Abstract
In this study, we explored the optimal treatment for cesarean scar pregnancy (CSP). One hundred three women diagnosed with CSP received 1 of the 3 treatments: local or systemic methotrexate (MTX) injection and surgery (MTX + Surg), uterine arterial embolization (UAE) and surgery (UAE + Surg) or surgery only (Surg only). We compared their therapeutic effects and their follow-up results. There was no significant difference between the groups in the baseline of clinical characteristic except for the initial β human chorionic gonadotropin levels, which was highest in the MTX + Surg group (median, [interquartile range]), (120,004 [16,720–181,727] mIU/mL), compared to the UAE + Surg group (38,219 [23,194–100,029] mIU/mL) and Surg only group (22,557 [9113–49,573] mIU/mL). There was no significant difference between groups in the sonographic characteristic of patients. The intraoperative hemorrhage was highest in the Surg-only group (7/42, 16.67%), compared to the MTX + Surg group (4/26, 15.38%) and the UAE + Surg group (0/35, 0%). The incidence of intrauterine adhesions was highest in the UAE + Surg group (20%), compared to the MTX + Surg group (0%) and the Surg only group (0%). The incidence of embryo residue was highest in Surg-only group (21.43%), compared to the MTX + Surg group (0%) and the UAE + Surg group (2.86%). To conclude, MTX injection plus surgery might be the best treatment for CSP patients.
Keywords: cesarean scar pregnancy, fertility complication, intrauterine adhesion, local MTX injection, systemic MTX injection, uterine artery embolization
1. Introduction
Cesarean scar pregnancy (CSP) refers to placental implantation on the scar of a previous cesarean delivery (CD).[1] The increasing numbers of cesarean deliveries in the last decades have led to an increased incidence of CSP.[1] The estimated incidence of CSP is approximately 1:1800 to 1:2000 pregnancies after CD.[1] Complications of CSP include morbidly adherent placenta, uterine rupture, severe hemorrhage, and preterm labor.[1–3]
With the development of sonography, especially the 3D ultrasound scan, the diagnostic accuracy of CSP has significantly increased. This enables doctors to provide appropriate and timely pregnancy options to the patients and prevent any life-threatening complications.
There are several treatment strategies for CSP. However, there are no universal guidelines for CSP management. Doctors usually select a therapeutic strategy according to their personal clinical experience or based on the hospital guidelines. Such practice based on habitual thinking limit clinicians to select the most effective treatment for CSP and avoid any potential complications. Available treatments for CSP include surgical managements, that is, dilatation and curettage (D&C), laparoscopy and/or hysteroscopy, systemic or local MTX injection and uterine artery embolization (UAE).[4–8] The objective of this study is to compare the therapeutic effects and follow-up results among 3 different treatments and to identify the most effective treatment strategy for CSP.
2. Materials and methods
2.1. Study population
This retrospective study was approved by the Ethics Committee of Renmin Hospital, Wuhan University (0130/2018). Informed consent was waived because this is a retrospective study. This study was carried out at the Obstetrics, Gynecology and Centre for Reproductive Science of Renmin Hospital. Data of the CSP patients admitted for treatment from September 2014 to May 2017 were obtained from their electronic medical records. All the patients included in this study met the selection criteria below. All patients had a history of cesarean section. All patients were diagnosed as CSP based on clinical manifestations and transvaginal ultrasound.[9] The main clinical manifestations include amenorrhea, vaginal bleeding, and lower abdominal pain.[1] Sonographic examinations were performed on a GE VOLUSON E8 imaging machine (General Electric Co., Boston, MA). The diagnosis of CSP met with the CSP criteria: no gestational sac appears in the uterus and cervical canal; a gestational sac or mass located in the anterior wall of the isthmic portion; a gestational sac embedded within the myometrium, with an absence or defect in the myometrium between the bladder and the sac.[10–11] Three-dimensional color ultrasonography was further performed when transvaginal ultrasound failed to provide a definitive diagnosis.[12]
2.2. Treatment
Three treatment strategies were compared in this study. Patients in the MTX + Surg group were treated with surgery including D&C, laparoscopy and/or hysteroscopy after local or systemic MTX pretreatment. For analysis, patients in the MTX + Surg group was further divided into 2 subgroups: Local MTX + Surg and systemic MTX + Surg. For the Local MTX + Surg group, 50 mg MTX was injected directly into the gestation sac (GS) under the guidance of ultrasound by an experienced gynecologist. In the systemic MTX + Surg group, 100 mg MTX was intramuscularly injected. The serum levels of β-human chorionic gonadotropin (β-HCG) were measured and pelvic ultrasound scan was performed 3 to 5 days after MTX followed by surgery. Patients in the UAE + Surg group were treated with surgery including D&C, laparoscopy and hysteroscopy after UAE. The UAE procedure was performed by a qualified radiologist. After local anesthesia, a 4F-angiographic catheter was inserted into the right femoral artery and extended bilaterally into the uterine arteries. 50–100 mg of gelatin sponge particle embolic agent for unequivocal embolization after the catheters were confirmed in their correct locations. Following UAE, D&C, laparoscopy and hysteroscopy were performed within 24 h after UAE. Patients in the Surg-only group were treated with surgery only.
Patients were discharged if pathological reports confirmed the presence of a conceptus; the serum β-HCG levels were below 5000 mIU/mL; and the patients were complication free. All patients’ serum β-HCG levels were measured weekly until they declined a normal value. Sonographic scan was carried out 1 month after discharge to detect whether there was any embryo residue in the uterus. All patients were followed up to 6 month. They either received a phone follow-up or visited the outpatient department for menstrual flow survey at 6 month. If patients felt a significant reduction in their menstrual flow, they were asked to return for follow-up examination as early as possible.
For the follow-up, a 3D sonographic scan was carried out for those who were suspected of intrauterine adhesion.
2.3. Statistical methods
All statistical analyses were performed using the SPSS 19.0 statistical package (SPSS Inc, Chicago, IL). Data were presented as medians (interquartile range [IQR]) for continuous data with non-normal distribution. Mann–Whitney U test was used to compare continuous data to a non-normal distribution. Categorical data were presented as frequencies and percentages. Pearson Chi-square (chi-squared) tests or Fisher's exact-probability test was performed when categorical variables were compared. Tests of significance were 2 tailed. P values of less than .05 were regarded as statistically significant.
3. Results
From September 2014 to May 2017, a total of 103 women were diagnosed with CSP in Renmin Hospital of Wuhan University. There was no significant difference among the 3 groups in terms of average age, gravidity, previous cesareans, length of gestation, vaginal bleeding, and lower abdominal pain. Major clinical characteristics in all patients are presented in Table 1. There was a significant difference in the average presurgery β-HCG levels among the 3 groups. It was 120,004 (16,720–181,727) mIU/mL in the MTX + Surg group, 38,219 (23,194–100,029) mIU/mL in the UAE + Surg group, and 22,557 (9113–49,573) mIU/mL in the Surg-only group (Table 1). The implantation may develop into either a normally shaped gestational sac with the basal decidua inserted into the scar (superficial type), a deformed sac semiembedded (partial type) or totally embedded (complete type) in the myometrium.[13] Sonographic evaluation of patients showed no significant difference among the 3 groups in terms of the mean sac diameter, remnant myometrial wall thickness, cases of thickness myometrial wall equal to or less than 3 mm, fetal heartbeat, and the location of the GS (Fig. 1 , Table 2).
Table 1.
Comparison of the clinical characteristic of patients treated with MTX + Surg, UAE + Surg, and Surg only.
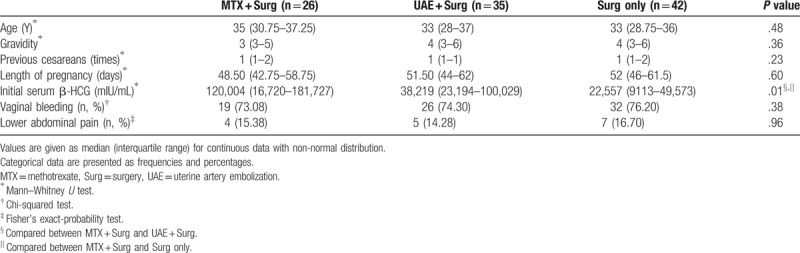
Figure 1.
Cesarean scar pregnancy was differentiated into 3 types. As the status of scar defects varies greatly, the implantation may develop either into a normally shaped gestational sac with the basal decidua inserting into the scar (superficial type, Figure1.1), into a deformed sac half-embedded (partial type, Figure1.2), or totally embedded (complete type, Figure1.3) in the myometrium. Color Doppler shows increased blood flow surrounding the gestation sac.
Table 2.
Comparison of sonographic characteristic of patients treated with MTX + Surg, UAE + Surg, and Surg only.
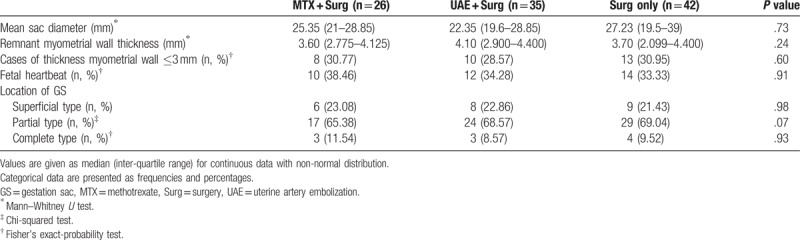
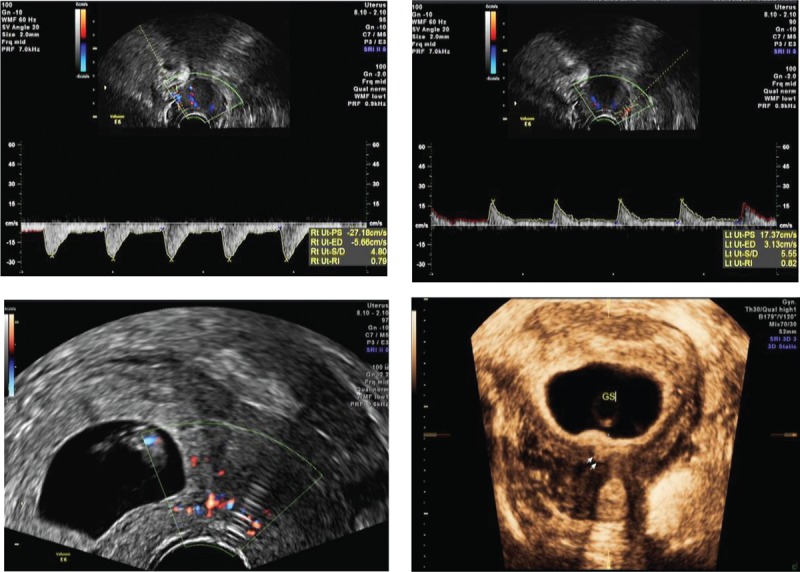
Figure 1.
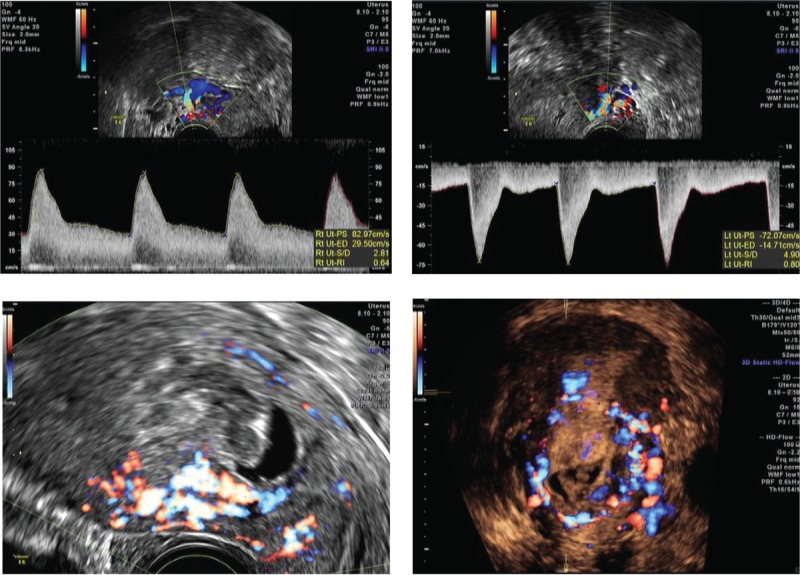
Cesarean scar pregnancy was differentiated into 3 types. As the status of scar defects varies greatly, the implantation may develop either into a normally shaped gestational sac with the basal decidua inserting into the scar (superficial type, Figure1.1), into a deformed sac half-embedded (partial type, Figure1.2), or totally embedded (complete type, Figure1.3) in the myometrium. Color Doppler shows increased blood flow surrounding the gestation sac.
Figure 1.
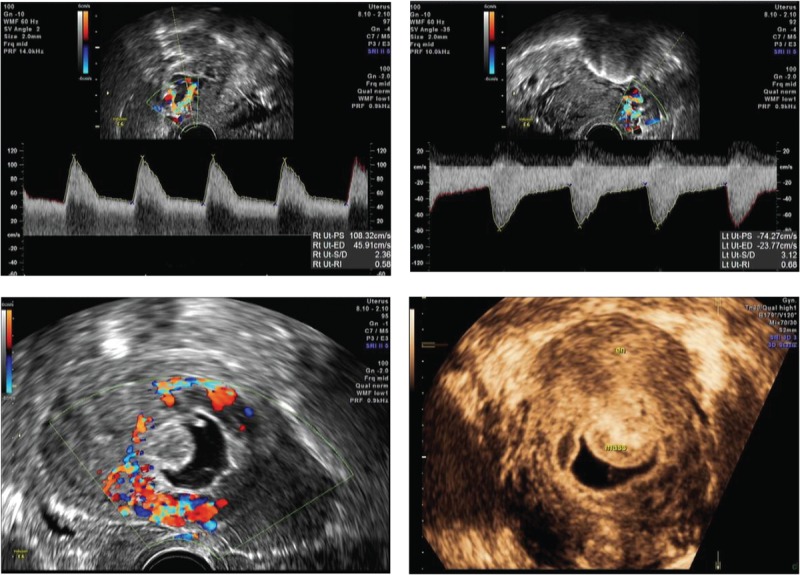
Cesarean scar pregnancy was differentiated into 3 types. As the status of scar defects varies greatly, the implantation may develop either into a normally shaped gestational sac with the basal decidua inserting into the scar (superficial type, Figure1.1), into a deformed sac half-embedded (partial type, Figure1.2), or totally embedded (complete type, Figure1.3) in the myometrium. Color Doppler shows increased blood flow surrounding the gestation sac.
The average length of hospital stay was significantly less in the UAE + Surg group and the Surg-only group, when compared to the MTX + Surg group (8[3.50], 10[3] vs. 12[3]; P < .01). However, the long hospital stays in the MTX + Surg group is because MTX treatment increased the length of stay. Because UAE might significantly reduce the blood supply of the uterus, heavy blood loss during surgery was significantly decreased in the UAE + Surg group (0%, 0/35), compared to the MTX + Surg group (15.38%, 4/26; P = .03), and the Surg-only group (16.67%, 7/42; P = .01). Remarkably, there was no heavy blood loss during surgeries in the MTX + Surg group (0%; 0/12) when MTX was injected directly into the GS. This demonstrated that pretreatment with UAE or local MTX injection significantly reduced the hemorrhage during surgery (Tables 3 and 4). The cases of laparoscopy and/or hysteroscopy in the MTX + Surg, UAE + Surg, and Surg-only group were 12 (46.15%, 12/26), 14 (40%, 14/35), and 23 (54.76%, 23/42), respectively. There was no significant difference among the 3 groups (P = .43, Table 3). The cases of laparoscopy and/or hysteroscopy in the MTX + Surg group were 4 (33.33%, 4/12). Although there was no significant difference between the UAE + Surg group and the Surg-only group (P = .28, Table 4), local MTX injection further reduced the ratio of laparoscopy and hysteroscopy to some extent (Table 4). The reduction in β-HCG levels in the Surg-only group (96.51%, IQR 1.94%) was the lowest, compared to the MTX + Surg group (98.82%, IQR 1.01%, P < .01) and the UAE + Surg group (98.39%, IQR 1.99%, P < .01). When compared to the Local MTX + Surg subgroup (99.19%, IQR 0.84), the difference was also statistically significant (Local MTX + Surg vs. UAE + Surg, P < .01; Local MTX + vs. Surg only, P < .01, Table 4).
Table 3.
Comparison of the therapeutic effects among patients treated with MTX + Surg, UAE + Surg, and Surg only.
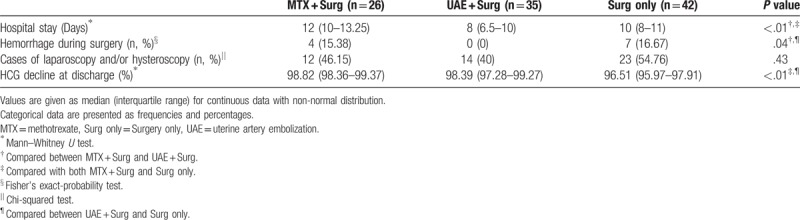
Table 4.
Comparison of the therapeutic effects among patients treated with local MTX + Surg, UAE + Surg, and Surg only.
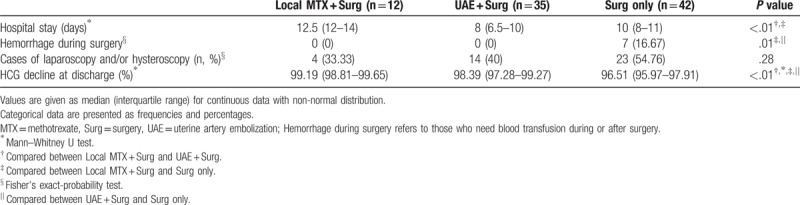
Our follow-up results showed these 103 women made a regular return visit to the outpatient department for sonographic scan 1 month after discharge. They either received a phone follow-up or visited the outpatient department for menstrual flow survey with a 6-month follow-up. If patients felt a significant reduction in their menstrual flow, they were asked to return for follow-up examination as early as possible. For the follow-up, a 3D sonographic scan was carried out for those who were suspected of intrauterine adhesion. There were 7 cases of intrauterine adhesions and 1 case of embryo residue in the UAE + Surg group (20%, 7/35; 2.86% 1/35). There were 9 cases of embryo residue (21.43%, 9/42) in the Surg-only group. In contrast, there was no intrauterine adhesions or embryo residue in the MTX + Surg group (0%, 0/26). The occurrence of intrauterine adhesions and embryo residue was significantly different among the 3 groups (Table 5).
Table 5.
Comparison of the Follow-up results among patients treated with MTX + Surg, UAE + Surg and Surg only.
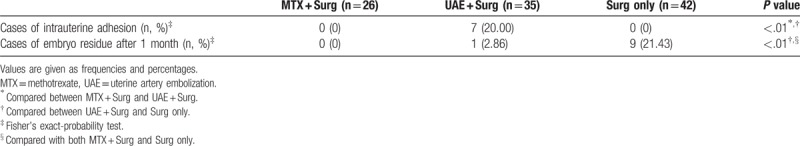
4. Discussion
CSP was first reported by Larsen and Solomon[14] referring to a special type of ectopic pregnancy in which embryos implant on the CD scar and can cause life-threatening complications. Severe complications and the increasing incidence worldwide argues for an urgent need for the identification of the optimal treatment for CSP.
However, there are no universal treatment guidelines for CSP available. Accumulating data suggested a variety of medical and surgical treatment modalities for CSP. The most common treatments include surgery such as D&C, laparoscopy and/or hysteroscopy, local or systemic MTX injection, UAE, and a combination of the methods above.[4,6,15–17] MTX was the most commonly used drug to treat CSP, and both systemic and local injections of MTX as the priority treatment have been previously used.[5–6] However, there are many side-effects of MTX injection including oral ulceration, bone marrow depression, and severe bleeding. This severely limited its application.[7] According to our clinical observation, the incidence of side-effects mentioned above is extremely low, especially for local MTX injection. In these cases, MTX was directly injected into the GS under the ultrasound guidance. This exposed the gestational sac to a higher MTX dose and effectively reduced the risk of rupture and heavy bleeding. Hence, intraoperative blood loss could be significantly reduced when surgery was performed.
The UAE treatment for CSP was first reported in 1999 and has been used widely to control hemorrhage and preserve the uterus. Gelfoam appears to promote clotting via physical effects by supporting thrombus development. Vascular occlusion with gelfoam causes acute necrotizing arteritis.[18] UAE followed by surgery is widely used by doctors because it is safe and effective. The inflammatory process of UAE eventually leads to the breakdown of the gelfoam within 1 to 3 weeks after embolization with subsequent vascular recanalization. A previous study showed there were no severe complications such as endometrial atrophy or permanent amenorrhea caused by UAE.[16] However, there are a few reports showing that UAE caused ischemia-related complications because UAE temporarily blocked the uterine arterial blood flow.[19–21]
In the present study, we observed that the serum β-HCG levels were highest in the MTX + Surg group. Because of this, the hospital stay of the MTX + Surg group was significantly longer compared with the UAE + Surg group and the Surg-only group. We also compared the ratio of laparoscopy and/or hysteroscopy in the 3 groups. It was 46.15% in the MTX + Surg group (33.33% in the Local MTX + Surg), 41.00% in the UAE + Surg group, and 54.76% in the Surg-only group. Although there was no significant difference among them, the combination of MTX or UAE and surgery slightly reduced the laparoscopy and/or hysteroscopy operation for CSP patients, which will reduce their hospital costs. This difference may be significant with a large sample size. The reduction in the β-HCG levels in the Surg-only group (96.51%, IQR 1.94%) was the lowest compared with the MTX + Surg group (98.82%, IQR 1.01%, P < .01) and the UAE + Surg group (98.39%, IQR 1.99%, P < .01). If compared to the Local MTX + Surg subgroup (99.19%, IQR 0.84), the difference was even more significant (Local MTX + Surg vs. UAE + Surg, P < .01; Local MTX + Surg vs. Surg only, P < .01). UAE effectively reduced the blood supply of the uterus. Heavy blood loss during surgery was significantly decreased in the UAE + Surg group (0%, 0/35) compared to the MTX + Surg group (15.38%, 4/26; P = .03) and the Surg-only group (16.67%, 7/42; P = .01). Remarkably, there was no heavy blood loss during surgery in the Local MTX + Surg (0%, 0/12) group and UAE + Surg group. In addition, local MTX injection into the GS under ultrasound guidance is less time-consuming, more convenient, and inexpensive compared to UAE and can be carried out in the ward.
There was no significant difference in clinical and sonographic characteristic of patients among the 3 groups in terms of average age, gravidity, previous cesareans, length of gestation, vaginal bleeding and lower abdominal pain, mean sac diameter, remnant myometrial wall thickness, cases of thickness myometrial wall ≤3 mm, fetal heartbeat, and the location GS.
The follow-up results highlighted the significant difference among the 3 groups. The incidence of intrauterine adhesions was significantly higher in the UAE + Surg group than the MTX + Surg group, and the Surg-only group (20% vs. 0% and 0%). The extremely high occurrence of intrauterine adhesions with UAE was inconsistent with a previous study[16] but consistent with other reports related to the UAE fertility complication.[22–24] The findings argue against using UAE as a routine strategy for CSP treatment. In the Surg-only group, there were 9 cases of embryo residue (21.43%). This might be due to surgeons’ lack of confidence in MTX injection.
There are several limitations in this study. First, because of the low rates of CSP pregnancy, the sample size of our study may not be big enough to draw a definite conclusion. Second, the longer hospital stay in the MTX + Surg group might be due to the MTX treatment. After MTX treatment, doctors need to wait 3 to 5 days for the results from the ultrasound examination and β-HCG test to determine whether they will perform a surgery. Third, because of the nature of retrospective studies, there might be a selection bias. So the results cannot be compared with the controlled studies. Fourth, the reason that the β-HCG levels declined more in the MTX + Surg group at discharge might be their relatively longer hospital stay. Last, we only followed the patients for 6 months and patients with complications were more likely to have a follow-up record. This may underestimate the difference between the groups. Longer follow-up periods are required to determine the long-term effects among these treatments. Multicentered prospective, controlled studies with large sample sizes are needed in the future to verify the results of this study and to establish a more reliable universal treatment guideline for CSP patients.
5. Conclusion
In summary, a combination of UAE and surgery should be selected carefully because of its potential fertility complication. Combination of Ultrasound-guided local MTX injection and surgery seems to be an optimal option for CSP because of its safety, convenience, economy, and validity.
Author contributions
Conceptualization: Zhuoni Xiao
Data analysis: Qingzhen Xie
Data collection: Dan Cheng, Jiao Chen
Data curation: Dan Cheng, Jiao Chen.
Formal analysis: Qingzhen Xie.
Funding acquisition: Zhuoni Xiao.
Methodology: Qingzhen Xie.
Project administration: Zhuoni Xiao.
Writing – original draft: Zhuoni Xiao.
Writing – review & editing: Jing Yang, Wangming Xu.
Zhuoni Xiao orcid: 0000-0002-5119-3012.
Footnotes
Abbreviations: CD = cesarean delivery, CSP = cesarean scar pregnancy, D&C = dilatation and curettage, GS = gestation sac, HCG = human chorionic gonadotropin, IQR = inter-quartile range, MTX = methotrexate, UAE = uterine arterial embolization.
This study was financially supported by the National Natural Science Foundation of China (Grant No. 81471455 and Grant No. 81100418).
The authors have no conflicts of interest to disclose.
References
- [1].Rotas MA, Haberman S, Levgur M. Cesarean scar ectopic pregnancies: etiology, diagnosis, and management. Obstet Gynecol 2006;107:1373–81. [DOI] [PubMed] [Google Scholar]
- [2].Wang YQ, Yin TL, Xu WM, et al. Reproductive outcomes in women with prior cesarean section undergoing in vitro fertilization: a retrospective case-control study. J Huazhong Univ Sci Technolog Med Sci 2017;37:922–7. [DOI] [PubMed] [Google Scholar]
- [3].Li SJ, Zhou DN, Li W, et al. Mental health status assessment in polycystic ovarian syndrome infertility patients: a pilot study. J Huazhong Univ Sci Technolog Med Sci 2017;37:750–4. [DOI] [PubMed] [Google Scholar]
- [4].Kanat-Pektas M, Bodur S, Dundar O, et al. Systematic review: what is the best first-line approach for cesarean section ectopic pregnancy? Taiwan J Obstet Gynecol 2016;55:263–9. [DOI] [PubMed] [Google Scholar]
- [5].Wang Q, Peng HL, He L, et al. Reproductive outcomes after previous cesarean scar pregnancy: follow up of 189 women Taiwan J Obstet Gynecol 2015;54:551–3. [DOI] [PubMed] [Google Scholar]
- [6].Maymon R. Ectopic pregnancies in Caesarean section scars: the 8 year experience of one medical centre. Hum Reprod 2004;19:278–84. [DOI] [PubMed] [Google Scholar]
- [7].Nawroth F, Foth D, Wilhelm L, et al. Conservative treatment of ectopic pregnancy in a cesarean section scar with methotrexate: a case report. Eur J Obstet Gynecol Reprod Biol 2001;99:135–7. [DOI] [PubMed] [Google Scholar]
- [8].Hehenkamp WJ, Volkers NA, Donderwinkel PF, et al. Uterine artery embolization versus hysterectomy in the treatment of symptomatic uterine fibroids (EMMY trial): peri- and post procedural results from a randomized controlled trial. Am J Obstet Gynecol 2005;193:1618–29. [DOI] [PubMed] [Google Scholar]
- [9].OuYang Z, Yin Q, Xu Y, et al. Heterotopic cesarean scar pregnancy: diagnosis, treatment, and prognosis. J Ultrasound Med Off J Am Inst Ultrasound Med 2014;33:1533–7. [DOI] [PubMed] [Google Scholar]
- [10].Jurkovic D, Hillaby K, Woelfer B, et al. First-trimester diagnosis and management of pregnancies implanted into the lower uterine segment Cesarean section scar. Ultrasound Obstet Gynecol 2003;21:220–7. [DOI] [PubMed] [Google Scholar]
- [11].Pascual MA, Hereter L, Graupera B, et al. Three-dimensional power Doppler ultrasound diagnosis and conservative treatment of ectopic pregnancy in a cesarean section scar. Fertil Steri 2007;l88:706. [DOI] [PubMed] [Google Scholar]
- [12].Shih JC. Cesarean scar pregnancy: diagnosis with three dimensional (3D) ultrasound and 3D power Doppler. Ultrasound Obstet Gynecol 2004;23:306–7. [DOI] [PubMed] [Google Scholar]
- [13].Sun QL, Wu XH, Luo L, et al. Characteristics of women with mixed mass formation after evacuation following uterine artery chemoembolization for cesarean scar pregnancy. Arch Gynecol Obstet 2018;doi:10.1007/s00404-018-4716-6. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [14].Larsen JV, Solomon MH. Pregnancy in a uterine scar sacculus: an unusual cause of postabortage hemorrhage. A case report. S Afr Med J 1978;53:142–3. [PubMed] [Google Scholar]
- [15].Liu G, Wu J, Cao J, et al. Comparison of three treatment strategies for cesarean scar pregnancy. Arch Gynecol Obstet 2017;296:383–9. [DOI] [PubMed] [Google Scholar]
- [16].Gao L, Hou YY, Sun F, et al. A retrospective comparative study evaluating the efficacy of adding intra-arterial methotrexate infusion to uterine artery embolisation followed by curettage for cesarean scar pregnancy. Arch Gynecol Obstet 2018;[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [17].Qian ZD, Weng Y, Du YJ, et al. Management of persistent caesarean scar pregnancy after curettage treatment failure. BMC Pregnancy Childbirth 2017;17:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Feng Y, Chen S, Li C, et al. Curettage after uterine artery embolization combined with methotrexate treatment for caesarean scar pregnancy. Exp Ther Med 2016;12:1469–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cottier JP, Fignon A, Tranquart F, et al. Uterine necrosis after arterial embolization for postpartum hemorrhage. Obstet Gynecol 2001;100:1074–7. [DOI] [PubMed] [Google Scholar]
- [20].Godfrey CD, Zbella EA. Uterine necrosis after uterine artery embolization for leiomyoma. Obstet Gynecol 2001;98:950–2. [DOI] [PubMed] [Google Scholar]
- [21].Sirkeci F, Narang L, Naguib N, et al. Uterine artery embolization for severe symptomatic fibroids: effects on fertility and symptoms. Hum Reprod 2014;29:1832–3. [DOI] [PubMed] [Google Scholar]
- [22].Karlsen K, Hrobjartsson A, Korsholm M, et al. Fertility after uterine artery embolization of fibroids: a systematic review. Arch Gynecol Obstet 2018;297:13–25. [DOI] [PubMed] [Google Scholar]
- [23].Berkane N, Moutafoff-Borie C. Impact of previous uterine artery embolization on fertility. Curr Opin Obstet Gynecol 2010;22:242–7. [DOI] [PubMed] [Google Scholar]
- [24].Tropeano G, Litwicka K, Di Stasi C, et al. Permanent amenorrhea associated with endometrial atrophy after uterine artery embolization for symptomatic uterine fibroids. Fertil Steril 2003;79:132–5. [DOI] [PubMed] [Google Scholar]


