1 primary-only pNETs were included, of which, 3955 were <75 years (85.8%, mean age 55.69 ± 11.82) and 653 patients ≥75 years (14.2%, mean age 80.24 ± 4.19). The baseline characteristics of both groups were compared using the Chi-squared analysis as shown in Table 1. Gender composition (P = .080), metastasis status (P = .475), and surgery of other region or disseminated site (P = .052) were compared between the 2 groups. Significant differences were detected regarding racial predilection (P = .014), tumor primary site (P = .048), histological grade (P < .001), T stage (P = .002), N stage (P = .007), primary site surgery (P < .001), resected number of regional lymph node (P < .001), and marital status (P < .001).
Table 1.
Chi-squared test of characteristics between elderly and younger patients.
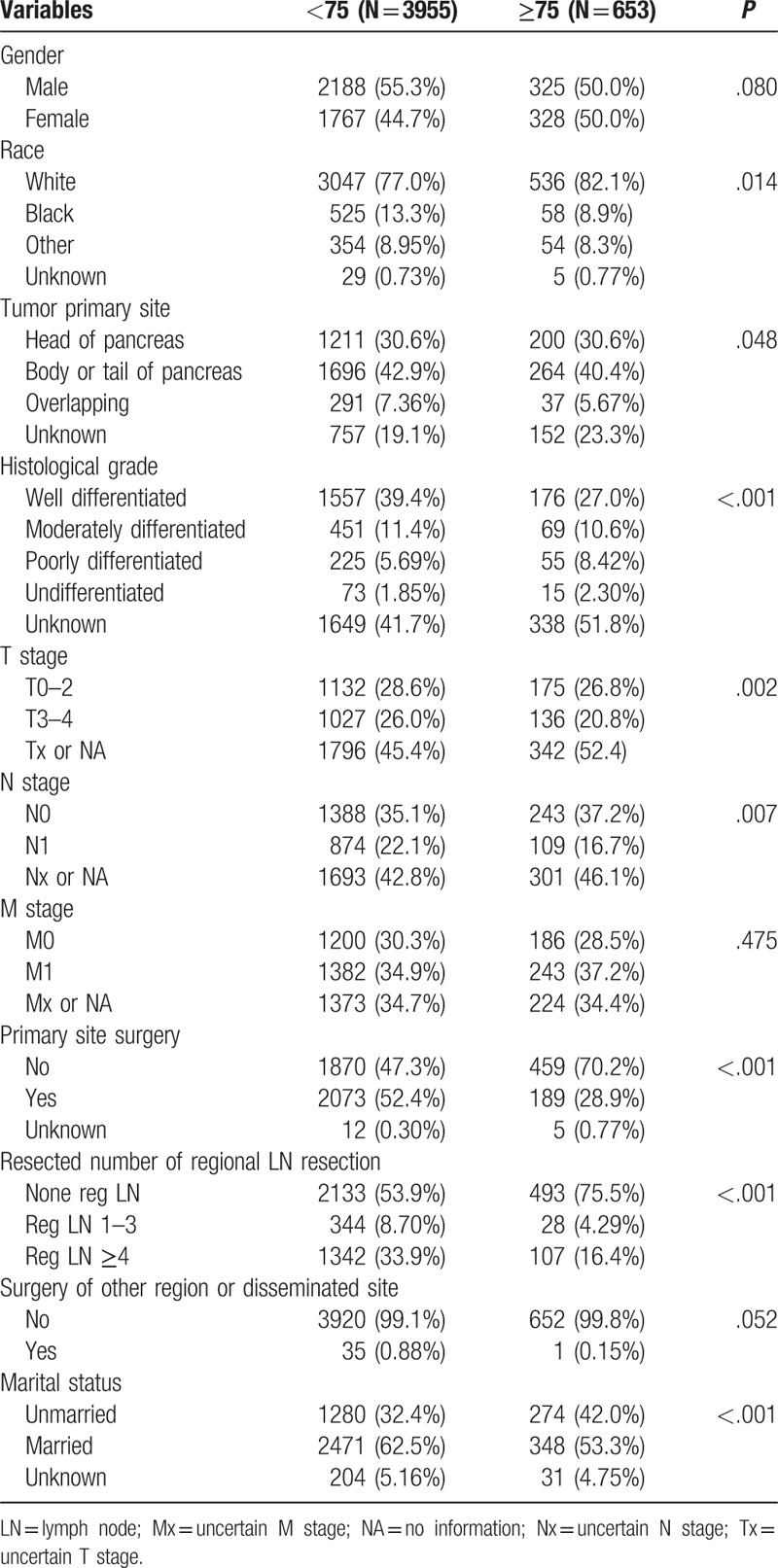
3.2. Multivariate logistic regression reveals characteristics associated with elder patients of pNETs
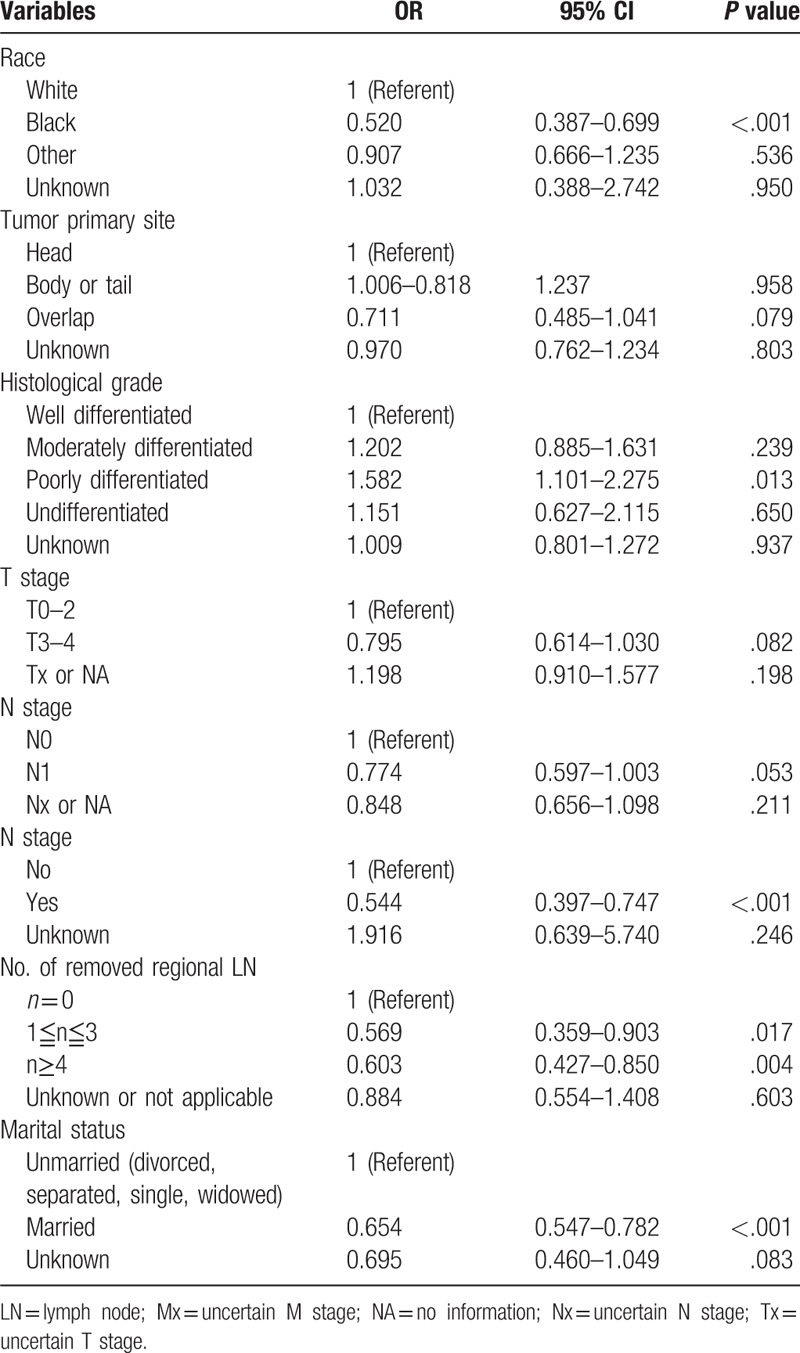
The differing variables between the 2 groups as revealed in the chi-squared test were selected and further analyzed using the multivariate logistic regression. The elderly patients showed decreased predilection for the Black race (P < .001, Odds ratio (OR) 0.520, 95% CI 0.387–0.699), and increased possibility of poorly differentiated tumor (P = .013, OR 1.582, 95% CI 1.101–2.275), primary site surgery (P < .001, OR 0.544, 95% CI 0.397–0.747), number of resected lymph nodes (1≤n≤3:P = .017, OR 0.569, 95% CI 0.359–0.903; n≥4: P = .004, OR 0.603, 95% CI 0.427–0.850), and married status (P < .001, OR 0.654, 95% CI 0.547–0.782) (Table 2).
Table 2.
Multivariate logistic regression analysis of clinicopathological characteristics.
3.3. CSS and OS outcomes and independent factors associated with survival
Younger patients with pNETs had better survival outcomes regarding CSS and OS than the elderly, according to the Kaplan–Meier mortality analysis with log-rank test P < .001 (Fig. 1). The 3 and 5-year CSS rates were 67.3% and 58.0% in the younger cohort, and 44.6% and 35.7% in the elderly patients, respectively. Similarly, the 3 and 5-year OS rates were 64.5% and 37.7% in the younger cohort, and 54.0% and 27.7% in the elderly, respectively.
Figure 1.
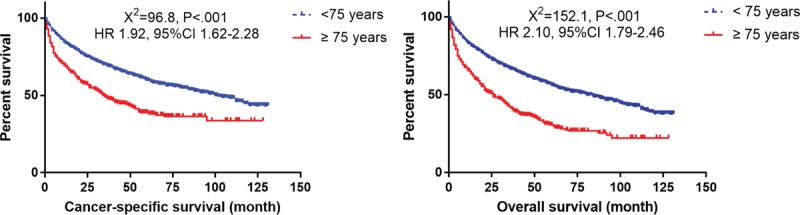
Survival curves with log-rank test between elderly patients (≥75) and younger patients (<75).
The elderly patients had worse CSS and OS rates than did the younger patients. The independent factors for CSS and OS in the elderly patients were analyzed using the multivariate Cox regression analysis (Table 3).
Table 3.
Multivariate analysis of cancer-specific survival (CSS) and overall survival (OS) in elderly patients.
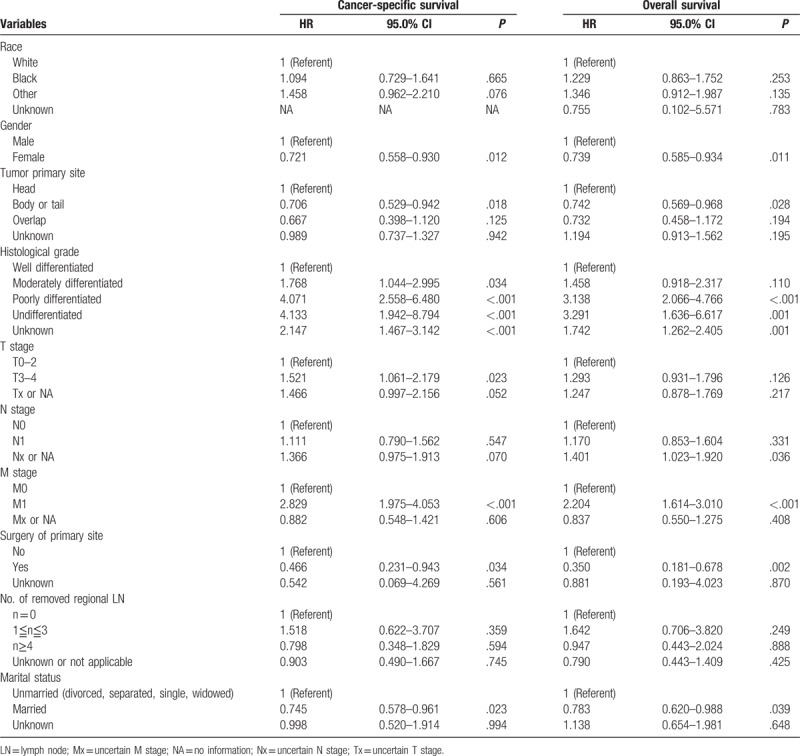
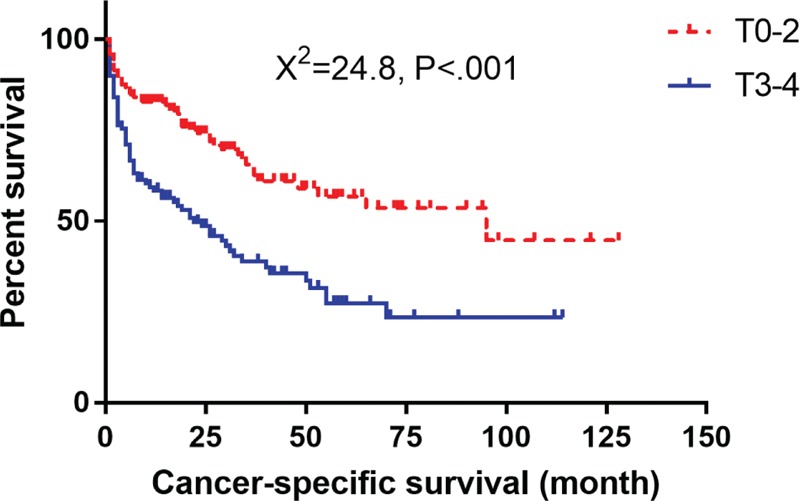
The results demonstrated that worse differentiation grade (moderately differentiated, P = 0.034, hazard ratio (HR) 1.768, 95% confidence interval (CI) 1.044–2.995; poorly differentiated P < .001, HR 4.071, 95% CI 2.558–6.480; and undifferentiated, P < .001, HR 4.133, 95% CI 1.942–8.794), T3–4 stage (P = .023, HR 1.521, 95% CI 1.061–2.179), and tumor metastasis (M1) (P < .001, HR 2.829, 95% CI 1.975–4.053) were associated with poor CSS, whereas female gender (P = .012, HR 0.721, 95% CI 0.558–0.930), primary tumor located in body/tail of pancreas (P = .018, HR 0.706, 95% CI 0.529–0.942), surgery of tumor primary site (P < .034, HR 0.466, 95% CI 0.231–0.943), and being married (P = .023, HR 0.745, 95% CI 0.578–0.961) were associated with better CSS in the elderly patients.
Considering the independent risk factors for OS, patients with higher histological grade (poor differentiated P < .001, HR 3.138, 95% CI 2.066–4.766; undifferentiated, P = .001 ,HR 3.291, 95% CI 1.636–6.617); and metastasis status (M1) (P < .001, HR 2.204, 95% CI 1.614–3.010) have poor OS, whereas female gender (P = .011, HR 0.739, 95% CI 0.585–0.934); being married (P = .039, HR 0.783, 95% CI 0.620–0.988); tumor location in the body/tail of pancreas (P = .028, HR 0.742, 95% CI 0.569–0.968); and tumor primary site surgery (P = .02, HR 0.35, 95% CI 0.181–0.678) have better OS.
Kaplan–Meier mortality analysis with log-rank test was conducted to analyze the independent risk factors and protective factors for CSS and OS, which are shown in Figures 2–8. Patients with the characteristics of worse differentiation grade and tumor metastasis (M1) were associated with poor CSS and OS, whereas female gender, location of primary tumor in the body/tail of pancreas, surgery of tumor primary site, and being married were associated with better CSS and OS. T3–4 stage was a risk factor for CSS but not OS.
Figure 2.
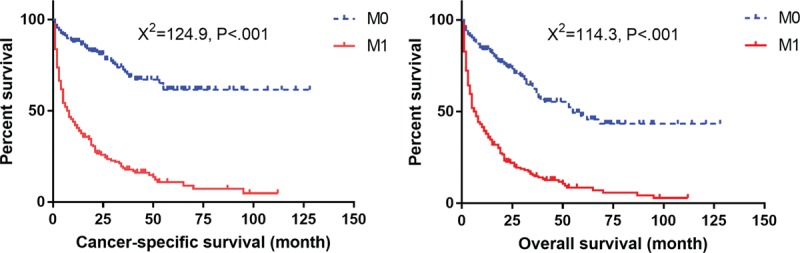
Survival curves with log-rank test for different M stage in elderly patients.
Figure 8.
Survival curves with log-rank test for T stage in elderly patients.
Figure 3.
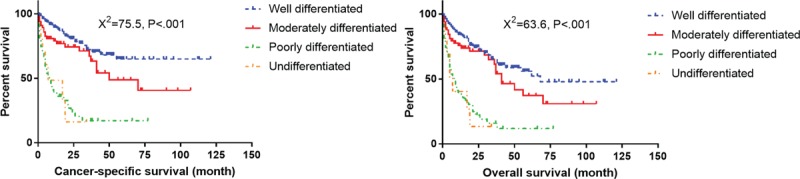
Survival curves with log-rank test for different histological grade in elderly patients.
Figure 4.
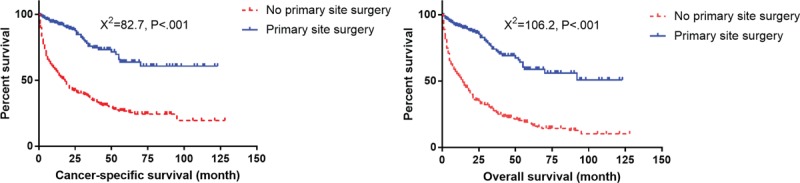
Survival curves with log-rank test for different surgery status in elderly patients.
Figure 5.
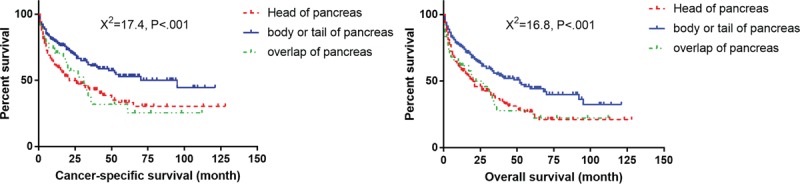
Survival curves with log-rank test for different tumor location in elderly patients.
Figure 6.
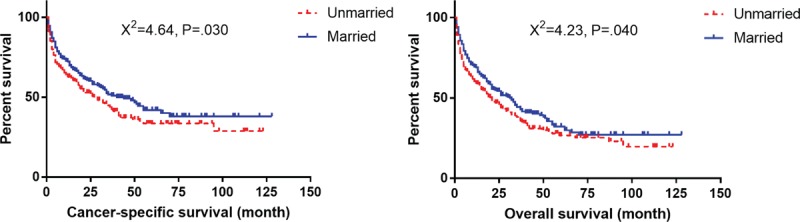
Survival curves with log-rank test for different marital status in elderly patients.
Figure 7.
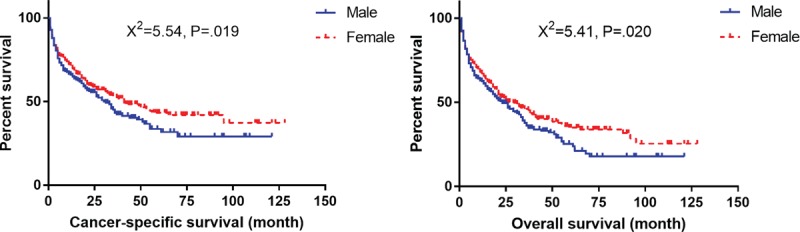
Survival curves with log-rank test for gender distribution in elderly patients.
3.4. Surgery of primary site improves CSS and OS for elderly patients with distant metastasis (M1 stage)
Total of 243 of 653 patients (37.2%) had distant metastasis and 224 patients had unknown M stage. Among the elderly patients with distant metastasis, 17 underwent primary site surgery, 225 did not undergo surgery, and for 1 patient the status was unknown. Out of 17 patients, 6 had liver metastasis, 1 bone metastasis, 1 lung metastasis, and 9 unknown. Patients who underwent surgery of primary site showed better CSS and OS than those without surgery (Fig. 9).
Figure 9.
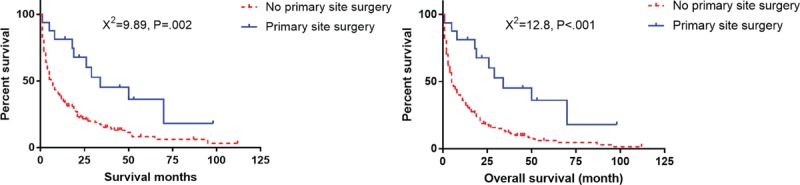
Survival curves with log-rank test for surgery of primary site in elderly patients with distant metastasis.
4. Discussion
Multivariate Cox regression analysis revealed that the worse differentiation grade, and distant metastasis (M1) were independent risk factors, whereas tumor location in the body/tail of pancreas, female patients, surgery, and being married were protective factors for both CSS and OS in the elderly cohort. The advanced T stage is also an independent risk factor for CSS, but not for OS. Surprisingly, the N stage and number of removed LN were not independent factors for prognosis.
According to our analysis, there are several possible explanations for the decreased CSS and OS in the elderly patients. First, the elderly patients possess more risk factors (worse differentiated grade) and less protective factors (surgery of tumor primary site and being married) than the younger cohort, which may partially contribute to the poor prognosis. Second, the elderly patients usually have a higher incidence of comorbidities, such as chronic obstructive pulmonary disease, hypertension, coronary artery disease, and diabetes mellitus.[15] These comorbidities would increase the nontumor-related death and reduce the possibility of surgery. Last, elderly patients are less likely to receive adjuvant chemotherapy, such as endocrine therapy, chemotherapy, or targeted therapy, in spite of a higher proportion of worse differentiated tumors.[15]
Liver is the most common metastatic site for pNETs. Up to 60–90% of patients develop neuroendocrine liver metastasis during the course of the disease and the presence of distant metastasis is one of the strongest predictors for survival. Previous studies have revealed that the 5-year survival rate (13–54%) was significantly worse in patients with distant metastasis than in those without (75–99%).[16–18] The patients with distant metastasis are mainly treated using systemic treatment, including surgery of the primary foci and/or metastasis, chemotherapy, endocrine therapy, and targeted therapy. Surgery of the primary tumor is still controversial regarding the patients with distant metastasis, especially for the elderly patients.[16–18] Bettini et al[19] have demonstrated that the benefit of resecting primary tumors was to prevent symptoms arising from the tumors, such as biliary or gastrointestinal obstruction and symptoms from functional tumors. No differences in progression free survival between the two groups were detected. However, in the studies from Bertani et al, a survival benefit was detected in patients undergoing primary site surgery for metastatic disease. The authors proposed that the resection of the primary tumor may enhance the efficacy of systemic therapy, even in the absence of complications or symptoms.[20,21] A recent retrospective study by Citterio et al[22] reveals similar results. In our study, 243 of 653 patients (37.2%) showed distant metastasis, of which, 6 had liver metastasis, 1 bone metastasis, 1 lung metastasis, and 9 unknown. Out of 243 patients, 17 underwent primary site surgery, whereas 225 did not, and the status was unknown in 1 patient. The results revealed that distant metastasis proved to be an independent risk factor for both CSS and OS. Patients who underwent surgery of the primary site had better CSS and OS than those who did not. However, due to the fewer cases and multitude of the metastatic organs in present study, further research is needed to confirm the benefit of primary tumor surgery in the elderly patients with distant metastasis.
Previous studies have reported the impact of race and ethnicity on tumors in recent years. African American patients are associated with poor OS in various tumors.[23,24] The study by Zhou et al[23] revealed that African American patients had a more advanced tumor stage at the time of diagnosis comparing to others. Additionally, they are more likely to be in the disadvantaged socioeconomic status (an economic, and sociological combined total measure), and tend to cluster in low-quality hospitals.[23] Moreover, Yao[5] and Dasari's[25] studies revealed similar results. However, we did not observed the difference in CSS and OS between African Americans and Caucasians. This may be explained by the present study focusing on elderly patients rather than all age groups. A lower proportion of African American patients was observed in the elderly cohort than among the younger patients in our results.
To analyze the influence of tumor location, a Pearson chi-squared test regarding baseline variables was performed to compare the patients with tumor located on the pancreatic head and body/tail. Well and moderately differentiated tumors account for 68.4% in the pancreatic head group, and 89.9% in the body and tail tumor group (χ2 = 20.6, P < .001). Nineteen percent of patients in the pancreatic head tumor group and 44.5% of patients in the latter group accepted surgery of the primary tumor (χ2 = 33.9, P < .001). Additionally, 17.3% of patients in the pancreatic head tumor group and 30.8% of patients in the latter group have more resected number of regional lymph nodes (χ2 = 16.6, P = .001). The patients with tumors located at body and tail of pancreas are likely to have better differentiated status, receipt of surgery, and more resected number of regional lymph nodes, which may contribute to better CSS and OS.
Undoubtedly, patients with malignancies are usually troubled by high levels of psychological distress, which is more common in unmarried patients.[26,27] Greater psychological distress can prolong the function of cortisol and lead to cytokine-mediated inflammation. These changes can disturb the normal immune function, and they have been recognized as the risk prognostic factors in patients with malignancies.[28,29] Previous studies have reported that married patients obtain more care, encouragement, and economic support from spouses and have a better socioeconomic status than the unmarried patients.[30] This better psychological and socioeconomic status explains better outcomes in the married cohort.[23,30–32] However, the exact mechanisms of marital status still need further investigation.
The present study revealed that females had better CSS and OS among the elderly patients. Kaplan RM has reported that sex has a potential interaction with marital status in a general population and the effect of marital status on survival outcomes varied by gender in some cancers.[32] Therefore, we performed the chi-squared test between the male and female patients in the elderly cohort, and the preliminary results showed that female patients of married status were lesser than their male counterparts (36.3% vs. 70.7%, χ2 = 86.64, P < .001). Further, Cox analysis showed that female patients have better survival outcome both in married and unmarried subgroups, which signified that the benefits in female patients on the prognosis had other underlying etiologies, rather than the marital status.
There are several limitations for this study. First, other variables, such as performance status, comorbidities, Ki-67, and surgical information (duration, blood loss, and postoperative complication) were not captured in the SEER database. Second, administration of adjuvant therapies (chemotherapy, targeted therapy, and endocrine therapy) may have contributed to a better analysis. However, this information is not provided in the SEER database.
5. Conclusion
Elderly patients showed increased possibility of poorly differentiated tumor, and decreased proportion of African American patients, receipt of surgery, number of resected lymph nodes, and married status. Poor differentiation and M1 stage were independent risk factors for both CSS and OS, while tumor location at the body/tail of pancreas, female patients, receipt of surgery, and being married were protective factors. Advanced T stage was also an independent risk factor for CSS, but not for OS.
Author contributions
Conceptualization: Gang Li, Maolin Tian, Yuntao Bing, Hangyan Wang, Bin Jiang, Chunhui Yuan, Dianrong Xiu.
Data curation: Gang Li.
Formal analysis: Lianyuan Tao.
Investigation: Gang Li, Bin Jiang.
Methodology: Lianyuan Tao, Hangyan Wang.
Resources: Yuntao Bing.
Software: Gang Li, Yuntao Bing, Chunhui Yuan, Dianrong Xiu.
Supervision: Maolin Tian, Dianrong Xiu.
Validation: Gang Li.
Writing – original draft: Gang Li, Maolin Tian.
Writing – review & editing: Gang Li, Dianrong Xiu.
Footnotes
Abbreviations: CSS = cancer-specific survival, ICD-O3 = International Classification of Diseases for Oncology third edition, NET = neuroendocrine tumor, OS = overall survival, pNET = pancreatic neuroendocrine tumor, SEER = Surveillance Epidemiology and End Results.
GL and M-lT contributed equally.
National Natural Science Foundation-Youth Fund, 81702855; National Natural Science Foundation, 81672862.
The authors have no conflicts of interest to disclose.
References
- [1].Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003;97:934–59. [DOI] [PubMed] [Google Scholar]
- [2].Yao JC, Eisner MP, Leary C, et al. Population-based study of islet cell carcinoma. Ann Surg Oncol 2007;14:3492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Halfdanarson TR, Rabe KG, Rubin J, et al. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol 2008;19:1727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lepage C, Bouvier AM, Phelip JM, et al. Incidence and management of malignant digestive endocrine tumours in a well defined French population. Gut 2004;53:549–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063–72. [DOI] [PubMed] [Google Scholar]
- [6].Hallet J, Law CH, Cukier M, et al. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2015;121:589–97. [DOI] [PubMed] [Google Scholar]
- [7].Zhu LM, Tang L, Qiao XW, et al. Differences and similarities in the clinicopathological features of pancreatic neuroendocrine tumors in china and the United States: a multicenter study. Medicine (Baltimore) 2016;95:e2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bilimoria KY, Talamonti MS, Tomlinson JS, et al. Prognostic score predicting survival after resection of pancreatic neuroendocrine tumors: analysis of 3851 patients. Ann Surg 2008;247:490–500. [DOI] [PubMed] [Google Scholar]
- [9].Fischer L, Bergmann F, Schimmack S, et al. Outcome of surgery for pancreatic neuroendocrine neoplasms. Br J Surg 2014;101:1405–12. [DOI] [PubMed] [Google Scholar]
- [10].Soto-Perez-de-Celis E, Cordoba R, Girones R, et al. Cancer and aging in Ibero-America. Clin Transl Oncol 2018;20:1117–26. [DOI] [PubMed] [Google Scholar]
- [11].Cheng KK, Lim EY, Kanesvaran R. Quality of life of elderly patients with solid tumours undergoing adjuvant cancer therapy: a systematic review. BMJ Open 2018;8:e18101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hidalgo M. Pancreatic cancer. N Engl J Med 2010;362:1605–17. [DOI] [PubMed] [Google Scholar]
- [13].Vlacich G, Samson PP, Perkins SM, et al. Treatment utilization and outcomes in elderly patients with locally advanced esophageal carcinoma: a review of the National Cancer Database. Cancer Med 2017;6:2886–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sakurai K, Tamura T, Toyokawa T, et al. Low preoperative prognostic nutritional index predicts poor survival post-gastrectomy in elderly patients with gastric cancer. Ann Surg Oncol 2016;23:3669–76. [DOI] [PubMed] [Google Scholar]
- [15].Shamali A, De’Ath HD, Jaber B, et al. Elderly patients have similar short term outcomes and five-year survival compared to younger patients after pancreaticoduodenectomy. Int J Surg 2017;45:138–43. [DOI] [PubMed] [Google Scholar]
- [16].Kulke MH, Shah MH, Benson AR, et al. Neuroendocrine tumors, version 1.2015. J Natl Compr Canc Netw 2015;13:78–108. [DOI] [PubMed] [Google Scholar]
- [17].Scigliano S, Lebtahi R, Maire F, et al. Clinical and imaging follow-up after exhaustive liver resection of endocrine metastases: a 15-year monocentric experience. Endocr Relat Cancer 2009;16:977–90. [DOI] [PubMed] [Google Scholar]
- [18].Frilling A, Modlin IM, Kidd M, et al. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol 2014;15:e8–21. [DOI] [PubMed] [Google Scholar]
- [19].Bettini R, Mantovani W, Boninsegna L, et al. Primary tumour resection in metastatic nonfunctioning pancreatic endocrine carcinomas. Dig Liver Dis 2009;41:49–55. [DOI] [PubMed] [Google Scholar]
- [20].Bertani E, Fazio N, Radice D, et al. Assessing the role of primary tumour resection in patients with synchronous unresectable liver metastases from pancreatic neuroendocrine tumour of the body and tail. A propensity score survival evaluation. Eur J Surg Oncol 2017;43:372–9. [DOI] [PubMed] [Google Scholar]
- [21].Bertani E, Fazio N, Botteri E, et al. Resection of the primary pancreatic neuroendocrine tumor in patients with unresectable liver metastases: possible indications for a multimodal approach. Surgery 2014;155:607–14. [DOI] [PubMed] [Google Scholar]
- [22].Citterio D, Pusceddu S, Facciorusso A, et al. Primary tumour resection may improve survival in functional well-differentiated neuroendocrine tumours metastatic to the liver. Eur J Surg Oncol 2017;43:380–7. [DOI] [PubMed] [Google Scholar]
- [23].Zhou H, Zhang Y, Wei X, et al. Racial disparities in pancreatic neuroendocrine tumors survival: a SEER study. Cancer Med 2017;6:2745–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mathur AK, Osborne NH, Lynch RJ, et al. Racial/ethnic disparities in access to care and survival for patients with early-stage hepatocellular carcinoma. Arch Surg 2010;145:1158–63. [DOI] [PubMed] [Google Scholar]
- [25].Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 2017;3:1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Goldzweig G, Andritsch E, Hubert A, et al. Psychological distress among male patients and male spouses: what do oncologists need to know? Ann Oncol 2010;21:877–83. [DOI] [PubMed] [Google Scholar]
- [27].Nipp RD, El-Jawahri A, Fishbein JN, et al. The relationship between coping strategies, quality of life, and mood in patients with incurable cancer. Cancer 2016;122:2110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Formica V, Luccchetti J, Cunningham D, et al. Systemic inflammation, as measured by the neutrophil/lymphocyte ratio, may have differential prognostic impact before and during treatment with fluorouracil, irinotecan and bevacizumab in metastatic colorectal cancer patients. Med Oncol 2014;31:166. [DOI] [PubMed] [Google Scholar]
- [29].Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychol 2002;21:531–41. [DOI] [PubMed] [Google Scholar]
- [30].Woods LM, Rachet B, Coleman MP. Origins of socio-economic inequalities in cancer survival: a review. Ann Oncol 2006;17:5–19. [DOI] [PubMed] [Google Scholar]
- [31].Shi RL, Qu N, Lu ZW, et al. The impact of marital status at diagnosis on cancer survival in patients with differentiated thyroid cancer. Cancer Med 2016;5:2145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kaplan RM, Kronick RG. Marital status and longevity in the United States population. J Epidemiol Community Health 2006;60:760–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


