Introduction
Ischemic heart disease is currently a leading cause of morbidity and mortality in the US 1 In recent decades, impressive advancements have been made in the diagnosis and treatment of coronary artery disease (CAD). Morbidity and mortality from CAD and its related complications, unfortunately, remain high in part due to the increasing prevalence of obesity and type 2 diabetes.
Nuclear cardiovascular imaging is a major player in the diagnosis and management of CAD. Myocardial perfusion imaging (MPI) in particular, fills an important role in its diagnosis, especially in patients of intermediate risk based on tradition risk factors, as well as assessment of risk for cardiac death or acute ischemic events in those with established disease. With its wide availability as well as wealth of data backing its utility, single photon emission computed tomography (SPECT) has been the dominant myocardial perfusion imaging modality used for the diagnosis of CAD as well as risk stratification of patients with known disease. However, positron emission tomography (PET)-based MPI (Figures 1 and 2) has increasing evidence to show its diagnostic utility and additive prognostic value. Further, a recent position statement from American Society of Nuclear Medicine/Society of Nuclear Medicine and Molecular Imaging advocates for PET MPI as the preferred stress imaging test for patients with known or suspected CAD who cannot undergo exercise stress testing2.
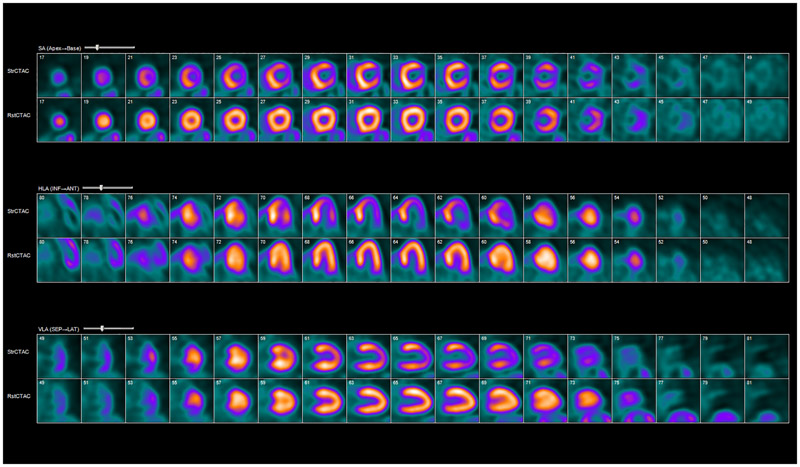
Figure 1:
Rubidium-82 PET myocardial perfusion imaging. Example of abnormal myocardial rest/stress perfusion imaging with rubidium-82 PET. Coregistered rest and stress images show a large reversible defect in the apex, apical anterior, and lateral walls consistent with ischemia. The patient had drop in ejection fraction and evidence of transient ischemic dilatation of the left ventricle with stress. StrCTAC = stress computed tomography attenuation correction; RstCTAC = rest computed tomography attenuation correction; HLA = horizontal long axis; VLA = vertical long axis; SA = short axis; INF = inferior; ANT = anterior; SEP = septal; LAT = lateral.
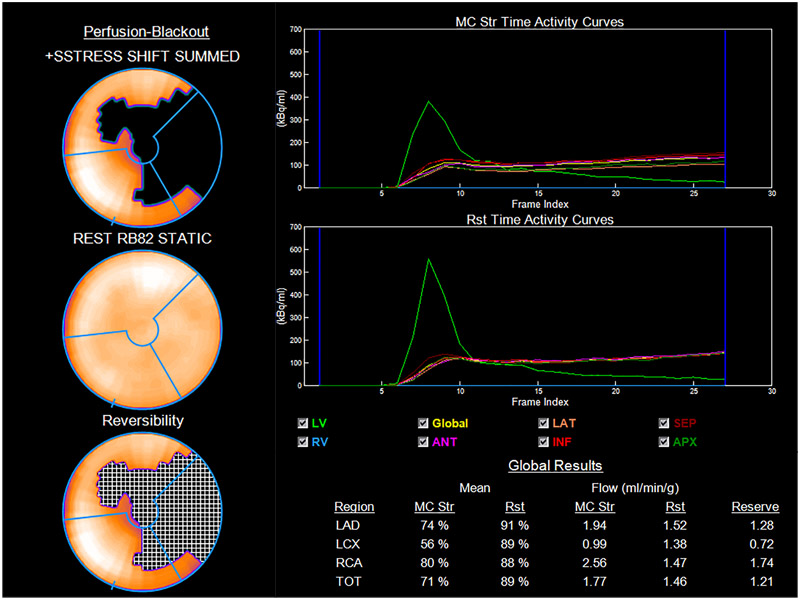
Figure 2:
Myocardial blood flows and flow reserve. Myocardial blood flow (MBF) and myocardial flow reserve (MFR) from patient in Figure 1. Perfusion data on 17-segment model on the left shows a large reversible defect mostly in the apex, apical anterior, and lateral walls. MFR is reduced in all three vascular territories especially in that of the left circumflex. Global MFR is severely reduced (1.21). Str = stress; Rst = rest; RV = right ventricle; LV = left ventricle; ANT = anterior; LAT = lateral; INF = inferior; SEPT = septal; APX = apex; TOT = total.
Current cardiac PET radiotracers
Three perfusions tracers are potentially available for PET MPI: 82-rubidium chloride (82Rb), 13N-ammonia and 15O-water (Table 1)3, and a few others are under development and evaluation (e.g., 18F-flupiridaz, 5-18F-fluoropentyltriphenylphosphonium, 6-18F-fluorohexyltriphenylphosphonium)4 Only 82Rb and 13N-ammonia are Food and Drug Administration (FDA)-approved for clinical use in the United States. In addition to freedom from requiring an on-site cyclotron, 82Rb PET MPI has significant advantages including lower radiation exposure to the patient compared to commonly used SPECT protocols and 13N-ammonia PET, and is the most commonly used PET radiotracer for MPI. 13N-ammonia may afford relatively higher sensitivity relative to 82Rb due to its higher myocardial extraction (up to 80%) and trapping inside the cardiomyocyte following irreversible conversion to glutamine. Its main limitation is the requirement of an on-site cyclotron which has precluded its widespread use. However, novel ‘bench top’ 13N-ammonia cyclotrons have recently become commercially available, potentially allowing its more widespread application. The main advantage of 15O-water is in providing near-perfect extraction fraction in comparison to myocardial blood flow.
Table 1:
Characteristics of PET radiotracers used for myocardial perfusion imaging
| Characteristics | 82Rubidium | 13N-Ammonia | 15O-water |
|---|---|---|---|
| Half-life | 78 sec | 9.8 min | 2.4 min |
| Extraction fraction* | ~60% | ~80% | ~95% |
| Cyclotron on-site | No | Yes | Yes |
| Data acquisition | Dynamic, static, gated | Dynamic, static, gated | Dynamic |
| Scan duration | 6 min | 20 min | 5 min |
| Dose (2-Dimensional PET) | 40-60 mCi | 15-25 mCi | 40 mCi |
| Dose (3-Dimensional PET) | 15-20 mCi 30-40 mCi 3D LSO |
15 mCi | 10 mCi |
| Interval between doses | 10 min | 30 min | 7 min |
| Image interpretation | Yes | Yes | No |
| Image quality | Good | Excellent | N/A |
LSO = lutetium oxyorthosilicate; MBF = myocardial blood flow; PET = positron emission tomography
Extraction fraction based on baseline myocardial blood flow (~1 ml/g/min).
Reproduced with permission from Schindler et al3.
Diagnostic Accuracy and Prognostic Potential
With increasing emphasis towards high value care, a premium is placed on delivering quality care that adds value at lower costs. In cardiovascular medicine, this can be achieved with diagnostic testing delivering superior accuracy for obstructive CAD, one which stratifies patients into more specific risk categories and correctly identifies those requiring further testing and/or interventions. PET MPI provides several advantages in this regard compared to conventional SPECT MPI5.
There is evidence supporting superior diagnostic accuracy of 82Rb PET perfusion imaging compared to conventional SPECT MPI. Bateman et al showed that compared to SPECT MPI, PET MPI had a higher accuracy for identifying coronary stenosis severity of 70% (89% Vs 79%, p = 0.03) and 50% (87% Vs 71%, p = 0.003) with coronary angiography designated as the gold standard, and can better identify patients with multi-vessel disease compared to SPECT. Further, PET imaging provided higher interpretive certainty, with more PET studies deemed as definitively abnormal or normal versus SPECT, likely due to superior image quality6. It should be noted that this study used risk-matched cohorts of patients for comparison and did not directly compare SPECT to PET. A meta-analysis by McArdle et al showed similar findings even when attenuation correction and ECG-gating were incorporated in SPECT MPI7 With the introduction of newer PET/CT systems, studies have investigated the sensitivity and specificity in detecting coronary stenosis (as compared to coronary angiography) 8-11. This data is summarized in Figure 3. More recently, Danand et al reported on a prospective study comparing 15O-water PET, 99mTc tetrofosmin SPECT and cardiac computed tomography angiography (CCTA) for the diagnosis of hemodynamically significant coronary artery disease (defined as fractional flow reserve <0.8) in symptomatic patients with suspicion of coronary disease. PET had the highest diagnostic accuracy of 85% (95%CI 80-90%) compared to CCTA (74%; 95%CI 67-79%, p = 0.03) and SPECT (77%, 95% CI 71-83%, p = 0.02)12.
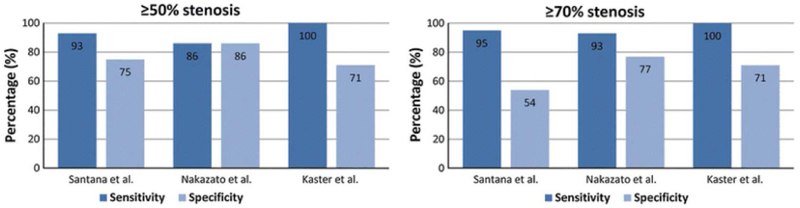
Figure 3:
Sensitivity and specificity of PET in detecting coronary artery disease. Results from three published studies8-10 showing specificity and sensitivity in detecting coronary stenosis of >=50% and >=70% using automated-relative-quantification PET myocardial perfusion imaging with comparison to normal database.
From Slomka P, Berman DS, Alexanderson E, Germano G. The role of PET quantification in cardiovascular imaging. Clin Transl Imaging. 2014;2(4):343-358; with permission.
Several reasons underlie the higher diagnostic accuracy of PET MPI. First, the use of coincidence detection imaging and high-count statistics in PET is associated with higher temporal and spatial resolution. Second, PET tracers have much higher photon energy (511 keV) compared to Tc-99m-labeled SPECT tracers (140 keV), reducing Compton scatter and non-uniform attenuation. This is supplemented by the fact that 82Rb has a short half–life allowing higher doses and thus improving count statistics, with low radiation exposure. Third, CT-based attenuation correction (AC) is more routinely available in PET MPI. In a patient population where obesity is increasingly the norm, the problem of non-uniform attenuation due to excessive body habitus can cause significant artifacts and AC can assist with improving specificity/image quality. Last, cardiac PET MPI provides additional diagnostic data such as peak stress ejection fraction, left ventricular ejection fraction (LVEF) reserve, and myocardial blood flow quantification.
These additive characteristics enable PET MPI’s use as a powerful prognostic tool. A normal PET MPI is associated with an exceedingly low risk (<1% annual rate of cardiac events) while an abnormal PET MPI results predicts higher rates of adverse cardiac events with risk proportional to the degree of abnormalities detected on perfusion imaging13-15 (Figure 4). Furthermore, given its higher image quality and accuracy, incremental changes in PET MPI may be easier to track throughout medical therapy for CAD. Sdringola et al showed that patients who received medical therapy along with lifestyle modifications exhibited improvement in outcomes as well as in previously detected perfusion imaging abnormalities in subsequent studies. In addition, higher risk scans prior to initiation of therapy or worsening abnormalities in subsequent studies were associated with worse prognosis16,17.
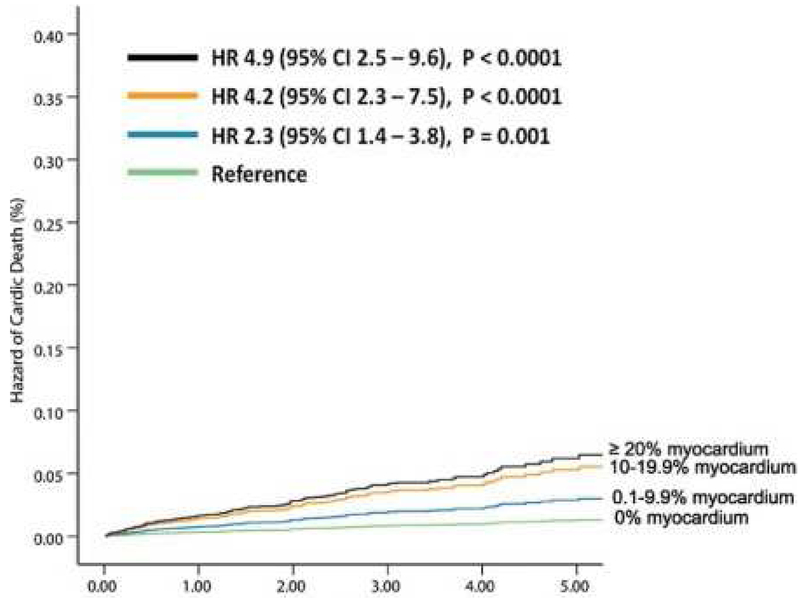
Figure 4:
Extent of perfusion abnormality and prognosis. Long term follow-up data showing, risk-adjusted hazard of cardiac death increases with increasing degree of perfusion abnormality (both ischemia and scar) on PET myocardial perfusion imaging. HR = Hazard Ratio.
From Dorbala S, Di Carli MF, Beanlands RS, et al. Prognostic value of stress myocardial perfusion positron emission tomography: results from a multicenter observational registry. J Am Coll Cardiol. 2013;61(2):176-184; with permission.
Quantification of myocardial flow and flow reserve
While most SPECT MPI studies are analyzed based on visual or at best semi-quantitative assessment of relative perfusion defects, the use of dynamic acquisition sequences with PET provides a unique opportunity for absolute quantification of myocardial blood flow (MBF). This affords several advantages to the clinician. Quantitative assessment of MBF and myocardial flow reserve (MFR), i.e., as the ratio of myocardial blood flow at hyperemic state and at rest, can provide information on total burden of both epicardial and microvascular disease. It can more reliably uncover multi-vessel disease, which, in cases of “balanced ischemia,” may be masked, leading misdiagnosis of the extent of CAD3. Furthermore, there is evidence showing the added prognostic utility of absolute myocardial flow and reserve. Murthy et al, demonstrated lower MFR correlated with higher cardiac mortality after adjusting for traditional cardiovascular risk factors, LVEF and summed stressed scores (SSS) (Hazard ratio (HR) = 5.6 for lowest MFR versus highest MFR tertiles, 95% CI 2.5-12.4, p < 0.0001; HR = 3.4 for middle versus highest MFR tertiles, 95% CI 1.5-7.7, p = 0.003). In addition, when MFR was incorporated into models assessing cardiac mortality risk with parameters such as LVEF and SSS, MFR re-classified 34% of patients at intermediate risk (1-3% annual rate of death; net reclassification index: 0.484, 95%CI 0.157-0.933)18. Ziadi et al similarly showed that lower myocardial flow and reserve in any class of summed stress score from relative perfusion imaging predicts higher risk of major cardiac adverse events 19 (Figure 5). Studies have also shown that in patients with cardiovascular risk factors, even with a normal PET/SPECT MPI or angiogram, an abnormal MBF/MFR independently identifies patients at risk of future cardiovascular events20,21. Information from MBF also allows the detection of early stages of CAD, prior to development of significant stenosis. As an example, changes in MFR in response to cold pressor testing which is mediated by the sympathetic nervous system activation allow the identification of patients who are at risk of CAD, and the opportunity to consider early aggressive medical therapy. For example, Schindler et al showed that impaired MFR in response to cold pressor testing was associated with increased risk of adverse cardiac events. When incorporated into multivariate analysis along with other traditional cardiovascular risk factors, however, this relationship was no longer statistically significant22 (Figure 6). Future prospective studies investigating this matter are warranted.
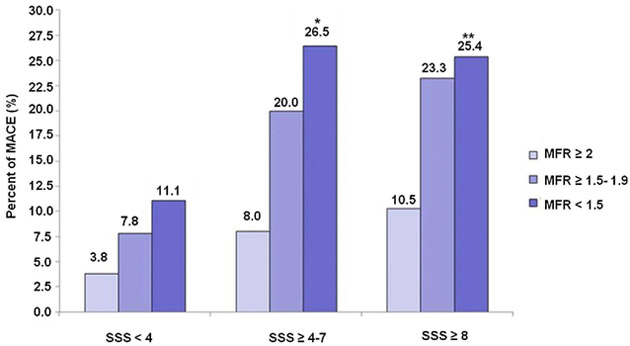
Figure 5:
Incremental prognostic potential of myocardial flow reserve (MFR). Bar graph showing reclassification in risk of major cardiac adverse events (MACE) with the incorporation of myocardial flow reserve in each category of summed stress scores (SSS) in PET myocardial perfusion imaging. *p = 0.028 for SSS ≥4 to 7 and MFR <1.5 versus MFR ≥2. **p = 0.002 for SSS ≥8 and MFR <1.5 versus MFR ≥2.
From Ziadi MC, Dekemp RA, Williams KA, et al. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol. 2011;58(7):740–748; with permission.
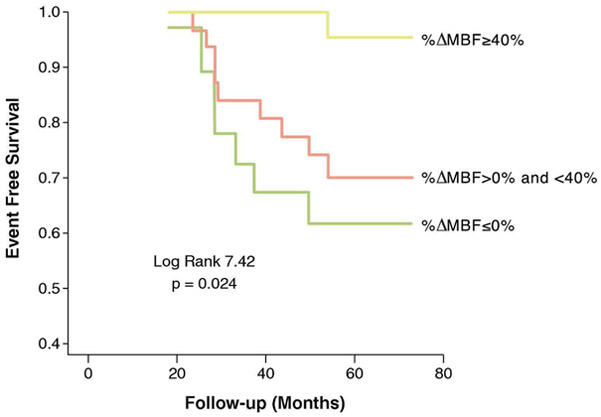
Figure 6:
Prognostic significance of coronary endothelial vasoreactivity. Kaplan Meier curves showing event free survival in patients with normal (group 1, ΔMBF ≥40%), impaired (group 2, ΔMBF >0% and <40%) and decreased (group 3, ΔMBF <0%) myocardial blood flow (MBF) to cold pressor testing. Patients with impaired and decreased MBF response to sympathetic stimulation were at increased risk of developing adverse cardiac events.
From Schindler TH, Schelbert HR, Quercioli A, Dilsizian V. Cardiac PET imaging for the detection and monitoring of coronary artery disease and microvascular health. JACC Cardiovasc Imaging. 2010;3(6):623–640; with permission.
Certain groups of patients, including diabetics, women, and post-heart transplant patients, may benefit from PET MBF assessment. Diabetic patients are at heightened risk of CAD and associated adverse events, but present a clinical challenge given their atypical and often asymptomatic presentations. Quinones et al found evidence of an inverse relationship between plasms glucose concentration and MFR23, and it may be possible that PET assessed MFR can identify diabetic individuals at the earliest stages of disease, even before onset of symptoms or changes in MPI or angiography. Women, particularly after menopause are another group of challenging patients who may benefit from PET assessment of MFR. Taqueti et al showed in symptomatic patients referred for coronary angiography after PET MPI, women had more major adverse cardiac events than men despite less obstructive disease on angiography, lower pretest clinical risk scores, and rates of prior myocardial infarctions. Sex-related differences in MFR accounted for the majority of this increased risk for adverse events seen in women 24. Thus, MFR may be a useful tool for evaluation of female patients at risk for developing CAD, and who may benefit from early aggressive medical therapy. Further prospective studies are needed to confirm these findings. Another population that may benefit from MBF are post-heart transplant patients at risk for coronary vasculopathy25.
Although the data regarding the relative usefulness of MBF/MFR are encouraging, one must be cognizant of their potential pitfalls. Patient motion can introduce errors in the calculation of MBF and MFR due to count spillover from the LV blood pool into the myocardium and vice versa. Unlike traditional SPECT MPI, rotating raw projection images are not available making detection of motion artifacts more difficult in PET. Further, the timing of radiotracer injection relative to vasodilator administration is important and can be a source of error from failure to capture maximal hyperemia due to the relatively short half-lives of vasodilators used, most commonly now regadenoson26. The issue or reproducibility is important in assessment of MFB/MFR. DiCarli et al showed that this is particularly the case in patients with coronary lesions of intermediate severity as there was high variability in MBF of these patients. Without prior knowledge of coronary anatomy in these patients, it may be difficult to delineate between purely epicardial disease and microvascular dysfunction as the true cause of reduction in MBF/MFR27. Finally, one should know the calculation of MBF and MFR requires the estimation of arterial input function and a model to correct for non-linear extraction of 82Rb. Several methods currently are used to estimate these two entities which can lead to variability of results28. In a retrospective study by Murthy et al, three methods, namely the region of interest (ROI), factor analysis and hybrid methods were selected to estimate arterial input function in patients referred for PET stress testing. Rest and stress MBF and MFR were calculated using each method with five of the most common extraction models for 82Rb. Results showed high variability in stress MBF in all three input function methods, particularly when using ROI, but calculations of MFR showed significantly less variability28. Consequently, there was high variability in the correlation between stress MBF and cardiac death, but much less so in MFR regardless of input function used28. These data and potential pitfalls in MBF assessment are codified in a recent ASNC position statement29.
Cost effectiveness with PET MPI
Several studies in the past have shown potential for PET MPI to reduce costs30. One prospective study showed that although the cost of PET MPI was greater than SPECT MPI, this was more than offset by a decrease in downstream testing, namely coronary angiography and subsequent revascularization, along with additional costs of procedural complications, without a significant difference in clinical outcomes at 1 year. This was largely attributed to a much lower rate of false positive testing in PET MPI versus its SPECT counterpart31. Other studies have shown the contrary, however. In a report from the SPARC study, Hlatky et al showed that in patients undergoing PET, SPECT and CTA to evaluate for suspected CAD, those undergoing PET were associated with the highest costs and mortality at 2 years compared to SPECT or CTA32. There is currently a lack of consensus in the optimal diagnostic protocol in MPI to maximize the cost/benefit ratio. Given the tremendous cost implications and the pressure to contain healthcare costs, however, future studies in this regard are needed.
Assessment of myocardial viability, ischemic memory and inflammation
B>esides the role it plays in myocardial perfusion imaging, PET has other applications in ischemic heart disease. In ischemic cardiomyopathy, FDG PET imaging is used to differentiate hibernating myocardium (the so-called viable myocardium) from scar, to identify the patients who might benefit from revascularization. However, several recent clinical trials have raised questions regarding the validity of this concept. In a sub-study of the STITCH trial33, a randomized trial of medical therapy with or without coronary artery bypass grafting (CABG) in patients with CAD and LV dysfunction, myocardial viability assessment did not identify patients with a differential survival benefit from CABG versus medical therapy alone34. The PARR-1 trial showed in patients with ischemic cardiomyopathy with EF ≤ 35%, the amount of scar on FDG PET was a significant predictor of EF recovery after revascularization35. However, this did not translate into positive outcomes in the PARR-2 trial which failed to show reduced adverse cardiac events when these patients were randomized to a PET-guided management strategy compared to standard care at one year follow up. Interestingly, post-hoc analysis of the data showed that patients who adhered to PET-guided recommendations benefited significantly36, supporting a potential role for viability assessment in this setting, which requires further investigation. Other data suggest that PET may identify myocardium at risk of adverse remodeling and cardiomyopathy after myocardial infarction. In a study by Rischpler et al, significant myocardial FDG uptake 5 days after an ST elevation myocardial infarction (STEMI) was associated with markers of poor cardiac function such as reduction in ejection fraction (EF) and increases in end diastolic volume (ESV) and end systolic volume (EDV) assessed by MRI 6 months after MI37. Finally, another potential application of PET is to identify myocardium which has recently experienced ischemia, a phenomenon called ischemic memory. This is due to metabolic changes of ischemic myocardium resulting in decreased metabolism of fatty acids, its usual energy source, and increased metabolism of glucose38.
PET/Computed Tomography Angiography (CTA) hybrid imaging
Hybrid PET/CTA imaging combines anatomical information from CTA with myocardial perfusion data obtained from PET, and may provide complementary data39. In a prospective study involving 107 patients with intermediate probability of CAD, Kajander et al showed hybrid quantitative PET with CTA had significantly greater diagnostic accuracy for detecting significant CAD (defined by >= 50% vessel stenosis) than either alone40. Gaemperli and Danad showed similar results purporting the diagnostic accuracy in PET/CTA hybrid imaging12,41. Currently, the use of PET/CTA hybrid imaging is rare, and several limitations including cost and increased exposure to radiation need to be addressed. As alternative, Maaniitty et al proposed imaging with PET MPI only after significant CAD is identified on CTA, as patients with normal CTA have very low rates of adverse outcomes42.
Future directions
Exciting new developments are currently underway for PET based imaging for ischemic heart disease. Among these is the development 18F-labeled flow tracers, e.g., 18F-flurpiridaz. Potential advantages of 18F-flurpiridaz include a higher extraction fraction compared to 82Rb, allowing higher sensitivity in detecting perfusion defects as well as better assessment of MBF/MFR43-45. The longer half-life of 18F also makes it compatible with exercise stress testing. In terms of logistics, the longer half-life of 18F necessitates only a regional cyclotron for production rather than an onsite cyclotron. Preliminary data from the first phase 3 trial showed 18F-flurpiridaz PET MPI was statistically superior compared to 99mTc-tetrofosmin or sestamibi SPECT MPI in sensitivity of CAD detection defined by gold standard coronary angiography as stenosis =>50% or documentation of prior myocardial infarction (71.9% Vs 53.7%, p<0.001). However, it failed to achieve its second primary outcome, non-inferiority in specificity compared to SPECT MPI (72.6% Vs 82.6%, p = 0.9450). Flurpiridaz MPI also exposed patients to less radiation compared to SPECT (6.1+/−0.4 mSv Vs 13.4 +/−3.2 mSv, p <0.01) and had higher diagnostic certainty (p <0.001) and superior image quality (p<0.001). In subgroup analysis, sensitivity of 18F-flurpiridaz PET MPI was superior in obese patients (body mass index > 30) and female patients46. Currently a second phase III trial is underway (ClinicalTrials.gov identifier: NCT03354273).
Another exciting development is the tremendous potential of PET-based molecular imaging in ischemic heart disease. Emerging molecular imaging techniques seek to image biological features of atherosclerotic lesions (e.g., inflammation and micro-calcification) which predispose coronary plaques to rupture. Rudd et al showed a differential uptake of FDG in carotid artery atherosclerotic plaques in symptomatic and asymptomatic patients47. Since this landmark study, multiple studies have shown an association between vascular FDG uptake and cardiovascular risk factors such as diabetes48 and metabolic syndrome49, as well as Framingham risk score50. These observations have been attributed to the ability of FDG PET to detect vascular inflammation51,52. However, FDG PET imaging of vascular inflammation suffers from poor specificity, as any metabolically active tissue using primarily glucose can take up FDG. Due to the small size of coronary arteries, cardiac motion, and myocardial uptake of the tracer, FDG PET imaging of coronary arteries remains challenging, and issues related to quantification methodology and optimal preparation of subjects prior to imaging remain to be fully addressed53. Several alternative PET-based strategies to imaging vascular inflammation are emerging, which may detect cardiac and coronary inflammation in ischemic heart disease54-57. Given the potential association of microcalcification with plaque vulnerability, 18F-sodium fluoride (NaF) PET may be of value in this setting. Joshi et al reported that in patients with myocardial infarction NaF PET can identify culprit coronary lesions58. Further studies are required to assess the ability of this agent in risk stratification and management of patients with CAD.
In the coming years, it is expected that PET based imaging of ischemic heart disease will increase in prevalence. Currently, 82Rb is the most widely used PET tracer for assessment of CAD, and although there are known limitations to the use of this radiotracer, evidence of the high diagnostic accuracy and added prognostic potential of 82Rb PET MPI make it an attractive alternative to its SPECT counterpart, especially in certain groups of patients such as those with obesity. Further, with exciting developments in new PET radiotracers, advances in molecular imaging, and progress in hybrid imaging with CT or MRI, PET based imaging will continue to play a prominent role in evaluation of ischemic heart disease.
Key points:
PET is an effective imaging modality for the diagnosis of coronary artery disease as well as assessing prognosis in patients with known disease.
PET is the most sensitive modality to assess myocardial viability and provides an important tool in assessing the benefit of revascularization in patients with ischemic cardiomyopathy.
Several exciting additions including molecular imaging and introduction of new imaging tracers are likely to increase the future use of cardiac PET
Synopsis.
PET-based cardiac nuclear imaging is increasingly playing a large role in the management of ischemic heart disease. Compared to conventional SPECT myocardial perfusion imaging, PET provides superior accuracy in the diagnosis of coronary artery disease, and with the incorporation of myocardial blood flow and coronary flow reserve it adds value in assessing prognosis for patients with established coronary and microvascular disease. This review aims to describe these and other uses of PET in ischemic heart disease, including its important role in assessing myocardial viability in ischemic cardiomyopathy. We also briefly describe exciting developments in novel PET flow tracers and molecular imaging tools to assess atherosclerotic plaque vulnerability, vascular calcification, and vascular remodeling.
Clinical Pearls for the Referring Provider.
PET myocardial perfusion imaging (MPI) is a powerful tool in the diagnosis of coronary artery disease and assessing prognosis for those with known disease. There is evidence showing its superior diagnostic and prognostic potential compared to conventional SPECT MPI in patients with coronary disease.
Information from myocardial blood flows allow early detection of coronary disease in certain groups of patients at high risk of CAD, such as diabetics and postmenopausal women.
PET MPI can produce higher image quality and interpretive certainty compared to SPECT MPI, and studies are done in less time with less radiation exposure to patients.
Due to short half-life of the tracer, 82Rb PET MPI studies cannot be done with exercise.
Patients referred for MPI and unable to exercise should be considered for PET. This is especially true for obese patients whose body habitus may be problematic with other MPI modalities.
PET is also useful for assessment of myocardial viability in patients with ischemic cardiomyopathy.
Exciting opportunities are arising in molecular imaging which may increase the use of PET imaging for ischemic heart disease in the future.
Acknowledgments
Funding Sources
This work was supported by grants from NIH (R01-HL138567) and Department of Veterans Affairs (I0-BX001750).
Footnotes
Disclosures:
EJM: Consultant (GE, Inc; Bracco, Inc.); Grant Support (Bracco, Inc)
MMS: Consultant (Bracco Research USA)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murphy SL, Xu J, Kochanek KD, Curtin SC, Arias E. Deaths: Final Data for 2015. Natl Vital StatRep. 2017;66(6):1–75. [PubMed] [Google Scholar]
- 2.Bateman TM, Dilsizian V, Beanlands RS, DePuey EG, Heller GV, Wolinsky DA. American Society of Nuclear Cardiology and Society of Nuclear Medicine and Molecular Imaging Joint Position Statement on the Clinical Indications for Myocardial Perfusion PET. J Nucl Med. 2016;57(10):1654–1656. [DOI] [PubMed] [Google Scholar]
- 3.Schindler TH, Schelbert HR, Quercioli A, Dilsizian V. Cardiac PET imaging for the detection and monitoring of coronary artery disease and microvascular health. JACC Cardiovasc Imaging. 2010;3(6):623–640. [DOI] [PubMed] [Google Scholar]
- 4.Brunken RC. Promising New 18F-Labeled Tracers for PET Myocardial Perfusion Imaging. J Nucl Med. 2015;56(10):1478–1479. [DOI] [PubMed] [Google Scholar]
- 5.Radiation dose to patients from cardiac diagnostic imaging. Circulation. 2007;116(11):1290–1305. [DOI] [PubMed] [Google Scholar]
- 6.Bateman TM, Heller GV, McGhie AI, et al. Diagnostic accuracy of rest/stress ECG-gated Rb-82 myocardial perfusion PET: comparison with ECG-gated Tc-99m sestamibi SPECT. J Nucl Cardiol. 2006;13(1):24–33. [DOI] [PubMed] [Google Scholar]
- 7.Mc Ardle BA, Dowsley TF, deKemp RA, Wells GA, Beanlands RS. Does rubidium-82 PET have superior accuracy to SPECT perfusion imaging for the diagnosis of obstructive coronary disease?: A systematic review and meta-analysis. J Am Coll Cardiol. 2012;60(18):1828–1837. [DOI] [PubMed] [Google Scholar]
- 8.Kaster T, Mylonas I, Renaud JM, Wells GA, Beanlands RS, deKemp RA. Accuracy of low-dose rubidium-82 myocardial perfusion imaging for detection of coronary artery disease using 3D PET and normal database interpretation. J Nucl Cardiol. 2012;19(6):1135–1145. [DOI] [PubMed] [Google Scholar]
- 9.Santana CA, Folks RD, Garcia EV, et al. Quantitative (82)Rb PET/CT: development and validation of myocardial perfusion database. J Nucl Med. 2007;48(7):1122–1128. [DOI] [PubMed] [Google Scholar]
- 10.Nakazato R, Berman DS, Dey D, et al. Automated quantitative Rb-82 3D PET/CT myocardial perfusion imaging: normal limits and correlation with invasive coronary angiography. J Nucl Cardiol. 2012;19(2):265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slomka P, Berman DS, Alexanderson E, Germano G. The role of PET quantification in cardiovascular imaging. Clin Transl Imaging. 2014;2(4):343–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danad I, Raijmakers PG, Driessen RS, et al. Comparison of Coronary CT Angiography, SPECT, PET, and Hybrid Imaging for Diagnosis of Ischemic Heart Disease Determined by Fractional Flow Reserve. JAMA Cardiol. 2017;2(10):1100–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorbala S, Di Carli MF, Beanlands RS, et al. Prognostic value of stress myocardial perfusion positron emission tomography: results from a multicenter observational registry. J Am Coll Cardiol. 2013;61(2):176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorbala S, Hachamovitch R, Curillova Z, et al. Incremental prognostic value of gated Rb-82 positron emission tomography myocardial perfusion imaging over clinical variables and rest LVEF. JACC Cardiovasc Imaging. 2009;2(7):846–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshinaga K, Chow BJ, Williams K, et al. What is the prognostic value of myocardial perfusion imaging using rubidium-82 positron emission tomography? J Am Coll Cardiol. 2006;48(5):1029–1039. [DOI] [PubMed] [Google Scholar]
- 16.Sdringola S, Nakagawa K, Nakagawa Y, et al. Combined intense lifestyle and pharmacologic lipid treatment further reduce coronary events and myocardial perfusion abnormalities compared with usual-care cholesterol-lowering drugs in coronary artery disease. J Am Coll Cardiol. 2003;41(2):263–272. [DOI] [PubMed] [Google Scholar]
- 17.Sdringola S, Gould KL, Zamarka LG, McLain R, Garner J. A 6 month randomized, double blind, placebo controlled, multi-center trial of high dose atorvastatin on myocardial perfusion abnormalities by positron emission tomography in coronary artery disease. Am Heart J. 2008;155(2):245–253. [DOI] [PubMed] [Google Scholar]
- 18.Murthy VL, Naya M, Foster CR, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124(20):2215–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ziadi MC, Dekemp RA, Williams KA, et al. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol. 2011;58(7):740–748. [DOI] [PubMed] [Google Scholar]
- 20.Herzog BA, Husmann L, Valenta I, et al. Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol. 2009;54(2):150–156. [DOI] [PubMed] [Google Scholar]
- 21.Taqueti VR, Hachamovitch R, Murthy VL, et al. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation. 2015;131(1):19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schindler TH, Nitzsche EU, Schelbert HR, et al. Positron emission tomography-measured abnormal responses of myocardial blood flow to sympathetic stimulation are associated with the risk of developing cardiovascular events. J Am Coll Cardiol. 2005;45(9):1505–1512. [DOI] [PubMed] [Google Scholar]
- 23.Quinones MJ, Hernandez-Pampaloni M, Schelbert H, et al. Coronary vasomotor abnormalities in insulin-resistant individuals. Ann Intern Med. 2004;140(9):700–708. [DOI] [PubMed] [Google Scholar]
- 24.Taqueti VR, Shaw LJ, Cook NR, et al. Excess Cardiovascular Risk in Women Relative to Men Referred for Coronary Angiography Is Associated With Severely Impaired Coronary Flow Reserve, Not Obstructive Disease. Circulation. 2017;135(6):566–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chih S, Chong AY, Erthal F, et al. PET Assessment of Epicardial Intimal Disease and Microvascular Dysfunction in Cardiac Allograft Vasculopathy. J Am Coll Cardiol. 2018;71(13):1444–1456. [DOI] [PubMed] [Google Scholar]
- 26.Johnson NP, Gould KL. Regadenoson versus dipyridamole hyperemia for cardiac PET imaging. JACC Cardiovasc Imaging. 2015;8(4):438–447. [DOI] [PubMed] [Google Scholar]
- 27.Di Carli M, Czernin J, Hoh CK, et al. Relation among stenosis severity, myocardial blood flow, and flow reserve in patients with coronary artery disease. Circulation. 1995;91(7):1944–1951. [DOI] [PubMed] [Google Scholar]
- 28.Murthy VL, Lee BC, Sitek A, et al. Comparison and prognostic validation of multiple methods of quantification of myocardial blood flow with 82Rb PET. J Nucl Med. 2014;55(12):1952–1958. [DOI] [PubMed] [Google Scholar]
- 29.Murthy VL, Bateman TM, Beanlands RS, et al. Clinical Quantification of Myocardial Blood Flow Using PET: Joint Position Paper of the SNMMI Cardiovascular Council and the ASNC. J Nucl Cardiol. 2018;25(1):269–297. [DOI] [PubMed] [Google Scholar]
- 30.Patterson RE, Eisner RL, Horowitz SF. Comparison of cost-effectiveness and utility of exercise ECG, single photon emission computed tomography, positron emission tomography, and coronary angiography for diagnosis of coronary artery disease. Circulation. 1995;91(1):54–65. [DOI] [PubMed] [Google Scholar]
- 31.Merhige ME, Breen WJ, Shelton V, Houston T, D'Arcy BJ, Perna AF. Impact of myocardial perfusion imaging with PET and (82)Rb on downstream invasive procedure utilization, costs, and outcomes in coronary disease management. J Nucl Med. 2007;48(7):1069–1076. [DOI] [PubMed] [Google Scholar]
- 32.Hlatky MA, Shilane D, Hachamovitch R, Dicarli MF, Investigators S. Economic outcomes in the Study of Myocardial Perfusion and Coronary Anatomy Imaging Roles in Coronary Artery Disease registry: the SPARC Study. J Am Coll Cardiol. 2014;63(10):1002–1008. [DOI] [PubMed] [Google Scholar]
- 33.Velazquez EJ, Lee KL, Deja MA, et al. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364(17):1607–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonow RO, Maurer G, Lee KL, et al. Myocardial viability and survival in ischemic left ventricular dysfunction. NEngl J Med. 2011;364(17):1617–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beanlands RS, Ruddy TD, deKemp RA, et al. Positron emission tomography and recovery following revascularization (PARR-1): the importance of scar and the development of a prediction rule for the degree of recovery of left ventricular function. J Am Coll Cardiol. 2002;40(10):1735–1743. [DOI] [PubMed] [Google Scholar]
- 36.Beanlands RS, Nichol G, Huszti E, et al. F-18-fluorodeoxyglucose positron emission tomography imaging-assisted management of patients with severe left ventricular dysfunction and suspected coronary disease: a randomized, controlled trial (PARR-2). J Am Coll Cardiol. 2007;50(20):2002–2012. [DOI] [PubMed] [Google Scholar]
- 37.Rischpler C, Dirschinger RJ, Nekolla SG, et al. Prospective Evaluation of 18F-Fluorodeoxyglucose Uptake in Postischemic Myocardium by Simultaneous Positron Emission Tomography/Magnetic Resonance Imaging as a Prognostic Marker of Functional Outcome. Circ Cardiovasc Imaging. 2016;9(4):e004316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jain D, He ZX, Lele V, Aronow WS. Direct myocardial ischemia imaging: a new cardiovascular nuclear imaging paradigm. Clin Cardiol. 2015;38(2):124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaemperli O, Bengel FM, Kaufmann PA. Cardiac hybrid imaging. Eur Heart J. 2011;32(17):2100–2108. [DOI] [PubMed] [Google Scholar]
- 40.Kajander S, Joutsiniemi E, Saraste M, et al. Cardiac positron emission tomography/computed tomography imaging accurately detects anatomically and functionally significant coronary artery disease. Circulation. 2010;122(6):603–613. [DOI] [PubMed] [Google Scholar]
- 41.Gaemperli O, Saraste A, Knuuti J. Cardiac hybrid imaging. Eur Heart J Cardiovasc Imaging. 2012;13(1):51–60. [DOI] [PubMed] [Google Scholar]
- 42.Maaniitty T, Stenstrom I, Bax JJ, et al. Prognostic Value of Coronary CT Angiography With Selective PET Perfusion Imaging in Coronary Artery Disease. JACC Cardiovasc Imaging. 2017;10(11):1361–1370. [DOI] [PubMed] [Google Scholar]
- 43.Huisman MC, Higuchi T, Reder S, et al. Initial characterization of an 18F-labeled myocardial perfusion tracer. J Nucl Med. 2008;49(4):630–636. [DOI] [PubMed] [Google Scholar]
- 44.Maddahi J. Properties of an ideal PET perfusion tracer: new PET tracer cases and data. J Nucl Cardiol. 2012;19 Suppl 1:S30–37. [DOI] [PubMed] [Google Scholar]
- 45.Yalamanchili P, Wexler E, Hayes M, et al. Mechanism of uptake and retention of F-18 BMS-747158-02 in cardiomyocytes: a novel PET myocardial imaging agent. J Nucl Cardiol. 2007;14(6):782–788. [DOI] [PubMed] [Google Scholar]
- 46.Abstracts of Original Contributions ASNC2015 The 20th Annual Scientific Session of the American Society of Nuclear Cardiology. J. Nucl. Cardiol. 2015;22(4):744–781. [Google Scholar]
- 47.Rudd JH, Warburton EA, Fryer TD, et al. Imaging atherosclerotic plaque inflammation with [18F]-fluorodeoxyglucose positron emission tomography. Circulation. 2002;105(23):2708–2711. [DOI] [PubMed] [Google Scholar]
- 48.Bucerius J, Mani V, Moncrieff C, et al. Impact of noninsulin-dependent type 2 diabetes on carotid wall 18F-fluorodeoxyglucose positron emission tomography uptake. J Am Coll Cardiol. 2012;59(23):2080–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tahara N, Kai H, Yamagishi S, et al. Vascular inflammation evaluated by [18F]-fluorodeoxyglucose positron emission tomography is associated with the metabolic syndrome. J Am Coll Cardiol. 2007;49(14):1533–1539. [DOI] [PubMed] [Google Scholar]
- 50.Noh TS, Moon SH, Cho YS, et al. Relation of carotid artery 18F-FDG uptake to C-reactive protein and Framingham risk score in a large cohort of asymptomatic adults. J Nucl Med. 2013;54(12):2070–2076. [DOI] [PubMed] [Google Scholar]
- 51.Myers KS, Rudd JH, Hailman EP, et al. Correlation between arterial FDG uptake and biomarkers in peripheral artery disease. JACC Cardiovasc Imaging. 2012;5(1):38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Menezes LJ, Kotze CW, Agu O, et al. Investigating vulnerable atheroma using combined (18)F-FDG PET/CT angiography of carotid plaque with immunohistochemical validation. J Nucl Med. 2011;52(11):1698–1703. [DOI] [PubMed] [Google Scholar]
- 53.Sadeghi MM. (18)F-FDG PET and vascular inflammation: time to refine the paradigm? J Nucl Cardiol. 2015;22(2):319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tavakoli S, Vashist A, Sadeghi MM. Molecular imaging of plaque vulnerability. J Nucl Cardiol. 2014;21(6):1112–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tarkin JM, Dweck MR, Evans NR, et al. Imaging Atherosclerosis. Circ Res. 2016;118(4):750–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Derlin T, Sedding DG, Dutzmann J, et al. Imaging of chemokine receptor CXCR4 expression in culprit and nonculprit coronary atherosclerotic plaque using motion-corrected [(68)Ga]pentixafor PET/CT. Eur J Nucl Med Mol Imaging. 2018, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tarkin JM, Joshi FR, Evans NR, et al. Detection of Atherosclerotic Inflammation by (68)Ga-DOTATATE PET Compared to [(18)F]FDG PET Imaging. J Am Coll Cardiol. 2017;69(14):1774–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Joshi NV, Vesey AT, Williams MC, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet. 2014;383(9918):705–713. [DOI] [PubMed] [Google Scholar]