Supplemental Digital Content is available in the text
Keywords: ACE inhibitors, angiotensin receptor blockers, diabetic kidney disease, randomized controlled trials, Tripterygium wilfordii Hook
Abstract
Background:
The present study aims to evaluate the clinical efficacy and safety of Tripterygium wilfordii Hook (TwH) combined with angiotensin receptor blockers/ACE inhibitors (ARB/ACEI) in the treatment of diabetic kidney disease (DKD) stage IV.
Methods:
We searched China National Knowledge Internet (CNKI), the Chinese Biomedical Database, Embase and PubMed for articles about TwH combined with ARB/ACEI in treating DKD stage IV and set the study inclusion and elimination standards.
Results:
A total of 22 randomized controlled trials (RCTs) with 1414 participants were collected for detailed evaluation. The meta-analysis results suggested that compared with the controls, the combined group showed significant effects in reducing 24-h urinary protein [mean difference (MD) = −0.87, 95% confidence interval (CI) = (−1.03, −0.71)], raising serum albumin [MD = 4.14, 95% CI (3.43, 4.85)] and the total efficiency [odds ratio (OR) = 4.84, 95% CI (3.33, 7.03)], with no statistical difference in serum creatinine between both groups [MD = −3.02, 95% CI (−6.40, 0.37), P > .05]. However, the risk of adverse reactions increased by 8% [Risk Difference (RD) = 0.08, 95% CI (0.05, 0.11)] in the combination.
Conclusions:
TwH combined with ARB/ACEI in the treatment of DKD stage IV is superior to the monotherapy of ARB/ACEI.
1. Introduction
Diabetes mellitus (DM) has been increasing at rapid speed. It has been estimated that in 2017 there are 425 million people (aged 20–79) suffering from diabetes worldwide and the number would rise to 629 million in 2045.[1] DM has become a serious burden and has significant impact on public health.[2] However, diabetic kidney disease (DKD) is one of the most devastating complications of DM in terms of patients’ quality of life and survival,[3] and DKD is the leading cause of chronic kidney disease (CKD) and end-stage renal disease (ESRD) as well.[4] Continuous proteinuria has been proved to be a clinical indicator and an independent risk predictor for the progression of DKD,[5] and utilizing angiotensin receptor blockers/ACE inhibitors (ARB/ACEI) could reduce proteinuria to prevent progression of DKD; however, it is not always adequate to alleviate proteinuria by using currently useful ARB/ACEI, so to prevent further deterioration of DKD, it is urgent for us to find other approaches.[6]
Tripterygium wilfordii Hook (TwH) has been widely used as a Chinese medicine for many years in many ways, especially in treating glomerulonephritis and organ transplantation.[7,8] Several trials have been confirmed that TwH markedly attenuated albuminuria and contributed to the prevention of DKD.[9–11] There is also one meta-analysis about the effect of TwH combined with ARB/ACEI in treating DKD stage IV,[12] but there is no well-designed meta-analysis of randomized controlled trials (RCTs) at present.
Therefore, we conducted the first meta-analysis of RCTs which included the largest sample size, and we especially aimed to further evaluate the clinical efficacy and safety of TwH combined with ARB/ACEI in treating DKD stage IV.
2. Methods
2.1. Search strategy
We searched China National Knowledge Internet (CNKI), the Chinese Biomedical Database, Embase and PubMed for articles from the establishment of databases to July 2018. The predefined key search terms included “diabetic kidney disease” or “diabetic nephropathy” or “diabetic glomerulosclerosis”, and “Tripterygium wilfordii Hook” or “tripterygium glycosides” or “triptolide”, and “rein-angiotensin” or “ARB” or “ACEI” and “efficacy” or “urinary protein”. At the same time, we also reviewed the related research references in order to prevent from neglecting any relevant studies.
2.2. Study criteria
The included criteria for studies were: (1) studies based on RCTs, (2) the patients of the original studies were diagnosed of DKD at clinical stage IV (albuminuria 300 mg/g Cr or more and Estimated Glomerular Filtration Rate (eGFR) is less than 30 ml/min/1.73 m2), (3) the treatment drug was ARB/ACEI alone or ARB/ACEI plus TwH, and (4) the subjects with outcomes included 24-h urinary protein (24 h UPr), serum albumin (Alb), serum creatinine (SCr), total efficiency and adverse reactions. In this meta-analysis, total efficiency was mainly defined as obvious effect plus effective according to Guidelines for clinical research of new Chinese medicine drugs.[13] Adverse reactions were mainly included: liver function damage, gastrointestinal reactions, myelosuppression, menstrual disorder, etc.
The exclusion criteria were: (1) duplicated publications, (2) studies of patients were not clearly diagnosed or patients with ESRD, tumor, kidney transplantation, acute and chronic nephritis, liver disease and other causes of hypoproteinemia, and (3) studies such as systemic reviews, meta-analysis, case reports, animal experimental studies, etc.
2.3. Data extraction
Data were extracted independently and cross checked by two investigators (Daijin Ren and Chao Zuo). All possible valid references that we searched were checked in detail to identify studies that satisfied the study criteria. Disagreements and differences in inclusion of studies were dealt with by consensus or discussion with a third person (Gaosi Xu). All of reference lists of researches that had been identified were checked out carefully again to prevent the omissions and the details of the selection process are shown in Figure 1.
Figure 1.
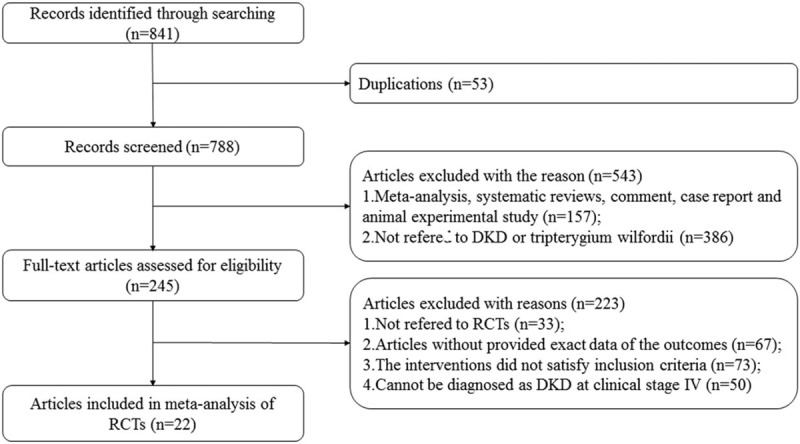
Study selection flow diagram.
Data extraction included the first authors’ name of the paper, the year of publication, study design, number of patients, the treatment methods were extracted from each of the included papers, course of treatment, urine protein baseline of inclusion criteria and the results for 24 h UPr, Alb, SCr, total efficiency and TwH-related toxicity: details are presented in Supplemental Table 1.
2.4. Quality assessment
The assessment of study quality was performed by using Review Manager (vision 5.3) risk-of-bias tool, including four sections: selection, performance/detection, attrition, and reporting bias (Fig. 2).
Figure 2.
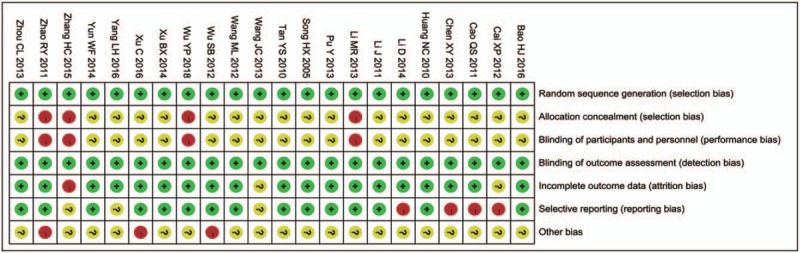
Risk of bias summary review authors’ judgments about each risk of bias item for each included study.
2.5. Statistical analysis
The data were abstracted and analyzed by using Review Manager (version 5.3) and STATA (version 12.0, Stata SE). Count data was summarized by forest plot, with expressed as odds ratio (OR) and risk difference (RD) with 95% confidence interval (CI). Measurement data was summarized by forest plot, with expressed as standard mean difference (SMD) and mean difference (MD) with 95% confidence interval (CI). Statistical heterogeneity across the studies was evaluated by the Q statistic and I2 tests. The heterogeneity is graded according to I2 thresholds of <25%, 25–75%, and >75%, separately represents low, moderate, and high heterogeneity.[36] A random-effects model was adopted to process data with heterogeneous results, whereas a fixed-effects model was applied to process data in poor heterogeneity. It is significant for P < .05 to evaluate all statistical tests. To explore the potential sources of heterogeneity, we analyzed the data by adopting subgroup and sensitivity analysis. Meta-regression was used to analyze and test the potential impact of the characteristics of included studies such as course of treatment, UPr baseline of inclusion criteria and duration of DKD. The publication bias was evaluated by Egger's and Begg's test funnel plots. For the occurrence of zero in the counting data, the automatic default in RevMan 5.3 software is 0.5, which does not affect the results of OR and RD.[37]
3. Results
3.1. Description of included studies
We identified 22 studies[14–35] with a total of 1414 (726 patients receiving TwH plus ARB/ACEI versus 688 patients receiving ARB/ACEI) that satisfied our criteria and all cases were treated with routine treatment such as reduce blood glucose and blood press. All the studies were conducted in China. The characteristics of included studies are shown in Supplemental Table S1. The quality of the 22 studies included based on RevMan 5.3 software risk-of-bias tool was as follows (Fig. 2): all the studies met random sequence generation, and six of them described the specific method of random.[18,19,24,26,29,33] Four of them referred to patients’ informed consent about treatment plan[21,22,26,33] and others did not report concealed allocation, blinding (participants and personnel). All studies met blinding (outcome assessment). Three of them mentioned the causes of missing and withdrawing cases[14,30,33] and others did not report incomplete outcome.
3.2. 24-hour urinary protein
Twenty-one trials demonstrated a difference in 24 h proteinuria between the combined and control group.[14–20,22–35] Three trials reported it after 3 and 6 months of treatment.[14,19,20] One trial reported it after 1, 2 and 3 months of treatment.[22] The results were included in RevMan 5.3 software. Heterogeneity testing showed that there was statistically significant difference between the studies (I2 = 89.0%, P < .05). Therefore, the random effect model was adopted. The combined group showed significant effect than the control in reducing the 24 h UPr [MD = −0.87, 95% CI (−1.03, −0.71)].
The subgroups were divided into t > 6 months, t < 6 months, and t < 3 months of combination compared to control treatment (Fig. 3). There was still obvious heterogeneity within each subgroup [t > 6 months: (I2 = 77%, P < .05); 3 < t < 6 months: (I2 = 88%, P < .05); t < 3 months: (I2 = 89%, P < .05)]. All subgroups indicated the combined group was superior to the control group in reducing the 24 h UPr [t > 6 months: SMD = −1.37, 95% CI (−1.73, −1.01); 3 < t < 6 months: SMD = −1.39, 95% CI (−2.03, −0.76); t < 3 months: SMD = −1.85, 95% CI (−2.56, −1.14)]. The results revealed that it was more significant in reducing proteinuria for the combined compared to the control when the course of treatment was t < 3 months.
Figure 3.
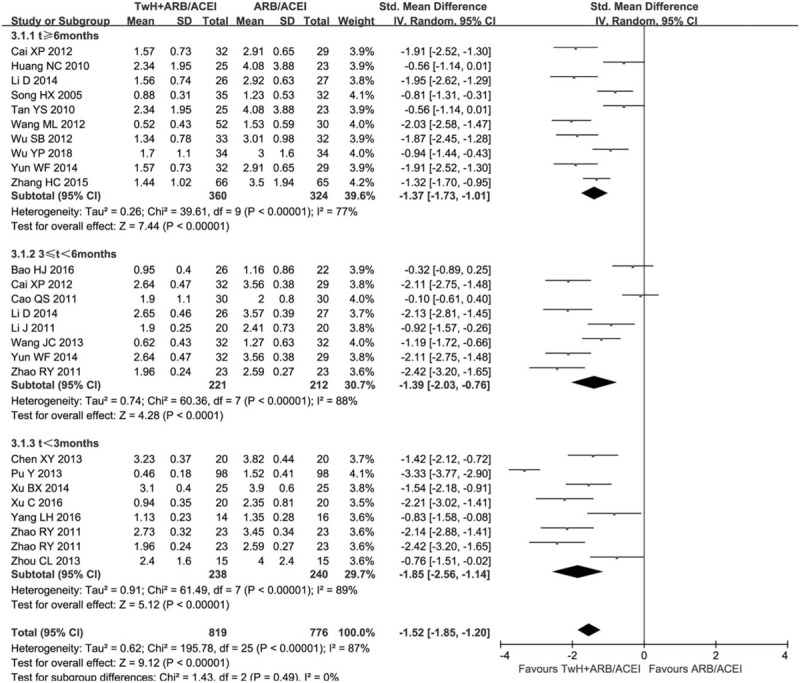
Subgroup analysis of TwH combined with ARB/ACEI in the treatment of DKD stage IV based on the course of treatment, outcome: 24 h UPr.
We also analyzed a subgroup based on UPr baseline of inclusion criteria (Fig. 4). There was still obvious heterogeneity within each subgroup [>3.5 g/24 h: (P = .09, I2 = 42%); >1.5 g/24 h: (P < .05, I2 = 92%); >1.0 g/24 h: (P < .05, I2 = 84%)]. All subgroups indicated the combined group was superior to the control group in reducing the 24 h UPr [>3.5 g/24 h: MD = −1.10, 95% CI (−1.26, −0.94); >1.5 g/24 h: MD = −0.72, 95% CI (−0.97, −0.47); >1.0 g/24 h: MD = −0.63, 95% CI (−1.00, −0.25)]. The results revealed that the combined showed more significant in decreasing proteinuria compared to the control when UPr baseline of inclusion criteria is >3.5 g/24 h.
Figure 4.
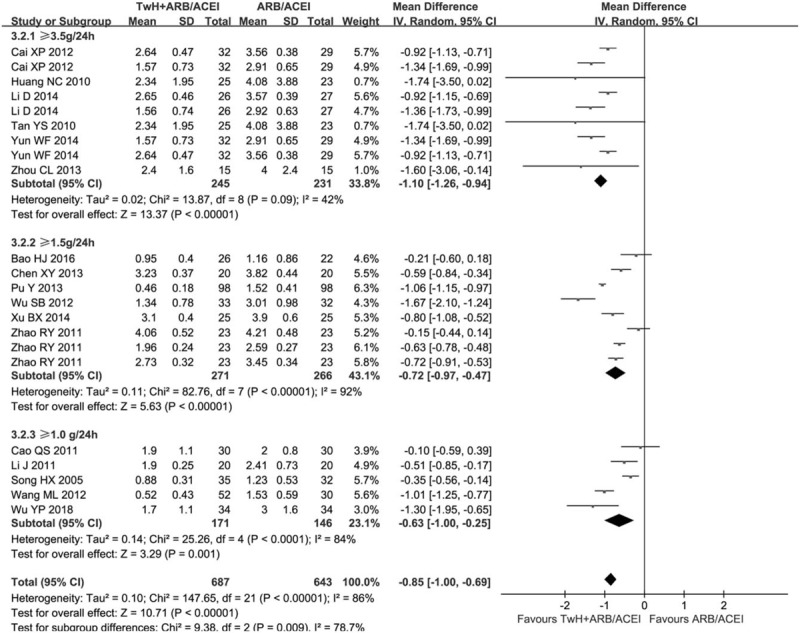
Subgroup analysis of TwH combined with ARB/ACEI in the treatment of DKD stage IV based on the UPr baseline of inclusion criteria, outcome: 24 h UPr.
In addition, we analyzed a subgroup based on TWH combined with ARB or ACEI (Supplemental Figure 1). There was still obvious heterogeneity within each subgroup [TWH + ARB: (P < .05, I2 = 89%); TWH + ACEI: (P < .05, I2 = 66%)]. All subgroups indicated the combined group was superior to the control in reducing the 24 h UPr [TWH + ARB: MD = −0.95, 95% CI (−1.13, −0.78); TWH + ACEI: MD = −0.50, 95% CI (−0.74, −0.26)]. The results revealed that TWH combined with ARB had a better effect in reducing proteinuria than combined with ACEI.
Furthermore, we performed a subgroup based on different ways of reducing blood sugar (oral hypoglycemic drugs and/or insulin VS isolated insulin) (Supplemental Figure 2). There was still obvious heterogeneity within each subgroup [oral hypoglycemic drugs and/or insulin: (P < .05, I2 = 88%); isolated insulin: (P < .05, I2 = 86%)]. All subgroups indicated the combined group was superior to the control group in reducing the 24 h UPr [oral hypoglycemic drugs and/or insulin: MD = −0.93, 95% CI (−1.16, −0.70); isolated insulin: MD = −0.80, 95% CI (−1.18, −0.43)]. The results revealed that the combined showed more significant in decreasing proteinuria compared to the control when way of reducing blood sugar is oral hypoglycemic drugs and/or insulin.
Finally, we performed meta-regression to explore the source of heterogeneity in Stata 12.0. The effect of reducing proteinuria was not influenced by the course of treatment (P > .851), either in proteinuria baseline of inclusion criteria (P > .46) or the duration of DKD (P > .372).
3.3. Serum albumin
Fifteen trials reported a difference in Alb between the combined and control group.[14–22,26,28–30,32,35] The only one trial with Alb over 40 g/L after treatment was excluded.[35] Three trials reported Alb after 3 and 6 months of treatment.[14,19,20] One trial reported Alb after 1, 2 and 3 months of treatment.[22] The results were included in RevMan 5.3 software. Statistical heterogeneity analysis indicated moderate heterogeneity across the studies (I2 = 47.0%, P < .05). Therefore, the data was pooled by a random effect model. The combined group was superior to the control group in elevating Alb [MD = 4.14, 95% CI (3.43, 4.85)].
We performed subgroup analysis based on the course of treatment (Fig. 5). There was significant heterogeneity between subgroups (P < .05, I2 = 91.0%). There was no heterogeneity within each subgroup [t > 6 months: (P = .42, I2 = 1.0%); 3 < t < 6 months: (P = .80, I2 = 0.0%); t < 3 months: (P = .86, I2 = 0.0%)], so it was pooled by a fixed effect model. All subgroups indicated the combined group was superior to the control in elevating Alb [t > 6 months: MD = 5.20, 95% CI (4.41, 6.00); 3 < t < 6 months: MD = 3.69, 95% CI (2.92, 4.46); t < 3 months: MD = 1.93, 95% CI (0.80, 3.06)]. The results revealed that there was a more significant elevation in Alb of the combined group compared to the control when the course of treatment was t > 6 months.
Figure 5.
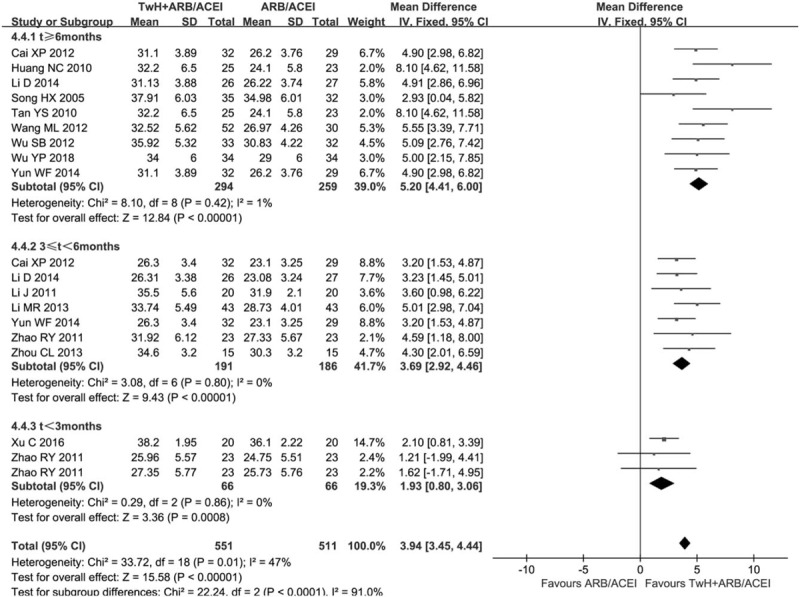
Subgroup analysis of TwH combined with ARB/ACEI in the treatment of DKD stage IV based on the course of treatment, outcome: Alb.
3.4. Serum creatinine
Eighteen trials demonstrated a difference in SCr between the combined and control group.[14,16,18–22,25–35] Three trials reported SCr after 3 and 6 months of treatment.[14,19,20] One trial reported SCr after 1, 2 and 3 months of treatment.[22] The results were included in RevMan 5.3 software. Statistical heterogeneity analysis indicated significant heterogeneity across the studies (I2 = 83.0%, P < .05). Therefore, the data was pooled by a random effect model. The combined group did not cause SCr elevation compared to the control because there was no significant difference between the combined and the control group [MD = −3.02, 95% CI (−6.40, 0.37), P > .05].
We performed subgroup analysis based on the course of treatment in RevMan 5.3 software (Supplemental Figure 3). There was still significant heterogeneity within each subgroup [t > 6 months: (P < .05, I2 = 89.0%); 3 < t < 6 months: (P < .05, I2 = 82.0%); t < 3 months: (P = .16, I2 = 37.0%)]. All subgroups indicated that there was no significant difference between the combined and the control group [t > 6 months: MD = −5.53, 95% CI (−13.06, 2.00); 3 < t < 6 months: MD = −1.16, 95% CI (−6.28, 3.96); t < 3 months: MD = −1.57, 95% CI (−5.21, 2.06)].
3.5. The total efficiency
Twelve trials reported a difference in the total efficiency between the combined and control group.[14,15,17,19,20,23–25,29,30,33,35] The data was included in RevMan 5.3 software (Fig. 6A). There was no significant statistical heterogeneity in the total efficiency (I2 = 0.0%, P = .52). Thus, the combined odds ratio (OR) was pooled by a fixed effect model. The total efficiency of the combined group was obviously higher than the control [OR = 4.84, 95% CI (3.33, 7.03)].
Figure 6.
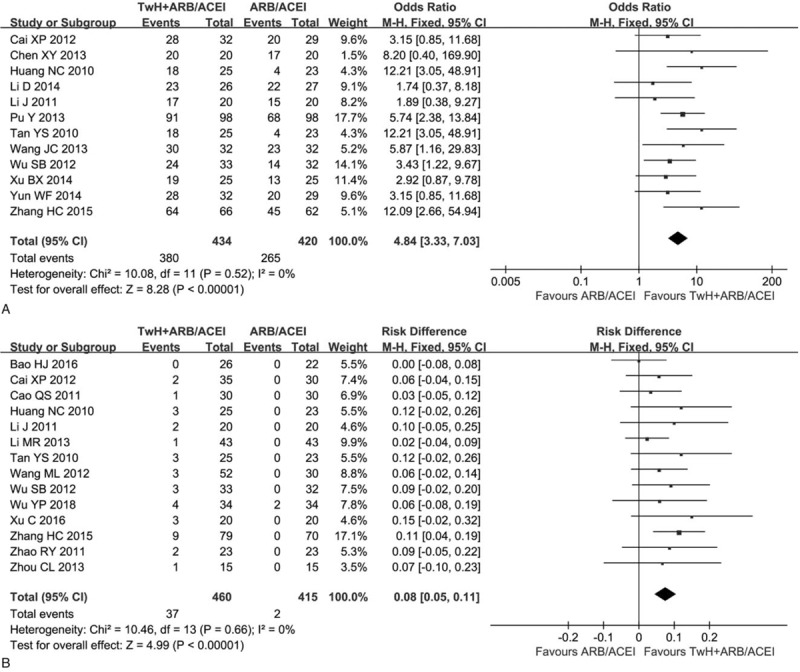
Forest plots of the total efficiency and adverse reactions of TwH combined with ARB/ACEI in the treatment of DKD stage IV, (a) the total efficiency and (b) adverse reactions.
3.6. Adverse reactions
Thirteen trials reported adverse reactions.[14–17,21,22,26–33] The combined group reported 25 cases with liver function damage, but recovered after treatment with hepatoprotective drugs and one of them withdrew from the experiment. Eight cases developed gastrointestinal reactions. Leukopenia was observed in three cases. One female had menstrual disorder. The combined reported two cases with hyperkalemia. The results were included in RevMan 5.3 software (Fig. 6B). There was no significant statistical heterogeneity in adverse reactions (I2 = 0.0%, P = .66). Thus, the risk difference (RD) was pooled by a fixed effect model. The adverse reaction was 8% higher in the combined group than the control [RD = 0.08, 95% CI (0.05, 0.11)].
3.7. Sensitivity analysis and publication bias
We only performed sensitivity analysis with 24 h UPr and SCr in Stata12.0 software for their significant heterogeneity. Sensitivity analysis indicated that the meta-analysis about them (Supplemental Figures 4 and 5) had low sensitivity and high stability in analysis of patients with DKD stage IV. In order to explore publication bias, we performed Begg's and Egger's test funnel plots with 24 h UPr in Stata12.0 software (Supplemental Figure 6). The Begg's test (Pr > |z| = 0.098) and the Egger's linear regression test (P > .792), which indicated that there was no evidence of substantial publication bias and the included studies were all small sample RCTs.
4. Discussion
DKD is the leading cause of ESRD[4] and one of the most important prognostic factor for its progression is persistent proteinuria.[5] As a result, reducing proteinuria level has been considered as a goal of DKD treatment. The benefit of reducing proteinuria by using ARB/ACEI is valid. However, the use of ARB/ACEI is not always enough to alleviate proteinuria, so other approaches have been put forward, for example, inflammation, oxidative stress and immune reactions have become quite significant as targets for new treatments.[6] TwH as a medicinal plant has been used for immune and inflammatory diseases in China to ameliorate proteinuria and prevent DKD by exerting immunosuppressive, anti-inflammatory, anti- oxidative stress and podocyte-protective effects.[9–11,38,39] Therefore, it is necessary to explore the efficacy and safety of TwH combined with ARB/ACEI in treating DKD.
In the present meta-analysis, we found the combined group showed significant effects in reducing proteinuria and increasing the total efficiency but the risk of adverse reactions is higher than the control, which is the same as another meta-analysis.[12] Furthermore, we found the combined showed significant effects in elevating Alb compared to the control, with no statistical difference in SCr. But in order to explore the heterogeneity of the meta-analysis, we performed different subgroup analyses in the process of analyzing different outcomes and there are also some original conclusions: (1) when the course of treatment is <3 months, the speed of reducing proteinuria is the fastest. Several studies mentioned the greatest reduction in proteinuria with ARB/ACEI used alone was obvious during the first 6.5 or 6 to 12 months and persisted for the remainder of the trial.[40,41] Therefore, we could draw a conclusion that TwH may help ARB/ACEI accelerate the speed of reducing proteinuria. But a trial mentioned that the greatest reduction in proteinuria was not clear.[42] So there are needed lots of trials to confirm it, (2) When UPr baseline of inclusion criteria is >3.5 g/24 h, the combined showed more significant effect in reducing proteinuria compared to the control, (3) TWH combined with ARB showed better effect in reducing proteinuria than TWH combined with ACEI, that is, ARB is better than ACEI in reducing proteinuria, which has been confirmed in a trial, Pathak JV et al.,[43] (4) When way of reducing blood sugar is oral hypoglycemic drugs and/or insulin, the combined showed more significant effect in reducing proteinuria compared to the control, (5) the combined group in elevating Alb is better than the control and the longer the course of treatment, the more the elevation of Alb, (6) the combined group does not cause serum creatinine elevation compared to the control, and (7) the risk of adverse reactions (mainly are liver function damage, gastrointestinal reaction, or menstrual disorders) was 8% higher in the combined group than the control, but adverse reactions are mild and do not influence the trial. We also performed meta-regression in the process of analyzing proteinuria including three variables, which were not heterogenous. The sensitivity analysis was carried out with 24 h Upr and SCr, which showed that our meta-analysis had low sensitivity and high stability, the Begg's and the Egger's test funnel plots with 24 h UPr showed no publication bias.
There were several limitations in this meta-analysis: (1) all the studies were small sample studies, (2) the included literatures lacked some data that might affect urinary protein, such as Body Mass Index (BMI), blood pressure control and etc, so that we could not analyze their effects on proteinuria, (3) there was no specific oral hypoglycemic drug or insulin in the included studies, (4) only Chinese and English studies were included in this meta-analysis, (5) significant statistical heterogeneity still existed in the included studies and should be further explored, and (6) some dosage of TwH was not uniform.
In conclusion, TwH combined with ARB/ACEI in the treatment of DKD stage IV shows significant effects than the monotherapy of ARB/ACEI, which has a good clinical application prospect. However, the sample size of the included studies is small and significant statistical heterogeneity still existed in the included studies, so it should be further explored and confirmed.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. H0517/81560132), the Supporting Project for the Foregoers of Main Disciplines of Jiangxi Province (No. 20162BCB22023), the “5511” Innovative Drivers for Talent Teams of Jiangxi Province (No. 20165BCB18018), and the Nature Science Foundation of JiangXi Province (No. 20181BAB205016).
Author contributions
Writing – original draft: Daijin Ren, Chao Zuo.
Writing – review & editing: Gaosi Xu.
Supplementary Material
Footnotes
Abbreviations: 24 h UPr = 24-hour urinary protein; Alb = serum albumin; ARB/ACEI = angiotensin receptor blockers/ACE inhibitors; DKD = diabetic kidney disease; RCTs = randomized controlled trials; SCr = serum creatinine TwH = Tripterygium wilfordii Hook.
DR and CZ contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].International Diabetes Federation. IDF Diabetes Atlas. 8th edition.2017. Available from: http://www.idf.org/diabetesatlas. [Google Scholar]
- [2].Lee S-Y, Choi ME. Urinary biomarkers for early diabetic nephropathy: beyond albuminuria. Pediatr Nephrol 2015;30:1063–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Robert C, Stanton M. Frontiers in diabetic kidney disease: introduction. Am J Kidney Dis 2014;63:S1–2. [DOI] [PubMed] [Google Scholar]
- [4].Reutens AT. Epidemiology of diabetic kidney disease. Med Clin North Am 2013;97:1–8. [DOI] [PubMed] [Google Scholar]
- [5].Remuzzi G, Ruggenenti P, Benigni A. Understanding the nature of renal disease progression. Kidney Int 1997;51:2–15. [DOI] [PubMed] [Google Scholar]
- [6].Quiroga B, Arroyo D, Arriba G. Present and future in the treatment of diabetic kidney disease. J Diabet Res 2015;2015doi: 10.1155/2015/801348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Liu ZH, Li SH, Wu Y, et al. Treatment of membranous nephropathy with Tripterygium wilfordii and steroid: a prospective randomized control trial. Chin J Nephrol Dial Transplant 2009;18:303–9. [Google Scholar]
- [8].Ji SM, Wang QW, Chen JS, et al. Clinical trial of Tripterygium wilfordii Hook F. in human kidney transplantation in China. Transplant Proc 2006;38:1274–9. [DOI] [PubMed] [Google Scholar]
- [9].Wu wei, Yang JJ, Yang HM, et al. Multi-glycoside of Tripterygium wilfordii Hook. f. attenuates glomerulosclerosis in a rat model of diabetic nephropathy by exerting anti-microinflammatory effects without affecting hyperglycemia. Int J Mol Med 2017;40:721–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ma RX, Liu LQ, Liu XM, et al. Triptolide markedly attenuates albuminuria and podocyte injury in an animal model of diabetic nephropathy. Exp Ther Med 2013;6:649–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Liu ZH, Chen ZH, et al. Triptolide: a potent inhibitor of NF-ΚB in T-lymphocytes. Acta Pharmacol Sin 2000;21:782–6. [PubMed] [Google Scholar]
- [12].Hong Y, Gui ZH, Cai XP, et al. Clinical efficacy and safety of tripterygium glycosides in treatment of stage IV diabetic nephropathy: a meta-analysis. De Gruyter Open 2016;11:611–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zheng XY. Guidelines for Clinical Research of New Chinese Medicine Drugs (trial). 2002;Beijing: China Medical Science Press, 156. [Google Scholar]
- [14].Cai XP. ARB combined with Tripterygium glycosides in the treatment of diabetic nephropathy. J Clin Med Pract 2012;16:112–4. [Google Scholar]
- [15].Huang NC. Clinical observation of Tripterygium wilfordii combined with Irbesartan in the treatment of diabetic nephropathy with large amount of proteinuria. Jilin Med J 2010;31:5312–3. [Google Scholar]
- [16].Wang ML, Zhang C. Clinical observation of Valsartan combined with Tripterygium wilfordii in the treatment of proteinuria in type 2 diabetic nephropathy. Chin J Clin Rational Drug Use 2012;5:84–5. [Google Scholar]
- [17].Tan YS, Wang M. Clinical observation of Tripterygium wilfordii combined with Irbesartan in the treatment of diabetic nephropathy with large amount of proteinuria. Natl Med Front China 2010;5:32–3. [Google Scholar]
- [18].Song HX, Gong J, Chen W, et al. Effects of Tripterygium glycosides on urinary monocyte chemotaxis protein-1 in diabetic nephropathy patients. Chin J Integr Traditional West Med 2005;25:416–8. [PubMed] [Google Scholar]
- [19].Li D. Tripterygium glycosides adjuvant treatment of diabetic nephropathy: reported of 53 cases. Heilongjiang Med J 2014;38:1029–30. [Google Scholar]
- [20].Yun WF. A clincial study of Tripterygium glycosides adjuvant treatment of diabetic nephropathy. Nei Mongol J Traditional Chin Med 2014;26:15. [Google Scholar]
- [21].Li MR. Clinical research of Tripterygium wilfordii glycosides tablets combined with Valsartan capsule in the treatment of diabetic nephropathy IV period China Health Care & nutrition. 2013; 9: 138. [Google Scholar]
- [22].Zhao RY, Tang BS, Shi XL, et al. Clinical observation on 46 cases of diabetic nephropathy treated with Tripterygium wilfordii and Valsartan. Chin J Integr Traditional West Nephrol 2011;12:811–3. [Google Scholar]
- [23].Chen XY. Clinical observation of Tripterygium wilfordii polyglycoside tablets combined with Benapril in the treatment of diabetic nephropathy with proteinuria. Shaanxi J Traditional Chin Med 2013;34:1016–7. [Google Scholar]
- [24].Pu Y, Zou QW, Pu. Min. Analysis of the effect of Valsartan combined with Tripterygium glycosides in the treatment of diabetic nephropathy: reported 78 cases. Jilin Med J 2013;34:5789–91. [Google Scholar]
- [25].Xu BX. Clinical analysis of tripterygium glycosides combined with benazepril in the treatment of diabetic nephropathy. J Bethune Military Med Coll 2014;12:90–1. [Google Scholar]
- [26].Wu YP, Shi NC. Clinical observation on the effect of Valsartan combined with Tripterygium glycosides in the treatment of diabetic nephropathy stage IV. Chin Rem Clin 2018;18:753–4. [Google Scholar]
- [27].Bao HJ. Clinical observation of Benapril comined with Tripterygium wilfordii in the treatment of diabetic nephropathy. J Pract Diabetol 2016;12:15–7. [Google Scholar]
- [28].Zhou CL. Clinical efficacy of Tripterygium wilfordii combined with Valsartan in the treatment of diabetic nephropathy with massive proteinuria. For all Health 2013;7:94–5. [Google Scholar]
- [29].Wu SB, Cao ZD, Zhang W, et al. Clinical observation of Tripterygium wilfordii combined with Valsartan in the treatment of diabetic nephropathy with massive proteinuria. Modern J Integr Traditional Chin West Med 2012;21:394–5. [Google Scholar]
- [30].Li J. Clinical study of Tripterygium glycosides combined with Valsartan in the treatment of diabetic nephropathy. Chin Commun Doctors 2011;13:43. [Google Scholar]
- [31].Cao QS. Clinical observation on the effect of Tripterygium glycosides combined with Valsartan tablets in the treatment of diabetic nephropathy. China Prac Med 2011;5:156–7. [Google Scholar]
- [32].Xu C, Qi AR, Fu B, et al. Clinical observation on treating diabetic nephropathy during IV period with the Tripterygium glycosides tablets. Clin J Chin Med 2016;8:95–6. [Google Scholar]
- [33].Zhang HC. A clincial study of tripterygium glycosides tablets adjuvant treatment of diabetic nephropathy during IV period. China Pharmaceut 2015;24:248–9. [Google Scholar]
- [34].Yang LH. Losartan combined with tripterygium glycosides in the treatment of diabetic nephropathy during IV period. Proc Clin Med 2016;25:553. [Google Scholar]
- [35].Wang JC. Clinical study of Tripterygium glycosides combined with Irbesartan in the treatment of diabetic nephropathy. China Prac Med 2013;8:182–3. [Google Scholar]
- [36].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Friedrich JO, Adhikari NKJ, Beyene J. Inclusion of zero total event trials in meta-analyses maintains analytic consistency and incorporates all available data. BMC Med Res Methodol 2007;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wu Y, Cui J, Bao X. Triptolide attenuate oxidative stress, NF-kB activation and multiple cytokine gene expression in murine peritoneal macrophage. Int J Mol Med 2006;17:141. [PubMed] [Google Scholar]
- [39].Wan YG, Sun W, Zhen YJ, et al. Multi-glycoside of Tripterygium wilfordii Hook. f. reduces proteinuria through improving podocyte slit diaphragm dysfunction in anti-Thy1.1 glomerulonephritis. J Ethnopharmacol 2011;136:322–33. [DOI] [PubMed] [Google Scholar]
- [40].Nakamura T, Fujiwara N, Sato E, et al. Renoprotective effects of various angiotensin II receptor blockers in patients with early-stage diabetic nephropathy. Kidney Blood Press Res 2010;33:213–20. [DOI] [PubMed] [Google Scholar]
- [41].Bakris G, Burgess E, Weir M, et al. Telmisartan is more effective than losartan in reducing proteinuria in patients with diabetic nephropathy. Kidney Int 2008;74:364–9. [DOI] [PubMed] [Google Scholar]
- [42].Ogawa S, Matsushima M, Mori T, et al. Identification of the stages of diabetic nephropathy at which angiotensin II receptor blockers most effectively suppress albuminuria. Am J Hypertens 2013;26:1064–9. [DOI] [PubMed] [Google Scholar]
- [43].Pathak JV, Dass EE. A retrospective study of the effects of angiotensin receptor blockers and angiotensin converting enzyme inhibitors in diabetic nephropathy. Indian J Pharmacol 2015;47:148–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


