Abstract
ALG11-Congenital Disorder of Glycosylation (ALG11-CDG, also known as congenital disorder of glycosylation type Ip) is an inherited inborn error of metabolism due to abnormal protein and lipid glycosylation. We describe two unrelated patients with ALG11-CDG due to novel mutations, review the literature of previously described affected individuals, and further expand the clinical phenotype. Both affected individuals reported here had severe psychomotor disabilities and epilepsy. Their fibroblasts synthesized truncated precursor glycan structures, consistent with ALG11-CDG, while also showing hypoglycosylation of a novel biomarker, GP130. Surprisingly, one patient presented with normal transferrin glycosylation profile, a feature which has not been reported previously in patients with ALG11-CDG. Together, our data expand the clinical and mutational spectrum of ALG11-CDG.
Keywords: ALG11, CDG, intellectual disability, GP130, LLO
Introduction
There are two main categories of glycosylation: N-glycosylation and O-glycosylation. Genetic defects in N-glycan assembly, i.e., the synthesis and attachment of glycans to glycoproteins and glycolipids, belong to the large family of Congenital Disorders of Glycosylation (CDG) [Peanne and others 2017]. Approximately 130 human disorders have been associated with defects in glycosylation causing protein or lipid dysfunction [Freeze and others 2015]. Abnormalities associated with CDG affect nearly every organ system. N-glycosylation defects are usually classified into two types. Type I CDG patients are deficient in either the synthesis or transfer of a lipid (dolichol) linked oligosaccharide (LLO) to proteins in the endoplasmic reticulum, while type II results from primarily Golgi-dependent processing of protein bound N-glycans [Dhamija and Chambers 2016].
Most CDG show autosomal recessive inheritance, and are characterized by broad multisystem effects ranging from developmental disability and hypotonia with multiple organ system involvement to hypoglycemia and protein-losing enteropathy [Freeze and others 2012; Freeze and others 2015; Ng and others 2016]. ALG11 (asparagine-linked glycosylation 11) (OMIM# 613666) is located on chromosome 13 and encodes the mannosyltransferase that uses GDP-mannose to sequentially add the fourth and the fifth of the nine mannoses to the LLO on the outer leaflet of the endoplasmic reticulum (ER). LLO biosynthesis is highly conserved among eukaryotes and is catalyzed by fourteen glycosyltransferases in an ordered stepwise manner [Rind and others 2010].
To date, only ten patients have been described with ALG11-CDG (OMIM# 613661) [Al Teneiji and others 2017; Pereira and others 2017; Regal and others 2015; Rind and others 2010; Thiel and others 2012]. We report two additional unrelated patients to expand the ALG11-CDG phenotype to include normal transferrin glycosylation as determined by mass spectrometry in one individual, together with functional studies supporting the pathogenicity of their novel ALG11 mutations. The characterization of these patients’ disease phenotype broadens the clinical and mutational spectrum of ALG11-CDG.
Informed consents were obtained from the patients’ parents.
Case reports:
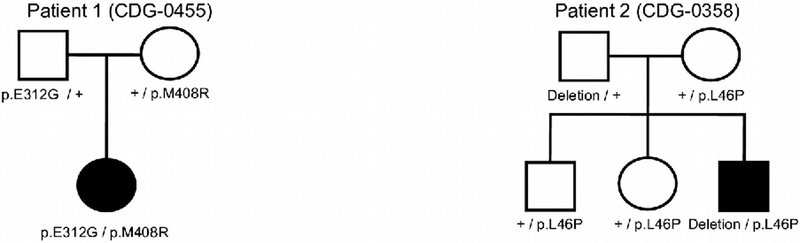
Patient 1 (CDG-0455)
This patient is a 29-month-old girl of mixed European ancestry with global developmental disability, central and peripheral hypotonia, slow/poor weight gain, and a history of infantile spasms (See Figure 1A). She was born at term, weight 3402 g. At examination at 26 months her head circumference was 45 cm, (−1.9SD); weight had fallen between 18–24 months to between (−3.05SD) and (−3.5SD), but had returned to 10.6 kg, (−1.57SD) by 26 months due to G-tube placement at 21 months; length was 87.5 cm, (−0.32SD). She was non-dysmorphic and had inverted nipples on physical examination. Her EEG was abnormal due to the presence of a modified hypsarrhythmia with repeated spikes bilaterally, and consistent with infantile spasms. Brain MRI showed subtly reduced diffusion along the periventricular parietal and temporal white matter tracts as well as the splenium of the corpus callosum (supplemental data). Oligo+SNP array CGH found her to be a heterozygous carrier of a 124bp interstitial deletion at 14q31.3 that involves the GALC gene (OMIM# 606890), which was inherited from her asymptomatic father. Galactocerebrosidase activity was lower than the normal range, but not in the affected range, consistent with her being a carrier for Krabbe disease (OMIM# 245200), and unrelated to her clinical phenotype. Mitochondrial DNA sequencing and deletion testing was normal. Whole exome sequencing (WES) results revealed that she was compound heterozygous for variants in ALG11 that were found to be in trans. Both variants were predicted to be pathogenic, resulting in a diagnosis of ALG11-CDG by the performing lab: c.935A>G, p.E312G was paternally inherited, and c.1223T>G, p.M408R, was inherited from the mother (See Figure 1C). In silico modeling of the variants was carried out using the Combined Annotation Dependent Depletion (CADD) (https://cadd.gs.washington.edu) scoring method [Kircher and others 2014] and predicted both variants to be in the top 0.5% of deleterious variants (p.E312G CADD score 28.9; p.M408R CADD score 29.9). The p.E312G and p.M408R variants were both absent from the Genome Aggregation Database (gnomAD) (http://gnomad.broadinstitute.org) of 125,748 exomes and 15,708 genomes (Ver2: accessed 12.13.2018), indicating the rarity of these three variants. Baseline ECG and echocardiogram at 26 months were both negative.
Figure 1A.
Patient 1 at age 2
Figure 1C.
Pedigrees for Patient 1 and Patient 2, showing the segregation of ALG11 variants in the families.
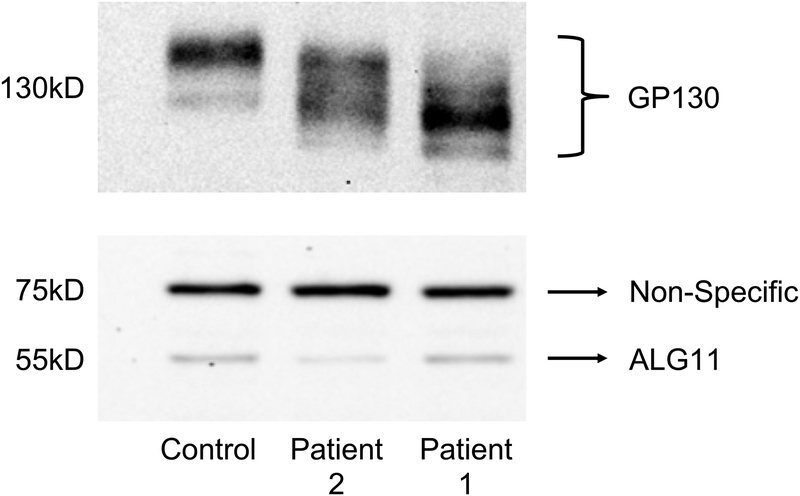
Due to the rarity of this disorder, carbohydrate-deficient transferrin (CDT) analysis was performed by electrospray ionization mass spectrometry (ESI-MS) to verify the pathogenicity of the mutations; however, the pattern was normal. Repeat analysis six weeks later was also normal. Skin fibroblasts were obtained and cultured to biochemically confirm the pathogenicity of the variants. Results showed that the size of LLO glycans from the patient’s fibroblasts were truncated and the size was consistent with a diagnosis of ALG11-CDG (supplemental data). Fibroblasts from the skin punch biopsy also yielded abnormally truncated GP130 isoforms on western blot (See Figure 2), in comparison to the isoform seen in an unaffected control fibroblast sample [Chan and others 2016]. This was consistent with the aberrant forms seen in at least one sample from a patient with previously confirmed ALG11-CDG, and similar to those seen in a sample from a patient with a different confirmed CDG. It appears that neither missense variant affected protein levels.
Figure 2.
Western blot analysis of GP130 and ALG11 protein
(Upper panel) Western blot analysis of the biomarker GP130 in a representative control and the two ALG11-CDG patients 1 and 2.
(Lower panel) western blot analysis showing the expression of ALG11 protein in patient 1 and in patient 2 compared to a control. The non-specific band shows equal protein loading.
Patient 2 (CDG-0358)
This is a 14-year-old Hispanic male with a past history of myoclonic epilepsy who presented with global developmental disability, hypertonia, visual inattention and microcephaly (See Figure 1B). He was born at term to healthy parents after an unremarkable pregnancy. The birth weight was 4,39kg (+2SD) and length was 54.61cm (+1.8SD). Both MRI and CT scan of the brain were within normal limits, but the EEG was abnormal. He was diagnosed with severe myoclonic epilepsy of infancy, epileptic encephalopathy, visual immaturity, myopia, and global developmental disability. Physical examination was otherwise unremarkable, except for long ears. Biochemical screening of plasma very long chain fatty acids, TPP1/PTT1 enzyme activity for ceroid lipofuscinosis, urine organic acids, plasma amino acids, and serum transaminases were normal. Microarray (44k oligo) was normal.
Figure 1B.
Patient 2 at age 14
CDG screening by ESI-MS revealed an abnormal profile suggestive of a diagnosis of a type I congenital disorder of glycosylation. PMM2 and PMI enzyme activities were normal. WES identified an apparently homozygous novel ALG11 variant, c.127T>C p.L46P (See Figure 1C). CADD scoring modeling of the variant’s pathogenicity predicted this variant to be in the top 0.5% of deleterious variants (CADD score of 26.8). The Genome Aggregation Database (gnomAD) of 125,748 exomes and 15,708 genomes (Ver2: accessed 12.13.2018) showed that the p.L46P had a single carrier in 251,448 alleles, indicating its rarity.
Though the mother was found to be heterozygous for the same mutation, the father did not have this mutation. Subsequent ALG11 gene deletion/duplication analysis found a paternally inherited whole gene deletion of ALG11 in the patient. LLO analysis also showed a truncated structure consistent with ALG11-CDG (data not shown). As seen in patient 1, fibroblasts from patient 2 were shown to have significant hypoglycosylation of GP130, supporting the presence of a glycosylation defect. As expected, patient 2 had slightly less ALG11 protein due to the paternally inherited whole gene deletion (Figure 2).
Discussion
Overall, finding a consensus phenotype for CDG can be difficult since there can be significant phenotypic overlap with other disorders, including mitochondrial disorders, lysosomal storage diseases, and other rare inborn errors of metabolism. Delineating common features associated with ALG11-CDG patients can be diagnostically challenging given the limited number of published cases. The most common features reported in patients with ALG11-CDG are summarized in Table 2. Intellectual disability and epilepsy are universally present, and all had visual problems, except our patient 1 (this information was not available for one patient). Strabismus and poor visual tracking were the most common ophthalmological findings. Dysmorphic features (such as long philtrum, retrognathia) and hypotonia were common, but not universal. Poor feeding, recurrent vomiting, and instability of body temperature were also present in several individuals.
Table 2.
Summary of clinical features of ALG11-CDG patients
| Developmental disability | Epilepsy | Dysmoprhic features | Microcephaly | Hypotonia | Hypertonia (peripheral) | Hyperreflexia | Deafness | Eye/visual problems | Feeding problems |
|---|---|---|---|---|---|---|---|---|---|
| 12/12 (100%) | 12/12 (100%) | 6/7 (86%), [5 pts NA] | 9/10 (90%) [2 pts NA] | 11/12 (92%) | 6/10 (60%) [2 pts NA] | 4/6 (66%) [6 pts NA] | 4/7 (57%) [5 pts NA] | 10/11 (91%) [1 pt NA] | 7/12 (58%) |
Previous publications [Regal and others 2015; Rind and others 2010] commented on verbal ability, but our patients are both nonverbal. Sensorineural deafness has been described as a frequent feature. Hearing loss data are missing or are not available for some patients. No audiologic test result is available for our patient 1, though she passed newborn hearing screen and routine pediatric office hearing screens. Sensorineural deafness is highly unusual in other types of CDG, except RFT1-CDG. This could be a clue feature to differentiate ALG11-CDG from the others. A plausible mechanism for sensorineural hearing loss may be that the cytoplasmic accumulation of one or more of the substrates of ALG11 and RFT1 has an otoneurotoxic effect.
All the previously reported ALG11-CDG patients have epilepsy. A recent paper by Pereira [Pereira and others 2017] describes that CDG can be associated with epileptic seizures showing particular features, such as absence of hypsarrhythmia, posterior EEG anomalies, and an unusual combination of epileptic seizures with myoclonus. Their patient with ALG11-CDG presented with typical epileptic seizures that were either in very long-lasting clusters or isolated, but also had focal clonic seizures and rare isolated massive jerks with a combination of myoclonus and spasm. Our two patients both have epileptic seizures. The parents of patient 1 noticed at age of 4 months that she has sudden onset of startling that was associated with eye rolling. EEG was markedly abnormal due to the presence of a modified hypsarrhythmia with repeated spikes in temporal and central regions bilaterally and infantile spasms with jerking arm extension. A couple of bursts of generalized epileptiform activity was noted. Patient 2 had his first seizures at age of 3–4 months. Seizures were initially startling or freezing episodes and then became grand mal seizures, involving all four limbs. The seizures occurred typically in the morning. At age of 4 years his seizures were the most severe and he was admitted twice with status epilepticus. With current medication he has been seizure free for 7 years.
While many confirmed CDG patients have abnormal CDT results, our patient 1 had two rounds of apparently normal CDT (Table 1). While this has been described on occasion for individuals with identified mutations and biochemically confirmed PMM2-CDG, it has not been previously reported in any ALG11-CDG patients. Also, it has not been observed in unpublished, but affected, individuals with ALG11-CDG screened by Mayo Medical Laboratories at the time patient 1 was evaluated [private communication]. We do not have a clear explanation for this, but note that because any potential mutation in ALG11 could show an inconsistent transferrin glycosylation pattern, analysis of fibroblast LLO and/or a cellular glycoprotein is important to confirm pathogenicity. We speculate that additional limitations of transferrin glycosylation analysis will become apparent as more individuals with rare CDG are identified.
Table 1.
Summary of demographics and mutations in ALG11-CDG patients
| Rind et al. 2010 (pt A) | Rind et al. 2010 (pt B) | Thiel et al. 2012 (pt A) | Thiel et al. 2012 (pt B) | Thiel et al. 2012 (pt C) | Regal et al. 2014 (pt A) | Regal et al. 2014 (pt B) | Teneiji et al. (2017) | Teneiji et al. (2017) | Pereira et al. (2017) | Present report (pt 1) | Present report (pt 2) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | F | M | F | F | M | M | M | F | F | F | F | M |
| Age at onset | 1 week | 6 weeks | 12 months | 2 months | 12 months | birth | 3 months | 4 months | 4 months | 5 months | 3–4 months | 3–4 months |
| Age | Died at 2 years | NA | 7 years at publication | 4.5years at publication | 8.5 years at publication | Died at 3 years | Died at 4 moths | 7 years at publication | 4 years at publication | 4 years at publication | 26 moths | 14 years |
| Ethnic origin | Turkish | Turkish | French-Canadian | Caucasian | Turkish | Belgian | Ducth | Canadian? | Canadian? | French? | Caucasian | Mexican |
| Other features | Oscillation of body temperature | NA | Cerebral atrophy and abnormal white matter | Oscillation of body temperature | Atactic movement disorder | Hypokinesia, burst suppression pattern on EEG, atrophy of white matter and cortex, subcortical heterotopia, retarded myelination | Burst suppression pattern on EEF, cerebral atrophy | Hypomyelination, brain atrophy | Pericerebral collection or enlarged subarachnoids spaces | |||
| Mutations | Homozygous c.257T>C, p.L86S | Homozygous c.257T>C, p.L86S | compound heterozygous c.623_624del/frame shift; c.836A>C, p.Y279S | Compound heterozygous c.1142T>C, P.L381S; c.1192G>A, p.E398K | Homozygous c.953A>C, p.Q318P | Compound heterozygous c.479G>T, p.G160V; c.45–2A>T | Compound heterozygous c.479G>T, p.G160V; c.36dupG | Homozygous c.1241T>A, p.I414N | Compound heterozygous c.1123_1126delAACA, p.N375FfsX6; c.986_988delAGA, p.K329del | NA | Compund heterozygous c.935A>G, p.E312G; c.1223T>G, p.M408R | Homozygous mutation c.127T>C p.L46P and whole gene deletion |
Biochemical analysis of primary fibroblasts from both our patients showed the hallmarks of ALG11-CDG, an accumulation of a N2M3 and N2M4 (N=N-acetylglucosamine, M=Mannose) LLO glycans. Importantly, we utilized a novel biomarker for CDG fibroblasts, GP130, and show that both affected individuals had hypoglycosylation.
Our 14-year-old patient (patient 2) is the oldest reported patient to date, with all others ranging between 2 months to 8 years. This patient is also the only one without hypotonia. While other reported cases are either homozygous or compound heterozygous for point mutations or indels, our patient is the only individual with a missense mutation in trans to a whole gene deletion. It is possible that partial residual activity of his protein, with haploinsufficiency of the other allele, results in a milder phenotype for our patient than would result from being compound heterozygous or homozygous for null or less functional/more severe mutations, or that the neurological phenotype may change over time to include more spasticity as patients age.
We advocate screening for CDG in any child with severe psychomotor impairment, microcephaly, and epileptic seizures, in the absence of characteristic features of an otherwise phenotypically recognizable disorder. On the other hand, whole exome sequencing will play an increasingly important role in the molecular diagnosis of CDG.
Supplementary Material
Acknowledgements
The authors would like to thank the families for their participation. This work was supported by The Rocket Fund and by NIH grant R01DK99551. MH is supported by grants from Sigrid Juselius Foundation and Instrumentarium Science Foundation.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Al Teneiji A, Bruun TU, Sidky S, Cordeiro D, Cohn RD, Mendoza-Londono R, Moharir M, Raiman J, Siriwardena K, Kyriakopoulou L, Mercimek-Mahmutoglu S. 2017. Phenotypic and genotypic spectrum of congenital disorders of glycosylation type I and type II. Mol Genet Metab 120(3):235–242. [DOI] [PubMed] [Google Scholar]
- Chan B, Clasquin M, Smolen GA, Histen G, Powe J, Chen Y, Lin Z, Lu C, Liu Y, Cang Y, Yan Z, Xia Y, Thompson R, Singleton C, Dorsch M, Silverman L, Su SM, Freeze HH, Jin S. 2016. A mouse model of a human congenital disorder of glycosylation caused by loss of PMM2. Hum Mol Genet 25(11):2182–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhamija R, Chambers C. 2016. Clinical and Molecular Characterization of ALG1-CDG. Pediatr Neurol Briefs 30(2):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeze HH, Eklund EA, Ng BG, Patterson MC. 2012. Neurology of inherited glycosylation disorders. Lancet Neurol 11(5):453–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeze HH, Eklund EA, Ng BG, Patterson MC. 2015. Neurological aspects of human glycosylation disorders. Annu Rev Neurosci 38:105–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kircher M, Witten DM, Jain P, O’Roak BJ, Cooper GM, Shendure J. 2014. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet 46(3):310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng BG, Shiryaev SA, Rymen D, Eklund EA, Raymond K, Kircher M, Abdenur JE, Alehan F, Midro AT, Bamshad MJ, Barone R, Berry GT, Brumbaugh JE, Buckingham KJ, Clarkson K, Cole FS, O’Connor S, Cooper GM, Van Coster R, Demmer LA, Diogo L, Fay AJ, Ficicioglu C, Fiumara A, Gahl WA, Ganetzky R, Goel H, Harshman LA, He M, Jaeken J, James PM, Katz D, Keldermans L, Kibaek M, Kornberg AJ, Lachlan K, Lam C, Yaplito-Lee J, Nickerson DA, Peters HL, Race V, Regal L, Rush JS, Rutledge SL, Shendure J, Souche E, Sparks SE, Trapane P, Sanchez-Valle A, Vilain E, Vollo A, Waechter CJ, Wang RY, Wolfe LA, Wong DA, Wood T, Yang AC, University of Washington Center for Mendelian G, Matthijs G, Freeze HH. 2016. ALG1-CDG: Clinical and Molecular Characterization of 39 Unreported Patients. Hum Mutat 37(7):653–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peanne R, de Lonlay P, Foulquier F, Kornak U, Lefeber DJ, Morava E, Perez B, Seta N, Thiel C, Van Schaftingen E, Matthijs G, Jaeken J. 2017. Congenital disorders of glycosylation (CDG): Quo vadis? Eur J Med Genet. [DOI] [PubMed] [Google Scholar]
- Pereira AG, Bahi-Buisson N, Barnerias C, Boddaert N, Nabbout R, de Lonlay P, Kaminska A, Eisermann M. 2017. Epileptic spasms in congenital disorders of glycosylation. Epileptic Disord 19(1):15–23. [DOI] [PubMed] [Google Scholar]
- Regal L, van Hasselt PM, Foulquier F, Cuppen I, Prinsen H, Jansen K, Keldermans L, De Meirleir L, Matthijs G, Jaeken J. 2015. ALG11-CDG: Three novel mutations and further characterization of the phenotype. Mol Genet Metab Rep 2:16–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rind N, Schmeiser V, Thiel C, Absmanner B, Lubbehusen J, Hocks J, Apeshiotis N, Wilichowski E, Lehle L, Korner C. 2010. A severe human metabolic disease caused by deficiency of the endoplasmatic mannosyltransferase hALG11 leads to congenital disorder of glycosylation-Ip. Hum Mol Genet 19(8):1413–1424. [DOI] [PubMed] [Google Scholar]
- Thiel C, Rind N, Popovici D, Hoffmann GF, Hanson K, Conway RL, Adamski CR, Butler E, Scanlon R, Lambert M, Apeshiotis N, Thiels C, Matthijs G, Korner C. 2012. Improved diagnostics lead to identification of three new patients with congenital disorder of glycosylation-Ip. Hum Mutat 33(3):485–487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.