Abstract
We compared intratumoral stromal proportions and positron emission tomography (PET) textural features between females and males with lung adenocarcinoma (ADC) or squamous cell carcinoma (SCC).
We retrospectively evaluated 167 consecutive patients (male 122, female 45) who underwent pretreatment fluorodeoxyglucose PET/CT and surgical resection. The tumor-stroma ratios (TSRs) of primary tumors were estimated on hematoxylin-and-eosin-stained histological sections, and higher-order textural features were extracted on PET. We compared the histological and PET features between the sexes.
More females than males had ADC. Age and pathological tumor size did not significantly differ between females and males. Females with ADC had more stroma-rich tumors than males with ADC (P = .016) or SCC (P = .047). In addition, some PET textural features significantly differed between females with ADC and males with ADC and SCC; short run emphasis, long run emphasis, coarseness, strength, short-zone emphasis, zone percentage and high-intensity large-zone percentage were the commonly differed textural features. However, the TSRs and PET textural features did not significantly differ between males with ADC or SCC.
Intratumoral heterogeneity in females with lung ADC differs from that in males with lung ADC or SCC.
Keywords: adenocarcinoma of lung, female, positron emission tomography, texture analysis, tumor-stroma ratio
1. Introduction
Non-small cell carcinoma (NSCLC) is a heterogeneous disease. The biological features and treatment-related outcomes of lung adenocarcinoma (ADC) and squamous cell carcinoma (SCC) differ, although both are categorized as NSCLCs. In addition, the clinical characteristics of NSCLC differ between females and males in terms of median age at diagnosis, smoking history, predominant histological subtype, and treatment outcomes.[1,2] Although the reasons are complex, a large and growing body of literature has emphasized the important roles of estrogen and estrogen receptors in lung cancer development.[3–7] The effects of estrogen on the tumor microenvironment have been investigated in several types of cancer including lung cancer.[5,8,9]
Interplay among cancer, stromal, and immune cells and extracellular molecules of the tumor microenvironment are critical in cancer growth. Increasing evidence indicates that interactions between cancer cells and the neighboring stroma are important in cancer progression and metastasis.[10–12] Some investigations have focused on the intratumoral stromal proportion as a key regulator of cancer biology. The clinical significance of a high stromal proportion (the so-called high tumor-stroma ratio) in terms of cancer progression has been investigated in many types of cancers including those of the esophagus, stomach, breast, colon, and lung.[13–17]
F-18 fluorodeoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT) measures tumor glucose metabolism noninvasively, and is widely used to stage NSCLC. In addition, PET textural analyses have emerged as novel tools for assessing intratumoral heterogeneity. Texture analysis involves a series of calculations that evaluate the position and intensity of a digital image and extract information about the relationship between adjacent pixels.[18,19] Increasing evidence suggests that intratumoral heterogeneity can be characterized by PET textural analysis and that these features are related to clinical outcome such as treatment response or survival prediction in lung cancer.[20–23]
We previously reported that heterogeneity imaging parameters are significantly associated with the intratumoral stromal proportion in head and neck squamous cell carcinoma.[24] We hypothesized that heterogeneity imaging parameters might reflect the tumor microenvironment, and thus differ between sexes in NSCLCs. Therefore, we measured tumor-stroma ratios (TSRs) and PET textural features of primary NSCLCs, and evaluated the differences between females and males.
2. Materials and methods
2.1. Patients
We retrospectively reviewed the pretreatment FDG PET/CT scans of 275 consecutive NSCLC patients who underwent surgical treatment at a single institution (Ajou University Hospital, Suwon, Korea). Those with small malignant nodules (<1 cm in diameter) and histological cancer subtypes other than adenocarcinoma (ADC) or squamous cell carcinoma (SCC) were excluded. We ultimately evaluated 167 patients with lung ADC or SCC. Our ethics committee approved this retrospective study and waived the requirement for informed patient consent.
2.2. FDG PET/CT acquisition
FDG PET/CT was performed using the Discovery STE PET/CT scanner (GE Healthcare, Milwaukee, WI). All patients fasted for at least 6 hours before FDG PET/CT; their serum glucose levels at the time of FDG injection were <150 mg/dL. Unenhanced CT was performed 60 minutes after injection of 5 MBq/kg FDG, using a 16-slice helical CT scanner (120 keV; 30–100 mA in the AutomA mode; section width=3.75 mm). Emission PET data were acquired from the thigh to the head for 3.0 minutes per frame in the 3-dimensional mode. Attenuation-corrected PET images using CT data were reconstructed by an ordered-subset expectation maximization algorithm (20 subsets, 2 iterations).
2.3. PET texture analyses
The PET texture analysis method was previously described.[25] For tumor delineation, we used the gradient-based segmentation method (PET Edge) in MIM version 6.4 (MIM Software Inc., Cleveland, OH). Texture analysis for the quantification of intratumor heterogeneity on PET was performed using the Chang-Gung Image Texture Analysis toolbox (CGITA, https://code.google.com/archive/p/cgita), an open-source software package implemented in MATLAB (version 2012a; MathWorks Inc., Natick, MA).[26] Of the many textural matrices that can be derived using CGITA, 27 higher-order textural features were analyzed, because these features are commonly evaluated by medical imaging researchers.[19,25] Higher order textural features included gray level run length matrix, neighborhood gray level difference matrix, and gray level size zone matrix. After resampling with 64 discrete bins, we derived 27 higher order textural features. We also measured conventional PET parameters including the maximum standardized uptake value (SUVmax), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) of the primary tumors.
2.4. Histopathology
Histological subclassification was performed by 2 pathologists (YWK and DL) based on the 2015 World Health Organization Classification of Lung Tumors.[27] To perform a differential diagnosis between ADC and SCC, we conducted immunohistochemical stainings for TTF-1, Napsin A and p40. TSRs were obtained by consensus. The stromal proportion was quantified as previously described.[16,24] In brief, the largest invasive tumor block was selected using the 1.25× objective. Subsequently, using the 10x objective, fields were only scored where both stroma and tumor were present, and the tumor cells were visible on all slides in the microscopic image field. Then the stromal proportion was estimated and scored in increments of 5 percentage points (e.g., 15%, 20%, and 25%). Representative photomicrographs are presented in Figure 1. All slides were reviewed in a blinded manner.
Figure 1.
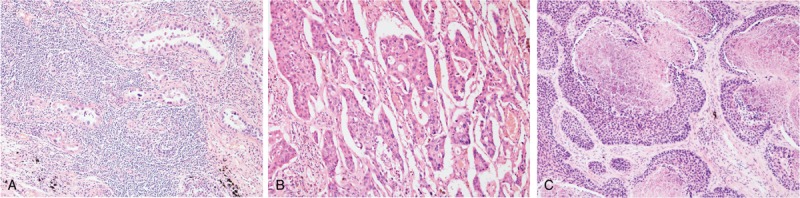
Representative photomicrographs of nonsmall cell carcinoma. (A) Female adenocarcinoma with a stromal proportion estimated as 60%. (B) Male adenocarcinoma with a stromal proportion estimated as 20%. (C) Male squamous cell carcinoma with a stromal proportion estimated as 20%.
2.5. Statistical analyses
We expressed data as means ± SDs for continuous variables and as percentages for categorical variables. All analyses were conducted using SPSS for Windows software (ver 18.0; IBM Inc., New York, NY). Differences between the 2 groups were compared using the t-test for continuous variables and the chi-square test for dichotomous variables. P values <.05 were considered to indicate statistical significance.
3. Results
3.1. Patient characteristics
Of the 167 patients, 45 were females (26.9%) and 122 males (73.1%) of a mean age of 63.3 ± 9.2 years. The mean diameter of resected primary tumors was 3.5 ± 1.3 cm (range, 1.2–7.0 cm). The clinical characteristics of females and males are summarized in Table 1. More males than females had smoking history. More females than males had ADC. Age, pathological tumor size, and TSR did not significantly differ between females and males. Stroma-rich and stroma-poor tumors were distinguished using the median TSR value (30). The proportion of stroma-rich tumors (TSR > 30) was significantly higher in females than in males (42.2 vs 24.6%, P = .026). We created 4 subgroups by sex and histology: females with ADC (FADC, n = 37), females with SCC (FSCC, n = 8), males with ADC (MADC, n = 58), and males with SCC (MSCC, n = 64). Figure 2 shows the differences in proportions of stroma-rich and stroma-poor tumors. We excluded the FSCC subgroup from statistical analyses because of the small number of patients. The FADC subgroup had more stroma-rich tumors than the MADC subgroup (45.9 vs 22.4%, P = .016) and the MSCC subgroup (45.9 vs 26.6%, P = .047); however, the MADC and MSCC subgroups did not significantly differ (P = .679).
Table 1.
Clinical characteristics of 167 patients.
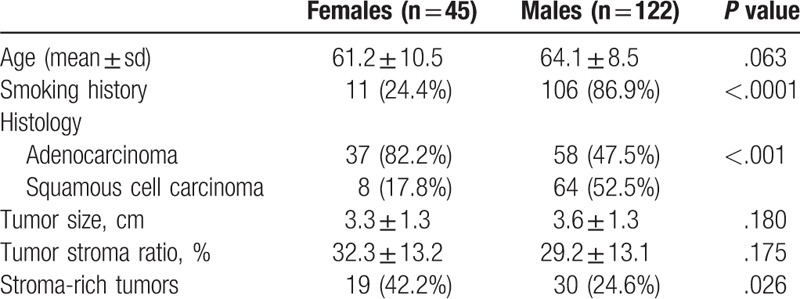
Figure 2.
The difference of stroma-rich tumors among 4 subgroups. FADC = females with adenocarcinoma, FSCC = females with squamous cell carcinoma, MADC = males with adenocarcinoma, MSCC = males with squamous cell carcinoma.
3.2. Differences in PET textural features according by sex and histology
The mean SUVmax, MTV, and TLG of females were 8.6 ± 4.8, 20.7 ± 30.5, and 107.1 ± 214.7, respectively; and 11.0 ± 6.6, 30.0 ± 38.4, and 181.9 ± 246.6, for males, respectively, who had a significantly higher SUVmax than females (P = .033). The MTV and TLG did not significantly differ between the sexes.
The higher order PET textural features of the 3 subgroups (FADC, MADC, and MSCC) are summarized in Tables 2–4 (gray-level run length matrix, neighborhood gray-level difference matrix, and gray-level size zone matrix, respectively). Significant differences were evident between FADC and MADC and FADC and MSCC, but not between MADC and MSCC. The textural features that differed commonly were the short run emphasis, long run emphasis, coarseness, strength, short-zone emphasis, zone percentage and high-intensity large-zone percentage.
Table 2.
Comparison of gray-level run length matrix.
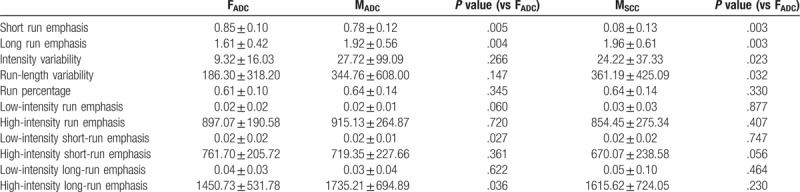
Table 4.
Comparison of gray-level size zone matrix.
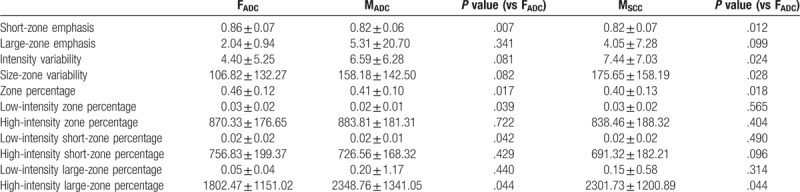
Table 3.
Comparison of neighborhood gray-level difference matrix.

4. Discussion
We found that intratumoral heterogeneity as measured using TSR and PET textural analyses significantly differed between females and males. Histopatholgically, FADC had more tumors with high proportions of stroma. Interestingly, we found no significant difference between MADC and MSCC. The PET data yielded similar results: some textural features significantly differed between FADC and MADC and FADC and MSCC, but not between MADC and MSCC.
Intratumoral heterogeneity measured by imaging modalities such as PET or CT may be associated with differences in regional tumor cellularity, proliferation, hypoxia, necrosis, and angiogenesis.[28,29] These heterogeneous biological characteristics may be related with the tumor–stroma interaction. Cancer-associated fibroblasts (CAFs), the main cellular components of the stroma, may play a critical role in the tumor–stroma interaction. The accumulation of fibrotic extracellular matrix is induced by CAFs and the structure of the extracellular matrix can be further stimulated and altered by proteases, which degrade the stroma. Together, these cause the destruction of epithelial tissue and remodeling of the extracellular matrix, thereby promoting the invasion of tumor cells. CAFs also secrete growth factors, inflammatory factors, and angiogenic factors, all of which contribute to intratumoral heterogeneity.[30,31] It has been suggested that increased image heterogeneity is associated with more aggressive tumor behavior, poorer response to treatment and worse prognosis.[19] Because clinical characteristics between sexes in NSCLC are apparent, we hypothesized that the intratumoral heterogeneity between females and males is different. Therefore, we chose TSR as a possible histopathologic feature to compare PET textural features between females and males although little is known what the biological correlates of textural features are.
NSCLC-specific research has demonstrated that stroma in NSCLC is associated with tumor invasiveness, metastasis and poor prognosis. Nakamura et al[32] showed that the expression of carbonic anhydrase IX by CAFs contributed to an increase in aggressive behavior in lung ADC with hypoxic regions. Takahashi et al[33] reported that SCC with a fibrous stroma displayed higher invasive phenotype and were associated with a significantly poor prognosis.
Recently, texture analysis has been identified as a tool for “radiomics” on medical images. Recent investigations using texture analyses by FDG PET and chest CT showed that some textural features of ADC and SCC differed, thus radiomic signature can be used to distinguish lung ADC from SCC.[18,34] Here, we found differences in PET textural features by sex and histological subtype, consistent with the different proportions of high-stroma tumors among the subgroups. Thus, tumor biology may differ by sex in addition to histological subtype; females with lung ADC differed from that of males with lung ADC or SCC. This result suggests that lung ADC and SCC may require a sex-specific PET radiomic approach.
Our study had several limitations. Patient numbers were relatively small and all had relatively early stage lung cancers; we only included surgically treated patients. We excluded females with SCC from statistical analyses because of the small number of patients; the female SCC incidence is rather low. Further studies with larger cohorts are required.
5. Conclusion
Intratumoral heterogeneity measured using TSR and PET textural analyses in females with lung ADC differed from that of males with lung ADC or SCC.
Author contributions
Conceptualization: Young Wha Koh, Dakeun Lee.
Data curation: Dakeun Lee.
Formal analysis: Young Wha Koh, Dakeun Lee, Su Jin Lee.
Funding acquisition: Su Jin Lee.
Methodology: Young Wha Koh, Dakeun Lee, Su Jin Lee.
Supervision: Su Jin Lee.
Visualization: Young Wha Koh, Su Jin Lee.
Writing – original draft: Young Wha Koh, Su Jin Lee.
Writing – review & editing: Dakeun Lee, Su Jin Lee.
Su Jin Lee orcid: 0000-0002-2096-6115.
Footnotes
Abbreviations: ADC = adenocarcinoma, CT = computed tomography, FDG = fluorodeoxyglucose, MTV = metabolic tumor volume, NSCLC = nonsmall cell lung carcinoma, PET = positron emission tomography, SCC = squamous cell carcinoma, SUV = standardized uptake value, TLG = total lesion glycolysis, TSR = tumor-stroma ratio.
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (NRF-2016R1C1B2011583).
The authors have no conflicts of interest to disclose.
References
- [1].De Matteis S, Consonni D, Pesatori AC, et al. Are women who smoke at higher risk for lung cancer than men who smoke? Am J Epidemiol 2013;177:601–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Donington JS, Colson YL. Sex and gender differences in non-small cell lung cancer. Semin Thorac Cardiovasc Surg 2011;23:137–45. [DOI] [PubMed] [Google Scholar]
- [3].Rodriguez-Lara V, Hernandez-Martinez JM, Arrieta O. Influence of estrogen in non-small cell lung cancer and its clinical implications. J Thorac Dis 2018;10:482–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hsu LH, Liu KJ, Tsai MF, et al. Estrogen adversely affects the prognosis of patients with lung adenocarcinoma. Cancer Sci 2015;106:51–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Siegfried JM, Stabile LP. Estrongenic steroid hormones in lung cancer. Semin Oncol 2014;41:5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Katcoff H, Wenzlaff AS, Schwartz AG. Survival in women with NSCLC: the role of reproductive history and hormone use. J Thorac Oncol 2014;9:355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Stabile LP, Dacic S, Land SR, et al. Combined analysis of estrogen receptor beta-1 and progesterone receptor expression identifies lung cancer patients with poor outcome. Clin Cancer Res 2011;17:154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Daniels G, Gellert LL, Melamed J, et al. Decreased expression of stromal estrogen receptor alpha and beta in prostate cancer. Am J Transl Res 2014;6:140–6. [PMC free article] [PubMed] [Google Scholar]
- [9].Pequeux C, Raymond-Letron I, Blacher S, et al. Stromal estrogen receptor-alpha promotes tumor growth by normalizing an increased angiogenesis. Cancer Res 2012;72:3010–9. [DOI] [PubMed] [Google Scholar]
- [10].Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013;19:1423–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 2013;501:346–54. [DOI] [PubMed] [Google Scholar]
- [12].Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer 2009;9:239–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Xi KX, Wen YS, Zhu CM, et al. Tumor-stroma ratio (TSR) in non-small cell lung cancer (NSCLC) patients after lung resection is a prognostic factor for survival. J Thorac Dis 2017;9:4017–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang K, Ma W, Wang J, et al. Tumor-stroma ratio is an independent predictor for survival in esophageal squamous cell carcinoma. J Thorac Oncol 2012;7:1457–61. [DOI] [PubMed] [Google Scholar]
- [15].de Kruijf EM, van Nes JG, van de Velde CJ, et al. Tumor-stroma ratio in the primary tumor is a prognostic factor in early breast cancer patients, especially in triple-negative carcinoma patients. Breast Cancer Res Treat 2011;125:687–96. [DOI] [PubMed] [Google Scholar]
- [16].Mesker WE, Junggeburt JM, Szuhai K, et al. The carcinoma-stromal ratio of colon carcinoma is an independent factor for survival compared to lymph node status and tumor stage. Cell Oncol 2007;29:387–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lee D, Ham IH, Son SY, et al. Intratumor stromal proportion predicts aggressive phenotype of gastric signet ring cell carcinomas. Gastric Cancer 2017;20:591–601. [DOI] [PubMed] [Google Scholar]
- [18].Ha S, Choi H, Cheon GJ, et al. Autoclustering of non-small cell lung carcinoma subtypes on (18)F-FDG PET using texture analysis: a preliminary result. Nucl Med Mol Imaging 2014;48:278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chicklore S, Goh V, Siddique M, et al. Quantifying tumour heterogeneity in 18F-FDG PET/CT imaging by texture analysis. Eur J Nucl Med Mol Imaging 2013;40:133–40. [DOI] [PubMed] [Google Scholar]
- [20].Koh YW, Park SY, Hyun SH, et al. Associations between PET textural features and GLUT1 expression, and the prognostic significance of textural features in lung adenocarcinoma. Anticancer Res 2018;38:1067–71. [DOI] [PubMed] [Google Scholar]
- [21].Kirienko M, Cozzi L, Antunovic L, et al. Prediction of disease-free survival by the PET/CT radiomic signature in non-small cell lung cancer patients undergoing surgery. Eur J Nucl Med Mol Imaging 2018;45:207–17. [DOI] [PubMed] [Google Scholar]
- [22].Lovinfosse P, Janvary ZL, Coucke P, et al. FDG PET/CT texture analysis for predicting the outcome of lung cancer treated by stereotactic body radiation therapy. Eur J Nucl Med Mol Imaging 2016;43:1453–60. [DOI] [PubMed] [Google Scholar]
- [23].Cook GJ, O’Brien ME, Siddique M, et al. Non-small cell lung cancer treated with erlotinib: heterogeneity of (18)F-FDG uptake at PET-association with treatment response and prognosis. Radiology 2015;276:883–93. [DOI] [PubMed] [Google Scholar]
- [24].Choi JW, Lee D, Hyun SH, et al. Intratumoural heterogeneity measured using FDG PET and MRI is associated with tumour-stroma ratio and clinical outcome in head and neck squamous cell carcinoma. Clin Radiol 2017;72:482–9. [DOI] [PubMed] [Google Scholar]
- [25].Hyun SH, Kim HS, Choi SH, et al. Intratumoral heterogeneity of (18)F-FDG uptake predicts survival in patients with pancreatic ductal adenocarcinoma. Eur J Nucl Med Mol Imaging 2016;43:1461–8. [DOI] [PubMed] [Google Scholar]
- [26].Fang YH, Lin CY, Shih MJ, et al. Development and evaluation of an open-source software package “CGITA” for quantifying tumor heterogeneity with molecular images. Biomed Res Int 2014;2014:248505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015;10:1243–60. [DOI] [PubMed] [Google Scholar]
- [28].Ganeshan B, Goh V, Mandeville HC, et al. Non-small cell lung cancer: histopathologic correlates for texture parameters at CT. Radiology 2013;266:326–36. [DOI] [PubMed] [Google Scholar]
- [29].Tixier F, Le Rest CC, Hatt M, et al. Intratumor heterogeneity characterized by textural features on baseline 18F-FDG PET images predicts response to concomitant radiochemotherapy in esophageal cancer. J Nucl Med 2011;52:369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2002;2:161–74. [DOI] [PubMed] [Google Scholar]
- [31].Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer 2006;6:392–401. [DOI] [PubMed] [Google Scholar]
- [32].Nakamura H, Ichikawa T, Nakasone S, et al. Abundant tumor promoting stromal cells in lung adenocarcinoma with hypoxic regions. Lung Cancer 2018;115:56–63. [DOI] [PubMed] [Google Scholar]
- [33].Takahashi Y, Ishii G, Taira T, et al. Fibrous stroma is associated with poorer prognosis in lung squamous cell carcinoma patients. J Thorac Oncol 2011;6:1460–7. [DOI] [PubMed] [Google Scholar]
- [34].Zhu X, Dong D, Chen Z, et al. Radiomic signature as a diagnostic factor for histologic subtype classification of non-small cell lung cancer. Eur Radiol 2018;28:2772–8. [DOI] [PubMed] [Google Scholar]


