Abstract
Previous studies have reached conflicting results regarding the possibility that hepatitis C virus (HCV) infection may increase the risk of non-Hodgkin lymphoma (NHL). We performed a meta-analysis to clarify the relationship between HCV infection and development of NHL. The PubMed, Web of Science, and Embase databases were searched for relevant studies estimating the association between HCV infection and NHL risk through October 31, 2017. Fixed effects or random effects models were used to calculate the pooled odds ratio (OR) and its 95% confidence interval (CI). A total of 18 studies met the inclusion criteria. We found a positive association between HCV infection and NHL (pooled OR 1.69, 95% CI 1.40–2.03, P < .05). In conclusion, our meta-analysis suggested that HCV infection was associated with increased risk of developing NHL.
Keywords: hepatitis C virus, meta-analysis, non-Hodgkin lymphoma, risk
1. Introduction
Non-Hodgkin lymphoma (NHL) is a malignant tumor of lymphoid tissue.[1] Several microbial infections (eg, hepatitis virus, human immunodeficiency virus, Epstein Barr virus, human T-cell leukemia virus, Helicobacter pylori), exposure to toxic pesticides, and lifestyle factors such as poor diet are closely related to the occurrence of NHL.[2,3] Hepatitis C virus (HCV) is an RNA virus of the family Flaviviridae,[4] and is involved in the development of primary mixed type cold globulin and lymphoid tissue hyperplasia, which can further evolve into lymphoma.[5] As far back as the early 1990s, studies identified that patients with NHL had a higher rate of HCV infection, suggesting that there was a close association between these 2 conditions, yet failed to clarify the causal relationship between them.[6] Subsequently, additional epidemiological studies were conducted to investigate the potential relationship between HCV infection and NHL, but yielded inconsistent results.[7–24] Some studies suggested that HCV infection is a risk factor for NHL[8,10,12,14–16,21,23,24] while others have reached the opposite conclusion.[7,9,11,13,17–20,22] Therefore, in order to clarify whether the HCV infection is a risk factor for NHL, it is first necessary to establish a theoretical basis for the causal relationship between these conditions. The purpose of this study was to systematically review the relationship between HCV infection and NHL, perform a meta-analysis, and reach more reliable conclusions regarding the potential association of these 2 conditions.
2. Materials and methods
2.1. Study selection
We searched the PubMed, Web of Science, and Embase databases to identify articles analyzing the association between HCV infection and NHL through October 31, 2017. Studies had to be indexed with a MeSH heading of “Lymphoma, non-Hodgkin” and “hepatitis C virus” in combination with any keywords. The references of all resulting studies were searched for other potentially relevant studies.
Because the data included in this study were retrieved from published literature, ethical approval was not required.
2.2. Study inclusion and exclusion criteria
Eligible studies included in this meta-analysis had to meet all of the following criteria:
-
(1)
the study analyzed the association between HCV infection and development of NHL using a case–control or cohort study design;
-
(2)
the study fully reported either relative risk (RR) or odds ratio (OR); and
-
(3)
the study was published in Chinese or English full text.
Studies meeting any of the following criteria were excluded:
-
(1)
the study design was a literature review, case report, basic animal experiment, conference paper, or letter;
-
(2)
the study analyzed exposures that were not HCV infection or outcomes that were not NHL;
-
(3)
the study reported RRs or ORs incompletely; or
-
(4)
the study was not published in Chinese or English full text.
2.3. Data abstraction
Three investigators abstracted the data independently using a standard information extraction form. Data abstracted from each study included: name of the first author, year of publication, study population, age distribution of the study population, the number of NHL cases, the total number of individuals studied, the OR or RR and their 95% confidence intervals (CIs), the date that participants entered the study, and the document quality score.
2.4. Statistical analysis
We evaluated the association between HCV infection and development of NHL using ORs and their 95% CIs. If no or minor heterogeneity was apparent (P > .05, I2 < 50%), a pooled OR was calculated using a fixed effects model. If significant heterogeneity was evident (P < .05, I2 > 50%), then the pooled OR was calculated using a random-effects model. Sensitivity analyses were conducted to explore the impact of each study on the pooled OR. Finally, to test for publication bias in the funnel plot, Begg and Egger tests were used. P values < .05 were considered statistically significant. All statistical analyses were conducted using STATA 12.0.
3. Results
3.1. Study retrieval
After searching and scanning 3048 study abstracts, we eliminated reviews, animal experiments, conference papers, letters or studies involving irrelevant exposures and outcomes. After reading the titles and abstracts, most of these were excluded, leaving 18 studies.[7–24] The study search process is shown in Figure 1.
Figure 1.
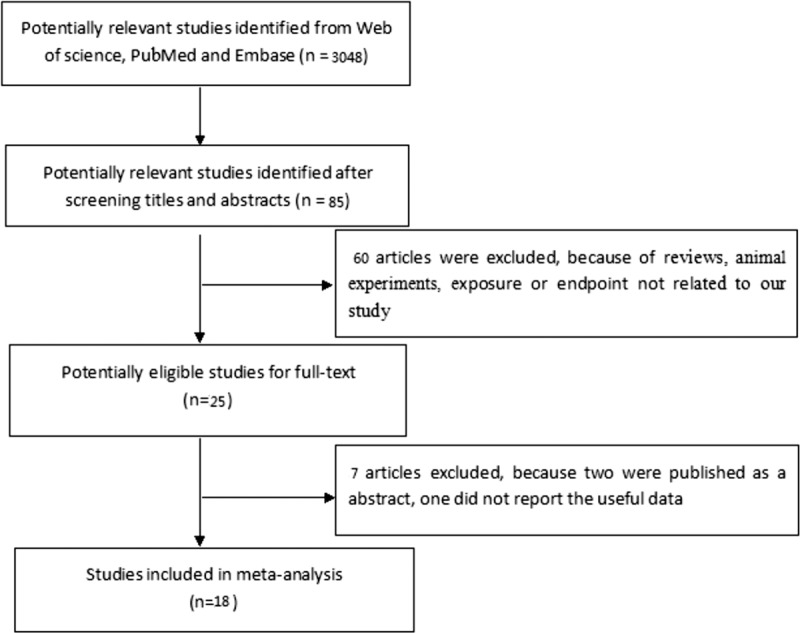
The flow chart of literature filtering.
3.2. Included studies
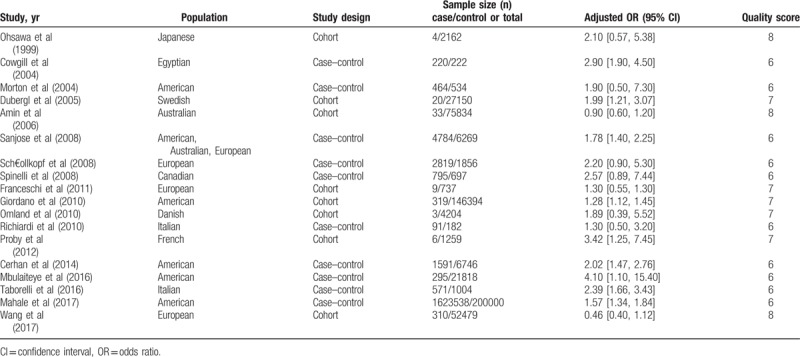
According to the inclusion and exclusion criteria, 18 studies were included in the meta-analysis, covering a time period from 1999 to 2017. Eight reports were of cohort studies[7–13,18] and the remaining 10 reports were of case–control studies.[14–17,19–24] The studies examined different populations in Western countries. All included studies had a high-quality score. The basic characteristics of the included studies are shown in Table 1.
Table 1.
The characteristics of included studies.
3.3. Meta-analysis
We conducted a meta-analysis of the 18 included studies. We found that HCV infection was significantly associated with risk of NHL in 8 studies,[8,10,12,14–16,21,23,24] while the remaining 10 studies suggested HCV infection was either not associated with increased risk of NHL or was associated with a reduced risk.[7,9,11,13,17–20,22] Significant heterogeneity was observed among studies (I2 = 68.5%, P < .05), and thus a random effects model was used to calculate a pooled OR. We found that HCV infection was associated with increased risk of NHL (pooled OR = 1.69, 95% CI 1.40–2.03, P < .05). This result suggested that patients with HCV infection were more likely to develop NHL. The results of the meta-analysis are shown in Figure 2.
Figure 2.
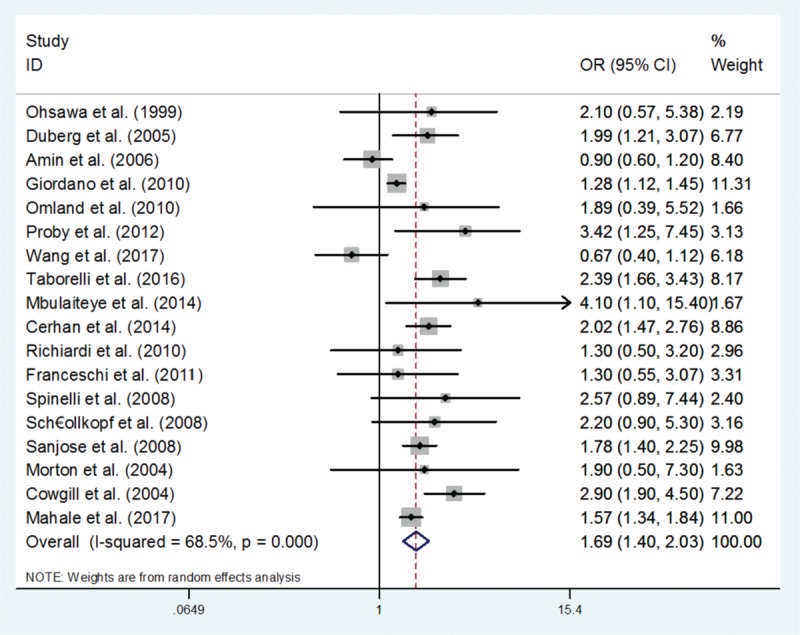
The effect of hepatitis C virus infection on the risk of non-Hodgkin lymphoma.
We also evaluated the association between HCV infection and risk of NHL using subgroup analyses. We found that HCV infection was associated with increased risk of NHL not only in cohort studies but also in case–control studies (OR = 1.26, 95% CI 1.12–1.40 and OR = 1.82, 95% CI 1.63–2.03, respectively). The results of subgroup analyses are shown in Figure 3.
Figure 3.
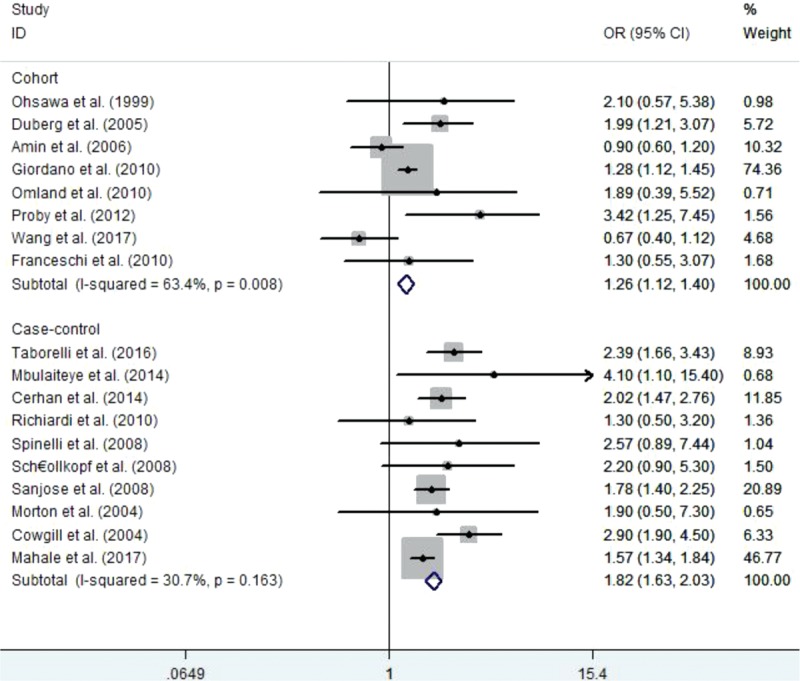
The subgroup analysis with the effect of hepatitis C virus infection on the risk of non-Hodgkin lymphoma according to study design.
3.4. Sensitivity analysis
In order to investigate the effect of each of the included studies on the pooled OR, we excluded the case–control studies, which had little effect on the overall result. This finding suggested that the risk of developing NHL in individuals with HCV infection was significantly higher than in uninfected individuals. The results of sensitivity analyses are shown in Figure 4.
Figure 4.
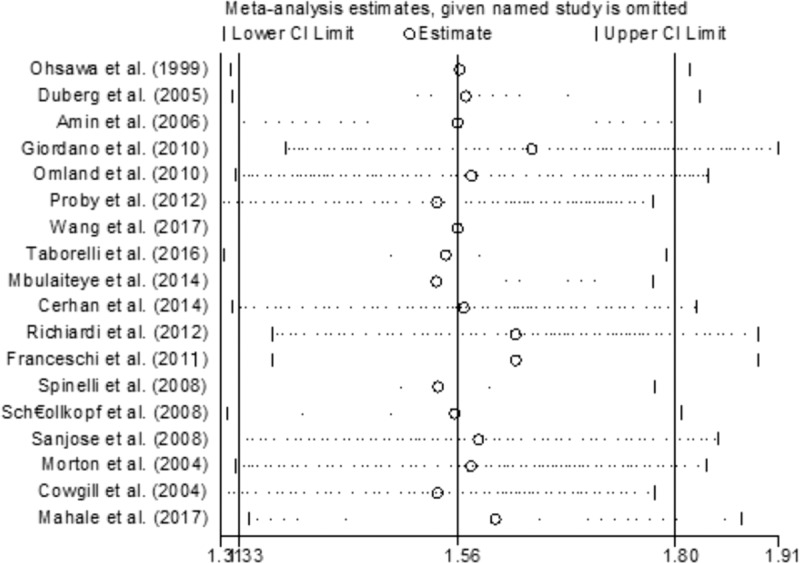
Sensitivity analysis forest plots.
3.5. Assessment of publication bias
We used a funnel plot, Begg test and Egger test to assess the presence of publication bias. As shown in Figure 5, the included studies were broadly distributed across the funnel area. Moreover, Egger test showed no evidence of publication bias (P = .16). In summary, there was relatively low potential of publication bias among the included studies.
Figure 5.
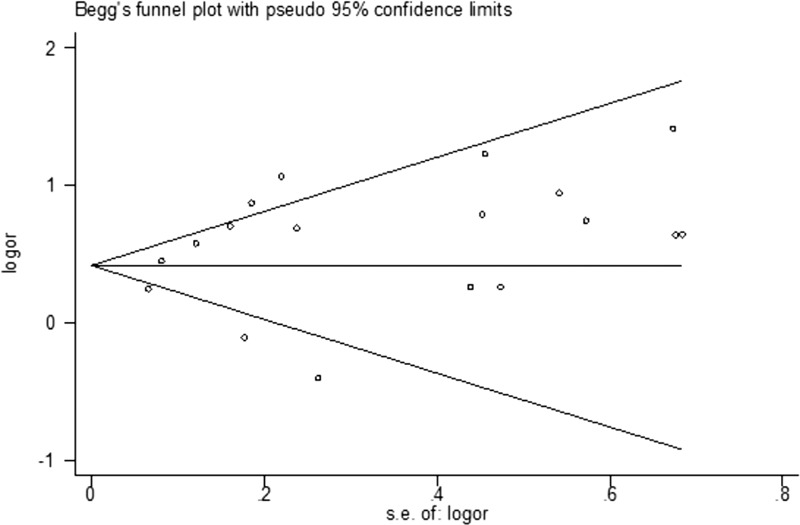
Forest plot for publication bias.
4. Discussion
In this systematic review meta-analysis, we found that HCV was significantly associated with risk of NHL. Compared with uninfected individuals, the risk of NHL was 69% higher among individuals with HCV infection.
The specific causal relationship between HCV infection and NHL remains unclear, but 2 possible biological mechanisms can be proposed. First, persistent HCV antigen stimulation during chronic infection may play an important role in the amplification of initial polyclonal B cells, which can lead to autonomous B-cell proliferation, immune dysfunction, and the eventual occurrence of B-cell malignancies.[25] Quinn et al found that the HCV E2 envelope glycoprotein was an important viral antigen that induced B cell proliferation.[26] In fact, the B-cell receptors (BCRs) of B-cells that cause HCV-associated lymphoma are often specific for the HCV E2 envelope glycoprotein. This result strongly supports the hypothesis that lymphocyte proliferation is involved in HCV-related NHL. B-cells respond to the E2 envelope glycoprotein of HCV through their specific BCRs, which can be activated by 2 signaling complexes (BCR and the CD19/CD21/CD81 complex), increasing the risk of B-cell malignancy. In addition to the dual stimulation hypothesis.[27] Levy et al also proposed the virus entry theory, in which B cells bind virions through these 2 receptors, effectively enabling viral internalization and leading to genomic instability, mutation of oncogenes, and development of malignant lesions. However, NHL patients with HCV infection receiving antiretroviral therapy still developed malignant tumor despite the reduction of virus load.
Second, cytokine imbalances may lead to some autoimmune and lymphoid tissue diseases. Cytokines may be the key factors in the pathogenesis of HCV-associated lymphoid tissue diseases. Mixed cryoglobulinemia is a predecessor of lymphoma, and this condition has been widely used for characterization of cytokine production in HCV-associated lymphoid tissue disease. CXCL13 is a positive regulator in patients with mixed cryoglobulinemia while BCA-1 and BLC regulate B cell transport regulator.[28] Antollie et al found different cytokines and chemokines in the serum of patients with mixed cryoglobulinemia and proposed that CXCL-10 and CXCL-11 have a potential role in the pathogenesis of mixed cryoglobulinemia.[29–31]
Compared with previous observational studies, our study had some advantages. First, 18 studies were included, which greatly increased the sample size, and made the conclusion more reliable. Second, all included studies were of high methodological quality. Thus, this meta-analysis should provide reliable evidence to inform epidemiological studies of the relationship between HCV infection and NHL.
However, our study also had some limitations. First, most of the included studies were conducted in European and American populations. People in different areas, with different dietary and lifestyle habits (for example, a high-fat diet) might have different baseline risk of NHL.[30] Thus, it is difficult to know whether our results can be generalized other populations. Second, most of the included studies did not distinguish between gender, but hormone levels also affect risk of NHL.[31] Third, HCV infection might have different influences on the distinct pathological types of NHL. Among HCV-infected individuals, the risk for diffuse large B-cell lymphoma was 4 times higher than the risk for follicular lymphoma. Fourth, previous epidemiological studies showed a significant association between HCV infection and NHL risk in areas with high HCV incidence (such as Japan and Italy) but there was no clear correlation observed in regions with low endemicity.[32] Therefore, there might be strong regionality in the association between HCV infection and NHL.[6] However, most of the included studies were conducted in European countries and had obvious geographical distributions. Fifth, significant heterogeneity was observed in the meta-analysis, and we considered the following factors might be potential explanations:
-
(1)
the included studies were based on different populations with different gender compositions;
-
(2)
the quality of the included studies differed; and
-
(3)
the pathological types of NHL studied were different.
5. Conclusions
In summary, this systematic review and meta-analysis suggested that HCV infection significantly increased the risk of NHL. However, multicenter, larger studies in other populations are still needed.
Author contributions
Data curation: Xiaofeng Zhu, Li Jing.
Formal analysis: Xiaofeng Zhu, Li Jing.
Investigation: Xiaofeng Zhu.
Methodology: Xiaoming Li, Xiaofeng Zhu, Li Jing.
Project administration: Xiaofeng Zhu.
Writing – original draft: Xiaofeng Zhu.
Writing – review and editing: Xiaoming Li.
Footnotes
Abbreviations: CI = confidence intervals, HCV = hepatitis C virus, OR = odds ratio, RR = relative risk.
The authors have no conflicts of interest to disclose.
References
- [1].Jaffe ES, Harris NL, Stein H, et al. World Health Organization Classifications of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2001. [Google Scholar]
- [2].Fisher SG, Fisher RI. The epidemiology of non-Hodgkin's lymphoma. Oncogene 2004;23:6524–34. [DOI] [PubMed] [Google Scholar]
- [3].Müller AM, Ihorst G, Mertelsmann R, et al. Epidemiology of non-Hodgkin's lymphoma (NHL): trends, geographic distribution, and etiology. Ann Hematol 2005;84:1–2. [DOI] [PubMed] [Google Scholar]
- [4].Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med 2001;345:41–52. [DOI] [PubMed] [Google Scholar]
- [5].Gasparotto D, DeRe V, Boiocchi M. Hepatitis C virus, B-cell proliferation and lymphomas. Leuk Lymphoma 2002;43:747–51. [DOI] [PubMed] [Google Scholar]
- [6].Yoshida EM, Shariff S, Shenkier T. Hepatitis C and B-cell non-Hodgkin's lymphoma: a geographically variable association? Am J Med 2000;108:350–1. [DOI] [PubMed] [Google Scholar]
- [7].Ohsawa M, Shingu N, Miwa H, et al. Risk of non-Hodgkin's lymphoma in patients with hepatitis C virus infection. Int J Cancer 1999;80:237–9. [DOI] [PubMed] [Google Scholar]
- [8].Duberg AS, Nordström M, Törner A, et al. Non-Hodgkin's lymphoma and other nonhepatic malignancies in Swedish patients with hepatitis C virus infection. Hepatology 2005;41:652–9. [DOI] [PubMed] [Google Scholar]
- [9].Amin J, Dore GJ, O’Connell DL, et al. Cancer incidence in people with hepatitis B or C infection: a large community-based linkage study. J Hepatol 2006;45:197–203. [DOI] [PubMed] [Google Scholar]
- [10].Giordano TP, Henderson L, Landgren O, et al. Risk of non-Hodgkin lymphoma and lympho proliferative precursor diseases in US veterans with hepatitis C virus. JAMA 2007;297:2010–7. [DOI] [PubMed] [Google Scholar]
- [11].Omland LH, Farkas DK, Jepsen P, et al. Hepatitis C virus infection and risk of cancer: a population-based cohort study. Clin Epidemiol 2010;2:179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Proby C, Minello A, Quantin C, et al. Chronic viral hepatitis and risk of lymphoid malignancies: a retrospective twelve-year population-based cohort study in Côte d’Or, France. Dig Liver Dis 2012;44:160–5. [DOI] [PubMed] [Google Scholar]
- [13].Wang Q, De Luca A, Smith C, et al. Chronic hepatitis B and C virus infection and risk for non-Hodgkin lymphoma in HIV-infected patients: a cohort study. Ann Intern Med 2017;166:9–17. [DOI] [PubMed] [Google Scholar]
- [14].Taborelli M, Polesel J, Montella M, et al. Hepatitis B and C viruses and risk of non-Hodgkin lymphoma: a case-control study in Italy. Infect Agent Cancer 2016;11:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mbulaiteye SM, Morton LM, Sampson JN, et al. Medical history, lifestyle, family history, and occupational risk factors for sporadic Burkittlymphoma/leukemia: the interlymph non-Hodgkin lymphoma subtypes project. J Natl Cancer Inst Monogr 2014;2014:106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cerhan JR, Kricker A, Paltiel O, et al. Medical history, lifestyle, family history, and occupational risk factors for diffuse large B-cell lymphoma: the interlymph non-Hodgkin lymphoma subtypes project. J Natl Cancer Inst Monogr 2014;2014:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Richiardi L, De Marco L, Gillio-Tos A, et al. Persistent infection by HCV and EBV in peripheral blood mononuclear cells and risk of non-Hodgkin's lymphoma. Cancer Epidemiol 2010;34:709–12. [DOI] [PubMed] [Google Scholar]
- [18].Franceschi S, Lise M, Trépo C, et al. Infection with hepatitis B and C viruses and risk of lymphoid malignancies in the European Prospective Investigation into Cancer and Nutrition (EPIC). Cancer Epidemiol Biomarkers Prev 2011;20:208–14. [DOI] [PubMed] [Google Scholar]
- [19].Spinelli JJ, Lai AS, Krajden M, et al. Hepatitis C virus and risk of non-Hodgkin lymphoma in British Columbia, Canada. Int J Cancer 2008;122:630–3. [DOI] [PubMed] [Google Scholar]
- [20].Schöllkopf C, Smedby KE, Hjalgrim H, et al. Hepatitis C infection and risk of malignant lymphoma. Int J Cancer 2008;122:1885–90. [DOI] [PubMed] [Google Scholar]
- [21].de Sanjose S, Benavente Y, Vajdic CM, et al. Hepatitis C and non-Hodgkin lymphoma among 4784 cases and 6269 controls from the International Lymphoma Epidemiology Consortium. Clin Gastroenterol Hepatol 2008;6:451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Morton LM, Engels EA, Holford TR, et al. Hepatitis C virus and risk of non-Hodgkin lymphoma: a population-based case-control study among Connecticut women. Cancer Epidemiol Biomarkers Prev 2004;13:425–30. [PubMed] [Google Scholar]
- [23].Cowgill KD, Loffredo CA, Eissa SA, et al. Case-control study of non-Hodgkin's lymphoma and hepatitis C virus infection in Egypt. Int J Epidemiol 2004;33:1034–9. [DOI] [PubMed] [Google Scholar]
- [24].Mahale P, Torres HA, Kramer JR, et al. Hepatitis C virus infection and the risk of cancer among elderly US adults: a registry-based case-control study. Cancer 2017;123:1202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Viswanatha DS, Dogan A. Hepatitis C virus and lymphoma. J Clin Pathol 2007;60:1378–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Quinn ER, Chan CH, Hadlock KG, et al. The B-cell receptor of a hepatitis C virus (HCV)-associated non-Hodgkin lymphoma binds the viral E2 envelope protein, implicating HCV in lymphomagenesis. Blood 2001;98:3745–9. [DOI] [PubMed] [Google Scholar]
- [27].Levy S. Alteration of B cell function by hepatitis C virus. Haematol Rep 2005;1:55–8. [Google Scholar]
- [28].Antonelli A, Ferri C, Fallahi P, et al. High values of CXCL10 serum levels in mixed cryoglobulinemia associated with hepatitis C infection. Am J Gastroenterol 2008;103:2488–94. [DOI] [PubMed] [Google Scholar]
- [29].Antonelli A, Fallahi P, Ferrari SM, et al. Circulating CXCL11 and CXCL10 are increased in hepatitis C-associated cryoglobulinemia in the presence of autoimmune thyroiditis. Mod Rheumatol 2012;22:659–67. [DOI] [PubMed] [Google Scholar]
- [30].Antonelli A, Ferri C, Ferrari SM, et al. High serum levels of CXCL11 in mixed cryoglobulinemia are associated with increased circulating levels of interferon-γ. J Rheumatol 2011;38:1947–52. [DOI] [PubMed] [Google Scholar]
- [31].De Vita S, Quartuccio L, Fabris M. Hepatitis C virus infection, mixed cryoglobulinemia and BLyS upregulation: targeting the infectious trigger, the autoimmune response, or both? Autoimmun Rev 2008;8:95–9. [DOI] [PubMed] [Google Scholar]
- [32].Kane EV, Bernstein L, Bracci PM, et al. Postmenopausal hormone therapy and non-Hodgkin lymphoma: a pooled analysis of inter lymph case-control studies. Ann Oncol 2013;24:433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]


