Abstract
Rationale:
Primary adrenal non-Hodgkin lymphomas are predominant diffuse large B cell lymphoma with frequently bilateral adrenal involvement, but the occurrence of nasal type extranodal NK/T cell lymphoma is relatively rare.
Patient concerns:
A 40-year-old woman complaining of left back pain for 2-month was admitted to our department.
Diagnosis:
Based on the feature of enhanced computed tomography (CT) images which showed huge bilateral well-defined adrenal masses with heterogeneous enhancement, she was tentatively diagnosed as having primary adrenal malignancy. Postoperative pathology revealed the diagnosis of primary adrenal Epstein-Barr virus-associated nasal type extranodal NK/T-cell lymphoma.
Interventions:
Then, she underwent 18F-fluoro-2-deoxy-D-glucose (18F-FDG) positron emission tomography (PET)/CT examination for staging, which showed homogeneously increased FDG uptake in the right adrenal gland and left thigh subcutaneous lesion, as well as heterogeneous increased FDG uptake in the left adrenal gland region with no abnormal uptake in the nasal cavity. Subsequently, the patient has performed 7 cycles of gemcitabine, L-asparaginase, ifosfamide, dexamethasone, etoposide (GLIDE) regimen and autologous stem cell transplantation.
Outcomes:
Fortunately, the subsequent 2 follow-up FDG PET/CT scans within 1 year revealed complete resolution with no abnormal FDG uptake in the initially involved sites after 7 cycles of GLIDE chemotherapy and autologous stem cell transplantation.
Lessons:
The enhanced CT and FDG PET/CT features of primary adrenal extranasal NK/T cell lymphoma are huge bilateral well-defined adrenal masses with heterogeneous enhancement, high FDG uptake, especially with subcutaneous involvement. And the awareness of this entity may help clinicians to differentiate it from other primary adrenal tumors and make reasonable therapeutic strategies. Besides, FDG PET/CT scan is very useful for the treatment follow-up of the primary adrenal extranasal NK/T cell lymphoma.
Keywords: 18F-FDG PET/CT, primary adrenal extranasal NK/T cell lymphoma
1. Introduction
Extranodal NK/T cell lymphoma, usually with an NK cell phenotype and positivity for Epstein-Barr virus (EBV), is an extranodal non-Hodgkin lymphoma (NHL).[1] And the most common involved extranodal site is a nasal cavity, and others include the Waldeyer ring, upper airway, skin, gastrointestinal tract, testis, lung, thyroid, or adrenal glands.[2–9] Primary adrenal NHLs are predominant diffuse large B cell lymphoma (>70%) with frequently bilateral adrenal involvement,[10–13] but the occurrence of nasal type extranodal NK/T cell lymphoma is relatively rare. Herein, we report a case with primary bilateral adrenal NK/T cell lymphoma with subcutaneous involvement.
2. Case report
A 40-year-old woman with a 2-month history of left back pain was admitted to our clinic. Physical examination revealed no abnormalities. Laboratory tests showed a slightly increased adrenaline and plasma renin activity (recumbent position) levels at 123 ng/L (reference range, 60–104 ng/L) and 2.6 ng/mL.h (reference range, 0.05–0.79 ng/mL.h), respectively. Abdominal enhanced computed tomography (CT) (Fig. 1A and C: axial; B and D: sagittal) presented well-defined bilateral adrenal masses with the diameter of 75 cm (Fig. 1 A and B, black arrows) and 31 cm (Fig. 1C and D, white arrows), showing heterogeneous enhancement. She was tentatively diagnosed as having primary adrenal malignancy based on the CT findings.
Figure 1.

Abdominal enhanced CT (A and C: axial; B and D: sagittal) presented well-defined bilateral adrenal masses with the diameter of 75 cm (A and B, black arrows) and 31 cm (C and D, white arrows), showing heterogeneous enhancement, suggestive of primary adrenal malignancy. CT = computed tomography.
Subsequently, the patient underwent left adrenalectomy and ipsilateral nephrectomy. Postoperative pathology showed proliferating lymphocytes with necrosis, infiltration of plasma cells and tissue cells. The immunohistochemical staining revealed that proliferative lymphocytes were positive for CD3ε, CD30, CD56, granzyme B, EBER1/2-ISH, as well as a high proliferation index (Ki-67) of about 70%, but negative for PCK, CD20, CD5, CD4, CD8, CD21, or CD79a. Background plasma cells were positive for CD79a, CD138, Igκ (P), Igλ (P). No rearrangement peaks of the TCRγ gene were detected by gene rearrangement detection. Combined with the above morphological findings, immunohistochemical staining and gene rearrangement results, the diagnosis of EBV-associated nasal type extranodal NK/T-cell lymphoma was confirmed.
Subsequently, she underwent 18F-fluoro-2-deoxy-D-glucose (18F-FDG) positron emission tomography (PET)/CT examination for staging. PET/CT images showed homogeneously increased FDG uptake in the right adrenal gland (maximal standardized uptake value (SUVmax) of 15.7, Fig. 2A, H, I, and J: thick arrows) and left thigh subcutaneous lesion (SUVmax of 12.5, Fig. 2A, K, L, and M: arrows), as well as heterogeneous increased FDG uptake in the left adrenal gland region (SUVmax of 8.3, Fig. 2A, E, F, and G: thin arrows). The whole body PET/CT revealed no abnormal uptake of FDG in the nasal cavity (Fig. 2A, B, C, and D), indicating that the bilateral adrenal masses might be primary lesions with subcutaneous involvement. Then, the patient has performed 7 cycles of gemcitabine, L-asparaginase, ifosfamide, dexamethasone, etoposide (GLIDE) regimen and autologous stem cell transplantation. Fortunately, the subsequent 2 follow-up FDG PET/CT scans within 1 year revealed complete resolution with no abnormal FDG uptake in the initially involved sites after chemotherapy and autologous stem cell transplantation (Fig. 2N and O).
Figure 2.
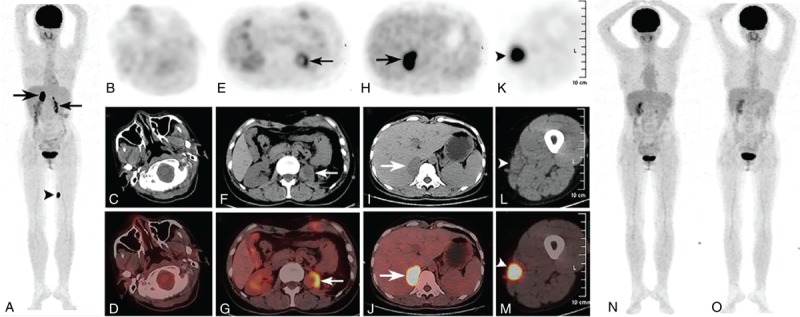
18F-PET/CT images showed homogeneously increased FDG uptake in the right adrenal gland (maximal SUVmax) of 15.7, A, H, I, and J: thick arrows) and left thigh subcutaneous lesion (SUVmax of 12.5, A, K, L, and M: arrows), as well as heterogeneous increased FDG uptake in the left adrenal gland region (SUVmax of 8.3, A, E, F, and G: thin arrows). The whole body PET/CT revealed no abnormal uptake of FDG in the nasal cavity (A, B, C, and D), indicating that the bilateral adrenal masses might be primary lesions with subcutaneous involvement. Fortunately, the subsequent 2 follow-ups FDG PET/CT scans revealed complete resolution with no abnormal FDG uptake in the initially involved sites after chemotherapy and autologous stem cell transplantation (N and O). CT = computed tomography, PET = positron emission tomography, SUVmax = standardized uptake value.
The Ethics Committee of West China Hospital of Sichuan University, Chengdu, China, waived the need to obtain informed consent.
3. Discussion
Extranodal NK/T-cell lymphoma is a subtype of mature T- and NK-cell lymphomas that most often involve the nasal region (nasal NK/T-cell lymphoma), with a broad range of morphologic appearances, frequent necrosis, and angioinvasion.[14] Extranodal NK/T cell lymphoma is most common in Asia (eg, China, Korea, and Japan) and in native populations of Central and South America (eg, Peru and Mexico), accounting for 5% to 10% of all NHL.[15–17] The median age at presentation is approximately 52 years with a male predominance.[17,18] Primary adrenal extranasal NK/T cell lymphoma is a very rare extranodal lymphoma mainly involving the adrenal glands, which is also associated with EBV.[1–3,19] The commonest presentation of patients with primary adrenal extranasal NK/T cell lymphoma is back pain with or without B symptoms (ie, fever, night sweats, and weight loss).[11,20–21] Imaging features of this disease are well-defined bilateral adrenal masses with heterogeneous enhancement, but without the regional lymph node involvement.[22] Differential diagnoses include metastatic disease, primary adrenal malignancy, such as adrenocortical carcinoma, primary adrenal benign disease like adenoma, phaeochromocytoma, or tuberculosis.[22] Certainly, the definitive diagnosis is made by an image-guided percutaneous biopsy or surgical excision.[23,24] This patient was diagnosed with primary adrenal extranasal NK/T cell lymphoma by surgical excision. The therapeutic strategies for this disease are diverse combinations of chemotherapy, surgery, radiation, or autologous stem cell transplantation.[9] In this case, the patient received 3 treatments, including surgery, chemotherapy, and autologous stem cell transplantation.
Recently, Kumar et al reviewed the imaging characteristics of diverse adrenal gland diseases on PET/CT using different tracers.[25]18F-Fluorodopa PET/CT and 11C-Hydroxyephedrine PET/CT have been used for imaging of adrenal medullary lesions, such as pheochromocytoma, and 11C-Metomidate PET/CT has been used for adrenocortical imaging like adrenocortical carcinoma.[25] While for imaging of adrenal lymphoma, 18F-FDG PET is very useful.[25] Apart from the demonstration of adrenal involvement, it can also show lymphomatous involvement at other sites. In this case, 18F-FDG PET/CT scan revealed not only the huge bilateral well-defined adrenal masses with marked FDG uptake, but also the left thigh subcutaneous involvement. This finding should be added to the list of primary adrenal tumors or adrenal infections with increased FDG uptake, commonly involving metastatic tumors, adrenocortical carcinoma, pheochromocytoma, neuroblastoma, or histoplasmosis, tuberculosis, and should be taken into account when interpreting similar images. Additionally, 18F-FDG PET/CT plays an important role in staging, predicting disease progression and response evaluation.[25–27]
4. Conclusions
In conclusion, we presented a rare case of primary adrenal extranasal NK/T cell lymphoma with subcutaneous involvement demonstrated on 18F-FDG PET/CT. Besides, 18F-FDG PET/CT is a useful diagnostic and follow up imaging modality in patients with an adrenal disease, which can not only help to do staging but also evaluate therapeutic response. Primary adrenal extranasal NK/T cell lymphoma, although a rare entity, should always be considered in patients with huge bilateral adrenal masses.
Author contributions
Data curation: Ping Dong, Li Wang.
Project administration: Ping Dong.
Supervision: Guohua Shen, Lin Li.
Writing – original draft: Ping Dong, Li Wang.
Writing – review and editing: Ping Dong, Guohua Shen, Lin Li.
Footnotes
Abbreviations: 18F-FDG = 18F-fluoro-2-deoxy-D-glucose, CT = computed tomography, EBV = Epstein-Barr virus, NHL = non-Hodgkin lymphomas, PET/CT = positron emission tomography/computed tomography, PRA = plasma renin activity, SUVmax = maximal standardized uptake value.
The authors declare that there is no conflict of interests.
References
- [1].Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016;127:2375–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Li YX, Fang H, Liu QF, et al. Clinical features and treatment outcome of nasal-type NK/T-cell lymphoma of Waldeyer ring. Blood 2008;112:3057–64. [DOI] [PubMed] [Google Scholar]
- [3].AlShemmari SH, Ameen RM, Sajnani KP. Extranodal lymphoma: a comparative study. Hematology 2008;13:163–9. [DOI] [PubMed] [Google Scholar]
- [4].Chim CS, Au WY, Shek TW, et al. Primary CD56 positive lymphomas of the gastrointestinal tract. Cancer 2001;91:525–33. [DOI] [PubMed] [Google Scholar]
- [5].Ye ZY, Cao QH, Liu F, et al. Primary esophageal extranasal NK/T cell lymphoma with biphasic morphology: a case report and literature review. Medicine (Baltimore) 2015;94:e1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhang WL, Ma S, Jug R, et al. Primary testicular natural killer/T-cell lymphoma: a CARE-case report and review of literature. Medicine (Baltimore) 2018;97:e0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zhang J, Wang M, Yang X, et al. Primary pulmonary extranodal NK/T-cell lymphoma of nasal type misdiagnosed as pneumonia: a case report and literature review. Medicine (Baltimore) 2017;96:e8914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Li JH, He HH, Cheng Y, et al. Primary thyroid extranasal NK/T-cell lymphoma associated with good outcome: a case report and literature review: a care-compliant article. Medicine (Baltimore) 2016;95:e3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hu L, Xu W, Wang M, et al. A case report of primary unilateral adrenal NK/T cell lymphoma: good clinical outcome with trimodality treatment. BMC Cancer 2017;17:15–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Al-Fiar FZ, Pantalony D, Shepherd F. Primary bilateral adrenal lymphoma. Leuk Lymphoma 1997;27:543–9. [DOI] [PubMed] [Google Scholar]
- [11].Grigg AP, Connors JM. Primary adrenal lymphoma. Clin Lymphoma 2003;4:154–60. [DOI] [PubMed] [Google Scholar]
- [12].Ozimek A, Diebold J, Linke R, et al. Bilateral primary adrenal non-Hodgkin's lymphoma — a case report and review of the literature. Eur J Med Res 2008;13:221–8. [PubMed] [Google Scholar]
- [13].Kim YR, Kim JS, Min YH, et al. Prognostic factors in primary diffuse large B-cell lymphoma of adrenal gland treated with rituximab-CHOP chemotherapy from the consortium for improving survival of lymphoma (CISL). J Hematol Oncol 2012;5:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yamaguchi M, Suzuki R, Oguchi M. Advances in the treatment of extranodal NK/T-cell lymphoma, nasal type. Blood 2018;131:2528–40. [DOI] [PubMed] [Google Scholar]
- [15].Li CC, Tien HF, Tang JL, et al. Treatment outcome and pattern of failure in 77 patients with sinonasal natural killer/T-cell or T-cell lymphoma. Cancer 2004;100:366–75. [DOI] [PubMed] [Google Scholar]
- [16].Au WY, Weisenburger DD, Intragumtornchai T, et al. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood 2009;113:3931–7. [DOI] [PubMed] [Google Scholar]
- [17].Laurini JA, Perry AM, Boilesen E, et al. Classification of non-Hodgkin lymphoma in Central and South America: a review of 1028 cases. Blood 2012;120:4795–801. [DOI] [PubMed] [Google Scholar]
- [18].Chim CS, Ma SY, Au WY, et al. Primary nasal natural killer cell lymphoma: long-term treatment outcome and relationship with the international prognostic index. Blood 2004;103:216–21. [DOI] [PubMed] [Google Scholar]
- [19].Shet T, Suryawanshi P, Epari S, et al. Extranodal natural killer/T cell lymphomas with extranasal disease in non-endemic regions are disseminated or have nasal primary: a study of 84 cases from India. Leuk Lymphoma 2014;55:2748–53. [DOI] [PubMed] [Google Scholar]
- [20].Mantzios G, Tsirigotis P, Veliou F, et al. Primary adrenal lymphoma presenting as Addison's disease: case report and review of the literature. Ann Hematol 2004;83:460–3. [DOI] [PubMed] [Google Scholar]
- [21].Levy NT, Young WF, Jr, Habermann TM, et al. Adrenal insufficiency as a manifestation of disseminated non-Hodgkin's lymphoma. Mayo Clin Proc 1997;72:818–22. [DOI] [PubMed] [Google Scholar]
- [22].Arora S, Vargo S, Lupetin AR. Computed tomography appearance of spontaneous adrenal hemorrhage in a pheochromocytoma. Clin Imaging 2009;33:314–7. [DOI] [PubMed] [Google Scholar]
- [23].Dunning KK, Wudhikarn K, Safo AO, et al. Adrenal extranodal NK/T-cell lymphoma diagnosed by fine-needle aspiration and cerebrospinal fluid cytology and immunophenotyping: a case report. Diagn Cytopathol 2009;37:686–95. [DOI] [PubMed] [Google Scholar]
- [24].Thompson MA, Habra MA, Routbort MJ, et al. Primary adrenal natural killer/T-cell nasal type lymphoma: first case report in adults. Am J Hematol 2007;82:299–303. [DOI] [PubMed] [Google Scholar]
- [25].Sharma P, Singh H, Dhull VS, et al. Adrenal masses of varied etiology: anatomical and molecular imaging features on PET-CT. Clin Nucl Med 2014;39:251–60. [DOI] [PubMed] [Google Scholar]
- [26].Berk V, Yildiz R, Akdemir UO, et al. Disseminated extranodal NK/T-cell lymphoma, nasal type, with multiple subcutaneous nodules: utility of 18F-FDG PET in staging. Clin Nucl Med 2008;33:365–6. [DOI] [PubMed] [Google Scholar]
- [27].Ko KY, Liu CJ, Ko CL, et al. Intratumoral heterogeneity of pretreatment 18F-FDG PET images predict disease progression in patients with nasal type extranodal Natural Killer/T-cell lymphoma. Clin Nucl Med 2016;41:922–6. [DOI] [PubMed] [Google Scholar]


