Abstract
This retrospective, nationwide, matched cohort study investigated the risk of central serous chorioretinopathy (CSCR) following end-stage renal disease (ESRD). The study cohort included 84722 ESRD patients who were registered between January 2000 and December 2009 at the Taiwan National Health Insurance Research Database. An age- and sex-matched control group comprised 84722 patients selected from the Taiwan Longitudinal Health Insurance Database 2000. We collected information for each patient from the index date until December 2011. During the follow-up period, we found a significantly elevated risk of CSCR in the ESRD patients compared with controls (incidence rate ratio = 1.51, 95% confidence interval = 1.24–1.84). After adjustment for potential confounders, including age, sex, coronary artery disease, peptic ulcer, and obstructive sleep apnea, ESRD patients were 1.41 times more likely to develop CSCR (adjusted hazard ratio = 1.41, 95% confidence interval = 1.14–1.73). In conclusion, we found that ESRD patients showed a significantly higher risk of developing CSCR and recommend regular retina examinations and education regarding CSCR for patients with ESRD.
Keywords: central serous chorioretinopathy, cohort study, end-stage renal disease, hazard ratio, International Classification of Diseases, Longitudinal Health Insurance Database 2000, Taiwan Longitudinal Health Insurance Database
1. Introduction
End-stage renal disease (ESRD), a leading cause of morbidity and mortality worldwide, is an important public health issue. Patients with ESRD require dialysis or transplant treatment because ESRD is the most severe form and the last stage of chronic kidney disease. Coresh et al[1] reported that the prevalence of ESRD has increased from approximately 0.21% to 0.35%. Recently, the prevalence and incidence of ESRD have increased rapidly, both in Western and Asian populations, and in developing and developed countries.[1,2] A particularly high prevalence and incidence of ESRD has been observed in Taiwan, compared with other countries.[3–5]
Central serous chorioretinopathy (CSCR), characterized by serous neurosensory retinal detachments and/or retinal pigment epithelium (RPE) detachment, is a major cause of vision threat.[6,7] Patients with CSCR often present with a sudden, mild reduction in visual acuity, micropsia, metamorphopsia, relative central scotoma, and reduced contrast sensitivity.[8] Although in some patients, CSCR develops acutely, with spontaneous resolution within 3 to 6 months; in other patients, CSCR presents chronically, with visual impairment and distinctive RPE changes as well as persistent shallow retinal detachment.[9–12] The exact molecular mechanisms and pathophysiology of CSCR are not clearly understood; however, advancements in imaging techniques have highlighted the contribution of changes in the choroidal circulation and RPE function in CSCR pathogenesis.[13,14] Although the aetiology and triggering factors of CSCR remain poorly understood, it is hypothesized that the condition is associated with activation of the mineralocorticoid receptor related to corticosteroids or hormones.[15,16] Furthermore, numerous reports showed familial CSCR suggesting that a genetic predisposition, such as complement factor H, may play a critical role in the pathogenesis of CSCR.[17–20] Finally, the inflammation related to oxidative stress increased may be related to the pathogenesis of CSCR.[21]
It has been postulated that the progression of kidney damage to ESRD is linked to an increase in blood pressure, proteinurea, and renal fibrosis resulting from vascular injury,[22] and that these factors may be associated with the activation of the mineralocorticoid receptor related to aldosterone, based on several animal studies.[23–26] Furthermore, it has been suggested that genetic variants in the complement system, particularly complement factor H, play a role in chronic kidney disease, such as membranoproliferative glomerulonephritis and atypical haemolytic uraemic syndrome.[27–29] In addition, the inflammation related to oxidative stress and endothelial dysfunction in ESRD patients has been shown in several reports.[30–35] We hypothesize that ESRD and CSCR share a common pathogenesis, furthermore. Therefore, it is clinically relevant to determine whether ESRD is a predictor of CSCR.
No study has discussed the association between ESRD and CSCR. Our study is the first study to investigate the risk of CSCR following ESRD in Taiwan using a nationwide population-based dataset.
2. Methods
2.1. Database
The National Health Insurance (NHI) of Taiwan, a mandatory social health insurance plan, provides extensive medical care coverage for all residents of Taiwan and was implemented on March 1, 1995. As of 2007, almost >98% of the total Taiwanese population of 23 million were enrolled in this program. The Taiwan National Health Insurance Research Database (NHIRD) provides enciphered patient identification numbers, as well as information regarding patient gender, birth date, admission and discharge dates, diagnoses and procedure codes, prescriptions details, and costs covered and paid by NHI.
2.2. Study design
This retrospective, nationwide, matched cohort study involved 2 groups of participants: a new onset ESRD group and a matched non-ESRD (control) group.
2.3. Study participants
The data of our cohort study were obtained from the Taiwan NHIRD and diagnosis codes were assigned according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Patients and controls were recruited between 2000 and 2009. In total, 84,722 ESRD patients who had started their first dialysis treatment after December 31, 2000 and had received a catastrophic illness certificate, with the ESRD (ICD-9-CM code 585), between January 1, 2000 and December 31, 2009, were included. Patients of unknown gender or with missing data were excluded. Patients diagnosed as having CSCR (ICD-9-CM code 362.41) prior to ESRD were also excluded. In addition, to control for other causes of RPE leaks among the differential diagnoses of CSCR, we also excluded those who had any of the following diagnoses before enrolment: degenerative myopia (ICD-9-CM code 360.21), hemorrhagic RPE detachment (ICD-9-CM code 362.43), exudative AMD (ICD-9-CM code 362.52), hereditary retinal dystrophies (ICD-9-CM code 362.7x), focal chorioretinitis (ICD-9-CM code 363.0x), disseminated chorioretinitis (ICD-9-CM code 363.1x), Harada's disease (ICD-9-CM code 363.22), angioid streak (ICD-9-CM code 363.43), malignant neoplasm of the choroid (ICD-9-CM code 190.6), optic disc pit (ICD-9-CM code 377.22), polypoidal choroidal vasculopathy (ICD-9-CM code 362.16), posterior scleritis (ICD-9-CM code 379.07), autoimmune disease such as lupus erythematosus (ICD-9-CM code 695.4, 710.0) and polyarteritis nodosa (ICD-9-CM code 446.0), malignant hypertension (ICD-9-CM code 402.0), and toxemia of pregnancy (ICD-9-CM code 642.2).
For each ESRD case, one control without ESRD was randomly selected from the Longitudinal Health Insurance Database 2000 (LHID2000), a data subset of NHIRD containing complete claim data for one million beneficiaries (4.34% of the total population) systematically randomly sampled in 2000. Differences in age, gender, and healthcare costs between the LHID2000 and NHIRD were insignificant. The 84722 controls were matched by sex and birth date. The index date for each ESRD patient was the date of their first dialysis, and the controls were assigned the same index dates as their matched ESRD patients. An SAS (SAS Institute, Inc., Cary, NC) matching macro, “%One-ToManyMTCH,” was used for the matching.[36] It allows propensity score matching from 1-to-1 to 1-to-N. We set a caliper for nearest-neighbor matching within the 4 to 8 digits.[35] Furthermore, controls diagnosed with CSCR before the index date were also excluded. Each patient was followed-up to determine the incidence of CSCR until the end of 2011.
To identify all patients who had developed CSCR (ICD-9-CM code 362.41), we tracked every patient from his or her index outpatient visit or hospitalization through December 2011. Demographic data (e.g., age and sex) were recorded.
2.4. Potential confounders
We identified potential comorbidities, including coronary artery disease (ICD-9-CM code 410–414),[37] peptic ulcer (ICD-9-CM code 531–534),[37,38] and obstructive sleep apnea (ICD-9-CM code 372.73 780.51 780.53 780.57),[39] because these conditions are critical factors that increase the risk of CSCR. In this study, the inclusion criterion for the conditions set out above was documentation of the condition at least once in the inpatient setting, or ≥3 times in the ambulatory setting, up to 1 year before the onset of ESRD, according to the dialysis medical service date.
2.5. Statistical analysis
SAS 9.4 for Windows (SAS Institute, Inc., Cary, NC) was used in this study. The significant differences in demographic characteristics and comorbid variables between the ESRD and control groups were determined using the Student's t-test for continuous variables and Pearson's chi-squared test for categorical variables. The median follow-up time was measured by the Mann–Whitney U test to determine differences between the 2 groups. The incidence rate was calculated as the number of CSCR cases identified during follow-up divided by the total person-years (PY) for each group by age, sex, and select comorbidities. The Poisson regression analysis was performed to calculate the incidence rate ratio (IRR), which demonstrated the risk of CSCR, in order to assess the differences between the ESRD and control groups. The hazard ratio (HR) was calculated to evaluate the overall and subgroup risk of developing CSCR using Cox proportional hazard regression analysis. The adjusted HR was also determined by adjusting the age group, sex, coronary artery disease, peptic ulcer, and obstructive sleep apnea in the Cox proportional hazard regression model. Besides, the proportionality assumption of the Cox proportional hazard model was checked by including time-dependent covariates of comorbidity in the model. The collective tests of all time-dependent variables were insignificant (P value=.1372), which indicated that the proportionality assumption was satisfied. Cumulative incidence rates for CSCR of ESRD were evaluated by Kaplan–Meier analysis, and differences in cumulative-incidence rate curves were analysed using the log-rank test. Additionally, we subdivided the patients into 3 age subgroups for further analysis: <50 years, 50–64 years, and ≥65 years. Data are presented as mean ± standard deviation (SD), and 95% confidence intervals (CIs) are provided when applicable. Statistical significance was defined as P < .05.
2.6. Ethics statement
This study was granted exemption from review by the Institutional Review Board of Chi-Mei Medical Center. Ethical approval and informed consent were waived because the public database was used for analysis and the data contained no identifiable personal information.
3. Results
3.1. Demographic data
Between 2000 and 2009, 84,722 ESRD patients and 84,722 controls were recruited after excluding ineligible subjects. Table 1 provides the demographic characteristics and comorbid disorders of ESRD patients and age- and sex-matched controls. There were no significant differences in age, and gender between the sample group and the controls. The mean age of all participants was 62.26 ± 14.51 years. ESRD patients exhibited a significantly higher prevalence of previously reported comorbidities, such as coronary artery disease, peptic ulcer, and obstructive sleep apnea than did the controls. The mean follow-up periods for the ESRD and control patients were 4.12 (interquartile range, 4.85) and 6.45 (interquartile range, 5.03) years, respectively.
Table 1.
Demographic characteristics and comorbid disorders in the end-stage renal disease (ESRD) and control groups.

3.2. Incidence rates of CSCR
During the follow-up period, there was a significant difference in the CSCR incidence between the 2 groups (ESRD patients = 5.05/10000 PY; control = 3.34/10000 PY), and the difference between IRRs in the ESRD and control groups was also statistically significant (IRR = 1.51, 95% CI = 1.24–1.84, P < .0001)
After the 2 groups were stratified according to age, we found that ESRD patients aged <50 years had the highest incidence rates of CSCR (6.32/10000 PY), followed by those aged 50–64 years and ≥65 years. We found significantly higher IRRs for ESRD patients aged <50 years (IRR = 1.46, 95% CI = 1.01–2.11, P = .0419) and 50–64 years old (IRR = 1.58, 95% CI = 1.14–2.18, P = .0056) compared with their age-matched controls (Table 2).
Table 2.
Risk of central serous chorioretinopathy (CSCR) between end-stage renal disease (ESRD) group and control group.

The incidence of CSCR in male ESRD patients was 5.30/10000 PY, whereas the incidence in male control patients was only 3.68/10000 PY, yielding a significant difference in the IRR between male ESRD and control patients (IRR = 1.43, 95% CI = 1.09–1.89, P = .0098). There was a significant difference in the CSCR incidence between the female ESRD patients (4.83/10000 PY) and their controls (3.02/10000 PY), and the IRR between the female ESRD and control groups was statistically significant (IRR = 1.60, 95% CI = 1.60–2.14, P = .0015; Table 2).
In the ESRD group, the incidence rates of CSCR stratified by disease were 3.62/10000 PY for coronary artery disease, and 2.58/10000 PY for peptic ulcers. After stratifying the 2 groups according to comorbidities, we found that the IRR for CSCR associated with all comorbidities did not indicate significantly greater risks in ESRD patients with the same condition compared with their controls. Whether obstructive sleep apnea in ESRD patients increases the risk of CSCR remains to be evaluated, because of the lack of CSCR incidence among patients with obstructive sleep apnea in both groups.
Table 3 provides the crude and adjusted HRs for CSCR, by cohort, during the follow-up period. After adjusting for age, sex, and select comorbid conditions, ESRD remained an independent risk factor for CSCR (adjusted HR = 1.41, 95% CI = 1.14–1.73). In addition, we found that ESRD patients <50 years (adjusted HR = 1.81, 95% CI = 1.39–2.35, P < .05) and patients aged 50 to 64 years (adjusted HR = 1.55, 95% CI = 1.22–1.98, P < .05) were independent risk factors for CSCR after adjusting for age, sex, and select comorbid conditions. All comorbidities were not significant risk factors for CSCR in both groups before and after adjusting for age, sex, and select comorbid conditions. We could not evaluate whether obstructive sleep apnea was a significant risk factor after adjusting for other confounding factors in the total cohort because of the lack of CSCR incidence among obstructive sleep apnea patients in both groups.
Table 3.
Crude and adjusted hazard ratios calculated using the Cox proportional hazard regression and 95% confidence interval for central serous chorioretinopathy (CSCR) during the follow-up period for the study cohort.

The Kaplan–Meier survival analyses revealed higher CSCR cumulative incidence rates in the ESRD patients than in the control patients, and the log-rank test was also significant (P < .001; Fig. 1).
Figure 1.
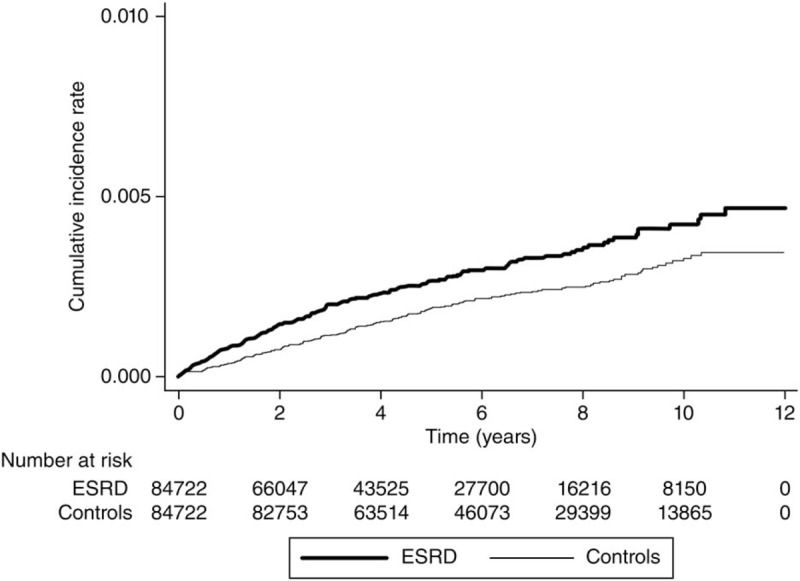
Kaplan–Meier curve of the cumulative incidence of central serous chorioretinopathy (CSCR) in patients with end-stage renal disease (ESRD) and controls during the follow-up period. CSCR = central serous chorioretinopathy, ESRD = end-stage renal disease.
4. Discussion
Our study is the largest-scale population-based study exploring the link between ESRD and subsequent CSCR. We analysed 84722 ESRD patients and 84722 control subjects. We found that the HR of CSCR in ESRD patients was 1.51 times higher than that in controls, and that the relative risk of CSCR for patients with ESRD was increased 1.41 times, after adjusting for age, sex, coronary artery disease, peptic ulcer, and obstructive sleep apnea.
CSCR is a frequent cause of vision threat in the middle-aged population, presenting as micropsia, metamorphopsia, and visual impairment. It is hypothesised that these are associated with hyperpermeability of choriocapillaris and disruption of the retinal pigment epithelium.[6,7] The exact pathophysiology is unclear but is thought to be multifactorial, and contributing factors may include mineralocorticoid receptor activation with corticosteroids or hormones,[15,37,40,41] genetic predisposition, such as complement factor H,[17–20] and the inflammation process triggered by oxidative stress. To the best of our knowledge, the link between ESRD and CSCR has never been explored previously. Our study is the largest nationwide population-based cohort study to investigate the risk of CSCR following ESRD in Taiwan. The multiple common pathogenic mechanisms of CSCR and ESRD will be discussed separately below.
The proposed pathogenic mechanism common in ESRD and CSCR is mineralocorticoid receptor activation related to corticosteroids or hormones. It appears that the major impact on the progression of kidney damage to ESRD results from the elevation in blood pressure, proteinuria, and renal fibrosis.[22] Several animal studies have demonstrated that activation of the mineralocorticoid receptor related to aldosterone may play an important role in hypertensive kidney disease, proteinuria, and renal fibrosis resulting from vascular injury.[23–26] Further experimental studies have shown that combining a mineralocorticoid receptor antagonist with angiotensin converting enzyme inhibitors and angiotensin receptor blocker agents could slow down the progression of kidney disease by reducing reduced proteinuria and blood pressure.[22] Furthermore, the mineralocorticoid receptor activation-related hypothesis has recently been examined in research on CSCR pathophysiology.[15,16] Animal data and clinical evidences suggest that oral mineralocorticoid antagonists show biological efficacy in chronic CSCR patients.[15,16] We have attempted to explain the association between ESRD and CSCR according to the mineralocorticoid receptor activation-related hypothesis. While corticosteroids or hormones, including cortisol and aldosterone, overactivate the mineralocorticoid receptor in the kidney in ESRD patients, the mineralocorticoid receptor of the choriocapillary might be activated at the same time and focal leakage related to choroidal vessel vasodilation might subsequently develop.[15,16] The hypothesis may explain why ESRD patients with mineralocorticoid receptor activation related to aldosterone, are susceptible to CSCR with hyperpermeability of the choriocapillaris and focal leakage.
Another proposed pathogenic mechanism common to CSCR and ESRD is the genetic variant in complement factor H. Complement factor H variation, as part of innate immunity, is associated with some forms of kidney disease, including type II membranoproliferative glomerulonephritis, a disease characterized by kidney failure, and atypical haemolytic uremic syndrome.[28,29,42,43] Complement factor H is an inhibitor in the complement system and a complement homeostasis controller, inhibiting the complement cascade over activation in local sites, such as capillaries of the glomerulus and choroids.[27] Recently, several reports have suggested evidence of genetic predisposition in the development of CSCR.[17,44,45] Potential associations have been found between complement factor H polymorphisms and CSCR.[19] It is hypothesised and the protein of complement factor H binds to adrenomedullin, a peptide belonging to the calcitonin family, eliciting a choroidcapillary vasodilator action.[6] The susceptibility of ESRD patients to CSCR may be explained by the fact the complement factor H variant in the ESRD population affects glomerular and choroid capillaries.
Increased inflammation is the likely common pathophysiologic cause of ESRD and CSCR. Many studies have found that that inflammation related to oxidative stress is increased in ESRD populations.[30–34] It is postulated that the systemic inflammatory responses and endothelial dysfunction, typical of ESRD, result from increased proinflammatory cytokines and other inflammatory factors.[33] The hypercytokinaemia in ESRD may be associated with conditions related to uraemia and dialysis, which result in decreased renal elimination and increased generation of pro-inflammatory cytokines, due to induction by oxidative stress, volume overload, or uremic toxin.[33,46,47] Of particular note is the fact that the inflammation process has been implicated in the pathogenesis of CSCR. The pathophysiology of CSCR remains poorly understood, and the major theories providing an account of the pathophysiology of CSCR, including those attributing the aetiology of CSCR to hyperpermeable choroids or compromised RPE pump function, explain this in terms of stasis, ischaemia, or inflammation.[21] The inflammatory process may play a role in the pathogenesis of CSCR. The common inflammation may explain the link between ESRD and subsequent CSCR formation.
CSCR is a common vision-threatening retinal disorder in the middle-aged population. Notably, ESRD patients aged <50 years had a statistically significant risk of CSCR compared with age-matched controls, and this constituted the highest statistically significant independent risk factor after adjustment for other confounding factors for the risk of CSCR in the ESRD groups (adjusted HR = 1.81, 95% CI = 1.39–2.35, P < 0.05). We postulate that this finding can be possibly explained with the fact that the onset of CSCR usually occurs between 30 and 50 years.
Many comorbidities have been linked to CSCR, including coronary artery disease, peptic ulcer, and obstructive sleep apnea. We evaluated these comorbidities in ESRD patients and controls and found that none were associated with a significantly higher risk of CSCR in the ESRD patients compared to that in the controls (Table 2). We also concluded that comorbidity is not a significant risk factor for CSCR in the overall cohort after adjusting for all confounding variables (Table 3).
The link between CSCR in ESRD is an interdisciplinary issue and close collaboration between nephrologists and ophthalmologists is essential in clinical practice. Nephrologists should be aware of this potentially vision-threatening retinal disorder, which typically presents as micropsia, metamorphopsia, and abrupt, mild decrement in visual acuity, in ESRD patients on chronic dialysis. One of the greatest concerns for ophthalmologists is distinguishing CSCR from other causes of maculopathy, such as age-related, pathologic myopia related, or serous detachment resulting from infection or inflammation. Multiple therapies have been attempted to treat CSCR, including halt external corticosteroid, focal retinal photocoagulation, intravitreal injections of anti–vascular endothelial growth factor agents,[48,49] and photodynamic therapy.[50] Recently, anti-mineraloreceptor therapy has also been proposed in a number of reports.[6] When dealing with CSCR in ESRD dialysis patients, close cooperation between nephrologists and ophthalmologists is important to reduce the risk of visual impairment.
There are several strengths in our study. This nationwide and population-based study has a high statistical power and precision of risk appraisal because the dataset includes a large sample of ESRD patients. Several previous studies using the same database have been published.[51,52] Additionally, with a maximum longitudinal data of 10 years, the cohort study monitors CSCR incidence in ESRD and control groups.
There are some limitations in our study. Unfortunately, in Taiwan, as in the other countries worldwide, the CSCR diagnosis may be confused with similar diagnoses, even by ophthalmologists. Therefore, misdiagnosis could possibly lead to misclassification bias. Second, selection bias might also have been involved in our study. Because the controls were sampled from patients who did not have ESRD during the entire study period, the controls would be relatively healthier than the “actual” general population because they were not only free of ESRD at matching but also were required to be free of ESRD during the entire study period. As a result, the incidence rate of CSCR would be lower in these relatively healthy controls than in the true general population. Therefore, selection of healthier controls could result in overestimation of the association between ESRD and CSCR risk. Third, it could not be confirmed whether the controls had a history of ESRD before January 1996, because the medical history of the cohort can only be traced back to the year 1996. Besides, in the control group, we exclude patients with recorded CSCR diagnosis in the dataset. However, our study is based on claims data and produces information bias because we might include controls, who really have the disease but without recorded CSCR diagnosis in the dataset. Additionally, the disease may be misclassified, because the diagnosis of ESRD, CSCR, and other comorbidity disorders depend on ICD-9-codes. Finally, several important confounding factors including steroid, stress, personality, smoking history, pregnancy, and alcohol consumption could not be evaluated. The results of our study may not apply to other populations, because this is a specific population (Taiwanese) study. Other studies should be conducted to determine further correlations between ESRD and CSCR due to the limitations of this study.
In summary, our study found that ESRD patients showed a significantly higher risk of developing CSCR after adjusting for age, sex, coronary artery disease, peptic ulcer, and obstructive sleep apnea. Additionally, ESRD patients aged <50 years showed higher incidence rate of CSCR. Furthermore, close cooperation between nephrologists and ophthalmologists is necessary when dealing with CSCR following ESRD.
Acknowledgments
Data from the National Health Insurance Research Database were provided by the Taiwan Bureau of National Health Insurance and Department of Health. The interpretation and conclusions contained herein do not represent those of the Bureau of National Health Insurance, Department of Health, or National Health Research Institutes.
Author contributions
Conceptualization: Yuh-Shin Chang, Shih-Feng Weng, Jhi-Joung Wang, Ren-Long Jan.
Data curation: Yuh-Shin Chang, Shih-Feng Weng, Ren-Long Jan.
Formal analysis: Yuh-Shin Chang, Shih-Feng Weng, Ren-Long Jan.
Funding acquisition: Jhi-Joung Wang.
Investigation: Yuh-Shin Chang, Shih-Feng Weng, Jhi-Joung Wang, Ren-Long Jan.
Methodology: Yuh-Shin Chang, Shih-Feng Weng.
Project administration: Yuh-Shin Chang, Shih-Feng Weng, Jhi-Joung Wang, Ren-Long Jan.
Resources: Jhi-Joung Wang.
Software: Shih-Feng Weng.
Supervision: Ren-Long Jan.
Validation: Yuh-Shin Chang, Shih-Feng Weng, Jhi-Joung Wang, Ren-Long Jan.
Visualization: Yuh-Shin Chang, Shih-Feng Weng, Jhi-Joung Wang, Ren-Long Jan.
Writing – original draft: Yuh-Shin Chang.
Writing – review & editing: Ren-Long Jan.
Footnotes
Abbreviations: CI = confidence interval, CSCR = central serous chorioretinopathy, ESRD = end-stage renal disease, HR = hazard ratio, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification, IRR = incidence rate ratio, LHID2000 = Longitudinal Health Insurance Database 2000, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, PY = patient-years, RPE = retinal pigment epithelium, SD = standard deviation.
The authors have no conflicts of interest to disclose.
References
- [1].Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007;298:2038–47. [DOI] [PubMed] [Google Scholar]
- [2].McClellan W, Warnock DG, McClure L, et al. Racial differences in the prevalence of chronic kidney disease among participants in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Cohort Study. J Am Soc Nephrol 2006;17:1710–5. [DOI] [PubMed] [Google Scholar]
- [3].Kuo HW, Tsai SS, Tiao MM, et al. Epidemiological features of CKD in Taiwan. Am J Kidney Dis 2007;49:46–55. [DOI] [PubMed] [Google Scholar]
- [4].Wen CP, Cheng TY, Tsai MK, et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet 2008;371:2173–82. [DOI] [PubMed] [Google Scholar]
- [5].Hsu CC, Hwang SJ, Wen CP, et al. High prevalence and low awareness of CKD in Taiwan: a study on the relationship between serum creatinine and awareness from a nationally representative survey. Am J Kidney Dis 2006;48:727–38. [DOI] [PubMed] [Google Scholar]
- [6].Daruich A, Matet A, Dirani A, et al. Central serous chorioretinopathy: recent findings and new physiopathology hypothesis. Prog Retin Eye Res 2015;48:82–118. [DOI] [PubMed] [Google Scholar]
- [7].Nicholson B, Noble J, Forooghian F, et al. Central serous chorioretinopathy: update on pathophysiology and treatment. Surv Ophthalmol 2013;58:103–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang M, Munch IC, Hasler PW, et al. Central serous chorioretinopathy. Acta Ophthalmol 2008;86:126–45. [DOI] [PubMed] [Google Scholar]
- [9].Fok AC, Chan PP, Lam DS, et al. Risk factors for recurrence of serous macular detachment in untreated patients with central serous chorioretinopathy. Ophthalmic Res 2011;46:160–3. [DOI] [PubMed] [Google Scholar]
- [10].Gemenetzi M, De Salvo G, Lotery AJ. Central serous chorioretinopathy: an update on pathogenesis and treatment. Eye (Lond) 2010;24:1743–56. [DOI] [PubMed] [Google Scholar]
- [11].Kim YY, Flaxel CJ. Factors influencing the visual acuity of chronic central serous chorioretinopathy. Korean J Ophthalmol 2011;25:90–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Deva R, Alias MA, Colville D, et al. Vision-threatening retinal abnormalities in chronic kidney disease stages 3 to 5. Clin J Am Soc Nephrol 2011;6:1866–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Maruko I, Iida T, Sugano Y, et al. One-year choroidal thickness results after photodynamic therapy for central serous chorioretinopathy. Retina 2011;31:1921–7. [DOI] [PubMed] [Google Scholar]
- [14].Roisman L, Lavinsky D, Magalhaes F, et al. Fundus autofluorescence and spectral domain OCT in central serous chorioretinopathy. J Opthalmol 2011;2011:706849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bousquet E, Beydoun T, Zhao M, et al. Mineralocorticoid receptor antagonism in the treatment of chronic central serous chorioretinopathy: a pilot study. Retina 2013;33:2096–102. [DOI] [PubMed] [Google Scholar]
- [16].Zhao M, Celerier I, Bousquet E, et al. Mineralocorticoid receptor is involved in rat and human ocular chorioretinopathy. J Clin Invest 2012;122:2672–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Weenink AC, Borsje RA, Oosterhuis JA. Familial chronic central serous chorioretinopathy. Ophthalmologica 2001;215:183–7. [DOI] [PubMed] [Google Scholar]
- [18].Park DW, Schatz H, Gaffney MM, et al. Central serous chorioretinopathy in two families. Eur J Ophthalmol 1998;8:42–7. [DOI] [PubMed] [Google Scholar]
- [19].Miki A, Kondo N, Yanagisawa S, et al. Common variants in the complement factor H gene confer genetic susceptibility to central serous chorioretinopathy. Ophthalmology 2014;121:1067–72. [DOI] [PubMed] [Google Scholar]
- [20].de Jong EK, Breukink MB, Schellevis RL, et al. Chronic central serous chorioretinopathy is associated with genetic variants implicated in age-related macular degeneration. Ophthalmology 2015;122:562–70. [DOI] [PubMed] [Google Scholar]
- [21].Yannuzzi LA. Central serous chorioretinopathy: a personal perspective. Am J Opthalmol 2010;149:361–3. [DOI] [PubMed] [Google Scholar]
- [22].Bolignano D, Palmer SC, Navaneethan SD, et al. Aldosterone antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev 2014;4:CD007004. [DOI] [PubMed] [Google Scholar]
- [23].Aldigier JC, Kanjanbuch T, Ma LJ, et al. Regression of existing glomerulosclerosis by inhibition of aldosterone. J Am Soc Nephrol 2005;16:3306–14. [DOI] [PubMed] [Google Scholar]
- [24].Greene EL, Kren S, Hostetter TH. Role of aldosterone in the remnant kidney model in the rat. J Clin Invest 1996;98:1063–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rocha R, Chander PN, Khanna K, et al. Mineralocorticoid blockade reduces vascular injury in stroke-prone hypertensive rats. Hypertension 1998;31:451–8. [DOI] [PubMed] [Google Scholar]
- [26].Silvestre JS, Robert V, Heymes C, et al. Myocardial production of aldosterone and corticosterone in the rat. Physiological regulation. J Biol Chem 1998;273:4883–91. [DOI] [PubMed] [Google Scholar]
- [27].Boon CJ, van de Kar NC, Klevering BJ, et al. The spectrum of phenotypes caused by variants in the CFH gene. Mol Immunol 2009;46:1573–94. [DOI] [PubMed] [Google Scholar]
- [28].Zipfel PF, Heinen S, Jozsi M, et al. Complement and diseases: defective alternative pathway control results in kidney and eye diseases. Mol Immunol 2006;43:97–106. [DOI] [PubMed] [Google Scholar]
- [29].Dragon-Durey MA, Fremeaux-Bacchi V, Loirat C, et al. Heterozygous and homozygous factor h deficiencies associated with hemolytic uremic syndrome or membranoproliferative glomerulonephritis: report and genetic analysis of 16 cases. J Am Soc Nephrol 2004;15:787–95. [DOI] [PubMed] [Google Scholar]
- [30].Oberg BP, McMenamin E, Lucas FL, et al. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int 2004;65:1009–16. [DOI] [PubMed] [Google Scholar]
- [31].Mezzano D, Pais EO, Aranda E, et al. Inflammation, not hyperhomocysteinemia, is related to oxidative stress and hemostatic and endothelial dysfunction in uremia. Kidney Int 2001;60:1844–50. [DOI] [PubMed] [Google Scholar]
- [32].Landray MJ, Wheeler DC, Lip GY, et al. Inflammation, endothelial dysfunction, and platelet activation in patients with chronic kidney disease: the chronic renal impairment in Birmingham (CRIB) study. Am J Kidney Dis 2004;43:244–53. [DOI] [PubMed] [Google Scholar]
- [33].Vidt DG. Inflammation in renal disease. Am J Cardiol 2006; 2006;97:20A–7A. [DOI] [PubMed] [Google Scholar]
- [34].Athyros VG, Mikhailidis DP, Papageorgiou AA, et al. The effect of statins versus untreated dyslipidaemia on renal function in patients with coronary heart disease. A subgroup analysis of the Greek atorvastatin and coronary heart disease evaluation (GREACE) study. J Clin Pathol 2004;57:728–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ramos LF, Shintani A, Ikizler TA, et al. Oxidative stress and inflammation are associated with adiposity in moderate to severe CKD. J Am Soc Nephrol 2008;19:593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Parsons LS. Ovation Research Group: Performing a 1:N Case-Control Match on Propensity Score. In: Proceedings of the 29th SAS Users Group International, Montreal, Canada; 2004:1–11. [Google Scholar]
- [37].Chang YS, Weng SF, Chang C, et al. Associations between topical ophthalmic corticosteroids and central serous chorioretinopathy: a Taiwanese population-based study. Invest Ophthalmol Vis Sci 2015;56:4083–9. [DOI] [PubMed] [Google Scholar]
- [38].Chen SN, Lian I, Chen YC, et al. Increased incidence of peptic ulcer disease in central serous chorioretinopathy patients: a population-based retrospective cohort study. Retina 2015;35:231–7. [DOI] [PubMed] [Google Scholar]
- [39].Chatziralli I, Kabanarou SA, Parikakis E, et al. Risk factors for central serous chorioretinopathy: multivariate approach in a case-control study. Curr Eye Res 2017;42:1069–73. [DOI] [PubMed] [Google Scholar]
- [40].Zakir SM, Shukla M, Simi ZU, et al. Serum cortisol and testosterone levels in idiopathic central serous chorioretinopathy. Indian J Ophthalmol 2009;57:419–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zhao M, Valamanesh F, Celerier I, et al. The neuroretina is a novel mineralocorticoid target: aldosterone up-regulates ion and water channels in Muller glial cells. FASEB J 2010;24:3405–15. [DOI] [PubMed] [Google Scholar]
- [42].Appel GB, Cook HT, Hageman G, et al. Membranoproliferative glomerulonephritis type II (dense deposit disease): an update. J Am Soc Nephrol 2005; 2005;16:1392–403. [DOI] [PubMed] [Google Scholar]
- [43].Abrera-Abeleda MA, Nishimura C, Smith JL, et al. Variations in the complement regulatory genes factor H (CFH) and factor H related 5 (CFHR5) are associated with membranoproliferative glomerulonephritis type II (dense deposit disease). J Med Genet 2006;43:582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lin E, Arrigg PG, Kim RY. Familial central serous choroidopathy. Ger J Ophthalmol 2000;238:930–1. [DOI] [PubMed] [Google Scholar]
- [45].Oosterhuis JA. Familial central serous retinopathy. Ger J Ophthalmol 1996;234:337–41. [DOI] [PubMed] [Google Scholar]
- [46].Kimmel PL, Phillips TM, Simmens SJ, et al. Immunologic function and survival in hemodialysis patients. Kidney Int 1998;54:236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Stenvinkel P, Ketteler M, Johnson RJ, et al. IL-10, IL-6, and TNF-alpha: central factors in the altered cytokine network of uremia--the good, the bad, and the ugly. Kidney Int 2005;67:1216–33. [DOI] [PubMed] [Google Scholar]
- [48].Artunay O, Yuzbasioglu E, Rasier R, et al. Intravitreal bevacizumab in treatment of idiopathic persistent central serous chorioretinopathy: a prospective, controlled clinical study. Curr Eye Res 2010;35:91–8. [DOI] [PubMed] [Google Scholar]
- [49].Schaal KB, Hoeh AE, Scheuerle A, et al. Intravitreal bevacizumab for treatment of chronic central serous chorioretinopathy. Eur J Ophthalmol 2009;19:613–7. [DOI] [PubMed] [Google Scholar]
- [50].Ma J, Meng N, Xu X, et al. System review and meta-analysis on photodynamic therapy in central serous chorioretinopathy. Acta Ophthalmol 2014;92:e594–601. [DOI] [PubMed] [Google Scholar]
- [51].Chang YS, Weng SF, Chang C, et al. Risk of nonarteritic anterior ischemic optic neuropathy following end-stage renal disease. Medicine 2016;95:e3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Chang YS, Weng SF, Chang C, et al. Risk of retinal artery occlusion in patients with end-stage renal disease: a retrospective large-scale cohort study. Medicine 2016;95:e3281. [DOI] [PMC free article] [PubMed] [Google Scholar]


