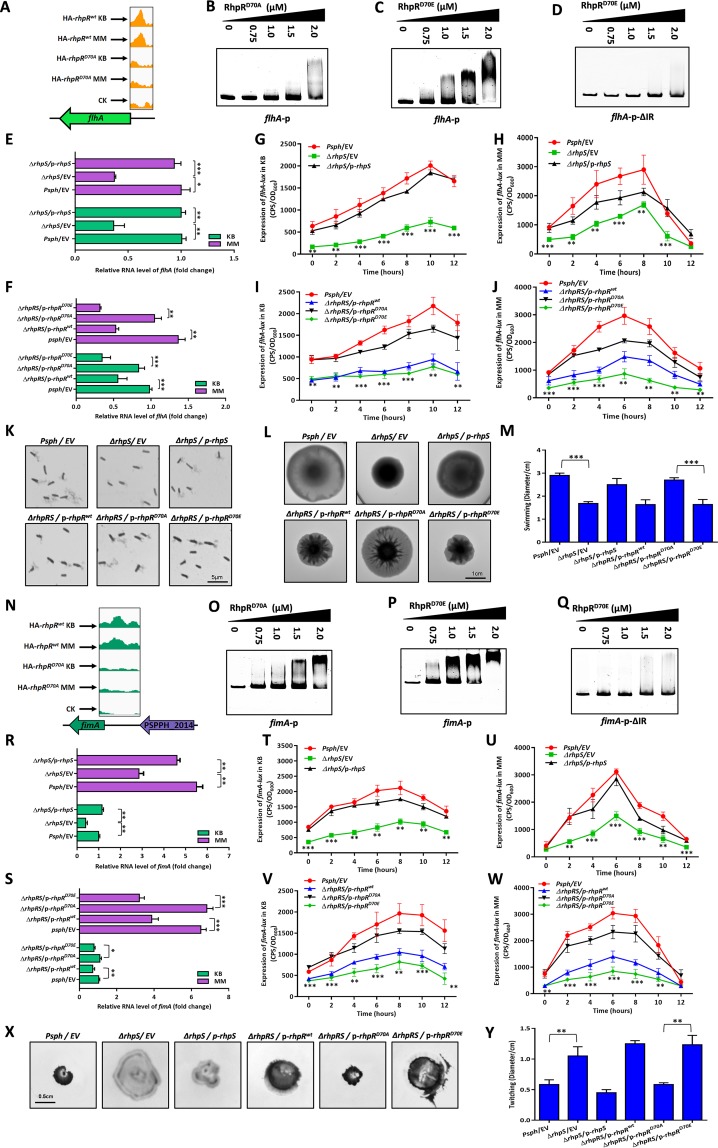
FIG 5.
RhpR negatively regulates swimming and positively regulates twitching motility. RhpR directly regulates swimming and twitching motility. (A) RhpR binds to the promoter region of flhA. (B and C) The phosphorylation of RhpR enhances the binding activity with promoter regions of flhA. PCR products containing the flhA promoter sequence were added to the EMSA reaction mixtures. (D) Validation of binding of RhpR to flhA-ΔIR promoter regions by EMSA. The flhA promoter without the putative IR element was subjected to EMSA with RhpRD70E. (E) RT-qPCR reveals that RhpR suppresses the mRNA level of flhA. The wild-type strain, ΔrhpS strain, and ΔrhpS strain carrying the pHM1-rhpS plasmid were grown in KB and induced in MM for 6 h. RT-qPCR was performed to measure the transcription level of flhA. (F) RT-qPCR shows that RhpR suppresses the expression of flhA. pHM1-RhpR, pHM1-RhpRD70A, pHM1-RhpRD70E, or pHM1 empty vector was transformed into the ΔrhpRS strain. RT-qPCR was performed to measure the transcription level of flhA. (G and H) RhpR directly suppresses the expression of flhA in vivo. The flhA-lux reporter was transformed into the wild-type strain, ΔrhpS strain, and complemented strain. A single colony was cultured in KB until it reached an OD600 of 0.6 and then transferred into MM. (I and J) Regulation of flhA promoters by RhpR in the ΔrhpRS strain. Activities of flhA-lux were introduced into the ΔrhpRS strain, ΔrhpRS strain carrying the pHM1-rhpR plasmid, ΔrhpRS strain carrying the pHM1-rhpRD70A plasmid, and ΔrhpRS strain carrying the pHM1-rhpRD70E plasmid. (K) Visualization of flagellar abundance in P. savastanoi pv. phaseolicola 1448A strains taken from KB motility plates. Shown are light microphotographs of cells from KB motility plates stained by the Leifson method. The scale bar represents 5 μm. (L and M) Effect of RhpR overexpression on swimming motility. Overnight cultures were spotted onto swimming plates (2-μl aliquots), and the plates were incubated at 28°C. The images were captured after 36 h of growth. (N) RhpR binds to the promoter region of fimA. (O and P) The phosphorylated RhpR has higher binding activity with the fimA promoter. The fimA promoter fragments were added to the EMSA reaction mixtures. RhpRD70A and RhpRD70E proteins were added to reaction buffer in lanes. (Q) Validation of binding of RhpR to fimA-ΔIR promoter regions by EMSA. The fimA promoter without the putative IR element was subjected to EMSA with RhpRD70E. (R) RT-qPCR reveals that RhpR suppresses the mRNA level of fimA. The wild-type strain, ΔrhpS strain, and ΔrhpS strain carrying the pHM1-rhpS plasmid were grown in KB and induced in MM for 6 h. RT-qPCR was performed to measure the transcription level of fimA. (S) RT-qPCR shows that RhpR suppresses the expression of fimA. pHM1-RhpR, pHM1-RhpRD70A, pHM1-RhpRD70E, or the pHM1 empty vector was transformed into the ΔrhpRS strain. RT-qPCR was performed to measure the transcription level of fimA. (T and U) RhpR directly suppresses the expression of fimA in vivo. The fimA-lux reporter was transformed into the wild-type, ΔrhpS, and complemented strains. A single colony was cultured in KB until it reached an OD600 of 0.6 and then transferred into MM. (V and W) Regulation of fimA promoters by RhpR in the ΔrhpRS strain. Activities of fimA-lux were introduced into the ΔrhpRS strain, ΔrhpRS strain carrying the pHM1-rhpR plasmid, ΔrhpRS strain carrying the pHM1-rhpRD70A plasmid, and ΔrhpRS strain carrying the pHM1-rhpRD70E plasmid. (X and Y) Effect of RhpR overexpression on swimming motility. Overnight cultures were inoculated into twitching plates (3-μl aliquots), and the plates were incubated at 28°C. The images were captured after 36 h of growth. The experiments were repeated at least three times, and similar results were observed. *, P < 0.05, **, P < 0.01, and ***, P < 0.001, compared to the ΔrhpS or ΔrhpRS/p-rhpRD70A strain by Student's t test. Data are representative of three independent experiments.