Abstract
Dysfunction in 24-h circadian rhythms is a common occurrence in aging adults, however, circadian rhythm disruptions (CRD) are more severe in people with age-related neurodegenerative diseases, including Alzheimer’s disease and related dementias (ADRD) and Parkinson’s disease (PD). Manifestations of CRD differ according to type and severity of neurodegenerative disease, and importantly, could occur before onset of typical clinical symptoms of neurodegeneration. Evidence from preliminary studies suggest that—in addition to being a symptom of neurodegeneration—CRD might also be a potential risk factor for developing ADRD and PD, although large, longitudinal studies are needed to confirm this. The mechanistic link between circadian rhythms and neurodegeneration is not fully understood, although proposed underlying pathways include alterations in protein homeostasis, and immune and inflammatory function. While preliminary clinical studies are promising, more studies of CRD and its mechanisms are needed, and treatment trials are required to determine whether circadian interventions may prevent or delay the onset of neurodegenerative diseases.
II. Introduction
Circadian rhythm activities change markedly as people age, and these changes might further accelerate the aging process (1). While it is recognized that circadian dysfunction in older adults can be partly attributed to the degeneration of the suprachiasmatic nucleus (SCN), known as the “master circadian clock” in mammals, the link between circadian rhythms and neurodegeneration is not fully understood. Patients with neurodegenerative diseases frequently experience circadian rhythm disruptions (CRD) in a much more severe form than typical age-related CRD (2–4). For example, they become more active during the night, less active during the day, and sometimes have complete reversal or loss of the 24-h rest-activity pattern (5, 6). Importantly, evidence suggests that disruptions of circadian functions could be early manifestations of neurodegeneration, and might even be a risk factor for the development of neurodegenerative diseases in healthy adults older than 60 years (7–9). Greater understanding of the relationship between circadian rhythms and neurodegeneration could be key to the early identification and management of neurodegenerative diseases.
This review discusses the association between circadian rhythms and neurodegenerative diseases by summarizing evidence from both human and animal studies. This review focuses on Alzheimer’s disease and related dementias (ADRD) and Parkinson’s disease (PD), as these are the most common neurodegenerative diseases and have been studied most in relation to circadian rhythms. We present both behavioral and biological circadian features in patients with ADRD and PD, summarize findings from clinical and longitudinal epidemiologic studies regarding the effects of CRD on the development of ADRD and PD, and discuss potential underlying mechanisms. Finally, we describe the results of different circadian interventions. For detailed discussion of studies published before 2013, we refer readers to previous reviews (10–12).
III. Circadian rhythms
A circadian rhythm is an approximately 24-hour cycle in the physiological processes of most organisms that is endogenously generated and can be modulated by external cues (13). A circadian cycle is characterized by several features. It is self-sustained, as the rhythm persists in the absence of any exogenous time signals (known as zeitgebers), including dark-light cycles, which indicates the presence of an intrinsic time-keeping mechanism (i.e. biological clock). Circadian cycles show rhythmicity, as they persist with a cycle of approximately 24 hours. Circadian cycles also show the ability to be synchronized by external cues, such as the dark-light cycle or other social and environmental modulators, like activity and temperature. The circadian rhythmicity is typically measured by three parameters: amplitude, phase and period. Amplitude is defined as the magnitude of a cycle, or the difference between crest and trough values. In relation to a hormonal cycle, for example, it would be the difference in the levels of the hormone from the trough to the peak within a time period (i.e. 24 hours). Phase (advanced or delayed) is defined as the timing of a reference point in the cycle relative to a fixed event. In relation to a sleep-wake cycle, for example, a phase advance (delay) would mean that sleep timing moves earlier (later). Period is the time interval between two reference points within a rhythm or recurring wave (for example between two hormonal peaks).
Circadian rhythms are generated in highly specialized cells of specific structures of the brain that control a complex network of coupled self-sustained clocks in the brain and in the peripheral organs. In mammals, the central or master clock of the circadian network is located in two groups of neurons called the SCN, in the anterior hypothalamus. The SCN consists of approximately 20,000 specialized neurons, which receive direct synaptic input from the retina, synchronizing activity to the external light- dark cycle (14). Light input serves to synchronize the core cellular clock machinery in SCN neurons, which keeps 24-hour time and in turn synchronize cellular clocks throughout the body via neurohormonal modulation. At the molecular level, the properties of circadian clocks are based on changes in the expression of certain genes and consist of proteins which form a transcriptional-translation feedback loop that is tuned to a 24-hour period (15). The clock proteins BMAL1 and Clock interact to drive transcription of clock-controlled genes, including their own negative feedback repressors, which include PERIOD, CRYTOCHROME, and REV-ERB proteins (16). This transcriptional feedback loop maintains 24-hour rhythms in gene expression which are required for behavioral and physiologic rhythmicity at the organismal level. While light is the primary circadian cue, resetting the circadian cycle in synchrony with the daily environmental and behavioral cycle (entrainment) is achieved through the 24-h cycle of light input (photic synchronizer) to the SCN and neurohormonal modulations (non-photic synchronizers) (e.g. temperature, food availability, social interactions) for the peripheral ones. Importantly, in the absence of external cues, such as in constant darkness, the circadian system retains a near 24-hour rhythm, while light cues that are out of phase with the SCN cause a gradual resetting of the clock to entrain to the new rhythm.
The pattern of one’s circadian rhythm can be measured with both biological and behavioral markers. Landmark experiments by Czeisler et al. (17) identified core body temperature (CBT), as well as melatonin and cortisol secretions, as circadian biomarkers, oscillations of which are controlled by the SCN. In normally entrained individuals, CBT has a rhythm that falls during the night and rises in the early hours of the morning; cortisol peaks in blood and saliva early in the morning, then regularly decreases throughout late morning and afternoon, to reach low values during evening and night, thereby availing sleep; melatonin is generated by the pineal gland, with its onset near sunset, peak during the nighttime hours and offset after sun rise, thereby stimulating wakefulness. The circadian rhythm of melatonin in saliva or plasma is one of the most commonly used circadian phase biomarkers in human beings (18). The onset time of melatonin secretion under dim light conditions, known as the dim light melatonin onset (DLMO), has been suggested as the single most accurate circadian phase marker in humans (19).
Behavioral markers of circadian rhythm mainly include sleep-wake cycles and rest-activity rhythms. The circadian system has powerful influence over the sleep-wake cycle, such that it is often difficult to distinguish the relative contributions of these two processes on behavior. The circadian clock regulates the timing of sleep, as mutations in core circadian clock genes in mice and humans manifest as abnormal sleep patterns, including short sleep time, early or late sleep phase, or fragmented sleep-wake rhythms (20, 21). Moreover, clock gene expression can be influenced by sleep deprivation, emphasizing that these systems are interrelated. While specific circadian analyses (such as cosinor analysis) can be used to parse out aspects of CRD from behavioral data, activity must be monitored around the clock for several days. Some circadian biomarkers, such as the timing of melatonin secretion or oscillation of expression of selected clock genes in blood, maintain their 24-hour oscillations even in the face of sleep deprivation (22, 23). Therefore, it is important for studies to include both behavioral and biological markers of circadian rhythms to more robustly identify CRD. Given the scope of this review, we include studies if they present information on biological markers or behavioral markers related to sleep timing, daytime sleep or sleepiness and rest-activity rhythm; studies that only present nocturnal sleep disruptions are excluded.
Age-related changes in any of the structures or processes involved in generating or entraining circadian rhythms may modify circadian rhythmicity. In particular, circadian phase has been shown in a study comparing 48 older adults (aged 77–89 years) with 36 younger adults (aged 20–52 years) to move earlier, or advance, with age (24), while the amplitude of the rhythms tend to decrease (25). For example, older adults have decreased peak melatonin, elevated nadir level of CBT, and a phase advance (earlier onset) in the peak of these rhythms compared with adults aged less than 60(1, 26), (27). Age-related changes in sleep-wake cycles may be related to circadian dysfunction and manifest as earlier bedtimes and rise times, increased sleep fragmentation, and increased daytime sleepiness that has been frequently suggested as an early indicator of declining health in the elderly (28, 29). Older adults are also more prone to several circadian rhythm sleep-wake disorders (CRSWDs)—characterized by the inability to fall asleep, remain asleep, or wake at the desired time—including advanced sleep-wake phase disorders (ASWPD), jet lag disorder, and shift-work disorder(30, 31). The circadian system is paramount for maintaining synchrony between internal physiology, behavior, and the cues deriving from the external environment. When this synchrony is lost, e.g. due to jet lag, shift work, or chronic sleep deprivation, a “circadian misalignment” occurs, leading to substantial health consequences affecting cardiovascular, metabolic, cognitive, immunological and oncogenic processes, with impact also on safety, performance and productivity (32–34).
IV. Circadian disruption in neurodegeneration
a. Alzheimer’s disease and related dementias
Compared to healthy adults of the same age, patients with moderate-to-severe AD have been considered to have much more severe circadian disruptions—including higher fragmentations, dampened amplitude and phase delay—as opposed to more typical advanced circadian phase associated with healthy aging (5). It was suggested that “sundowning”, known as the increasing behavioral and neuropsychiatric symptoms in AD patients around the time of sunset, could also partly be attributed to the phase delay of temperature and hormone rhythms in AD (35, 36). The most common CRSWD seen in AD patients is irregular sleep-wake rhythm disorder (ISWRD), as opposed to ASWRD in healthy older adults. ISWRD is defined as a lack of clear 24-hour sleep-wake pattern, usually with long periods of wakefulness during the night and irregular bouts of sleep throughout the day which might get worse in severe AD (37, 38).
Over the past five years, a growing number of studies observed patients of various levels of cognitive impairment and found their circadian patterns differed from those reported in previous studies that focused on moderate to severe AD (5). This could be due to the different type or severity of cognitive impairment reported in these more recent studies. These studies included patients with pre-clinical AD (7), mild cognitive impairment (MCI) (39) (40), mild AD (2) (41) (42), moderate to severe AD (42), global AD(43), as well as early onset dementia (EOD) (3). All of these studies have reported on behavioral markers of CRD, including disruptions of rest-activity rhythms and sleep timing (table 1). Overall, studies have found high rest-activity rhythm fragmentation(3) (7) but only a slight reduction or no change in the amplitude of rest-activity or melatonin rhythms (2, 3, 7, 41, 43). One US study in 189 cognitive healthy older adults (mean age 66.6 years; 50 with preclinical AD pathology as measured by PET) showed decreased rhythm amplitude associated with aging, but not with AD pathology(7). Another study in 16 mild-to-moderate AD patients (mean age 70.3 years) from Italy found large variability among individual actigraphic profiles compared with 10 age-matched neurologically healthy controls, which could have also contributed to the overall minor changes in the amplitude of rhythms in these patients (41). There are mixed findings with regard to changes in circadian phases. Data from the ongoing Rush University Memory and Aging Project suggested a statistically significant phase delay in rest-activity rhythm among 7 AD patients (mean age 90.5 years) compared to 10 age-matched controls (43), whereas a study of 48 AD patients (mean age 70.6 years) from Italy and 29 age-matched controls without dementia showed an advanced bedtime in AD, especially for moderate to severe cases of AD (42). Meanwhile, two studies of MCI patients both found a phase advance, one in melatonin and sleep onset 30 patients with MCI (mean age 65.9 years) compared with 28 healthy age-matched controls (39), and another in CBT and activity rhythm in 21 patients with MCI (mean age 74.1 years) compared with 19 healthy age-matched controls(40). The differences among these findings could be due to the different characteristics of the participants, including age, and severity of cognitive impairment (AD vs. MCI). XX
Table 1.
Case-control studies of circadian rhythm disruptions among patients with dementia or mild cognitive impairment
| First author, year | Participants | Type of circadian markers | Measure of circadian markers | Results |
|---|---|---|---|---|
| Musiek, 2018(7) | 189 cognitively healthy older adults (mean age 66.6y, 50 with preclinical amyloid pathology and 139 amyloid negative) | Rest-activity rhythm | 7 to 14-day actigraphy | Those with preclinical AD had increased rest-activity rhythm fragmentation (p=0.008) but no significant difference in the amplitude or phase, after adjusting for age and sex, compared with healthy controls. |
| Weissova, 2016(2) | 13 mild AD patients (mean age 78.9 y) and 13 age-matched controls | Rest-activity rhythm; melatonin rhythm; peripheral clock gene expression | 21-day actigraphy; sleep diary; saliva melatonin assay; real-time PCR | There was a higher mean number of daytime naps among AD patients than among controls(p=0.04); AD patients had dampened melatonin profiles and slightly reduced amplitude of melatonin rhythm; There was no significant difference in PER1 and BMAL1 expression. |
| La Morgia, 2016(41) | 16 mild-moderate AD patients and 10 age-matched controls (mean age 70.3y) | Rest-activity rhythm | 7-day actigraphy | AD patients had a reduced rhythm amplitude (p=0.04), were less active during the wake period (p=0.04) but more active during the night (p=0.12) compared with controls; there were large individual variabilities among AD patients. |
| Wang, 2015(43) | 7 AD patients and 10 age-matched controls (mean age 90.5 y at death) | Rest-activity rhythm | ≥7-day actigraphy within the 18 months prior to death | AD patients had a phase delay (activity nadirs and acrophases occurred 2.9 hours later than in controls; p=0.002); there was no significant difference in rhythm amplitude. |
| Hooghiemstra, 2015(3) | 61 patients with EOD and 67 controls (mean age 61.9y) | Rest-activity rhythm | 7-day actigraphy | Patients with EOD had increased rest-activity rhythm fragmentation (p=0.03) but no significant difference in the amplitude or regularity. |
| Liguori, 2014(42) | 48 drug-naïve AD patients (mean age 70.6y; 21 mild and 27 moderate to severe) and 29 controls | Sleep timing | Polysomnography | Those who had AD, especially moderate to severe AD had earlier bedtimes (10:15pm and 9:45pm, respectively, compared to controls (11:30pm, p<0.01) and those with mild AD (10:45, p=0.04); there was no difference in rise times. |
| Naismith, 2014(39) | 26 MCI patients (mean age 65.9y) and 26 age-matched controls | Sleep timing; melatonin rhythm | 14-day actigraphy; saliva melatonin assay | MCI patients had earlier melatonin (p=0.001) and sleep onset (p=0.01) compared to controls; there was no significant difference in melatonin levels. |
| Ortiz-Tudela, 2014(40) | 21 MCI patients (mean age 74.1y) and 19 age-matched controls | Rest-activity rhythm; temperature rhythm | 7-day actimeter; wrist temperature sensor | MCI patients showed a phase advance in both activity rhythm (p=0.04. and temperature (p=0.01). |
AD: Alzheimer’s disease; EOD: Early-onset dementia; MCI: mild cognitive impairment
In general, studies that focused on severe AD found more circadian disruptions, while studies in MCI, preclinical AD and mild AD suggested moderate circadian changes (2) (7) (39). However, Weissova et al. found no correlation between circadian features and severity measures of AD in 16 mild to moderate AD patients (mean age 70.3 years) compared with 10 age-matched neurologically healthy controls (41). This might be because of the small sample size and relatively small range of AD severity between participants in this single study. XX No study to date has prospectively examined change in circadian rhythms with the progression of AD symptoms. Few studies have examined molecular perturbations in circadian clock oscillations in ADRD, though alteration in clock gene methylation and expression have been described in fibroblast cultures from post mortem tissue (44), and altered clock gene expression noted in varying brain regions of post-mortem tissue (45). Further, evidence specifically pertaining to circadian disruptions among patients with non-AD dementia is sparse. Larger and longitudinal studies are needed to determine the correlation between both behavioral and biological markers of CRD and severity or progression of AD. Additional studies designed to establish circadian markers and features specific to each type of dementia might help with the differential diagnosis of the disease.
b. Parkinson’s disease
Both motor and non-motor manifestations of PD show disruptions in their typical 24-h oscillations. Unlike patients with ADRD, CRD among PD patients is featured by a reduction in the amplitude of the circadian rhythm but no statistically significant shift in circadian phases (4) (46) (47) (48) (49) (50). Sleep-wake disturbances as a whole are the most common non-motor symptom of PD patients, affecting up to 80% of PD patients (51). Indeed, five of the six studies that examined circadian features in PD patients reported on either excessive daytime sleepiness (EDS) (4) (46) (47) or changes in sleep timing (table 2) (47) (50) (48). It has been reported that PD patients were at least twice as likely to experience EDS compared to healthy older adults (4, 46). Only one study reported slightly later sleep onset time in 30 PD patients (mean age at diagnosis 68.0 years) compared to 15 healthy age-matched controls from England (47), while the others did not find significant differences in sleep timing (48, 50). One Australian study found among 12 PD patients (mean age 62.2 years) a significant reduction in the mesor (mean value around which the rhythm oscillates) and amplitude of their CBT rhythm, compared to 11 healthy age-matched controls(48). Three studies examined rhythms of melatonin secretion, using plasma (4), serum (47) and saliva melatonin (50), respectively. While none of these studies found a difference in the timing of melatonin onset, most found significantly reduced circulating melatonin levels among PD patients compared with age-matched healthy controls (4) (47). Importantly, the usual circadian dip in blood pressure during the night may be lost in PD, putting patients at significantly higher risk for cardiovascular complications including nocturnal hypertension (49). For example, a study of 111 PD patients (mean age 67.8years) from Spain reported that 71.1% of patients did not have the usual dip in blood pressure as measured by 24-h ambulatory blood pressure monitoring (49).
Table 2.
Case-control studies of circadian rhythm disruptions among patients with Parkinson’s disease
| First author, year | Participants | Type of circadian markers | Measure of circadian markers | Results |
|---|---|---|---|---|
| Tholfsen, 2015 (46) | 153 drug-naive PD patients (mean age 66.3y) and 169 age- and sex-matched controls | EDS | ESS | 11.8% PD patients and 4.7% controls had EDS at baseline; after 5 years on PD medication, 23.4% PD patients and 8% controls had EDS. |
| Videnovic, 2014 (4) | 20 PD patients and 15 age-matched controls (mean age 64.1y) | Melatonin rhythm; EDS | Plasma melatonin by 24-h repeated blood sampling; ESS | PD patients had reduced melatonin rhythm amplitude (p<0.001) and 4-fold decrease in 24-h AUC for circulating melatonin levels compared with controls; there was no significant difference in DLMO. 60% PD patients and 27% controls had EDS (p<0.01). |
| Breen, 2014 (47) | 30 PD patients (mean age at diagnosis 68.0y) and 15 age- and sex-matched controls | Sleep timing; EDS; melatonin rhythm; cortisol rhythm; peripheral clock gene expression | 14-day actigraphy; ESS; serum melatonin and cortisol by 24-h repeated blood sampling | PD patients had more fragmented motor activity during 24-h (p=0.01) and later sleep onset time (p=0.04); they had reduced circulating melatonin levels (p=0.05), increased cortisol levels (p<0.001), and altered Bmal1 expression (p=0.04) compared with controls. There was no difference in the timing of melatonin or cortisol onset or offset. |
| Bolitho, 2014 (50) | 29 PD patients (mean age 64.2y; 16 medicated and 13 non-medicated) and 28 age-matched controls | Sleep timing; melatonin rhythm; phase angle of entrainment | 14-day actigraphy and saliva melatonin assay | There were no differences in sleep timing or DLMO; dopaminergic treatment more than doubled (p=0.001)the melatonin secretion and the phase angle of entrainment in patients with PD compared with controls. |
| Zhong, 2013 (48) | 12 PD patients (mean age 62.2y) and 11 age-matched controls | Sleep timing; core-body temperature profiling | 14-day actigraphy; temperature profile recorded by 24-h ingestible capsule sensor | There was no significant difference in sleep timing. PD patients had lower temperature mesor (p=0.02) and reduced nocturnal temperature amplitude (p=0.04) compared with controls. |
| Berganzo, 2013 (49) | 111 PD patients (mean age 67.8y) and XX | Blood pressure | 24-h ambulatory blood pressure monitoring | 71.1% of PD patients had no usual dip in nocturnal blood pressure; PD patients had a great burden of nocturnal hypertension. |
AUC: Area under the curve; DLMO: Dim light melatonin onset; EDS: Excessive Daytime Sleepiness; ESS: Epworth Sleepiness Scale; PD: Parkinson’s disease. Mesor=mean value around which the rhythm oscillates.
Despite the consistently reported CRD among PD patients, it remains unclear whether these circadian changes result from dopaminergic treatment or PD disease progression itself. Studies have reported that the dopaminergic treatment might lead to phase advance of the melatonin rhythm(52, 53), however, another study including 29 PD patients (mean age 64.2 years; 16 medicated and 13 non-medicated) and 28 healthy age-matched controls from Australia found more than double the melatonin secretion and uncoupling of circadian and sleep-wake regulations in the group receiving dopamine treatment (50). EDS is another potential consequence of dopaminergic treatment (46). One study in Norway suggested a doubled frequency of EDS among 153 drug-naive patients with early PD (mean age 66.3 years), compared to 169 age- and sex-matched controls at baseline, and a tripled frequency of EDS among these patients after 5 years of dopaminergic treatment compared to the controls (46). Larger studies with other circadian markers (eg, cortisol secretion, CBT, etc are needed to help clarify the effects of dopaminergic treatment on circadian rhythms, relative to neurodegeneration per se.
V. Circadian disruption and risk of neurodegeneration
A critical question is whether CRD is a cause or consequence of neurodegeneration, or both. If CRD were contributing to neurodegeneration, it would be expected to occur early in disease course (or precede disease), and would increase disease risk or rate of progression. While this question is still unanswered, growing evidence suggests that CRD might precede the development of clinical symptoms of neurodegenerative diseases. One study of 189 cognitively healthy older adults (mean age 66.6 years; 50 with preclinical AD pathology as measured by PET) reported that circadian rest-activity rhythm fragmentation appeared very early on in the preclinical phase of AD compared with XX, and correlated with AD-related pathology as assessed with PET imaging and cerebrospinal fluid (CSF) phosphorylated tau to amyloid β (Aβ)42 ratio (7). Several studies have found a correlation between sleep-wake disturbances and increased levels of AD-related biomarkers or brain structural change in cognitively healthy adults aged 60 years and older, though other biological markers of CRD such as CBT or cortisol rhythm were not specifically examined (9, 54, 55). Alterations in circadian melatonin rhythm measured in saliva were also found in 24 cognitively healthy men (mean age 57.5 years) compared with 26 men (mean age 57.3 years) who were cognitively impaired (56). These cross-sectional findings suggested that CRD could be a result of preclinical AD pathology and may be a prodromal symptom.
Several longitudinal studies with long follow-up periods (5–41 years) also reported greater cognitive decline, increased risk of all-cause dementia and increased risk of PD among those with circadian disturbances, including shift work, compared with those without CRD (57–61). Table 3 shows longitudinal studies investigating CRD and risk of developing ADRD or PD published over the past five years. These studies all examined behavioral indicators of CRD, including actigraphy-measured rest-activity rhythm and daytime napping (8, 59, 62) and self-reported sleep timing(63). Two studies both found an association between lower baseline circadian amplitude and greater cognitive decline at 3–5 years follow up in 2754 cognitively healthy men (mean age 76.0 years) (62) and 1287 cognitively healthy women (mean age 82.8 years) (59) from the USA. Bokenberger et al. reported in 11,247 individuals (mean age 72.5 years at baseline) from the Swedish Twin Registry that delayed rising time predicted dementia incidence after 17 years of follow up (63). Another study of 2920 men (mean age 76.0 years) from the USA suggested that those who napped for at least 1h per day were twice as likely to develop PD after 11 years of follow up (8). While all together these studies suggest that reduced circadian amplitude and circadian phase shifts precede the risk of ADRD, and that daytime inactivity precedes the risk of PD in healthy older adults, the number of published studies is small, especially for PD. Additional confirmatory studies with a long follow-up period are needed to determine whether CRD is a risk factor for ADRD and PD. Comprehensive and repeated measures of CRD with simultaneous assessment of preclinical disease biomarkers (such as amyloid and tau pathology) will also help understand the nature of this association.
Table 3.
Longitudinal studies on CRD and subsequent risk of neurodegenerative diseases
| First author, year | Participants | Length of follow-up | Type of circadian marker | Measure of circadian marker | Primary outcome | Results |
|---|---|---|---|---|---|---|
| Cognitive impairment including Alzheimer’s disease and related dementias | ||||||
| Rogers-Soeder,2018 (62) | 2754 men (mean age 76.0y) | 3.4y (mean) | Rest-activity rhythm | Actigraphy | Cognitive decline by tests of global cognition (3MS) and executive function (Trails B) | Lower circadian amplitude (p<0.001) and phase advance was associated with greater cognitive decline. |
| Bokenberger, 2017(63) | 11,247 adults (mean age 72.5y) | 17y | Sleep timing | Karolinska Sleep Questionnaire | Incident dementia by ICD-10 codes | Delayed rising time was associated with increased dementia risk. |
| Walsh, 2014 (59) | 1287 women (mean age 82.8y) | 5y | Rest-activity rhythm | Actigraphy | Cognitive decline by tests of global cognition (3MS), memory (CVLT-II recall, Digits span backward) and executive function (Trails B, Categorical fluency and Letter fluency) | Lower circadian amplitude was associated with worse cognitive function, especially executive function (p<0.05). |
| Parkinson’s disease | ||||||
| Leng, 2018 (8) | 2920 men (mean age 76.3y) | 11y | Daytime sleepiness and napping | Actigraphy | Incident PD defined by self-report or PD medication use | Long daytime napping was associated with increased risk of PD (p=0.001). |
AD: Alzheimer’s disease; PD: Parkinson’s disease.
VI. Underlying mechanisms
The mechanisms by which neurodegenerative pathology affects circadian function likely vary by specific disease. In AD, human post-mortem neuropathological studies have demonstrated loss of critical neuronal populations in the SCN, including those expressing arginine vasopressin (AVP) or vasoactive intestinal peptide (VIP) (43, 64). Both age- and AD-associated loss of VIP-expressing neurons in the SCN were correlated with pre-mortem circadian dysfunction (Fig.1). However, the mechanisms driving SCN neuronal loss are unclear, as it is not a major site of amyloid plaque or neurofibrillary pathology. Circadian abnormalities are observed in transgenic mouse models of AD, including those expressing human mutant amyloid precursor protein (APP), tau, or both. However, there is great heterogeneity across mouse models, and little correlation with pathology, obscuring any definitive mechanistic conclusions (65–67). Aβ peptide has been implicated as a mediator of circadian dysfunction, and in cultured cells it can induce degradation of the master clock protein BMAL1 (68, 69). However, this direct interaction between Aβ and the circadian clock has not been demonstrated in vivo in animals, or in humans. Altered methylation of the BMAL1 promoter, leading to altered BMAL1 expression and disrupted circadian rhythms, was described in fibroblasts from AD patients and in post-mortem AD brain samples, suggesting an underlying epigenetic mechanism of circadian disruption in AD (Fig.1) (44).
Figure 1. Proposed bi-directional relationship between CRD and neurodegeneration.
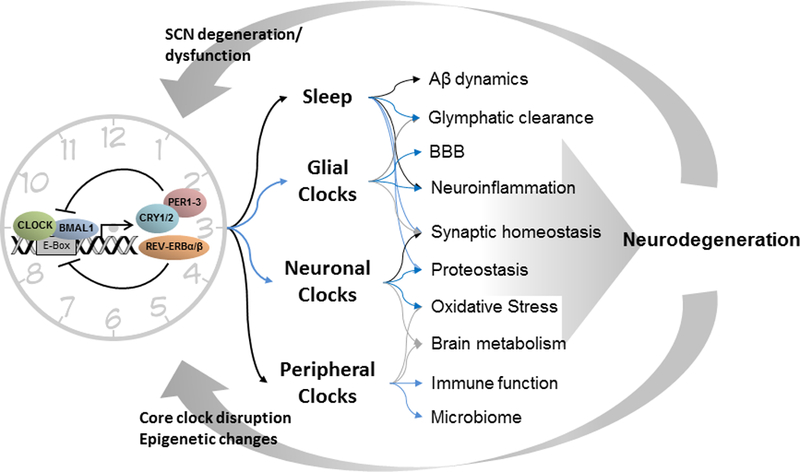
The core circadian clock is present in most cells, including those of the central circadian pacemaker in the suprachiasmatic nucleus (SCN), and consists of a transcriptional-translational feedback loop involving the positive transcriptional regulators CLOCK and BMAL1, and their negative feedback inhibitors PERIOD, CRYTOCHROME, and REV-ERB proteins. The circadian clock influences sleep timing, which has been shown to directly control Aβ dynamics (in humans (90) and mice (87,88)) and glymphatic clearance of toxic proteins (in animals (76)). Sleep disruption also alters a host of other factors, from synaptic homeostasis to inflammation. Circadian clocks in microglia and astrocytes (glial clocks) may regulate the blood-brain barrier (BBB), inflammation, and synaptic function (78, 83, 92, 93)(109), though the evidence is too preliminary to draw a strong conclusion. Animal studies suggest that circadian clocks in neurons influence brain oxidative stress (78), and could affect brain metabolic function and synaptic homeostasis (78). Finally, peripheral clocks in organs such as the gut, liver, and immune tissue impact peripheral metabolism, the microbiome, and immune function (79). It is proposed that this multi-system perturbation could promote toxic protein aggregation and neurodegeneration, which in turn could disrupt circadian clocks in the SCN and periphery. Black arrows=supported by human data, Blue arrows=supported by animal data, Grey arrows=data is suggestive but too preliminary to draw firm conclusions.
Conversely, there are several proposed mechanisms by which the circadian clock influences neurodegenerative disease (Fig. 1). Circadian dysfunction could promote neurodegeneration by altering sleep timing, leading to less consolidated nighttime sleep and increased daytime napping. Sleep deprivation causes altered Aβ dynamics in humans (70) and increased Aβ and tau pathology in mouse models(71, 72), and can increase inflammatory and neuronal injury markers in human cerebrospinal fluid (73). Sleep deprivation has also been shown in mouse models to impact other aspects of neurodegeneration including protein clearance from the brain, inflammation, and synaptic homeostasis (74, 75). In this case, intervention to promote sleep should overcome any effect of circadian disruption. However, in mouse models, clock gene deletion in the brain can cause neuropathology (eg, astrogliosis) without altering sleep, suggesting that altered sleep patterns alone may not explain the brain effects of circadian disruption (76).
Circadian regulation of immune responses may also contribute to the effects of circadian dysfunction on neurodegeneration. The circadian system strongly modulates the peripheral immune response to inflammogens in mice, as the degree of inflammation is highly dependent on time-of-day of exposure (77, 78). In a mouse model of experimental autoimmune encephalitis, the time of day of immunization has a striking impact on disease severity weeks later, while deletion of Bmal1 in myeloid cells exacerbates pathology (79, 80). In the brain, microglia and astrocytes represent the primary innate immune cells, and in rodents both cell types possess functional circadian clocks which regulate inflammatory activation (81, 82). Deletion of Bmal1 in the mouse brain, which disrupts all circadian clock function, causes widespread astrocyte activation and synaptic degeneration, emphasizing the importance of core clock function in maintaining innate immune homeostasis in the brain (76). In mouse models of Amyotrophic Lateral Sclerosis and PD, circadian disruption using non-24 hour light dark cycles led to increased glial activation and neuroinflammation and exacerbated neuropathology (83, 84). Thus, circadian dysfunction appears to promote aspects of neuroinflammation, which could influence neurodegeneration in many disease states.
The circadian clock could directly regulate protein homeostasis and quality control, thereby influencing protein aggregation in neurodegenerative diseases (85). In a mouse AD model, levels of interstitial fluid Aβ peptide in the hippocampus show clear diurnal oscillation, which require an intact circadian system (86, 87). Similar diurnal oscillations in Aβ are observed in human cerebrospinal fluid (88). Moreover, disruption of the circadian clock in a mouse β-amyloidosis model of AD leads to accelerated amyloid plaque deposition (86). Circadian regulation in protein quality control systems, such as autophagy, may contribute to the circadian influence on protein aggregation in general (89, 90) . Bulk removal of aggregated proteins from the brain by the glymphatic system, a glia-mediated perivascular fluid flow, has been associated with sleep, but its relation to the circadian system and the role of glial clocks in the process are still unclear(74). Recent animal studies demonstrating circadian clock control of blood-brain barrier permeability may also have implications for protein aggregates clearance from the brain (91, 92). Finally, numerous mouse studies reveal a complex, bidirectional relationship between the circadian clock and oxidative stress, a key pathogenic process in neurodegeneration (76, 93–96). Thus, a number of potential identified mechanisms, as well as those which are not yet known, could link the circadian clock to neurodegenerative diseases.
VII. Circadian Interventions
If circadian dysfunction is a risk factor contributing to the development of neurodegenerative diseases, one of the appealing testable hypotheses is that restoring regular circadian rhythms might prevent or halt the progress of these diseases as well as mitigating their related symptoms. Several earlier studies have tested this hypothesis using timed light and/or melatonin treatments but provided inconsistent results (97). For instance, a double-blind, placebo-controlled, randomized trial of 189 residents of group care facilities in the Netherlands (mean age 85.8 years; 164 [87%] had dementia) examined the effects of daily treatment with whole-day bright light (1000 lux) as compared to dim light (300 lux), and daily evening melatonin treatment as compared to placebo, and found that the long-term light treatment (up to 3.5 years) attenuated cognitive decline with aging and improved depressive symptoms (98). However, another randomized controlled trial of 48 patients (mean age 83.4years) in two nursing homes in the UK with diagnosed dementia, sleep disruption, and agitated behavior did not find similar cognitive benefit of bright light (99). The discrepancy may be attributed to differences in treatment dose, such as exposure duration and intensity of light, that are especially important for the elderly who have reduced responses of the circadian system to light exposure (100); future studies should examine these possibilities.
In the last five years, only two published circadian intervention studies examined patients with ADRD or PD. In a multicenter (one in the UK and four in the USA), double-blinded, parallel-group study (101), 80 patients diagnosed with mild to moderate AD dementia (mean age 75.3 years; 13 of them had insomnia) were randomized to receiving daily treatment of a prolonged-release melatonin formulation for 24 weeks or placebo. In the 60 participants that completed the trial, there was a positive effect of melatonin treatment on cognitive performance, especially for those with insomnia, compared with placebo. Another study was performed in two PD centers in the USA, where 31 patients (mean age 63.2 years) with PD and coexistent excessive daytime sleepiness who received stable dopaminergic therapy underwent a 14-day light intervention with twice 1-h exposure to bright (10000 lux) or dim (<300 lux) light each day (102). The light intervention improved daily activity rhythms and reduced daytime sleepiness, and the effects were stronger with bright light than with dim light.
The application of circadian interventions in neurodegenerative diseases is a promising but emerging field. Many questions and concerns remain to be addressed. First, circadian rhythms can also be entrained or shifted by many other non-photic time cues or zeitgebers (103), including food (104), caffeine consumption (105) and exercise (106). These zeitgebers affect circadian rhythms likely through direct influences on the peripheral clocks and their feedback to the central circadian clock (107). How to appropriately implement these time cues in circadian interventions requires better understanding of the interactions between the central and peripheral clocks. Second, the intrinsic properties such as the period of the central circadian clock can be different between individuals, leading to different chronotypes (i.e., evening- and morning-types) and different circadian timings (relative to time of day) of behavior and physiological functions including melatonin secretion. Thus, individuals of different chronotypes have different responses even when light exposure and melatonin are scheduled at the same time of day (108). However, no clinical trials have incorporated chronotype into personalized circadian interventions. Third, though circadian control and sleep regulation are tightly coupled, they have different underlying mechanisms. Understanding these specific mechanistic pathways in addition to distinguishing whether the observed beneficial effects of interventions are through the influences on the circadian clocks or directly on the neural circuitry of sleep homeostasis may improve strategies for future drug and therapeutic design. Fourth, despite the association between CRD and cognitive impairment, more evidence for the impacts of circadian interventions on cognitive decline and the progression of neurodegenerations over a long term (e.g., >5 years), especially after the intervention period, is required. Fifth, no circadian intervention study has yet considered neuropathological biomarkers. Using structural MRI or PET scans of the brain and examining longitudinal changes in CSF Aβ and tau levels will help clarify the contributions of CRD to neuropathological and anatomical changes in the brain, which may provide insights into potential mechanisms. Lastly, previous studies have been exclusively focused on the stages of neurodegenerative diseases after the clinical onset of the diseases. It will be important to test the benefits of circadian therapies for the prevention of the diseases and related symptoms at preclinical stages.
VIII. Conclusions and future directions
People with ADRD or PD frequently experience disruptions in both behavioral and biological markers of CRD, including disrupted sleep-wake cycles, impaired hormonal and body temperature rhythms, and dysregulation of the autonomic system. CRD associated with neurodegeneration often presents in a much more severe form than typical age-related CRD and also has distinct features. Unlike healthy older adults who usually have reduced circadian amplitude and advanced circadian phase, patients with ADRD tend to have high fragmentation and slightly reduced amplitude of circadian rhythms. There are mixed findings regarding phase shift among these patients, and they are likely to have irregular sleep-wake patterns. PD patients tend to have reduced circadian amplitude but no change in circadian phases. In general, behavioral CRD markers such as sleep timing, daytime sleepiness and rest-activity rhythms have been examined more often than biological markers such as CBT and melatonin or cortisol secretion rhythms. Recent evidence has also suggested that the stage and severity of the disease, as well as the treatment, increase variation in markers of CRD. Large longitudinal clinical studies are needed to examine the change in circadian rhythms associated with the progression of neurodegeneration, including non-AD dementias, and to separate the potentially interacting effects of disease progression and dopaminergic treatment on circadian rhythms in patients with PD. The integration of non-behavioral circadian biomarkers into these studies would help disentangle CRD from sleep/behavioral confounds (panel 2). This will help identify circadian features that are important for differentiating various types and stages of neurodegenerative diseases, and is important for the management of circadian symptoms in these diseases.
Panel 2: Directions for future research.
Studies of CRD in neurodegeneration should incorporate the assessment of both biological (eg, CBT, melatonin and cortisol rhythms) and behavioral (eg, rest-activity rhythms) markers of CRD.
Large, longitudinal studies are needed to determine circadian features for different types and severities of ADRD, and clarify the link between the progression of ADRD and change in circadian rhythm disruptions.
The interaction between PD disease progression, dopaminergic treatment, and circadian changes should be clarified.
Additional studies with long-term (eg, over 20–30 years) follow-up periods are needed to confirm the effects of CRD on subsequent cognitive decline and risk of developing ADRD or PD.
Underlying mechanisms for the bi-directional relationship between circadian rhythms and neurodegeneration need to be understood to help draw causal inference and inform therapeutic targets.
The use of circadian interventions in patients with neurodegenerative diseases should be further explored, and personalized circadian treatment should be explored, taking the large between-individual differences (ie, differing chronotypes) into consideration.
Randomized controlled trials of individuals at preclinical stages are needed to test the benefits of circadian therapies for the prevention of neurodegenerative diseases.
Several epidemiologic studies suggested the presence of CRD at the preclinical stage of ADRD. CRD might be considered as a useful preclinical marker or prodrome for neurodegenerative diseases and help with the early detection of the disease. Emerging evidence from longitudinal studies also showed that CRD precedes the development of ADRD or PD. Additional confirmatory studies with longer follow-up are needed to examine the relationship between different circadian markers and subsequent risk of developing neurodegenerative diseases, and should consider the use of biomarkers to help understand potential mechanisms. For example, using structural MRI or PET scans of the brain and examining longitudinal changes in CSF Aβ and tau levels will help clarify if CRD might contribute to AD pathology or structural change in the brain. Studies of biological mechanisms and intervention trials are required to determine if CRD is a cause of neurodegenerative diseases.
Finally, personalized multicomponent circadian intervention should be developed and tested for benefits on circadian synchronization as well as symptom management of ADRD or PD. In addition, large longitudinal clinical trials with long follow-up periods are needed to examine the long-term benefits of these interventions, and especially to determine whether these interventions might help prevent or delay the onset of neurodegenerative diseases among healthy older adults. In this way, CRD may be a promising therapeutic target for the prevention and management of neurodegenerative diseases.
VIIII. Search strategy and selection criteria
We identified references for this Review by searches of PubMed between Jan 1, 2013 and Oct 31, 2018, and by hand searches of reference lists from relevant articles. We used the search terms: “dementia”, “Alzheimer’s disease”, “cognitive function”, “cognitive decline”, “cognition”, “Parkinson disease”, “neurodegeneration” and “circadian rhythm”, “circadian clock”, “twenty-four-hour rhythm”, “sleep-wake”, “melatonin” or “chronotherapy”. There were no language restrictions. We included only references published within the past 5 years, except for key or landmark studies in the field. The final reference list was made based on relevance to the theme of this review.
Acknowledgements
Y.L. is supported by National Institute on Aging (NIA) 1K99AG056598–01. E.S.M is supported by NIA R01AG054517 and the Coins for Alzheimer’s Research Trust (CART) Fund. K.H. is supported by NIA R01AG048108 and NIA R01AG059867. K.Y. is supported in part by NIA K24AG031155 and NIA R01AG026720. These organizations had no direct input on any aspect of the paper.
Footnotes
Declaration of interests
E.S.M receives personal fees from Eisai Pharmaceuticals and GLG Consulting.
F.P.C. receives book royalties from Oxford University Press.
K.Y. serves on DSMBs for Takeda, Eli Lilly, and an NIH sponsored study and is a member of the Beeson Scholars in Aging Scientific Advisory Board and a Senate member of the German Center for Neurodegenerative Diseases.
All other authors declare not competing interests.
References
- 1.Hood S, Amir S. The aging clock: circadian rhythms and later life. J Clin Invest 2017. February 1;127(2):437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weissova K, Bartos A, Sladek M, Novakova M, Sumova A. Moderate Changes in the Circadian System of Alzheimer’s Disease Patients Detected in Their Home Environment. PloS one 2016;11(1):e0146200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hooghiemstra AM, Eggermont LH, Scheltens P, van der Flier WM, Scherder EJ. The rest-activity rhythm and physical activity in early-onset dementia. Alzheimer Dis Assoc Disord 2015. Jan-Mar;29(1):45–9. [DOI] [PubMed] [Google Scholar]
- 4.Videnovic A, Noble C, Reid KJ, Peng J, Turek FW, Marconi A, et al. Circadian melatonin rhythm and excessive daytime sleepiness in Parkinson disease. JAMA Neurol 2014. April;71(4):463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Videnovic A, Lazar AS, Barker RA, Overeem S. ‘The clocks that time us’--circadian rhythms in neurodegenerative disorders. Nat Rev Neurol 2014. December;10(12):683–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witting W, Kwa IH, Eikelenboom P, Mirmiran M, Swaab DF. Alterations in the circadian rest-activity rhythm in aging and Alzheimer’s disease. Biological psychiatry 1990. March 15;27(6):563–72. [DOI] [PubMed] [Google Scholar]
- 7.Musiek ES, Bhimasani M, Zangrilli MA, Morris JC, Holtzman DM, Ju YS. Circadian Rest-Activity Pattern Changes in Aging and Preclinical Alzheimer Disease. JAMA Neurol 2018. May 1;75(5):582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leng Y, Goldman SM, Cawthon PM, Stone KL, Ancoli-Israel S, Yaffe K. Excessive daytime sleepiness, objective napping and 11-year risk of Parkinson’s disease in older men. Int J Epidemiol 2018. June 4. [DOI] [PMC free article] [PubMed]
- 9.Ju YS, Ooms SJ, Sutphen C, Macauley SL, Zangrilli MA, Jerome G, et al. Slow wave sleep disruption increases cerebrospinal fluid amyloid-beta levels. Brain 2017. August 1;140(8):2104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Musiek ES, Holtzman DM. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science (New York, NY 2016. November 25;354(6315):1004–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Videnovic A, Zee PC. Consequences of Circadian Disruption on Neurologic Health. Sleep Med Clin 2015. December;10(4):469–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mattis J, Sehgal A. Circadian Rhythms, Sleep, and Disorders of Aging. Trends Endocrinol Metab 2016. April;27(4):192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vitaterna MH, Takahashi JS, Turek FW. Overview of circadian rhythms. Alcohol Res Health 2001;25(2):85–93. [PMC free article] [PubMed] [Google Scholar]
- 14.Herzog ED. Neurons and networks in daily rhythms. Nat Rev Neurosci 2007. October;8(10):790–802. [DOI] [PubMed] [Google Scholar]
- 15.Menet JS, Pescatore S, Rosbash M. CLOCK:BMAL1 is a pioneer-like transcription factor. Genes Dev 2014. January 1;28(1):8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buhr ED, Takahashi JS. Molecular components of the Mammalian circadian clock. Handb Exp Pharmacol 2013(217):3–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science (New York, NY 1999. June 25;284(5423):2177–81. [DOI] [PubMed] [Google Scholar]
- 18.Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE. Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms 2002. April;17(2):181–93. [DOI] [PubMed] [Google Scholar]
- 19.Pandi-Perumal SR, Smits M, Spence W, Srinivasan V, Cardinali DP, Lowe AD, et al. Dim light melatonin onset (DLMO): a tool for the analysis of circadian phase in human sleep and chronobiological disorders. Prog Neuropsychopharmacol Biol Psychiatry 2007. January 30;31(1):1–11. [DOI] [PubMed] [Google Scholar]
- 20.Toh KL, Jones CR, He Y, Eide EJ, Hinz WA, Virshup DM, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science (New York, NY 2001. February 9;291(5506):1040–3. [DOI] [PubMed] [Google Scholar]
- 21.Laposky A, Easton A, Dugovic C, Walisser J, Bradfield C, Turek F. Deletion of the mammalian circadian clock gene BMAL1/Mop3 alters baseline sleep architecture and the response to sleep deprivation. Sleep 2005. April;28(4):395–409. [DOI] [PubMed] [Google Scholar]
- 22.Davies SK, Ang JE, Revell VL, Holmes B, Mann A, Robertson FP, et al. Effect of sleep deprivation on the human metabolome. Proc Natl Acad Sci U S A 2014. July 22;111(29):10761–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lech K, Ackermann K, Revell VL, Lao O, Skene DJ, Kayser M. Dissecting Daily and Circadian Expression Rhythms of Clock-Controlled Genes in Human Blood. J Biol Rhythms 2016. February;31(1):68–81. [DOI] [PubMed] [Google Scholar]
- 24.Monk TH, Buysse DJ, Reynolds CF 3rd, Kupfer DJ, Houck PR. Circadian temperature rhythms of older people. Exp Gerontol 1995. Sep-Oct;30(5):455–74. [DOI] [PubMed] [Google Scholar]
- 25.Duffy JF, Zitting KM, Chinoy ED. Aging and Circadian Rhythms. Sleep Med Clin 2015. December;10(4):423–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carrier J, Paquet J, Morettini J, Touchette E. Phase advance of sleep and temperature circadian rhythms in the middle years of life in humans. Neurosci Lett 2002. March 1;320(1–2):1–4. [DOI] [PubMed] [Google Scholar]
- 27.Duffy JF, Zeitzer JM, Rimmer DW, Klerman EB, Dijk DJ, Czeisler CA. Peak of circadian melatonin rhythm occurs later within the sleep of older subjects. Am J Physiol Endocrinol Metab 2002. February;282(2):E297–303. [DOI] [PubMed] [Google Scholar]
- 28.Mander BA, Winer JR, Walker MP. Sleep and Human Aging. Neuron 2017. April 5;94(1):19–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leng Y, Stone K, Ancoli-Israel S, Covinsky K, Yaffe K. Who Take Naps? Self-Reported and Objectively Measured Napping in Very Old Women. J Gerontol A Biol Sci Med Sci 2018. March 2;73(3):374–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JH, Duffy JF. Circadian Rhythm Sleep-Wake Disorders in Older Adults. Sleep Med Clin 2018. March;13(1):39–50. [DOI] [PubMed] [Google Scholar]
- 31.Sack RL, Auckley D, Auger RR, Carskadon MA, Wright KP Jr., Vitiello MV, et al. Circadian rhythm sleep disorders: part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. An American Academy of Sleep Medicine review. Sleep 2007. November;30(11):1484–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris CJ, Purvis TE, Hu K, Scheer FA. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci U S A 2016. March 8;113(10):E1402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris CJ, Purvis TE, Mistretta J, Scheer FA. Effects of the Internal Circadian System and Circadian Misalignment on Glucose Tolerance in Chronic Shift Workers. J Clin Endocrinol Metab 2016. March;101(3):1066–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kecklund G, Axelsson J. Health consequences of shift work and insufficient sleep. BMJ 2016. November 01;355:i5210. [DOI] [PubMed] [Google Scholar]
- 35.Volicer L, Harper DG, Manning BC, Goldstein R, Satlin A. Sundowning and circadian rhythms in Alzheimer’s disease. Am J Psychiatry 2001. May;158(5):704–11. [DOI] [PubMed] [Google Scholar]
- 36.Canevelli M, Valletta M, Trebbastoni A, Sarli G, D’Antonio F, Tariciotti L, et al. Sundowning in Dementia: Clinical Relevance, Pathophysiological Determinants, and Therapeutic Approaches. Front Med (Lausanne) 2016;3:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Auger RR, Burgess HJ, Emens JS, Deriy LV, Thomas SM, Sharkey KM. Clinical Practice Guideline for the Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders: Advanced Sleep-Wake Phase Disorder (ASWPD), Delayed Sleep-Wake Phase Disorder (DSWPD), Non-24-Hour Sleep-Wake Rhythm Disorder (N24SWD), and Irregular Sleep-Wake Rhythm Disorder (ISWRD). An Update for 2015: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med 2015. October 15;11(10):1199–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abbott SM, Zee PC. Irregular Sleep-Wake Rhythm Disorder. Sleep Med Clin 2015. December;10(4):517–22. [DOI] [PubMed] [Google Scholar]
- 39.Naismith SL, Hickie IB, Terpening Z, Rajaratnam SM, Hodges JR, Bolitho S, et al. Circadian misalignment and sleep disruption in mild cognitive impairment. J Alzheimers Dis 2014;38(4):857–66. [DOI] [PubMed] [Google Scholar]
- 40.Ortiz-Tudela E, Martinez-Nicolas A, Diaz-Mardomingo C, Garcia-Herranz S, Pereda-Perez I, Valencia A, et al. The characterization of biological rhythms in mild cognitive impairment. Biomed Res Int 2014;2014:524971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.La Morgia C, Ross-Cisneros FN, Koronyo Y, Hannibal J, Gallassi R, Cantalupo G, et al. Melanopsin retinal ganglion cell loss in Alzheimer disease. Annals of neurology 2016. January;79(1):90–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liguori C, Romigi A, Nuccetelli M, Zannino S, Sancesario G, Martorana A, et al. Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease. JAMA Neurol 2014. December;71(12):1498–505. [DOI] [PubMed] [Google Scholar]
- 43.Wang JL, Lim AS, Chiang WY, Hsieh WH, Lo MT, Schneider JA, et al. Suprachiasmatic neuron numbers and rest-activity circadian rhythms in older humans. Annals of neurology 2015. August;78(2):317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cronin P, McCarthy MJ, Lim ASP, Salmon DP, Galasko D, Masliah E, et al. Circadian alterations during early stages of Alzheimer’s disease are associated with aberrant cycles of DNA methylation in BMAL1. Alzheimers Dement 2017. June;13(6):689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cermakian N, Lamont EW, Boudreau P, Boivin DB. Circadian clock gene expression in brain regions of Alzheimer ‘s disease patients and control subjects. Journal of biological rhythms 2011. April;26(2):160–70. [DOI] [PubMed] [Google Scholar]
- 46.Tholfsen LK, Larsen JP, Schulz J, Tysnes OB, Gjerstad MD. Development of excessive daytime sleepiness in early Parkinson disease. Neurology 2015. July 14;85(2):162–8. [DOI] [PubMed] [Google Scholar]
- 47.Breen DP, Vuono R, Nawarathna U, Fisher K, Shneerson JM, Reddy AB, et al. Sleep and circadian rhythm regulation in early Parkinson disease. JAMA Neurol 2014. May;71(5):589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhong G, Bolitho S, Grunstein R, Naismith SL, Lewis SJ. The relationship between thermoregulation and REM sleep behaviour disorder in Parkinson’s disease. PLoS One 2013;8(8):e72661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berganzo K, Diez-Arrola B, Tijero B, Somme J, Lezcano E, Llorens V, et al. Nocturnal hypertension and dysautonomia in patients with Parkinson’s disease: are they related? J Neurol 2013. July;260(7):1752–6. [DOI] [PubMed] [Google Scholar]
- 50.Bolitho SJ, Naismith SL, Rajaratnam SM, Grunstein RR, Hodges JR, Terpening Z, et al. Disturbances in melatonin secretion and circadian sleep-wake regulation in Parkinson disease. Sleep Med 2014. March;15(3):342–7. [DOI] [PubMed] [Google Scholar]
- 51.Chahine LM, Amara AW, Videnovic A. A systematic review of the literature on disorders of sleep and wakefulness in Parkinson’s disease from 2005 to 2015. Sleep Med Rev 2017. October;35:33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bordet R, Devos D, Brique S, Touitou Y, Guieu JD, Libersa C, et al. Study of circadian melatonin secretion pattern at different stages of Parkinson’s disease. Clin Neuropharmacol 2003. Mar-Apr;26(2):65–72. [DOI] [PubMed] [Google Scholar]
- 53.Fertl E, Auff E, Doppelbauer A, Waldhauser F. Circadian secretion pattern of melatonin in de novo parkinsonian patients: evidence for phase-shifting properties of l-dopa. J Neural Transm Park Dis Dement Sect 1993;5(3):227–34. [DOI] [PubMed] [Google Scholar]
- 54.Sprecher KE, Koscik RL, Carlsson CM, Zetterberg H, Blennow K, Okonkwo OC, et al. Poor sleep is associated with CSF biomarkers of amyloid pathology in cognitively normal adults. Neurology 2017. August 1;89(5):445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Someren EJW, Oosterman JM, Van Harten B, Vogels RL, Gouw AA, Weinstein HC, et al. Medial temporal lobe atrophy relates more strongly to sleep-wake rhythm fragmentation than to age or any other known risk. Neurobiol Learn Mem 2018. June 1. [DOI] [PubMed]
- 56.Waller KL, Mortensen EL, Avlund K, Fagerlund B, Lauritzen M, Gammeltoft S, et al. Melatonin and cortisol profiles in late midlife and their association with age-related changes in cognition. Nat Sci Sleep 2016;8:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tranah GJ, Blackwell T, Stone KL, Ancoli-Israel S, Paudel ML, Ensrud KE, et al. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol 2011. November;70(5):722–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schlosser Covell GE, Dhawan PS, Lee Iannotti JK, Hoffman-Snyder CR, Wellik KE, Caselli RJ, et al. Disrupted daytime activity and altered sleep-wake patterns may predict transition to mild cognitive impairment or dementia: a critically appraised topic. Neurologist 2012. November;18(6):426–9. [DOI] [PubMed] [Google Scholar]
- 59.Walsh CM, Blackwell T, Tranah GJ, Stone KL, Ancoli-Israel S, Redline S, et al. Weaker circadian activity rhythms are associated with poorer executive function in older women. Sleep 2014. December 01;37(12):2009–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gao J, Huang X, Park Y, Hollenbeck A, Blair A, Schatzkin A, et al. Daytime napping, nighttime sleeping, and Parkinson disease. Am J Epidemiol 2011. May 1;173(9):1032–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bokenberger K, Sjolander A, Dahl Aslan AK, Karlsson IK, Akerstedt T, Pedersen NL. Shift work and risk of incident dementia: a study of two population-based cohorts. Eur J Epidemiol 2018. October;33(10):977–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rogers-Soeder TS, Blackwell T, Yaffe K, Ancoli-Israel S, Redline S, Cauley JA, et al. Rest-Activity Rhythms and Cognitive Decline in Older Men: The Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc 2018. August 23. [DOI] [PMC free article] [PubMed]
- 63.Bokenberger K, Strom P, Dahl Aslan AK, Johansson AL, Gatz M, Pedersen NL, et al. Association Between Sleep Characteristics and Incident Dementia Accounting for Baseline Cognitive Status: A Prospective Population-Based Study. J Gerontol A Biol Sci Med Sci 2017. January;72(1):134–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou JN, Hofman MA, Swaab DF. VIP neurons in the human SCN in relation to sex, age, and Alzheimer’s disease. Neurobiology of aging 1995. Jul-Aug;16(4):571–6. [DOI] [PubMed] [Google Scholar]
- 65.Oyegbami O, Collins HM, Pardon MC, Ebling FJ, Heery DM, Moran PM. Abnormal clock gene expression and locomotor activity rhythms in two month-old female APPSwe/PS1dE9 mice. Curr Alzheimer Res 2017. March 17. [DOI] [PubMed]
- 66.Stevanovic K, Yunus A, Joly-Amado A, Gordon M, Morgan D, Gulick D, et al. Disruption of normal circadian clock function in a mouse model of tauopathy. Experimental neurology 2017. August;294:58–67. [DOI] [PubMed] [Google Scholar]
- 67.Duncan MJ, Smith JT, Franklin KM, Beckett TL, Murphy MP, St Clair DK, et al. Effects of aging and genotype on circadian rhythms, sleep, and clock gene expression in APPxPS1 knock-in mice, a model for Alzheimer’s disease. Experimental neurology 2012. August;236(2):249–58. [DOI] [PubMed] [Google Scholar]
- 68.Schmitt K, Grimm A, Eckert A. Amyloid-beta-Induced Changes in Molecular Clock Properties and Cellular Bioenergetics. Front Neurosci 2017;11:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Song H, Moon M, Choe HK, Han DH, Jang C, Kim A, et al. Abeta-induced degradation of BMAL1 and CBP leads to circadian rhythm disruption in Alzheimer’s disease. Mol Neurodegener 2015;10:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lucey BP, Hicks TJ, McLeland JS, Toedebusch CD, Boyd J, Elbert DL, et al. Effect of sleep on overnight CSF amyloid-beta kinetics. Annals of neurology 2017. December 8. [DOI] [PMC free article] [PubMed]
- 71.Di Meco A, Joshi YB, Pratico D. Sleep deprivation impairs memory, tau metabolism, and synaptic integrity of a mouse model of Alzheimer’s disease with plaques and tangles. Neurobiology of aging 2014. August;35(8):1813–20. [DOI] [PubMed] [Google Scholar]
- 72.Rothman SM, Herdener N, Frankola KA, Mughal MR, Mattson MP. Chronic mild sleep restriction accentuates contextual memory impairments, and accumulations of cortical Abeta and pTau in a mouse model of Alzheimer’s disease. Brain research 2013. September 5;1529:200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Benedict C, Cedernaes J, Giedraitis V, Nilsson EK, Hogenkamp PS, Vagesjo E, et al. Acute sleep deprivation increases serum levels of neuron-specific enolase (NSE) and S100 calcium binding protein B (S-100B) in healthy young men. Sleep 2014. January;37(1):195–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science (New York, NY 2013. October 18;342(6156):373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Vivo L, Bellesi M, Marshall W, Bushong EA, Ellisman MH, Tononi G, et al. Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science (New York, NY 2017. February 3;355(6324):507–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Musiek ES, Lim MM, Yang G, Bauer AQ, Qi L, Lee Y, et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Invest 2013. December 2;123(12):5389–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Curtis AM, Bellet MM, Sassone-Corsi P, O’Neill LA. Circadian clock proteins and immunity. Immunity 2014. February 20;40(2):178–86. [DOI] [PubMed] [Google Scholar]
- 78.Curtis AM, Fagundes CT, Yang G, Palsson-McDermott EM, Wochal P, McGettrick AF, et al. Circadian control of innate immunity in macrophages by miR-155 targeting Bmal1. Proceedings of the National Academy of Sciences of the United States of America 2015. June 9;112(23):7231–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Druzd D, Matveeva O, Ince L, Harrison U, He W, Schmal C, et al. Lymphocyte Circadian Clocks Control Lymph Node Trafficking and Adaptive Immune Responses. Immunity 2017. January 17;46(1):120–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sutton CE, Finlay CM, Raverdeau M, Early JO, DeCourcey J, Zaslona Z, et al. Loss of the molecular clock in myeloid cells exacerbates T cell-mediated CNS autoimmune disease. Nat Commun 2017. December 12;8(1):1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hayashi Y, Koyanagi S, Kusunose N, Okada R, Wu Z, Tozaki-Saitoh H, et al. The intrinsic microglial molecular clock controls synaptic strength via the circadian expression of cathepsin S. Sci Rep 2013;3:2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fonken LK, Frank MG, Kitt MM, Barrientos RM, Watkins LR, Maier SF. Microglia inflammatory responses are controlled by an intrinsic circadian clock. Brain Behav Immun 2015. March;45:171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang Z, Liu Q, Peng Y, Dai J, Xie Y, Chen W, et al. Circadian Rhythm Dysfunction Accelerates Disease Progression in a Mouse Model With Amyotrophic Lateral Sclerosis. Front Neurol 2018;9:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lauretti E, Di Meco A, Merali S, Pratico D. Circadian rhythm dysfunction: a novel environmental risk factor for Parkinson’s disease. Mol Psychiatry 2017. February;22(2):280–6. [DOI] [PubMed] [Google Scholar]
- 85.Hastings MH, Goedert M. Circadian clocks and neurodegenerative diseases: time to aggregate? Curr Opin Neurobiol 2013. October;23(5):880–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kress GJ, Liao F, Dimitry J, Cedeno MR, FitzGerald GA, Holtzman DM, et al. Regulation of amyloid-beta dynamics and pathology by the circadian clock. J Exp Med 2018. January 30. [DOI] [PMC free article] [PubMed]
- 87.Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science (New York, NY 2009. November 13;326(5955):1005–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang Y, Potter R, Sigurdson W, Santacruz A, Shih S, Ju YE, et al. Effects of age and amyloid deposition on Abeta dynamics in the human central nervous system. Arch Neurol 2012. January;69(1):51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Woldt E, Sebti Y, Solt LA, Duhem C, Lancel S, Eeckhoute J, et al. Rev-erb-alpha modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nature medicine 2013. August;19(8):1039–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang G, Zhang F, Ye Q, Wang H. The circadian clock regulates autophagy directly through the nuclear hormone receptor Nr1d1/Rev-erbalpha and indirectly via Cebpb/(C/ebpbeta) in zebrafish. Autophagy 2016. August 2;12(8):1292–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang SL, Yue Z, Arnold DM, Artiushin G, Sehgal A. A Circadian Clock in the Blood-Brain Barrier Regulates Xenobiotic Efflux. Cell 2018;173(1):130–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nakazato R, Kawabe K, Yamada D, Ikeno S, Mieda M, Shimba S, et al. Disruption of Bmal1 Impairs Blood-Brain Barrier Integrity via Pericyte Dysfunction. J Neurosci 2017. October 18;37(42):10052–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stangherlin A, Reddy AB. Regulation of circadian clocks by redox homeostasis. The Journal of biological chemistry 2013. September 13;288(37):26505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kuintzle RC, Chow ES, Westby TN, Gvakharia BO, Giebultowicz JM, Hendrix DA. Circadian deep sequencing reveals stress-response genes that adopt robust rhythmic expression during aging. Nat Commun 2017. February 21;8:14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pekovic-Vaughan V, Gibbs J, Yoshitane H, Yang N, Pathiranage D, Guo B, et al. The circadian clock regulates rhythmic activation of the NRF2/glutathione-mediated antioxidant defense pathway to modulate pulmonary fibrosis. Genes & development 2014. March 15;28(6):548–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rey G, Valekunja UK, Feeney KA, Wulund L, Milev NB, Stangherlin A, et al. The Pentose Phosphate Pathway Regulates the Circadian Clock. Cell Metab 2016. August 17. [DOI] [PMC free article] [PubMed]
- 97.Forbes D, Blake CM, Thiessen EJ, Peacock S, Hawranik P. Light therapy for improving cognition, activities of daily living, sleep, challenging behaviour, and psychiatric disturbances in dementia. Cochrane Database Syst Rev 2014. February 26(2):CD003946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Riemersma-van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJ, Van Someren EJ. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA 2008. June 11;299(22):2642–55. [DOI] [PubMed] [Google Scholar]
- 99.Burns A, Allen H, Tomenson B, Duignan D, Byrne J. Bright light therapy for agitation in dementia: a randomized controlled trial. Int Psychogeriatr 2009. August;21(4):711–21. [DOI] [PubMed] [Google Scholar]
- 100.Figueiro MG. Light, sleep and circadian rhythms in older adults with Alzheimer’s disease and related dementias. Neurodegener Dis Manag 2017. April;7(2):119–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wade AG, Farmer M, Harari G, Fund N, Laudon M, Nir T, et al. Add-on prolonged-release melatonin for cognitive function and sleep in mild to moderate Alzheimer’s disease: a 6-month, randomized, placebo-controlled, multicenter trial. Clin Interv Aging 2014;9:947–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Videnovic A, Klerman EB, Wang W, Marconi A, Kuhta T, Zee PC. Timed Light Therapy for Sleep and Daytime Sleepiness Associated With Parkinson Disease: A Randomized Clinical Trial. JAMA Neurol 2017. April 1;74(4):411–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mrosovsky N Locomotor activity and non-photic influences on circadian clocks. Biol Rev Camb Philos Soc 1996. August;71(3):343–72. [DOI] [PubMed] [Google Scholar]
- 104.Yoshizaki T, Tada Y, Hida A, Sunami A, Yokoyama Y, Yasuda J, et al. Effects of feeding schedule changes on the circadian phase of the cardiac autonomic nervous system and serum lipid levels. Eur J Appl Physiol 2013. October;113(10):2603–11. [DOI] [PubMed] [Google Scholar]
- 105.Burke TM, Markwald RR, McHill AW, Chinoy ED, Snider JA, Bessman SC, et al. Effects of caffeine on the human circadian clock in vivo and in vitro. Sci Transl Med 2015. September 16;7(305):305ra146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Youngstedt SD, Kline CE, Elliott JA, Zielinski MR, Devlin TM, Moore TA. Circadian Phase-Shifting Effects of Bright Light, Exercise, and Bright Light + Exercise. J Circadian Rhythms 2016. February 26;14:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schibler U, Gotic I, Saini C, Gos P, Curie T, Emmenegger Y, et al. Clock-Talk: Interactions between Central and Peripheral Circadian Oscillators in Mammals. Cold Spring Harb Symp Quant Biol 2015;80:223–32. [DOI] [PubMed] [Google Scholar]
- 108.Duffy JF, Rimmer DW, Czeisler CA. Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behav Neurosci 2001. August;115(4):895–9. [DOI] [PubMed] [Google Scholar]
- 109.Lananna BV, Nadarajah CJ, Izumo M, Cedeno MR, Xiong DD, Dimitry J, et al. Cell-Autonomous Regulation of Astrocyte Activation by the Circadian Clock Protein BMAL1. Cell Rep 2018. October 2;25(1):1–9 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]


