Abstract
Immune-mediated glomerulonephritis is a serious end organ pathology that commonly affects patients with systemic lupus erythematosus (SLE). A classic murine model used to study lupus nephritis (LN) is nephrotoxic serum nephritis (NTN) in which mice are passively transferred nephrotoxic antibodies. We have previously shown that macrophages are important in the pathogenesis of LN. To further investigate the mechanism by which macrophages contribute to the pathogenic process, and to determine if this contribution is mediated by NF-κB signaling, we created B6 mice which had RelA knocked out in myeloid cells, thus inhibiting classical NF-κB signaling in this cell lineage. We induced NTN in this strain to assess the importance of macrophage derived NF-κB signaling in contributing to disease progression.
Myeloid cell RelA knock out (KO) mice injected with nephrotoxic serum had significantly attenuated proteinuria, lower BUN levels, and improved renal histopathology compared to control injected wildtype B6 mice (WT). Inhibiting myeloid NF-κB signaling also decreased inflammatory modulators within the kidneys. We found significant decreases of IL-1a, IFNg, and IL-6 in kidneys from KO mice, but higher IL-10 expression. Flow cytometry revealed decreased numbers of kidney infiltrating classically activated macrophages in KO mice as well.
Our results indicate that macrophage NF-κB signaling is instrumental in the contribution of this cell type to the pathogenesis of NTN. While approaches which decrease macrophage numbers can be effective in immune mediated nephritis, more targeted treatments directed at modulating macrophage signaling and/or function could be beneficial, at least in the early stages of disease.
Keywords: Lupus nephritis, nephrotoxic serum nephritis, macrophages, RelA, NF-kappa B
1. INTRODUCTION
Lupus nephritis (LN), or the kidney involvement in systemic lupus erythematosus (SLE), is a serious end organ complication. Treatment options remain far from ideal; many patients suffer unfavorable side effects, and 15–25% of patients still progress to end stage renal disease. The pathogenesis of disease needs to be further explained, in the hope that greater understanding of the key mediators will lead to discovery of novel therapeutic targets [1].
Macrophages are cells of the innate immune system which are present in nearly every tissue. Macrophages are highly plastic cells, which can adapt various functional phenotypes to respond to the stimuli surrounding them in the microenvironment. Broadly speaking, macrophage phenotypes can be divided into two subclasses, M1 and M2, with M1 being the classically activated, infiltrating, and inflammatory macrophages, and M2 being the alternatively active, tissue resident, and trophic macrophages [2,3].
LN pathogenesis is associated with immune complex deposition within the kidney, as well as activation of resident cell types, infiltration of immune cells, and expression of inflammatory cytokines [1]. There are several lines of evidence conclusively linking macrophages to the development of LN. The degree of macrophage infiltration correlates with disease severity in both mice and humans [4,5]. Furthermore, macrophage depletion by a variety of approaches ameliorates LN both in inducible models and spontaneous models, correlating with decreased expression of inflammatory mediators [2,6,7].
Macrophages can contribute to disease pathogenesis through the large number of inflammatory mediators they express which can damage local tissue, activate resident cells, and further recruit immune cells to the tissue enhancing the inflammatory process. Many of these inflammatory mediators are the result of activation of the NF-kB pathway, which is a key transcription factor for M1 polarization [8]. Classical NF-kB pathway activation is dependent on the protein p65, also known as RelA. RelA is part of the heterodimer of NF-kB which translocates into the nucleus, and transcribes multiple strongly pro-inflammatory genes including IL-1, IL-6, IL-12 and TNFα [9].
In this study we used an inducible model of LN known as nephrotoxic serum nephritis (NTN), in which mice are passively transferred with nephrotoxic antibodies that induce an immune complex mediated disease that mimics LN. We generated mice which have RelA knocked out specifically from their myeloid cells to assess the role of classical NF-kB signaling in macrophages in NTN. These studies were intended to help further elucidate why macrophages are so vital to the pathogenesis of immune complex mediated nephritis, including LN.
2. MATERIALS AND METHODS
2.1. Mice and disease induction
Myeloid specific RelA knock out mice were generated by crossing B6 RelA flox/flox mice (a kind gift from Dr. Baldwin of the University of North Carolina Lineberger Comprehensive Cancer Center) with B6 LysM cre/cre mice (Jackson Laboratories, Bar Harbor, ME) to generate mice homozygous for both the RelA flox and cre under the myeloid specific LysM promoter. The mice were bred at the Albert Einstein College of Medicine. The genotype of the founder mice were validated by PCR, and the purity of the colony was assured by continued genotyping via Transnetyx (Cordova, TN), with validated probes. Wild type B6 mice were obtained from Jackson at 3–4 weeks of age, and housed at Einstein while they were aged until the time of experiment. Female mice were immunized intraperitoneally with CFA and rabbit IgG starting at 10–12 weeks of age, and five days later given serum containing nephrotoxic antibodies to induce disease via an intravenous injection as previously described [6,10]. For comparison, in a separate experiment age matched female B6 mice were immunized with rabbit IgG on day 0 but were not given nephrotoxic serum (healthy control (HC)). Disease was followed over the course of the experiment and mice were sacrificed on day 14, which was 9 days post nephrotoxic serum transfer. At sacrifice, urine, serum, and tissue was collected for analysis. All studies were approved by the Institutional Animal Care Committee.
2.2. Assessment of kidney function
Proteinuria was assessed semi-quantitatively throughout the experiment by Uristix where 1+ = 30 mg/dl, 2+ = 100 mg/dl, 3+ = 300 mg/dl, and 4+ = 2000 mg/dl. Midrange colors were assigned an intermediate value. These results were further quantified by measuring albumin:creatinine ratios in the terminal urine. Albumin levels were measured using the Mouse Albumin ELISA Kit from Bethyl Laboratories (Montgomery, TX), according to the manufacturer’s instructions. Creatinine levels were measured using QuantiChrom Creatinine Assay Kit (BioAssay Systems, Hayward, CA). Blood urea nitrogen (BUN) levels were measured in terminal serum to assess kidney function via the QuantiChrom Urea Assay Kit (BioAssay Systems).
2.3. Renal histopathology
Kidney sections were deparaffinized and stained with hematoxylin and eosin (H&E) and periodic acid Schiff (PAS) by the Histology and Comparative Pathology Core at Albert Einstein College of Medicine. Kidney sections were then analyzed and scored by an experienced nephropathologist (L.H.) who was blinded to the experimental groups. Scoring was assigned as described [10].
2.4. Measurement of mouse anti-rabbit antibodies
Total levels of mouse anti-rabbit IgG antibodies were measured in terminal serum as previously described [6]. Additionally, we assessed the relative abundance of various subtypes of antibodies, including IgG1, IgG2a, IgG2c, and IgG3. We followed a similar protocol to that as the total IgG ELISA, however the secondary antibodies used were goat anti-mouse IgG1, IgG2a, IgG2c and IgG3, respectively. Values were normalized to serum from an unimmunized mouse.
2.5. Rabbit anti-mouse glomerular basement (GBM) antibodies
Titers of anti-GBM antibodies in terminal serum were measured as previously described [6].
2.6. Immunofluorescent staining
Kidney sections were deparaffinized and rehydrated, followed by antigen retrieval in antigen retrieval buffer (citrate buffer pH 6 or Tris-EDTA buffer pH 9) for 10 minutes. Slides were then washed with PBS, and blocked with 20% NHS in 0.1% triton in PBS for 1 hour at room temperature. Following the block, sections were incubated overnight at room temperature with the primary antibody cocktails which were diluted in 2% NHS/0.1% triton in PBS. The following day, the sections were washed, and incubated with the secondary antibodies at room temperature for 1 hour, washed, stained with DAPI and mounted. Table 1 provides additional details on the staining schemes.
Table 1.
Antibodies used for staining.
| IF STAINING | ||||
|---|---|---|---|---|
| primary | dilution | secondary | dilution | |
| Stain 1 | goat anti- mouse C3 | 1:100 | donkey anti-goat AF594 | 1:100 |
| rabbit anti-mouse IBA-1 | 1:250 | donkey anti-rabbit AF488 | 1:250 | |
| donkey anti-mouse IgG | 1:500 | donkey anti-mouse IgG | 1:500 | |
| Stain 2 | rat anti- mouse B220 | 1:100 | donkey anti-rat AF594 | 1:100 |
| rabbit anti-mouse CD4 | 1:100 | donkey anti-rabbit AF488 | 1:100 | |
| Stain 3 | rat anti-mouse Ly6G | 1:100 | donkey anti-rat AF594 | 1:100 |
| FLOW CYTOMETRY | ||||
| Stain 1 | Ly6C (PE) | 1:1000 | ||
| F480 (PerCP) | 1:100 | |||
| CD45 (FITC) | 1:100 | |||
| CD11b (AF700) | 1:100 | |||
| CD11c (Pacific Blue) | 1:100 | |||
| Stain 2 | CD8 (FITC) | 1:101 | ||
| B220 (PE) | 1:102 | |||
| CD45 (PerCP) | 1:103 | |||
| CD4(APC) | 1:104 | |||
2.7. Flow cytometry
At sacrifice, kidneys were harvested and made into a single cell suspension. Cells were then incubated with Fc block for 30 minutes on ice, followed by a 30-minute incubation with the antibody cocktails. Samples were run on an LSR II. For each sample, 50,000 events were collected, and each cell type was assessed as a percent of single cells.
2.8. Measurement of cytokine protein expression
Protein was isolated from snap frozen kidneys using Tper Buffer, according to the manufacturer’s instructions (Thermo). Protein concentration was measured using Coomassie (Thermo). The isolated protein was analyzed using Biolegend’s Legendplex mouse inflammation panel kit, according to the manufacturer’s instructions. Protein concentrations for the cytokines were then adjusted for total protein in each sample, as described [7].
2.9. Macrophage isolation, culture, and stimulation
Peritoneal macrophages were collected from unmanipulated, female WT and KO mice at an age of 10–12 weeks via a peritoneal lavage using cold PBS with 5mM of EDTA. Collected cells were spun down, washed, and then re-suspended in complete RPMI with 10% FBS and plated in 12 well plates overnight to allow the macrophages to settle. The following day, non-adherent cells were washed away, and macrophages were stimulated with 100 ng/ml of LPS for 18 hours. Supernatants were collected, spun down to remove debris, and then used with Biolegend’s Legendplex mouse inflammation panel kit, according to the manufacturer’s instructions.
2.10. RNA isolation, cDNA synthesis, and RT-PCR
RNA was isolated from snap frozen kidneys by homogenizing the tissue in TRIzol (ThermoFisher, Waltham, MA) and applying Qiagen’s RNeasy Mini Kit (Hilden, Germany), according to the manufacturers’ instructions. RNA was reversed transcribed into cDNA using the ThermoFisher Scientific’s Superscript III First-Strand Synthesis System. Real time PCR was performed in triplicate, and values normalized to GAPDH as described [6].
112.11. Statistics
Data was analyzed using Graphpad Prism 7. All groups were first tested for normality; when comparing data with only two groups, a student’s T-Test was used if the data was normally distributed. With two groups and nonparametric data, a Mann-Whitney test was used to determine significance. For comparing three groups, an ANOVA was performed. Tukey or Dunn’s test was used multiple comparisons of normal and non-parametric data, respectively. Grubb’s test was used to assess for outliers. Throughout the results, *p <0.05, **p<0.01, ***p<0.001, and ****p<0.0001. All error bars are reported as SEM.
3. RESULTS
3.1. Myeloid-specific RelA knock out mice have attenuated kidney disease following induction of NTN
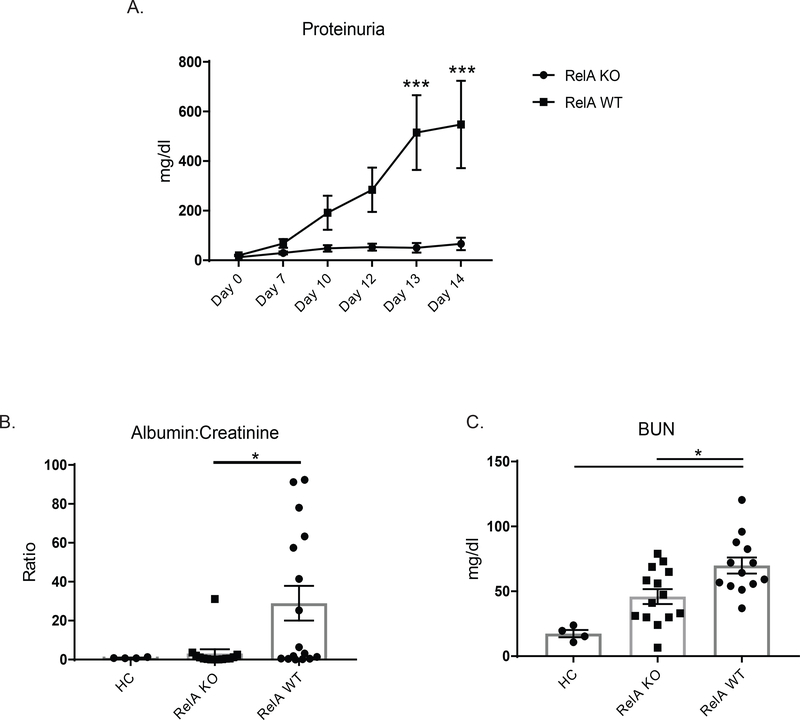
We induced nephrotoxic serum nephritis in 10–12-week-old female B6 wildtype mice (WT mice) and age and sex matched myeloid-specific RelA knock out mice (KO mice), to assess the effect of classical NF-kB signaling in the pathogenesis of antibody-mediated nephritis. As expected, WT mice progressively developed high levels of proteinuria, an indicator of kidney damage. By day 13 of the experiment, there was a significant difference between KO and WT mice (Figure 1A), with KO mice protected from developing significant proteinuria. To confirm the protection of myeloid RelA deficiency seen with the semiquantitative method, we measured albumin:creatinine ratios in terminal urine, and found significantly lower ratios in myeloid RelA KO versus WT mice (KO mice = 3.12 ± 2.17, WT mice = 28.96 ± 8.96; p<0.05, Figure 1B). Next, blood urea nitrogen (BUN) levels were measured in terminal serum to assess kidney function. Although BUN levels did not fully normalize in KO mice relative to healthy control mice (HC), they were significantly lower compared to their WT controls (KO mice = 33.62 ± 5.71 mg/dL, WT mice = 69.91 ± 6.17 mg/dL, p<0.01; Figure 1C).
Figure 1. Assessment of kidney function.
(A) Proteinuria was measured over the course of the experiment using Uristix. Myeloid RelA KO mice were significantly protected from increased levels of proteinuria compared to RelA WT mice. (B) Results in (A) were confirmed by measuring albumin:creatinine ratios in urine obtained at the time of sacrifice. (C) Myeloid RelA KO mice had lower blood urea nitrogen (BUN) levels compared to WT mice. Shown are the combined results from 2 cohorts (HC, n = 4, RelA KO, n = 14; RelA WT, n = 13). Outliers were assessed using Grubb’s test. Data was analyzed using an ordinary one-way ANOVA, and multiple comparisons were determined using Tukey’s test.
3.2. Histology
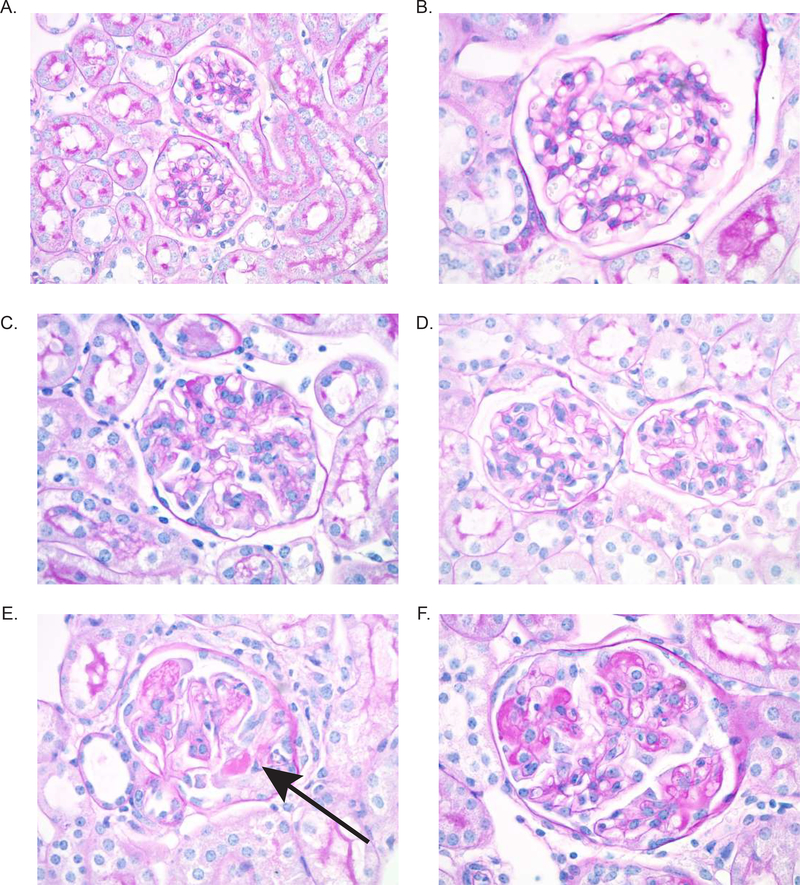
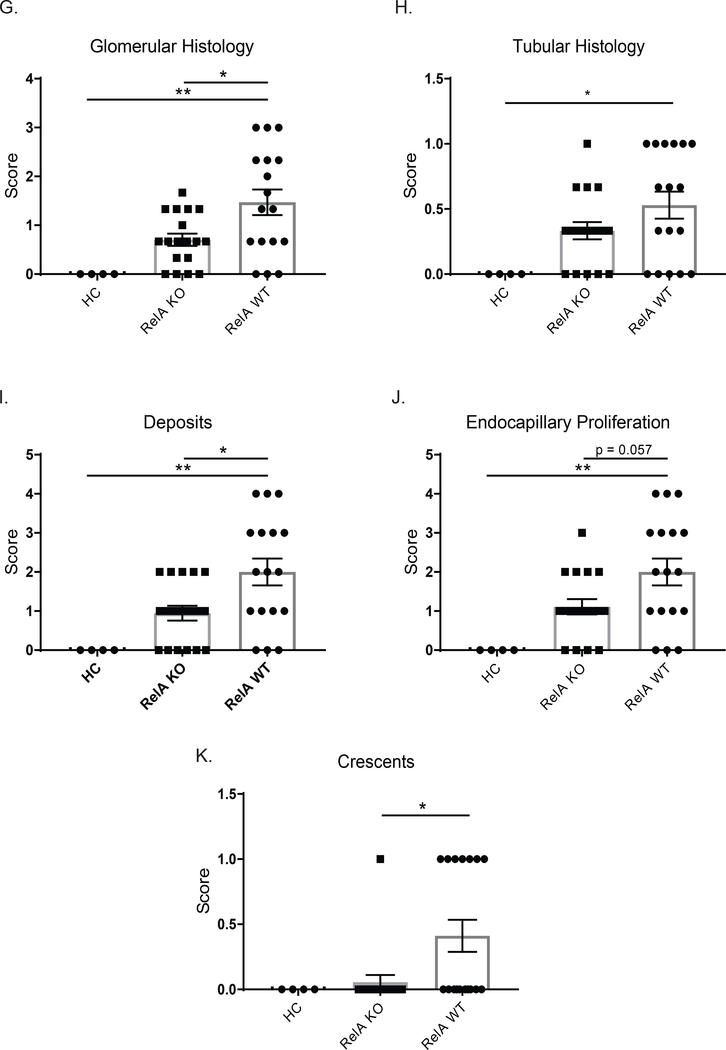
Following NTN induction, susceptible mouse strains including B6 mice develop a crescentic proliferative glomerulonephritis. We found that myeloid RelA KO mice had significantly improved glomerular histology compared to matched WT control mice following induction of NTN. As shown in Figure 2E and F, WT mice had significant crescent formation, as well as endocapillary proliferation and glomerular immune deposits. These pathologic features were significantly attenuated in myeloid RelA KO mice (Figure 2C, D), which looked more similar to HC mice (Figure 2A and B), than WT mice. Scoring for glomerular parameters confirmed significant improvement in KO mice (Figure 2G); however, there was not a significant difference in tubular histology (Figure 2H), most likely because significant tubular disease was not present. Looking more closely at glomerular histology, we specifically found a significant improvement in glomerular deposits, endocapillary proliferation, and crescent formation in KO compared to WT mice (Figure 2I-J).
Figure 2. Renal histology.
Kidney sections were stained with PAS, and images were taken at 600x. Representative images were selected from HC (A and B), KO (C and D), and WT (E and F) mice. WT mice showed significant crescent formation (E, arrow), as well as endocapillary proliferation and immune deposits (F). Myeloid RelA KO mice, however, displayed much more normal histology, despite minimal endocapillary proliferation (C and D). There was significant improvement in the glomerular histology in KO mice (G), but neither group had a significant degree of tubular disease (H). Specifically, KO mice showed improvements in deposits, endocapillary proliferation, and crescents (I-K). Sections from 3 separate cohorts were scored by a renal pathologist blinded to the genotype (HC, n=4; RelA KO, n = 18; RelA WT, n = 17). Normally distributed data (glomerular histology, deposits, and endocapillary proliferation) were analyzed using an ordinary one-way ANOVA, and multiple comparisons were determined using Tukey’s test. Nonparametric data (Tubular histology and crescents) were analyzed using a Kruskal-Wallis test, and multiple comparisons were assessed with Dunn’s test.
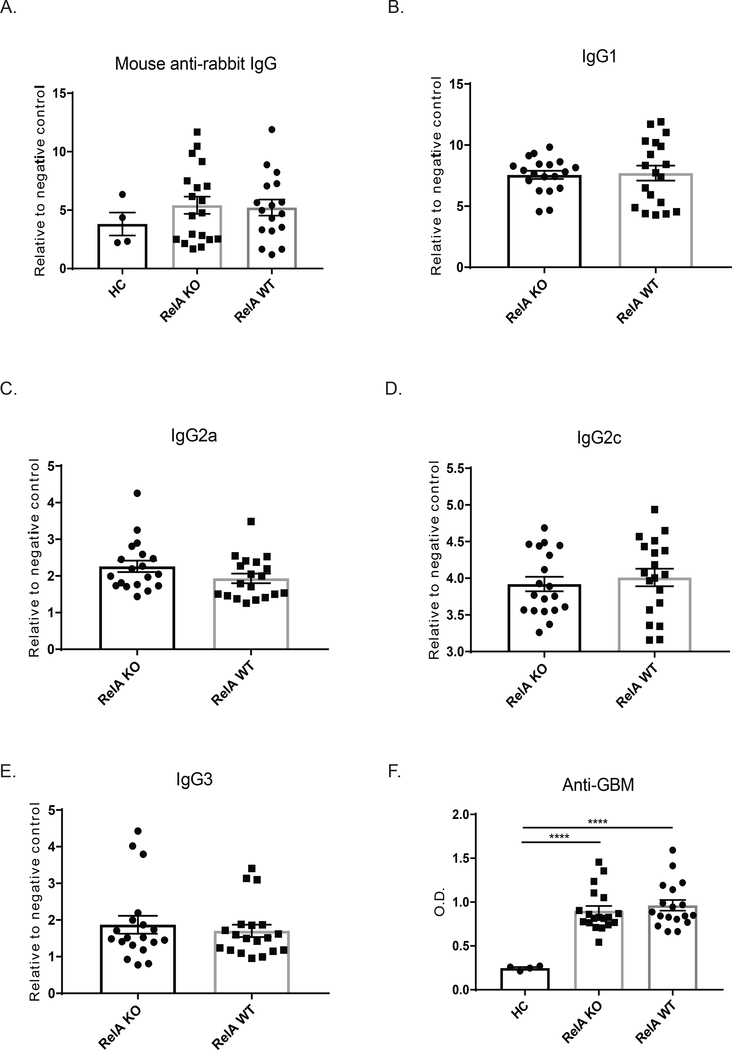
3.3. RelA knock out mice did not exhibit altered antibody responses
To ensure that knocking out RelA in myeloid cells did not alter the ability of KO mice to respond to the initial immunization with rabbit IgG (as the induced anti-rabbit IgG antibodies bind the subsequently administered nephrotoxic antibodies, enhancing the subsequent disease), we measured total levels of mouse anti-rabbit IgG antibodies (Figure 3A) and found no difference between the strains. Additionally, there were no differences in the levels of IgG1, IgG2a, IgG2c, or IgG3 specific mouse anti-rabbit antibodies, indicating that myeloid RelA knockdown did not affect the subclass of antibodies generated during the initial immunization (Figure 3B-E). Serum levels of the passively transferred nephrotoxic antibodies were also not different between KO and WT mice, further indicating that the NTN model was successfully induced in the KO strain (Figure 3F).
Figure 3. NTN is successfully induced in myeloid RelA KO mice.
To ensure that knocking out RelA did not interfere with disease induction, we measured antibodies generated in response to the initial rabbit IgG immunization in WT and KO mice in terminal serum(A). Isotype specific mouse anti-rabbit IgG antibody titers (IgG1, IgG2a, IgG2c, and IgG3 levels) are shown in (B-E), with no difference seen in any of the isotypes. Anti-GBM antibody titers were measured following passive transfer of the nephrotoxic serum to ensure that both mouse genotypes received similar doses and had similar clearance (F). Shown are the results from 3 separate cohorts (HC, n=4; RelA KO, n = 19, RelA WT, n = 17). Data for mouse anti-rabbit IgG and anti-GBM titers were analyzed using an ordinary one-way ANOVA, and multiple comparisons were determined using Tukey’s test. To assess the various IgG isotypes, which were normally distributed, a student’s T-test was used.
3.4. Renal macrophage infiltration is reduced in myeloid RelA KO mice
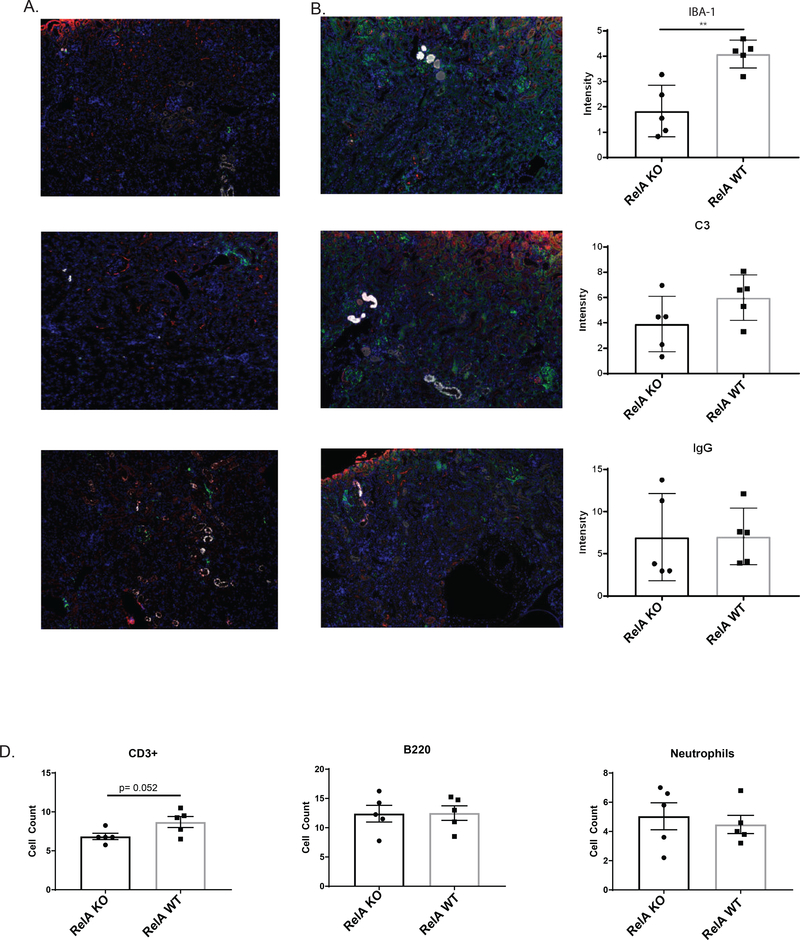
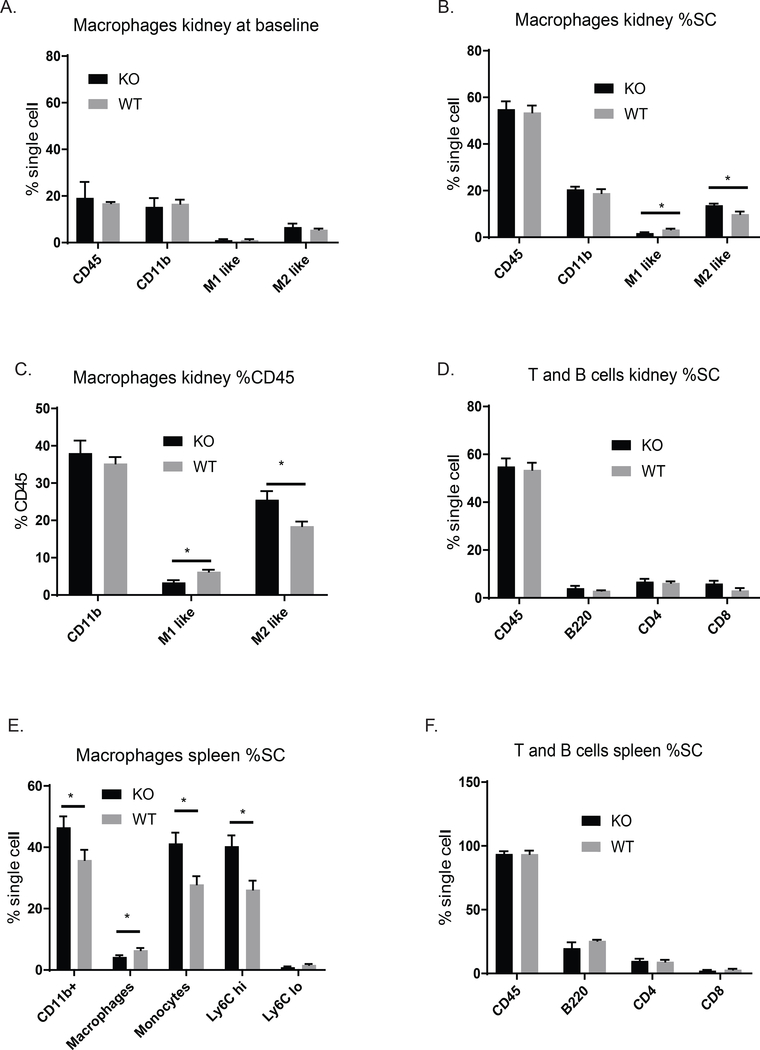
Proliferative glomerulonephritis is accompanied by glomerular IgG and complement deposition, as well as by infiltration of inflammatory cells [1,11]. To determine the effect of the myeloid RelA KO on local inflammation, we stained kidney sections for IBA-1 (macrophages), C3, and IgG. While there was no difference in C3 and IgG deposition, there was a significant reduction in kidney IBA-1+ macrophages (Figure 4A-C). To further characterize the macrophage populations during immune-mediated nephritis, flow cytometry was performed to assess the effect of RelA KO in myeloid cells. We found a significant reduction in M1 (CD11b+, F4/80 lo, Ly6C hi), classically activated cells, whereas M2 macrophages (CD11b+, F4/80 hi, Ly6C lo) were significantly increased in KO compared to WT (Figure 5B) when looked at as a percent of single cells. For absolute numbers, we found 743 ± 297 M1-like cells in KO mice compared to 1343 ± 244 M1-like cells in the WT mice. We also looked at the percent of CD45 cells to assess the composition of the immune cell accumulations, and found similar and significant trends in the relative kidney infiltration by M1 and M2 macrophages (Figure 5C). It should be noted that cell populations, including M1 and M2 macrophages, neutrophils, myeloid DCs, T cells, and B cells, were not different between WT and KO mice at baseline in either the spleen or kidney (Figure 5A).
Figure 4. Renal cell infiltration.
Kidney sections were stained for macrophages (IBA-1, green), C3 (red), and IgG (white) in myeloid RelA KO mice (A) and WT mice (B). There was a significant reduction in macrophage infiltration in KO mice, but no difference in IgG and C3 deposition (C). CD3+ T cells trended towards being lower in KO mice (D); however; there was no difference in B cells (B220+) and neutrophils (Ly6G+). Shown are the results from one cohort (RelA KO, n = 5, RelA WT, n = 5). Significance was assessed using a student’s T-Test.
Figure 5. Cytometric analysis of renal immune cells and splenic cell populations.
(A) Myeloid-specific RelA KO mice had similar populations of kidney myeloid cells as WT mice, when assessed by flow cytometry at baseline (pre-disease induction). (B) A significant reduction of M1 inflammatory macrophages was found in the kidneys of myeloid RelA KO mice compared to WT mice. In contrast, increased M2 tissue resident macrophages were found in the former strain. This trend was not only true for total cell number, but also when looking at proportions of immune cells in the kidneys (% CD45+), as shown in C. There was no difference in T and B cell renal populations (D). In the spleen, there were increased numbers of M1 monocytes in myeloid RelA KO mice, indicating that the splenic reservoir was not being depleted in this strain (E). There was no difference in T and B cells in the spleen (F). Shown are results from one cohort (RelA KO, n = 5; RelA WT, n = 5). %SC, % single cells. Significance was assessed using a student’s T-Test.
Immunofluorescent staining for B and T cells (Figure 4D), as well as flow cytometric analysis (Figure 5D), found no difference in kidney infiltration of B and T cells (CD4+ and CD8+) between WT and KO mice. Staining, however, revealed a nearly significant decrease in CD3+ T cells in KO kidney (Figure 4D, p = 0.052). There was also no difference in neutrophil numbers as determined by staining for Ly6G (Figure 4D).
3.5. KO mice have reduced trafficking of monocytes out of the spleen
The spleen is a known reservoir for monocytes which can quickly be recruited to sites of inflammation. When assessing myeloid populations in the spleen at the time of sacrifice when the nephritic process was still active, we found that WT mice had significantly less Ly6C hi, or inflammatory monocytes, which would be consistent with the increased recruitment of these cells to the kidney compared to the KO mice, which displayed attenuated kidney inflammation (Figure 5E). T and B cell numbers in the spleen were not different between the two groups (Figure 5F).
3.6. KO mice have decreased expression of inflammatory cytokines
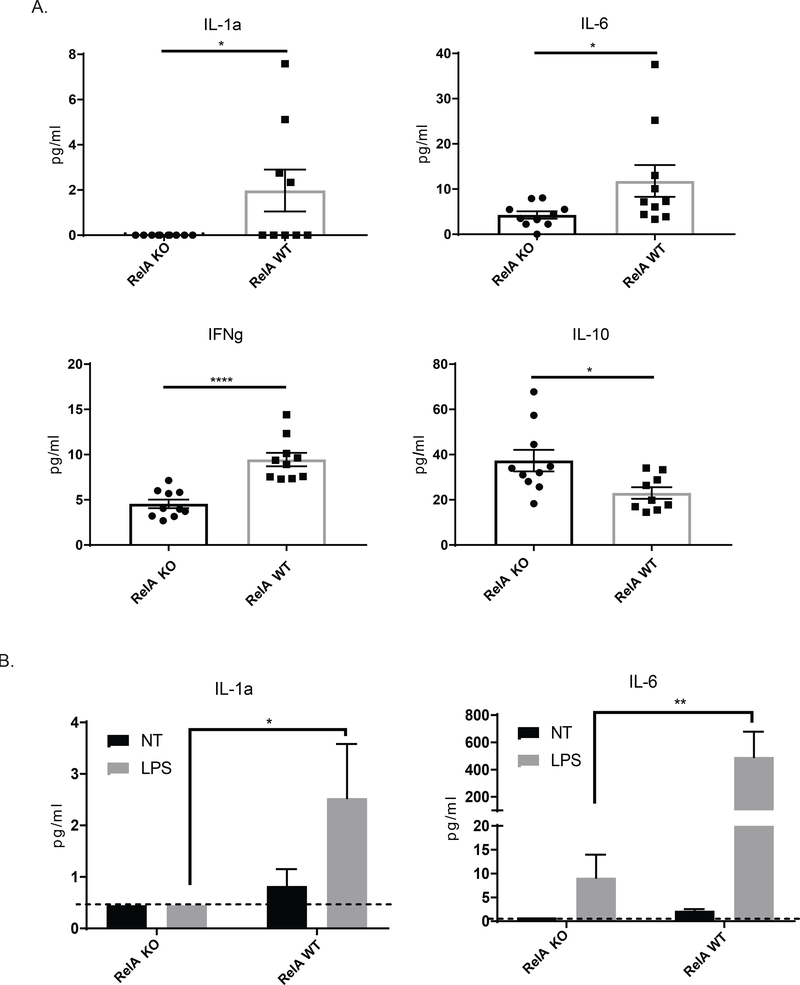
NF-kB signaling is important for macrophage mediated inflammation [12]. To assess the expression of inflammatory cytokines in the kidneys after disease induction, we assessed the differences between the KO and WT mice. We found that myeloid RelA KO mice had significantly reduced IL-1a and IL-6, two cytokines typically associated with M1 macrophages. IFNg was also significantly reduced in KO mice. Interestingly, concentrations of IL-10 were significantly higher in KO compared to WT mice (Figure 6A), which is consistent with the significantly increased numbers of kidney M2 macrophages observed in the former strain by flow cytometry.
Figure 6. Expression of inflammatory cytokine proteins in the kidney.
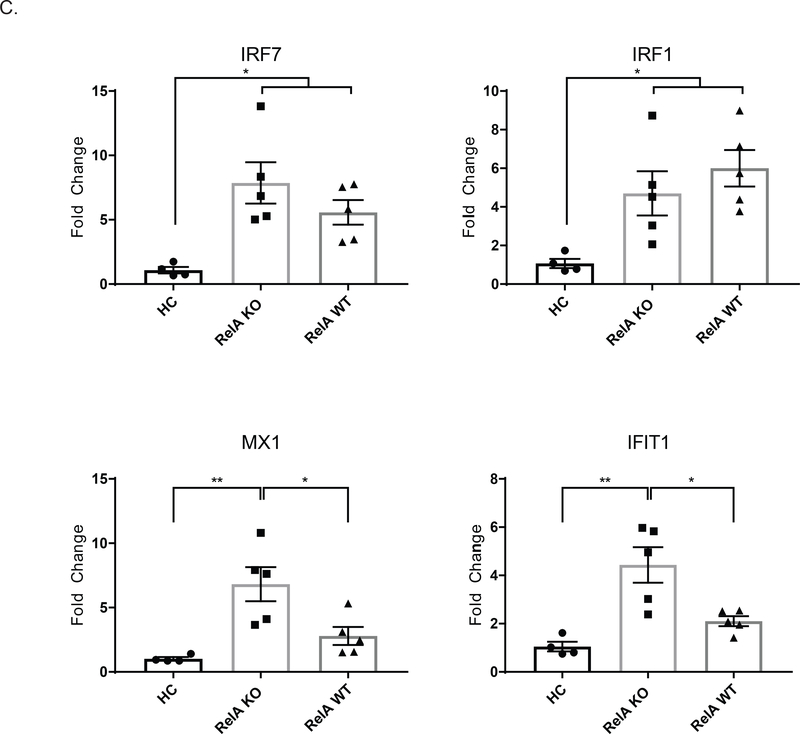
(A) We measured protein cytokine levels in whole kidney protein extracts using Legendplex. There was decreased expression of IL-1a, IFNg, and IL-6 in KO mice, whereas IL-10 was increased. This pattern of cytokine expression is consistent with the larger population of resident macrophages as opposed to inflammatory macrophages. Outliers were assessed using Grubb’s test. Data was analyzed using a student’s T-test (B) Macrophages were isolated from KO and WT mice, cultured, and stimulated with LPS. Legendplex was performed on the supernatants. Cells were isolated from 3 mice per group, and plated in duplicate for stimulation. Data was analyzed using a two-way ANOVA and Tukey’s test for multiple comparisons. (C) RT-PCR was performed on RNA isolated from whole kidneys to assess the expression of IFN response genes. Data was analyzed using an ordinary one-way ANOVA and Tukey’s test for multiple comparisons. (For the kidney protein levels and RT-PCR, shown are the results from 2 cohorts; HC, n = 4; RelA KO, n = 10, RelA WT, n = 10)
To establish whether the reduction of IL-1a and IL-6 in kidneys of myeloid RelA KO mice was due to inhibited classical NF-kB signaling in macrophages as opposed to other cell types, we isolated peritoneal macrophages and stimulated them with LPS, a potent M1-phenotype inducer. As shown in Figure 6B, we found that stimulated macrophages from myeloid RelA KO mice have significantly abrogated levels of both IL-1a and IL-6 compared to WT mice, similar to the kidney expression profile of these inflammatory cytokines.
We also assessed the expression of type 1 IFN response genes in the kidney via RT-PCR. As shown in Figure 6C, knocking out relA in myeloid cells either had no effect compared to diseased WT mice, as with the genes, IRF7 and IRF1; or myeloid specific relA knock out mice had increased levels of certain genes, such as MX1 and IFIT1, compared to WT mice.
4. DISCUSSION
Lupus nephritis is a serious end organ manifestation that afflicts up to 60% of patients with SLE. Current treatment options are less than ideal, and more targeted therapies are needed to better serve patients. With current treatment options largely based on non-specific immunosuppression, a significant number of patients still progress to end stage renal disease and require renal replacement therapy. Additionally, patients are often subjected to negative side effects from their treatments [11].
Here, we sought to elucidate if NF-kB signaling mediates the macrophage contribution to disease pathogenesis. We generated myeloid specific RelA knock out mice and induced NTN to study the effect of myeloid derived classical NF-kB signaling in contributing to LN pathogenesis.
We found that knocking out RelA in myeloid cells markedly (albeit not completely) abrogated disease. Specifically, we saw an improvement in proteinuria, as well as improved kidney function and histopathology. Importantly, this was not due to a defect in disease induction. All mice regardless of genotype responded similarly to the initial IgG immunization, and likewise, all received similar levels of nephritic serum and cleared it at the same rate.
Mechanistically, myeloid RelA KO mice had a significant reduction in kidney M1 inflammatory macrophages, but an increase in M2, despite starting with similar resident kidney cell populations at baseline. Each strain had similar levels of CD11b+ cells overall indicating that KO mice had an increased absolute number of M2 cells, likely creating a protective environment that favored resolution over the progression of disease seen in the WT mice.
We found that myeloid RelA knockdown had no effect on B or T cells, either in the kidney or the spleen, but there was a significant depletion of the splenic monocyte reservoir in the WT mice, suggesting the cells were recruited to the inflamed kidney. KO mice were likely protected because of the reduced inflammation in their kidneys. Consistent with the observed changes in cell numbers, we noted several M1 associated cytokines having higher expression in kidneys of WT mice, whereas IL-10 was significantly increased in KO mice. To determine whether this difference in kidney cytokine profile is due to macrophages rather than other myeloid cell subsets in which NF-kB signaling was affected as well, we isolated peritoneal macrophages from both KO mice and WT mice. These cells were stimulated with LPS, a potent M1 inducer; similar to what was seen in the kidney, macrophage RelA KO cells had attenuated responsiveness and exhibited reduced expression of IL-1a and IL-6. Taken together, these data indicate that classical NF-kB signaling in macrophages is instrumental in the pathogenesis of antibody mediated nephritis.
NF-kb can regulate many different aspects of both innate and adaptive immunity. For example, NF-kB signaling is important to maturation of DCs, activation and differentiation of T cells, survival and recruitment of neutrophils, and inflammatory signaling and polarization within macrophages [13]. Functionally, canonical NF-kB signaling is important to nearly every aspect of an immune response, whereas the non-canonical, or alternative pathway of NF-kB, regulates specific functions of the adaptive immune system. Specifically, non-canonical NF-kB is important for LTβR, BAFFR, CD40, and RANK signaling [13].
Dysregulation of NF-kB can lead to chronic inflammation, like that seen in SLE and other autoimmune diseases. Specifically, the NF-kB pathway has been linked to LN pathogenesis [14]. In lupus patients with nephritis, there is increased expression of NF-kB, especially within the glomerular endothelial cells and mesangial cells [15,16], and many of the inflammatory cytokines seen within the kidney are downstream of NF-kB activation. Furthermore, genetic studies implicate both A20 and ABIN1, negative regulators of NF-kB activity, in lupus and nephritis, respectively [17–20]. An additional study found that a selective inhibitor of IKK was able to decrease nephritis within a murine model of LN via inhibition of the NLRP3 inflammasome [21]. Taken together, these studies highlight the role NF-kB may be playing in disease pathogenesis.
Macrophages present themselves as promising therapeutic target within LN. They have been shown to contribute to several murine models of LN, both inducible and spontaneous. We had previously demonstrated the importance of macrophages in the nephrotoxic serum nephritis model used in this paper. By depleting macrophages with GW2580, a CSF-1 kinase inhibitor, disease development was prevented. Furthermore, delayed treatment until nephritis was established was also shown to be effective [6]. In the MRL/lpr mouse model, a spontaneous and severe model of LN, we also demonstrated the benefit of macrophage depletion on kidney disease using the same inhibitor [7]. Other studies have reinforced these findings. Activated macrophages can be used as markers for disease onset and remission in the NZB/w F1 model of LN [22], and congenital deletion of CSF-1 or its receptor (CSF-1R) in the MRL/lpr mouse ameliorated disease [23,24]. Furthermore, systemic deletion of macrophages or inhibition of trafficking also prevented disease development in nephritis models [25–27].
Studies strongly support a role for macrophages in the pathogenesis of LN; although macrophages have been investigated previously in immune-mediated nephritis, up until now the primary approaches used to modulate this cell type have been rather non-selective. Studies involved macrophage depletion overall regardless of phenotype (e.g. using kinase inhibitors which target the CSF-1R) [2,6,7]), or involved inhibitors with off-target effects which affected other cell types as well (e.g. BTK inhibition preferentially targeting M1-type macrophages, but also significantly affecting B cells) [2]. The approach within this paper circumvents these confounding factors by modulating a signaling pathway most closely associated with M1 macrophages, allowing us to specifically assess the effect of classically activated macrophages.
Macrophages are important in human lupus as well. Renal mononuclear phagocyte infiltration is associated with a poor clinical outcome in human LN [4], and proliferating macrophages are associated with more severe glomerulonephritis [28]. These CD169+ macrophages are not found in glomeruli from healthy patients; however, they are found in LN patients and positively correlate with proteinuria and the severity of the pathologic lesions. Moreover, CD169+ macrophages were reduced in patients that responded to treatment. Overall, the data linking macrophages to LN pathogenesis is strong [2,4,28,29].
Because of this strong association of macrophages with LN pathogenesis, we wanted to further elucidate how macrophages are contributing to disease. As mentioned above, noncanonical NF-kB signaling is often associated with particular aspects of the adaptive immune system. Therefore, we examined classical NF-kB signaling specifically within macrophages, where it contributes to the effects of the inflammatory M1 phenotype. Using induction of nephrotoxic serum nephritis in myeloid RelA deficient mice, we could determine the importance of M1 macrophages, or macrophage-derived classical NF-kB signaling, to disease pathogenesis within an inducible model of LN that relies on the passive transfer of nephrotoxic antibodies.
Classical NF-kB signaling is also relevant to pattern recognition receptor (PRR) signaling (such as TLRs), as well as Fc receptor signaling [13], both of which may be important within this model of LN. Therefore, this experimental set-up also allowed us to determine the likely importance of macrophage activation through these specific receptors, although whether the benefit in immune nephritis is effected through a single dominant pathway cannot yet be stated with certainty.
The strain we generated knocks out classical NF-kB signaling within myeloid cells. Consequently, it is important to consider whether other myeloid cells types may be affected, such as dendritic cells. However, there is not a single standardized accepted method to distinguish between renal dendritic cells and macrophages, and similar functions in acute kidney disease have been attributed to both cell types (Gottschalk 2015). These two cells types can be quite difficult to differentiate, as they can share many different functional phenotypes and cell markers [30,31]. Nevertheless, there are several reasons we believe that the protective effect of RelA deficiency in our study is due to macrophages, rather than dendritic cells. Dendritic cell depletion using CD11c-DTR mice indicated a protective effect for renal dendritic cells in nephrotoxic nephritis (Rogers), while ablating mostly macrophages in CD11b-DTR mice was protective (Duffield, 2005). Moreover, the cytokine milieu in nephritic kidneys could be replicated by our studies of macrophages in vitro.
Neutrophils are thought to also be involved in NTN [32], and are another cell type that could have been affected by our myeloid specific RelA knockout. In inflammatory kidney disease, neutrophils can have multiple possible contributions including (among others) release of inflammatory mediators (e.g. reactive oxygen species, cytokines, and proteases), as well as orchestrating the recruitment of infiltrating leukocytes. Additionally, neutrophils can produce NETs (neutrophil extracellular traps), which can contribute to the end organ damage and disease progression in lupus [33].
However, we found no differences in kidney neutrophil number in KO versus WT mice. Additionally, although neutrophils are important effectors in the early stages of nephrotoxic nephritis, macrophages are the dominate myeloid cell later on [30,34,35]. Because our study focuses on the later stage of the disease process, the so-called autologous phase, it is much more likely to conclude that any effects seen in the mutant strain were due to predominant effects on macrophages rather than neutrophils. Indeed, GM-CSF deficiency during the autologous phase of NTN, resulting in a significantly reduced population of macrophages but not neutrophils, had much improved disease despite no change in the neutrophil number [35]. Therefore, based on our results and previous studies taken together, we conclude that classical NF-kB signaling in macrophages rather than other cell types played a primary pathogenic role in this model of immune mediated nephritis.
We also assessed type 1 interferon response within our kidney by RT-PCR. Classical NF-kB signaling does have some potential to regulate different IFN response genes [13,36,37]. We found varied responses with different genes related to type I IFNs. For example, classical NF-kB can negatively regulate certain IFN response genes, such as MX1; and MX1 was found to be increased in relA KO mice relative to WT mice, both healthy and diseased. Other genes, such as IRF1 and IRF7, however, were not different between relA KO mice and WT diseased mice, although KO mice did have increased levels compared to WT healthy mice. In both cases, despite relA KO mice have increased levels of these response genes relative to HC, we can surmise that the IFN response genes are not contributing heavily to disease pathogenesis, as the relA KO have improved kidney disease overall.
5. CONCLUSIONS
Our data indicate that M1 macrophages play an important role in the pathogenesis of nephritis mediated by pathogenic antibodies. Nevertheless, kidney disease in the myeloid RelA KO mice following induction of nephrotoxic serum nephritis was not completely abrogated, suggesting the role of macrophages is more complicated than just a dominance of M1 cells. M2b, which are immunity-regulating cells, have also been implicated within LN, which may explain why some kidney macrophage infiltration was still present even in the myeloid RelA KO strain. Nevertheless, disease was significantly reduced, indicating that targeting inflammatory signaling of macrophages early in disease could hold promise therapeutically. Macrophage depletion shows promise in treating LN; targeting macrophages more selectively through inhibition of particular signaling pathways might further increase the expected benefits of such an approach by potentially increasing efficacy and minimizing off target effects.
HIGHLIGHTS.
Myeloid RelA deficient mice develop attenuated glomerulonephritis following administration of nephrotoxic sera;
Kidneys of myeloid RelA deficient mice expressed less IL-1, IFNg, and IL6 and more IL-10;
There was decreased infiltration of inflammatory macrophages in myeloid RelA deficient mice;
Macrophage NF-kappa B signaling is central to the pathogenesis of immune mediated nephritis.
Acknowledgments
FUNDING
These studies were supported by nephrology and MSTP training grants to S. Chalmers and J. Reynolds from the NIH; and a R01 grant from the National Institute of Arthritis and Musculoskeletal Diseases (3R01AR065594) to C. Putterman.
Footnotes
STATEMENT OF COMPETING FINANCIAL INTERESTS
None.
REFERENCES
- [1].Davidson A, What is damaging the kidney in lupus nephritis?, Nat Rev Rheumatol. 12 (2016) 143–153. doi: 10.1038/nrrheum.2015.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chalmers SA, Chitu V, Ramanujam M, Putterman C, Therapeutic targeting of macrophages in lupus nephritis, Discov Med. 20 (2015) 43–49. [PubMed] [Google Scholar]
- [3].Maria NI, Davidson A, Renal Macrophages and Dendritic Cells in SLE Nephritis, Curr Rheumatol Rep. 19 (2017) 81. doi: 10.1007/s11926-017-0708-y. [DOI] [PubMed] [Google Scholar]
- [4].Hill GS, Delahousse M, Nochy D, Rémy P, Mignon F, Méry JP, Bariéty J, Predictive power of the second renal biopsy in lupus nephritis: significance of macrophages, Kidney Int. 59 (2001) 304–316. doi: 10.1046/j.1523-1755.2001.00492.x. [DOI] [PubMed] [Google Scholar]
- [5].Bergtold A, Gavhane A, D’Agati V, Madaio M, Clynes R, FcR-bearing myeloid cells are responsible for triggering murine lupus nephritis, J. Immunol. 177 (2006) 7287–7295. [DOI] [PubMed] [Google Scholar]
- [6].Chalmers SA, Chitu V, Herlitz LC, Sahu R, Stanley ER, Putterman C, Macrophage depletion ameliorates nephritis induced by pathogenic antibodies, J. Autoimmun. 57 (2015) 42–52. doi: 10.1016/j.jaut.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chalmers SA, Wen J, Shum J, Doerner J, Herlitz L, Putterman C, CSF-1R inhibition attenuates renal and neuropsychiatric disease in murine lupus, Clin. Immunol. 185 (2017) 100–108. doi: 10.1016/j.clim.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang N, Liang H, Zen K, Molecular Mechanisms That Influence the Macrophage M1– M2 Polarization Balance, Front Immunol. 5 (2014). doi: 10.3389/fimmu.2014.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lawrence T, The Nuclear Factor NF-κB Pathway in Inflammation, Cold Spring Harb Perspect Biol. 1 (2009). doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chalmers SA, Doerner J, Bosanac T, Khalil S, Smith D, Harcken C, Dimock J, Der E, Herlitz L, Webb D, Seccareccia E, Feng D, Fine JS, Ramanujam M, Klein E, Putterman C, Therapeutic Blockade of Immune Complex-Mediated Glomerulonephritis by Highly Selective Inhibition of Bruton’s Tyrosine Kinase, Sci Rep. 6 (2016) 26164. doi: 10.1038/srep26164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Saxena R, Mahajan T, Mohan C, Lupus nephritis: current update, Arthritis Res Ther. 13 (2011) 240. doi: 10.1186/ar3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Baker RG, Hayden MS, Ghosh S, NF-κB, inflammation and metabolic disease, Cell Metab. 13 (2011) 11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liu T, Zhang L, Joo D, Sun S-C, NF-κB signaling in inflammation, Signal Transduction and Targeted Therapy. 2 (2017) 17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhang H, Sun S-C, NF-κB in inflammation and renal diseases, Cell Biosci. 5 (2015). doi: 10.1186/s13578-015-0056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zheng L, Sinniah R, Hsu SI-H, In situ glomerular expression of activated NF-kappaB in human lupus nephritis and other non-proliferative proteinuric glomerulopathy, Virchows Arch. 448 (2006) 172–183. doi: 10.1007/s00428-005-0061-9. [DOI] [PubMed] [Google Scholar]
- [16].Zheng L, Sinniah R, Hsu SI-H, Pathogenic Role of NF-κB Activation in Tubulointerstitial Inflammatory Lesions in Human Lupus Nephritis, J Histochem Cytochem. 56 (2008) 517–529. doi: 10.1369/jhc.7A7368.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ma A, Malynn BA, A20: linking a complex regulator of ubiquitylation to immunity and human disease, Nat Rev Immunol. 12 (2012) 774–785. doi: 10.1038/nri3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shembade N, Harhaj EW, Regulation of NF-κB signaling by the A20 deubiquitinase, Cell Mol Immunol. 9 (2012) 123–130. doi: 10.1038/cmi.2011.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Caster DJ, Korte EA, Nanda SK, McLeish KR, Oliver RK, G’Sell RT, Sheehan RM, Freeman DW, Coventry SC, Kelly JA, Guthridge JM, James JA, Sivils KL, Alarcon-Riquelme ME, Scofield RH, Adrianto I, Gaffney PM, Stevens AM, Freedman BI, Langefeld CD, Tsao BP, Pons-Estel BA, Jacob CO, Kamen DL, Gilkeson GS, Brown EE, Alarcon GS, Edberg JC, Kimberly RP, Martin J, Merrill JT, Harley JB, Kaufman KM, Reveille JD, Anaya J-M, Criswell LA, Vila LM, Petri M, Ramsey-Goldman R, Bae S-C, Boackle SA, Vyse TJ, Niewold TB, Cohen P, Powell DW, ABIN1 Dysfunction as a Genetic Basis for Lupus Nephritis, J Am Soc Nephrol. 24 (2013) 1743–1754. doi: 10.1681/ASN.2013020148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nanda SK, Venigalla RKC, Ordureau A, Patterson-Kane JC, Powell DW, Toth R, Arthur JSC, Cohen P, Polyubiquitin binding to ABIN1 is required to prevent autoimmunity, J Exp Med. 208 (2011) 1215–1228. doi: 10.1084/jem.20102177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bay11–7082 attenuates murine lupus nephritis via inhibiting NLRP3 inflammasome and NF-κB activation. - PubMed - NCBI, (n.d.). https://www.ncbi.nlm.nih.gov/pubmed/23770281/ (accessed August 20, 2018).
- [22].Schiffer L, Bethunaickan R, Ramanujam M, Huang W, Schiffer M, Tao H, Madaio MM, Bottinger EP, Davidson A, Activated Renal Macrophages Are Markers of Disease Onset and Disease Remission in Lupus Nephritis, The Journal of Immunology. 180 (2008) 1938–1947. doi: 10.4049/jimmunol.180.3.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Menke J, Iwata Y, Rabacal WA, Basu R, Stanley ER, Kelley VR, Distinct Roles of CSF-1 Isoforms in Lupus Nephritis, J Am Soc Nephrol. 22 (2011) 1821–1833. doi: 10.1681/ASN.2011010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Menke J, Amann K, Cavagna L, Blettner M, Weinmann A, Schwarting A, Kelley VR, Colony-Stimulating Factor-1: A Potential Biomarker for Lupus Nephritis, J Am Soc Nephrol. 26 (2015) 379–389. doi: 10.1681/ASN.2013121356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Han Y, Ma FY, Tesch GH, Manthey CL, Nikolic-Paterson DJ, c-fms blockade reverses glomerular macrophage infiltration and halts development of crescentic anti-GBM glomerulonephritis in the rat, Lab. Invest. 91 (2011) 978–991. doi: 10.1038/labinvest.2011.61. [DOI] [PubMed] [Google Scholar]
- [26].Hasegawa H, Kohno M, Sasaki M, Inoue A, Ito MR, Terada M, Hieshima K, Maruyama H, Miyazaki J, Yoshie O, Nose M, Fujita S, Antagonist of monocyte chemoattractant protein 1 ameliorates the initiation and progression of lupus nephritis and renal vasculitis in MRL/lpr mice, Arthritis Rheum. 48 (2003) 2555–2566. doi: 10.1002/art.11231. [DOI] [PubMed] [Google Scholar]
- [27].McIntosh LM, Barnes JL, Barnes VL, McDonald JR, Selective CCR2-targeted macrophage depletion ameliorates experimental mesangioproliferative glomerulonephritis, Clin. Exp. Immunol. 155 (2009) 295–303. doi: 10.1111/j.1365-2249.2008.03819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yang N, Isbel NM, Nikolic-Paterson DJ, Li Y, Ye R, Atkins RC, Lan HY, Local macrophage proliferation in human glomerulonephritis, Kidney International. 54 (1998) 143–151. doi: 10.1046/j.1523-1755.1998.00978.x. [DOI] [PubMed] [Google Scholar]
- [29].Ikezumi Y, Suzuki T, Hayafuji S, Okubo S, Nikolic-Paterson DJ, Kawachi H, Shimizu F, Uchiyama M, The sialoadhesin (CD169) expressing a macrophage subset in human proliferative glomerulonephritis, Nephrol. Dial. Transplant. 20 (2005) 2704–2713. doi: 10.1093/ndt/gfi105. [DOI] [PubMed] [Google Scholar]
- [30].Rogers NM, Ferenbach DA, Isenberg JS, Thomson AW, Hughes J, Dendritic cells and macrophages in the kidney: a spectrum of good and evil, Nat Rev Nephrol. 10 (2014) 625–643. doi: 10.1038/nrneph.2014.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gottschalk C, Kurts C, The Debate about Dendritic Cells and Macrophages in the Kidney, Front Immunol. 6 (2015). doi: 10.3389/fimmu.2015.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ryan J, Ma FY, Kanellis J, Delgado M, Blease K, Nikolic-Paterson DJ, Spleen tyrosine kinase promotes acute neutrophil-mediated glomerular injury via activation of JNK and p38 MAPK in rat nephrotoxic serum nephritis, Lab. Invest. 91 (2011) 1727–1738. doi: 10.1038/labinvest.2011.137. [DOI] [PubMed] [Google Scholar]
- [33].Kaplan MJ, Neutrophils in the pathogenesis and manifestations of SLE, Nat Rev Rheumatol. 7 (2011) 691–699. doi: 10.1038/nrrheum.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kurts C, Panzer U, Anders H-J, Rees AJ, The immune system and kidney disease: basic concepts and clinical implications, Nat. Rev. Immunol. 13 (2013) 738–753. doi: 10.1038/nri3523. [DOI] [PubMed] [Google Scholar]
- [35].Kitching AR, Ru Huang X, Turner AL, Tipping PG, Dunn AR, Holdsworth SR, The requirement for granulocyte-macrophage colony-stimulating factor and granulocyte colonystimulating factor in leukocyte-mediated immune glomerular injury, J. Am. Soc. Nephrol. 13 (2002) 350–358. [DOI] [PubMed] [Google Scholar]
- [36].Wei L, Sandbulte MR, Thomas PG, Webby RJ, Homayouni R, Pfeffer LM, NFκB Negatively Regulates Interferon-induced Gene Expression and Anti-influenza Activity, J. Biol. Chem. 281 (2006) 11678–11684. doi: 10.1074/jbc.M513286200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Pfeffer LM, The Role of Nuclear Factor κB in the Interferon Response, J Interferon Cytokine Res. 31 (2011) 553–559. doi: 10.1089/jir.2011.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]