Abstract
How nonalcoholic fatty liver disease (NAFLD) is linked to atherosclerosis is still disputed. This study aimed to explore the association between NAFLD and atherosclerosis among adults in Shandong province, China.
A total of 6849 individuals were enrolled in the final analyses for a community-based study. The relationship between NAFLD and atherosclerosis was evaluated after adjusting for common confounding factors.
Hypertension, diabetes, and higher serum low-density lipoprotein cholesterol (LDL-c) level were positively correlated with NAFLD. An odds ratio (OR) (95% confidence interval [CI]) of 1.325 (range 1.157–1.518) for hypertension, 2.153 (range 1.814–2.555) for diabetes, and 1.161 (range 1.071–1.259) for LDL-c was noticed. These factors also were positively correlated with atherosclerosis, with an OR (95% CI) of 1.501 (range 1.286–1.751) for hypertension, 1.716 (range 1.414–2.084) for diabetes, and 1.344 (range 1.231–1.466) for LDL-c. The prevalence of metabolic syndrome was higher in the atherosclerosis+NAFLD group (81.8%) when compared with the NAFLD-only (30.3%), atherosclerosis-only (32.2%), and control (20.3%) groups (P <.01).
NAFLD and atherosclerosis have common metabolic characteristics, such as hypertension, diabetes, and higher serum LDL-c level. Patients with NAFLD in combination with atherosclerosis were found to have a more severe metabolic burden and greater chances of having hypertension, diabetes, dyslipidemia, and higher metabolic syndrome scores than those in the other groups.
Keywords: atherosclerosis, dyslipidemia, metabolic syndrome, nonalcoholic fatty liver disease
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) encompasses a wide spectrum of liver diseases, including simple steatosis, nonalcoholic steatohepatitis (NASH), liver fibrosis, liver cirrhosis, and hepatocellular carcinoma.[1] NAFLD is associated mainly with obesity, diabetes, dyslipidemia, and insulin resistance, which are the main features of metabolic syndrome also. Therefore, NAFLD is considered to be the hepatic manifestation of metabolic syndrome.[2]
NAFLD is associated not only with liver-related morbidity and mortality but also with an increasing risk of cardiovascular disease (CVD).[3] The majority of deaths in NAFLD patients are related to CVD.[4] NAFLD is closely related to the risk factors for CVD, but it is unclear whether NAFLD is independently related to atherosclerosis. How NAFLD is linked to atherosclerosis is still disputed. It is very interesting to know whether NAFLD can act as a causal risk factor for atherosclerosis or an intermediate phenotype in the metabolic syndrome pathway, or whether it is just an isolated disease sharing common metabolic characters with atherosclerosis.
Carotid intima-media thickness (CIMT) measurement obtained using ultrasound is a noninvasive imaging method used to directly identify clinical and preclinical atherosclerosis in human arteries, and to identify the presence of carotid plaques.[5,6] The presence of carotid plaques reflects the overall atherosclerotic burden,[6] with CIMT being a marker of early atherosclerosis.[5] This study was performed to investigate whether NAFLD is independently associated with atherosclerosis, and also to explore the metabolic characteristics of patients with NAFLD and atherosclerosis.
2. Methods
2.1. Participants
This community-based study was designed and used to investigate the epidemiology of metabolic diseases in Shandong province, China between July and December 2014. Eligible residents from each community or village were those who were aged ≥18 years and had been living in their current residence for 5 years or longer. They were invited to participate in the screening examination. Individuals with excessive alcohol consumption (≥140 g/w for men and ≥70 g/w for women) were excluded. Individuals with viral hepatitis or other liver diseases were also excluded from this study. Other exclusion factors included participants with diseases that could affect their metabolic state and thyroid status or diseases that made the individuals unsuitable for this study, such as pregnancy, lactation, mental illness, severe cardiac diseases, pulmonary or renal insufficiency, and cancer.
A total of 8922 subjects were invited to participate in the screening examination. Among them, 278 subjects were excluded for missing vital data such as age, gender, body mass index (BMI), liver and carotid artery ultrasound, or blood specimen; 1723 subjects were excluded from analysis because of excessive alcohol consumption; 46 subjects were excluded for chronic liver diseases such as viral hepatitis, liver cancer, autoimmune liver disease, and similar conditions; 18 subjects were excluded for chronic renal failure; and 8 subjects were excluded for chronic heart failure; 45 subjects were excluded for abnormal of thyroid function or taking any thyroid hormone, anti-thyroid drugs in the past 3 months; 12 subjects were excluded for cancer and 9 subjects were excluded for pregnancy or lactation. Finally, 6849 individuals were enrolled in the final analysis.
The study was approved by the local ethics committee and was done in accordance with the Helsinki Declaration of 1975. Written consent was obtained from each participant before data collection.
2.2. Data collection
Data collection was conducted in examination centers of local health stations in the residential areas of the participants. Trained medical staff interviewed all participants face to face and collected data concerning demographic characteristics, medical history, and health-related lifestyle behaviors using a well-established questionnaire. The questionnaire was made according to cardiovascular survey methods (World Health Organization, Geneva, 2004). Current smoking was defined as those smoking cigarettes at the time of the survey. Current drinking was defined as alcohol intake of more than once per month during the last 12 months. Physical activity was assessed using the Global Physical Activity Questionnaire and measured using the metabolic equivalent.[7]
Weight was in kilogram and height in meter. The participants were required to be in light clothing, footwear removed, and pockets emptied. Waist circumference (WC) was measured in centimeters midway between the lower edge of the costal arch and the upper edge of the iliac crest of participants in the standing position. BMI was calculated as weight (kg) divided by square of height (m). Resting blood pressure was measured 3 times at 2-minute intervals using an electronic sphygmomanometer (HEM-7117; Omron, Kyoto, Japan). Participants were in a sitting position with arms at the height of heart. The average of 3 readings was required for further data analysis.
Blood samples were collected after an overnight fast of at least 10 hours. Each participant without a self-reported history of diabetes was given an oral glucose tolerance test. Plasma glucose was measured at 0 and 2 hours after administration during the test. Blood specimens were centrifuged and measured on site within 2 hours after collection. Serum samples on dry ice were shipped to the central laboratory. Hemoglobin A1c (HbA1c) was measured by high-performance liquid chromatography using the VARIANT II Hemoglobin Testing System (Bio-Rad Laboratories). The participants’ serum lipid profiles and renal function were measured using the ARCHITECT ci16200 Integrated System (Abbott, IL).
Ultrasound examinations of the participants’ livers and carotid arteries were conducted. All the ultrasound examinations were performed by an experienced radiologist who was blinded to the laboratory and clinical data during the procedure. Liver ultrasounds were performed to assess the presence of hepatic steatosis. Common carotid arteries were scanned bilaterally by a color ultrasonic apparatus equipped with a 9-MHz linear-array transducer (Toshiba Aplio 500 Ultrasound Scanner). CIMT was defined as the distance between the blood-intima and media-adventitia interfaces on the wall of the artery. A carotid plaque was defined as a focal structure that encroached into the arterial lumen of at least 0.5 mm or 50% of the surrounding CIMT value or demonstrated a thickness >1.5 mm as measured from the media-adventitia interface to the intima-lumen interface.[8] Atherosclerosis was defined as the presence of focal plaques or diffuse thickening of the carotid wall (CIMT >0.9 mm).[9,10]
Research subjects rested in the supine position with head tilted backward, while the study was performed in a temperature-controlled room (25°C). Two different longitudinal-view observations of the bilateral carotid artery were made at the following points: common carotid artery (10 mm proximal to the bulb) and bulb (10 mm cranial to the start of the bulb). During the process, the anterior and posterior walls of both sides of the carotid artery were measured 3 times, and the average was taken for use. Hence, a total of eight readings were obtained. The average CIMT (CIMTave) were used for analysis for it's less susceptible to outliers.
2.3. Disease definition
This study defined diabetes, impaired fasting glucose (IFG), and impaired glucose tolerance (IGT) according to the 2010 criteria of the American Diabetes Association.[11] Hypertension was based on the Seventh Report of the Joint National Committee.[12] Dyslipidemia was defined according to the American Association of Clinical Endocrinologists’ guidelines. Based on these guidelines, serum lipid concentration levels were classified into 3 categories: optimal, borderline, and high-risk.[13] Normal weight was defined as a BMI of 18.5 to 23.9. Overweight was defined as a BMI of 24.0 to 27.9, and obesity was defined as a BMI of 28.0 or higher. The metabolic syndrome was categorized according to the criteria defined by the National Cholesterol Education Program, Adult Treatment Panel III (NCEP, ATPIII).[14] NAFLD was defined as the presence of hepatic steatosis on ultrasonography[15,16] in the absence of elevated alcohol consumption. Atherosclerosis in this study was defined as the presence of carotid artery plaque and/or CIMTave >0.9 mm scanned by ultrasound.
2.4. Statistical analysis
Research subjects were classified into 2 groups according to presence or absence of NAFLD. Demographic and biochemical indexes were described in the overall population and subgroups according to gender, by using counts (percentages) for categorical variables and means ± standard deviation (SD) or median (interquartile range) for continuous variables. Differences between the 2 groups were analyzed by using the independent-samples t test (normal distribution, independent samples) or the Wilcoxon Mann–Whitney test (skewed data) for continuous variables and the Chi-square test (χ2-test) for categorical variables. Odds ratios (ORs) for atherosclerosis in each group were determined based on logistic regression analyses. Multivariable logistic regression was used to analyze the associations between NAFLD and atherosclerosis for different thresholds.
Independent risk factors of NAFLD and atherosclerosis were determined with univariate and multivariate logistic regression analyses. Variables, including gender, age, household income, physical activity, drinking, smoking, BMI, wWC, total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), uric acid (UA), alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyltransferase (GGT), alkaline phosphatase (ALP), creatinine, hypertension, and diabetes, were drawn into the univariate logistic regression model to choose statistically significant variables. Selected variables were then drawn into multivariate logistic regression, and independent risk factors were found.
The subjects were further divided into 4 groups according to the presence or absence of NAFLD alone or in combination with atherosclerosis. The differences in the demographic and biochemical indexes between the 4 groups were analyzed using analysis of variance for continuous variables and Chi-squared tests (χ2-test) for categorical variables.
Data were analyzed with SPSS 23 software. A 2-sided P value less than .05 was considered significant.
3. Results
3.1. General characteristics of the participants
A total of 6849 subjects participated in the study. Their average age was 51.67 (±13.17) years, and 26.8% of them were males. Based on the ultrasound criteria, 2347 subjects (34.3%) were found to have NAFLD, and 1538 subjects (22.5%) were found to have atherosclerosis. The proportions of participants with diabetes, hypertension, and obesity in this cohort were 24.8%, 43.7%, and 22.7%, respectively.
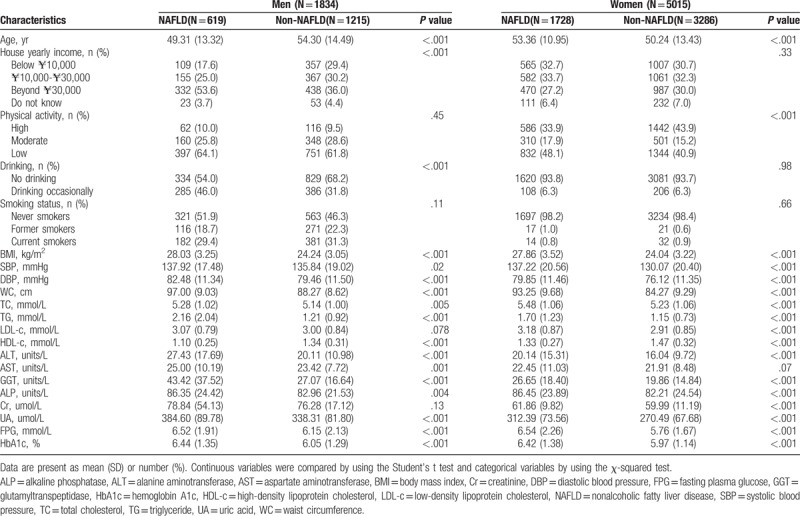
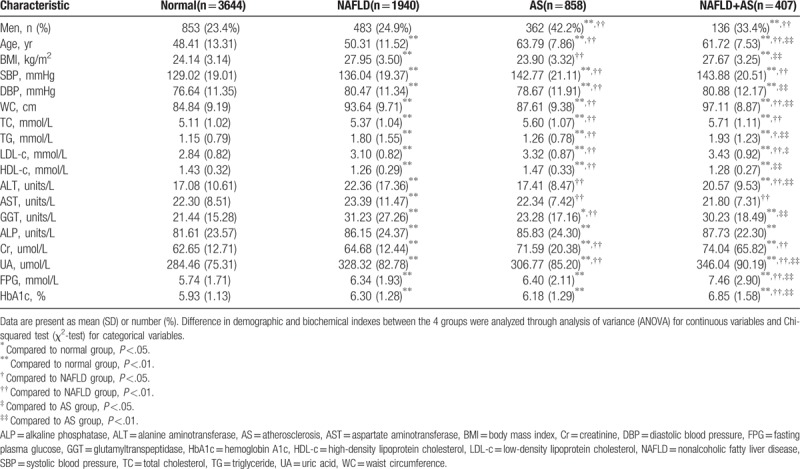
Table 1 shows the clinical, anthropometric, and biochemical characteristics of all subjects, as well as the detailed results of the comparative analysis among patients with and without NAFLD according to gender. When compared with people without NAFLD, patients with NAFLD had features of higher values for BMI, systolic blood pressure, diastolic blood pressure, LDL-c, TG, ALT and AST levels, and lower HDL-c levels.
Table 1.
Comparison of clinical characteristics for subjects with and without nonalcoholic fatty liver disease in men and women respectively.
3.2. Comparison of atherosclerosis risk factors for subjects with and without NAFLD in men and women
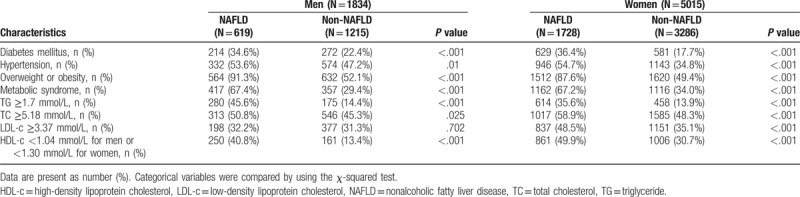
As shown in Table 2, patients with NAFLD had a greater prevalence of diabetes, dyslipidemia, hypertension, obesity, and metabolic syndrome. Patients with NAFLD had a higher prevalence of arteriosclerotic dyslipidemia with lower HDL-c and higher TG and cholesterol levels. NAFLD patients had more risk factors for atherosclerosis.
Table 2.
Comparison of atherosclerosis risk factors for subjects with and without nonalcoholic fatty liver disease in men and women respectively.
3.3. Comparison of the risk factors for the presence of NAFLD and atherosclerosis, according to logistic regression analyses
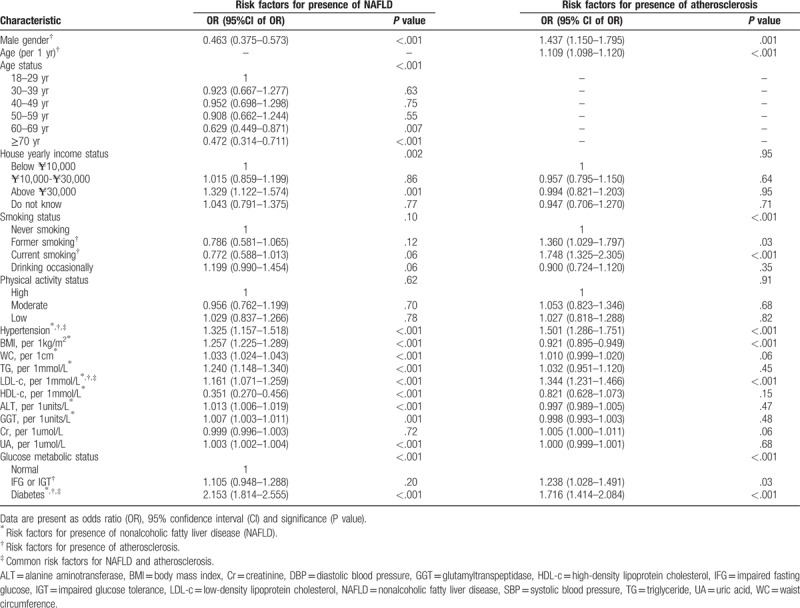
A logistic regression analysis was performed to investigate factors that independently related to the prevalence of NAFLD and atherosclerosis in patients. Hypertension, BMI, WC, TG, LDL-c, HDL-c, ALT, GGT, UA, and diabetes were found to have a significant influence on NAFLD. Meanwhile, age, male, smoking, diabetes, IFG, IGT, hypertension, and LDL-c significantly contributed to the prevalence of atherosclerosis. Hypertension, diabetes, and higher serum LDL-c level were positively correlated with NAFLD with an OR (95% CI) of 1.325 (range 1.157–1.518) for hypertension, an OR of 2.153 (range 1.814–2.555) for diabetes, and an OR of 1.161 (range 1.071–1.259) for LDL-c. Hypertension, diabetes, and higher LDL-c were positively correlated with atherosclerosis. An OR (95% CI) of 1.501 (range 1.286–1.751) for hypertension, 1.716 (range 1.414–2.084) for diabetes, and 1.344 (range 1.231–1.466) for LDL-c was noticed (Table 3).
Table 3.
Comparisons of risk factors for presence of nonalcoholic fatty liver disease and atherosclerosis.
3.4. Multivariable-adjusted analyses and the absence of an independent association between atherosclerosis and NAFLD
Univariate and multivariate logistic regressions were performed to assess whether an independent association existed between NAFLD and atherosclerosis. There was no independent correlation between NAFLD and atherosclerosis after adjusting for age, sex, presence of hypertension or diabetes, smoking status, creatinine level, BMI, WC, alcohol consumption, and lipid level (Table 4).
Table 4.
Logistical regression analysis of nonalcoholic fatty liver disease and risk for presence of atherosclerosis.
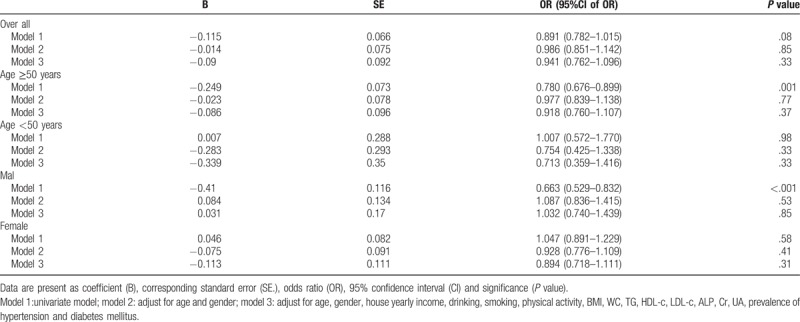
Given the significant differences in age and sex in the distribution of atherosclerosis, age- and sex-stratified regression analyses were also performed. No independent association was observed between atherosclerosis and NAFLD in each stratified analysis (Table 4).
3.5. Comparison of the metabolic characteristics for subjects in the 4 groups according to the presence or absence of NAFLD alone or in combination with atherosclerosis
Patients with NAFLD in combination with atherosclerosis were found to have a more severe metabolic burden and chances of having hypertension, diabetes, dyslipidemia, and higher metabolic syndrome scores than those in the other groups (Figs. 1 and 2; Table 5).
Figure 1.
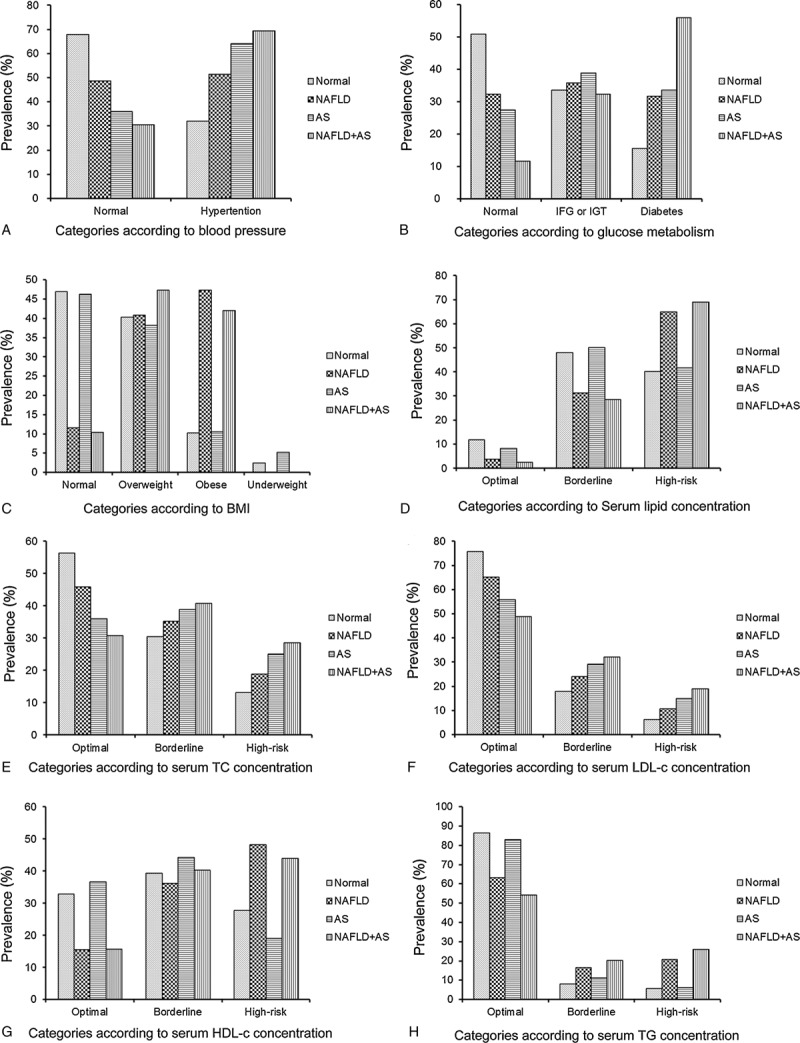
Prevalence of metabolic alterations for subjects in the 4 groups according to the presence or absence of NAFLD alone or in combination with AS. AS = atherosclerosis, NAFLD = nonalcoholic fatty liver disease.
Figure 2.
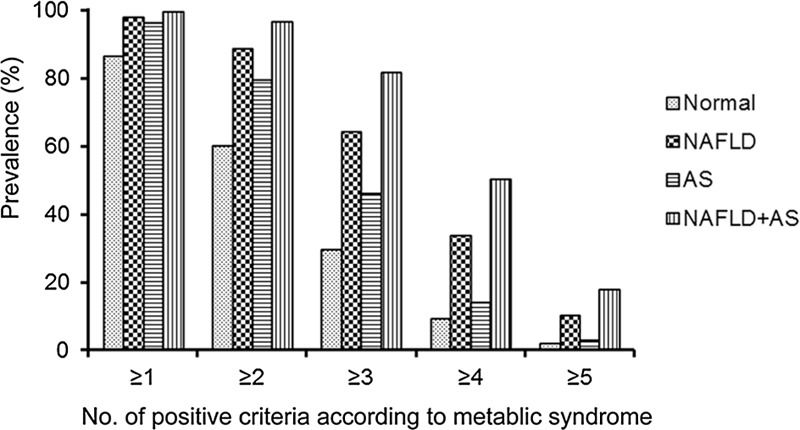
Prevalence of positive criteria of the metabolic syndrome for subjects in the 4 groups according to the presence or absence of NAFLD alone or in combination with atherosclerosis. NAFLD = nonalcoholic fatty liver disease.
Table 5.
Comparisons of clinical characteristics for subjects in the 4 groups according to the presence or absence of nonalcoholic fatty liver disease alone or in combination with atherosclerosis.
4. Discussion
Whether NAFLD is an independent risk factor for CVD is an interesting and controversial topic. Recent survival studies suggest that NAFLD patients have a significantly higher all-cause and CVD-related mortality as compared with the general population.[17,18] Cardiac-related death is one of the leading causes of death in patients with NAFLD.[4,19,20] Moreover, NAFLD has been associated with a greater risk of non-fatal CVD events and 26% higher 5-year overall health care costs, mainly due to cardiometabolic diseases.[21] Therefore, some researchers have pointed out that NAFLD is likely associated with increasing CVD risk and have raised the possibility that NAFLD might be not only a marker but also an early mediator of atherosclerosis.[22,23] There is some epidemiological evidence linking NAFLD to CVD. Besides that, some studies provide the evidence of a strong association between NAFLD and subclinical manifestations of atherosclerosis (thickened intima-media layer, endothelial dysfunction, arterial stiffness, impaired left ventricular function, and coronary calcification).[18] But the observation has not always remained constant, as some studies have had conflicting inferences.[24–28] Based on CIMT evaluation and the presence of carotid artery plaques, this study observed that NAFLD was not independently associated with carotid atherosclerosis. Different conclusions might be attributed to differences in the study designs and the population enrolled. Some findings suggest that the degree of liver fibrosis is related to clinical outcomes in NAFLD patients, and simple steatosis has a benign clinical course.[29] This helps to partly explain why no independent association between NAFLD and CVD was concluded in some epidemiological investigation of the general population, and why NAFLD can exert more deleterious influence on the risk of CVD in patients with comorbid type 2 diabetes mellitus who are often worse in terms of histopathological changes.[30]
This study further showed that NAFLD and atherosclerosis have common but distinct metabolic characteristics. Typical atherogenic dyslipidemia was detected in patients with NAFLD, characterized by higher serum TG and LDL-c levels and lower serum HDL-c level. Moreover, a higher serum LDL-c level was more dominant in patients with atherosclerosis. Also, hypertension and diabetes were common risk factors. Age and smoking were crucial for atherosclerosis but not for NAFLD. However, obesity and higher serum UA concentration were more crucial for NAFLD. As observed in this study, the majority of patients with NAFLD were overweight or obese or had type 2 diabetes or atherogenic dyslipidemia. NAFLD is not only a hepatic manifestation of metabolic syndrome[2,31] but is also bidirectionally linked with metabolic syndrome as a consequence of metabolic abnormalities and is a trigger of further metabolic abnormalities.[32] This study found that NAFLD adversely influences the metabolic burden and also verified that atherosclerosis accompanied by NAFLD had higher risk for metabolic syndrome, dyslipidemia, diabetes, and hypertension. Patients with NAFLD should be candidates for not only the aggressive treatment of their liver disease but also for the aggressive treatment of the underlying risk factors.
Although CVD risk level is not the same across the entire histological and clinical spectrum of NAFLD,[33] both NAFLD and atherosclerosis share common pathological mechanisms in their disease progression. An imbalance of lipolysis and lipogenesis is considered to be the initiating mechanism of metabolic disorders, including NAFLD and atherosclerosis.[34] The multiple parallel hit theory has been suggested, in which mechanisms such as ectopic fat accumulation, insulin resistance, oxidative stress, inflammation, and imbalanced secretion of cytokines act in both parallel and cascade ways in the development of NAFLD;[35] these mechanisms also facilitate the development of atherosclerosis and CVDs. Interesting new evidence has led researchers to consider NAFLD as an early marker of endothelial dysfunction, and they have suggested that the evolution of NAFLD might worsen endothelium-dependent vasodilation responses in metabolic syndrome-related hypertension.[36] Shared pathological mechanisms link atherosclerosis and NAFLD together. It is plausible to hypothesize that the same pathways generating NAFLD might also lead to atherosclerosis. As these conditions proceed, the liver may be involved as target of metabolic abnormalities on 1 hand, and on the other hand is responsible for an adverse metabolic burden, with a consequent vicious circle. Although no “cause-and-effect” relationship for NAFLD and atherosclerosis was drawn from this study, the development and evolution of NAFLD may accelerate the progression of atherosclerosis in many ways.
Steatosis is considered a benign reversible event, whereas NASH has the potential to lead to cirrhosis and substantially influence overall mortality, mainly due to higher CVD events.[37] As many as 10% to 25% of patients with liver steatosis progress to NASH.[1] The reversible characteristics of steatosis have led to the belief that NAFLD might be a key point in the development of metabolic disease. Abdominal ultrasonography has been shown to have a sufficient degree of diagnostic accuracy for fatty liver.[38] It is convenient to perform in clinical practice and is noninvasive. Early detection of NAFLD and subsequent intervention should be encouraged, especially for patients with CVD risk factors, to reduce metabolic burden.
Several limitations of this study should, however, be acknowledged. First, this was an observational study on a cross-sectional population, which could demonstrate only an association but not causality. Second, this study used ultrasonography to detect hepatic steatosis. Although this technique is widely used in epidemiological surveys of NAFLD, because of its safety and economical and practical utility, it has limited accuracy in detecting mild steatosis compared with liver biopsy, which is considered the gold standard. Third, Insulin plasma levels and HOMA index were not evaluated in this study for too much missing data of insulin plasma levels regrettably. In the future study, we will further introduce indicators that reflect insulin resistance, oxidative stress, inflammation, and imbalanced secretion of cytokines for further investigation of pathophysiological hypothesis. Besides, we will further investigate the association between different stages of NAFLD and atherosclerosis in populations in a future longitudinal study.
In conclusion, NAFLD and atherosclerosis share similar pathological mechanisms and have common but also proprietary metabolic characteristics. Moreover, atherosclerosis accompanied by NAFLD has more adverse metabolic burdens than atherosclerosis alone. In clinical work, early detection and intervention of NAFLD should be encouraged, especially for patients with CVD risk factors, to reduce their metabolic burdens. In NAFLD patients, more attention should be focused on systemic metabolic states and CVD risk levels in addition to liver disease.
Author contributions
Conceptualization: Jie Han, Xu Zhang.
Data curation: Jie Han, Lu Liu, Meng Zhao.
Formal analysis: Yong Wang, Ling Gao.
Funding acquisition: Ling Gao, Jiajun Zhao.
Investigation: Qingbo Guan, Haiqing Zhang.
Methodology: Jie Han, Lu Liu, Haiqing Zhang, Ling Gao, Xu Zhang.
Project administration: Yong Wang, Zhongshang Yuan, Meng Zhao, Jiajun Zhao.
Resources: Qiu Li.
Software: Jie Han, Yong Wang, Lu Liu, Qiu Li, Xu Zhang.
Supervision: Zhongshang Yuan, Qingbo Guan, Ling Gao, Jiajun Zhao.
Validation: Jie Han, Yong Wang, Meng Zhao, Qingbo Guan, Haiqing Zhang, Qiu Li.
Visualization: Zhongshang Yuan, Xu Zhang.
Writing – original draft: Jie Han, Yong Wang.
Writing – review & editing: Jie Han, Jin Xu, Ling Gao.
Footnotes
Abbreviations: ALT = alanine aminotransferase, AST = aspartate aminotransferase, BMI = body mass index, CIMT = carotid intima-media thickness, CVD = cardiovascular disease, GGT = glutamyltranspeptidase, HDL-c = high-density lipoprotein cholesterol, IFG = impaired fasting glucose, IGT = impaired glucose tolerance, LDL-c = low-density lipoprotein cholesterol, NAFLD = nonalcoholic fatty liver disease, NASH = nonalcoholic steatohepatitis, ORs = odds ratios, TG = triglyceride, UA = uric acid, WC = waist circumference.
This work was supported in part by grants from the National Natural Science Foundation of China (No. 81230018, No. 81370891, No. 81270869, and No.81541023), the Scientific & Technologic Development Program of Shandong Province (No. 2016GSF201013 and No. 2012GSF11824), and the Scientific Research Foundation of Shandong Province of China (No. 2006BS03012, No.2013GSF11814).
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants included in the study.
There are no conflicts of interest to disclose in this paper.
References
- [1].Willebrords TJ, Pereira IVA, Maes M, et al. Strategies, models and biomarkers in experimental non-alcoholic fatty liver disease research. Prog Lipid Res 2015;59:106–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 2003;37:917–23. [DOI] [PubMed] [Google Scholar]
- [3].Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of non-alcoholic fatty liver disease–meta-analytic assessment of prevalence, incidence and outcomes. Hepatology 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- [4].Rafiq N, Bai C, Fang Y, et al. Long-term follow-up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol 2009;7:234–8. [DOI] [PubMed] [Google Scholar]
- [5].O’Leary DH, Polak JF. Intima-media thickness: a tool for atherosclerosis imaging and event prediction. Am J Cardiol 2002;90suppl 10C:18L–21L. [DOI] [PubMed] [Google Scholar]
- [6].Sirimarco G, Amarenco P, Labreuche J, et al. Carotid atherosclerosis and risk of subsequent coronary event in outpatients with atherothrombosis. Stroke J Cerebral Circ 2013;44:373–9. [DOI] [PubMed] [Google Scholar]
- [7].Gao N, Yuan Z, Tang X, et al. Prevalence of CHD-related metabolic comorbidity of diabetes mellitus in Northern Chinese adults: the REACTION study. J Diabetes Complications 2016;30:199–205. [DOI] [PubMed] [Google Scholar]
- [8].Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima-media thickness consensus (2004–2006)—an update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis 2007;23:75–80. [DOI] [PubMed] [Google Scholar]
- [9].Zhang X, Shao S, Geng H, et al. Expression profiles of six circulating microRNAs critical to atherosclerosis in patients with subclinical hypothyroidism: a clinical study. J Clin Endocrinol Metab 2014;99:E766–74. [DOI] [PubMed] [Google Scholar]
- [10].Doria A, Shoenfeld Y, Wu R, et al. Risk factors for subclinical atherosclerosis in a prospective cohort of patients with systemic lupus erythematosus. Ann Rheum Dis 2003;62:1071–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].American Diabetes Association. Standards of medical care in diabetes–2010. Diabetes Care 2010;33suppl 1:S11–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure—the JNC 7 report. JAMA 2003;289:2560–72. [DOI] [PubMed] [Google Scholar]
- [13].Jellinger PS, Smith DA, Metha AE, et al. American Association of Clinical Endocrinologists’ guidelines for management of dyslipidemia and prevention of atherosclerosis. Endocrine Pract 2012;18suppl 1:1–78. [DOI] [PubMed] [Google Scholar]
- [14].Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 2005;112:2735–52. [DOI] [PubMed] [Google Scholar]
- [15].Mazhar SM, Shiehmorteza M, Sirlin CB. Noninvasive assessment of hepatic steatosis. Clin Gastroenterol Hepatol 2009;7:135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Saverymuttu SH, Joseph AEA, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J 1986;292:13–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Stepanova M, Rafiq N, Makhlouf H, et al. Predictors of all-cause mortality and liver-related mortality in patients with non-alcoholic fatty liver disease (NAFLD). Dig Dis Sci 2013;58:3017–23. [DOI] [PubMed] [Google Scholar]
- [18].Fargion S, Porzio M, Fracanzani AL. Nonalcoholic fatty liver disease and vascular disease: state-of-the-art. World J Gastroenterol 2014;20:13306–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Younossi ZM, Otgonsuren M, Venkatesan C, et al. In patients with non-alcoholic fatty liver disease, metabolically abnormal individuals are at a higher risk for mortality while metabolically normal individuals are not. Metabolism 2013;62:352–60. [DOI] [PubMed] [Google Scholar]
- [20].Ekstedt M, Hagstrom H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology 2015;61:1547–54. [DOI] [PubMed] [Google Scholar]
- [21].Baumeister SE, Volzke H, Marschall P, et al. Impact of fatty liver disease on health care utilization and costs in a general population: a 5-year observation. Gastroenterology 2008;134:85–94. [DOI] [PubMed] [Google Scholar]
- [22].Gastaldelli A, Kozakova M, Hojlund K, et al. Fatty liver is associated with insulin resistance, risk of coronary heart disease, and early atherosclerosis in a large European population. Hepatology 2009;49:1537–44. [DOI] [PubMed] [Google Scholar]
- [23].Targher G, Day C, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fattly liver disease. N Engl J Med 2010;363:1341–50. [DOI] [PubMed] [Google Scholar]
- [24].Kim S, Choi YJ, Huh BW, et al. Nonalcoholic fatty liver disease is associated with increased carotid intima-media thickness only in type 2 diabetic subjects with insulin resistance. J Clin Endocrinol Metab 2014;99:1879–84. [DOI] [PubMed] [Google Scholar]
- [25].Petit JM, Guiu B, Terriat B, et al. Nonalcoholic fatty liver is not associated with carotid intima-media thickness in type 2 diabetic patients. J Clin Endocrinol Metab 2009;94:4103–6. [DOI] [PubMed] [Google Scholar]
- [26].Dick TJ, Lesser IA, Leipsic JA, et al. The effect of obesity on the association between liver fat and carotid atherosclerosis in a multi-ethnic cohort. Atherosclerosis 2013;226:208–13. [DOI] [PubMed] [Google Scholar]
- [27].Huang Y, Bi Y, Xu M, et al. Nonalcoholic fatty liver disease is associated with atherosclerosis in middle-aged and elderly Chinese. Arterioscler Thromb Vasc Biol 2012;32:2321–6. [DOI] [PubMed] [Google Scholar]
- [28].Jacobs K, Brouha S, Bettencourt R, et al. Association of nonalcoholic fatty liver disease with visceral adiposity but not coronary artery calcification in the elderly. Clin Gastroenterol Hepatol 2016;14:1337–44. [DOI] [PubMed] [Google Scholar]
- [29].Kim D, Kim WR, Kim HJ, et al. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology 2013;57:1357–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol 2013;10:330–44. [DOI] [PubMed] [Google Scholar]
- [31].Kotronen A, Yki-Jarvinen H. Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol 2008;28:27–38. [DOI] [PubMed] [Google Scholar]
- [32].Yki-Jarvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lacent Diabetes Endocrinol 2014;2:901–10. [DOI] [PubMed] [Google Scholar]
- [33].Dowman JK, Tomlinson JW, Newsome PN. Systematic review: the diagnosis and staging of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2011;33:525–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Saponaro C, Gaggini M, Carli F, et al. The subtle balance between Lipolysis and Lipogenesis: a critical point in metabolic homeostasis. Nutrients 2015;7:9453–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology 2010;52:1836–46. [DOI] [PubMed] [Google Scholar]
- [36].Perticone M, Cimellaro A, Maio R, et al. Additive effect of non-alcoholic fatty liver disease on metabolic syndrome-related endothelial dysfunction in hypertensive patients. Int J Mol Sci 2016;17:456–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Athyros VG, Tziomalos K, Katsiki N, et al. Cardiovascular risk across the histological spectrum and the clinical manifestations of non-alcoholic fatty liver disease: an update. World J Gastroenterol 2015;21:6820–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hernaez R, Lazo M, Bonekamp S, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology 2011;54:1082–90. [DOI] [PMC free article] [PubMed] [Google Scholar]


