Abstract
Background:
Current physical frailty assessment tools are often time-consuming withlimited feasibility.
Objective:
To address these limitations, an instrumented trail-making task (iTMT) platform was developed using wearable technology to computerize quantification of frailty phenotypes without the need of a frailty walking test.
Methods:
Sixty-one older adults (age=72.8±9.9 years, body-mass-index=27.4±4.9kg/m2) were recruited. According to the Fried Frailty Criteria, 39% of participants were determined as robust and 61% as non-robust (pre-frail or frail). In addition, 17 young subjects (age=29.0±7.2years, body-mass-index=26.2±4.6kg/m2), were recruited to determine the healthy benchmark. The iTMT included reaching to 5 indexed circles (including numbers 1-to-3 and letters A&B placed in random orders), which virtually appeared on a computer-screen, by rotating one’s ankle-joint while standing. By using an ankle-worn inertial sensor, 3D ankle-rotation was estimated and mapped into navigation of a computer-cursor in real-time (100Hz), allowing subjects to navigate the computer-cursor to perform the iTMT. The ankle-sensor was also used for quantifying ankle-rotation velocity (representing slowness), its decline during the test (representing exhaustion), and ankle-velocity variability (representing movement inefficiency), as well as the power (representing weakness) generated during the test. Comparative assessments included Fried frailty phenotypes and gait assessment.
Results:
All subjects were able to complete the iTMT, with an average completion time of 125±85 seconds. The iTMT-derived parameters were able to identify the presence and absence of slowness, exhaustion, weakness, and inactivity phenotypes (Cohen’s d effect size=0.90–1.40). The iTMT Velocity was significantly different between groups (d=0.62–1.47). Significant correlation was observed between the iTMT Velocity and gait speed (r=0.684 p<0.001). The iTMT-derived parameters and age together enabled significant distinguishing of non-robust cases with area-under-curve of 0.834, sensitivity of 83%, and specificity of 67%.
Conclusion:
This study demonstrated a non-gait-based wearable platform to objectively quantify frailty phenotypes and determine physical frailty, using a quick and practical test. This device may be able to address the hurdles of conventional physical frailty phenotypes methods by reducing the time to assess, computerizing the test to improve objectivity, and replacing the conventional frailty walking test with a practical test that requires less space and is potentially more practical in those with mobility limitations.
Keywords: frailty, instrumented trail-making task, frailty phenotype, gait, wearable, virtual-reality, cognitive-motor test
Introduction
Frailty is a geriatric syndrome that increases an older-adult’s vulnerability to fall incidence, hospitalization, institutionalization, and mortality [1]. Recent epidemiologic studies show that, in the United States, there are around 3.6 million people with frailty [2]. The total cost related to frailty each year is approximately $18.5 billion or 15% of the total healthcare expenditure in the United States [3]. Early diagnosis of frailty can help patients and physicians make more informed medical decisions of modifiable risk factors to reduce post-operation adverse or poor functional outcomes [4]. Although several studies have suggested that frailty is not an irreversible process, it has been hypothesized that the early detection of frailty stages may provide a window of opportunity for timely preventive or therapeutic interventions, which may delay the progression of frailty and even reverse it [5]. Thus, a practical and quick tool to determine frailty stages irrespective of setting (e.g., at home or clinic) is desperately needed.
Currently, there is no gold standard for diagnosing frailty, and “old age” itself does not define frailty [6]. One of the most widely accepted biological syndrome models to assess frailty was proposed by Fried’s group in 2001 (Fried Frailty Criteria, FFC) [1]. Five different phenotypes (slowness, weakness, exhaustion, inactivity, and shrinking or involuntary weight loss) are suggested as criteria to determine different frailty stages. Subjects who are positive for 1 or 2 phenotypes are considered pre-frail, while those with 3 or more positive phenotypes are considered frail. Subjects who are negative for all 5 phenotypes are considered robust. The FFC has been proved to be accurate for identifying older adults with low resilience and high vulnerability, and poor health outcomes can be predicted with it independently of comorbidities. However, it has limited feasibility and reliability in routine clinical applications [7]. In addition, it is not sensitive to track changes in frailty stages over time [8]. Specifically, certain self-reported criteria used in the FFC are subjective and prone to self-report bias, as well as other non-objective parameters [9]. Furthermore, one of the most important criteria of the FFC is slowness, assessed by a 15-foot (~5 meter) walking test. This test could be challenging to administer in busy clinics and among frail patients. There are some other operational assessments for frailty [9, 10]. Unfortunately, all of these tools share similar limitations as the FFC.
Recent advances in designing wearable, virtual-reality, and interactive-interface technologies have opened up new opportunities to design practical and time-efficient tools, which provide objective metrics to quantify motor functional performance, identify cognitive impairment, and track health status, irrespective of setting and across disciplines [11–15]. Recently we have designed a non-gait based cognitive-motor assessment tool named the instrumented trail-making task (iTMT). In our previous studies [16, 17], we have demonstrated that the iTMT is sensitive enough to determine cognitive function in older adults. To continue these efforts, in the current study, we examined whether the iTMT is able to distinguish different frailty stages (robust, pre-frail, and frail), as determined by the FFC. In addition, we examined whether the iTMT-derived parameters could describe physical frailty phenotypes. Our basic premise was that older adults with frailty or pre-frailty will have poorer performance, as measured by the iTMT, than robust or young subjects.
Methods
Study Population
Sixty-one older adults were recruited in this study from specialized outpatient clinics (e.g., Cancer Center, Alzheimer’s Disease and Memory Disorders Center, Geriatric Clinic, Endocrine Surgery Clinic, etc.). To be eligible, subjects had to be ambulatory, aged at least 60 years, and willing to participate in this study. Subjects were excluded if they were unable to walk 20 meters with or without walking assistance; had significant visual problems, limiting their ability to interact with a computer-screen with or without visual correction; had lower-extremity problems, limiting their ability to perform ankle-rotation needed for the purpose of this study; or had severe balance impairment, limiting their ability to independently stand for at least 1 minute. Those who could stand behind a chair and perform the iTMT test by holding the chair were not excluded. To compare the iTMT results between young and older adults, as well as to determine a healthy benchmark, 17 young ambulatory subjects with ages ranging from 20 to 35 years were also recruited. This study was approved by the local IRBs.
Clinical and Motor Performance Measurements
We first applied the FFC to all older subjects. According to the FFC, 24 subjects (39%) were classified as robust, 29 subjects (48%) were classified as pre-frail, and 8 subjects (13%) were classified as frail. Since the sample size of the frail group was small, we further combined the pre-frails and frails as a non-robust group. Subjects’ demographics, including age, gender, weight, height, body-mass-index (BMI), daily number of prescription medicines, daily number of over-the-counter medicines, use of walking assistance, and fall history were collected. All subjects underwent clinical assessments, including with the Falls Self-Efficacy Scale (FES-I) and Center for Epidemiologic Studies Depression (CES-D) scale. The FES-I and cutoff score suggested by Delbaere et al. [18] was used to identify subjects with high concern about falling. The CES-D short-version scale was used to measure self-reported depression symptoms. A cutoff of CES-D score of 16 or greater was used to identify subjects with depression [19]. Gait performance was measured using wearable sensors (LegSys™, BioSensics, MA, USA) attached to both left and right lower legs. Subjects were asked to walk with their habitual gait speed for 20 meters without any distraction. Using a validated model (double inverse pendulum model representing motion around ankle and knee joint), we calculated gait speed [20, 21].
iTMT Platform Design
We designed the iTMT platform (Figure 1) based on a single inertial wearable sensor (LEGSys™, BioSensics, MA, USA), which includes a triaxial accelerometer, a triaxial gyroscope, and a triaxial magnetometer. This configuration enabled estimation of 3D oint angles [21]. Sensor data were acquired and transmitted at 100Hz frequency for real-time feedback.
Figure 1.
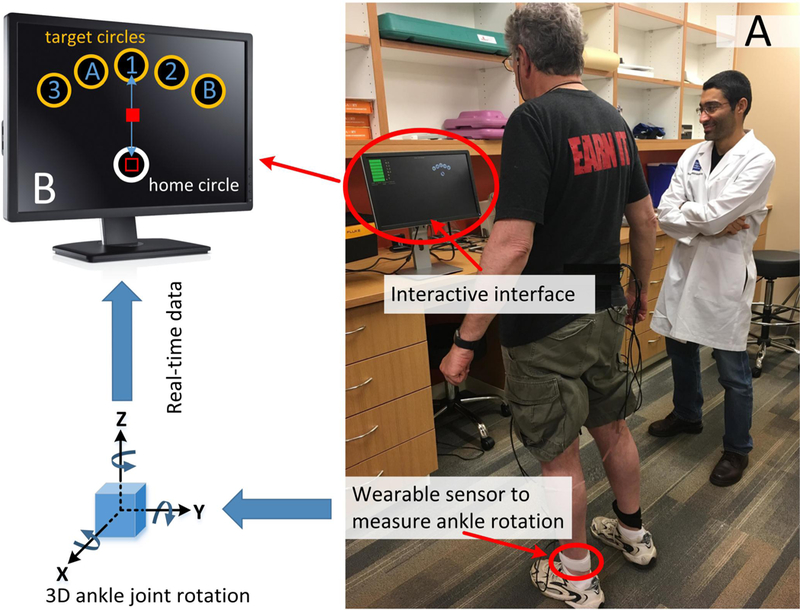
The iTMT included standing in front of a standard computer and performing a series of virtual trail-making tasks by rotating ankle joint (Fig. 1A). Subjects were allowed to hold a chair or table for support if needed. A shin-worn sensor enabled measuring 3D rotation of ankle 638 joint and mapped it into movement of a computer-cursor in real-time (sample frequency of100Hz). This allowed a smooth navigation of the computer-cursor by rotation of ankle joint. The iTMT reaching tasks include bringing the cursor back and forth to 5 targets indexed with numbers (1–3) and letters (A&B) in the sequential number-letter order (i.e. 1, A, 2, B, and 3, Fig. 1B). Audio and visual feedbacks were provided to assist accurate execution of the tasks in the correct order.
The sensor was attached to the subject’s lower shin of the dominant leg (Figure 1A), using an elastic strap. The sensor enabled tracking ankle-rotation in 3D. The data were transmitted to an interactive interface installed on a standard computer (Figure 1B). By rotating the ankle, the subject could navigate a cursor on the computer-screen from a home circle to target circles appearing on the same screen. In summary, the sensor provided quaternion components of ankle-rotation with a sample frequency of 100Hz.Using the method described in our previous studies [22, 23], we calibrated the sensor at the beginning of the test, when the subject was in the upright position and resting (no movement). The calibration process corrected misalignment of the sensor with respect to gravity, bringing the cursor to the home circle (Figure 1B) at the beginning of each iTMT trial. In addition, it saved the coordinates of the body landmarks (reference axes), representing frontal, sagittal, and transverse planes for the subject. The code updated reference axes at the beginning of each iTMT trial to reduce the effect of potential drift. All subsequent quaternion components during movement were subsequently converted to Euler angles with respect to the reference axes. These angles were used to describe a sequence of 3 rotations, determining the orientation of the shank segment in 3 dimensions, including yaw, pitch, and roll. The yaw and pitch components were used to estimate components of ‘X’ (horizontal movement) and ‘Y’ (vertical movement) of the cursor in real time. The estimated X and Y cursor positions were updated on the screen with a sample frequency of 100Hz. The cursor velocity, representing the ankle-rotation velocity, in directions of X and Y were then estimated from the change of the position of the cursor. A code was developed to identify the “start” and “end” points for each Itmt trial. The “start” point was determined when the cursor was inside of home circle, and cursor velocity exceeded a pre-defined threshold (initiation of movement). The “end” point was determined when the cursor reached to one of the target circles, and cursor velocity was below a pre-defined threshold (cursor stopped in the target circle). In our previous study, we demonstrated that all calculation processes and visualization of the cursor could be executed in real time with a sample frequency of 100Hz [24]. At the end of the iTMT test, the platform recorded all sensor data and the iTMT key variables for the post-processing phase.
Definitions of the iTMT protocol and iTMT-derived parameters are summarized in Table 1 and will be described in the following sections.
Table 1.
Definition of the iTMT terms.
| iTMT Protocol | Unit | Description |
|---|---|---|
| Start Point | NA | When the computer-cursor was inside of the home circle and cursor velocity exceeded a pre-defined threshold (initiation of movement) |
| End Point | NA | When the computer-cursor reached to one of the target circle and cursor velocity was below a pre-defined threshold (stopped in the target circle) |
| iTMT Trial | NA | A target reaching including moving the computer-cursor from the home circle to one of the target circle |
| iTMT Round | NA | Five iTMT trials including successfully completing reaching to all five targets (1-A-2-B-3) |
| iTMT Test | NA | The whole iTMT test including successfully completing three iTMT rounds |
| iTMT Derived Parameters | Unit | Description |
| iTMT Time | s | The time needed to successfully complete the iTMT test |
| iTMT Velocity | unit/s | Maximum ankle-rotation velocity, averaged by the first 15 iTMT trials |
| iTMT Power | Unit2/s3 | Maximum multiplication of ankle-rotation velocity and ankle-rotation acceleration, averaged by the first 15 iTMT trials |
| iTMT Exhaustion | % | Percentage of decline in maximum ankle-rotation velocity from the 1-to-5 iTMT trials toward the 11-to-15 iTMT trials |
| iTMT Variability | % | Coefficient of variation (CoV) of ankle-rotation velocity during the first 15 iTMT trials |
iTMT: instrumented trail-making task
iTMT Protocol
The subject was instructed to stand in front of the computer-screen, while wearing the sensor on the lower shin of the dominant leg (Figure 1A). For safety purposes, a research coordinator was in the room supervising the iTMT test at all times. Subjects were given the option to use the support of a sturdy chair or table placed in front of them to maintain balance during ankle-rotation, which required weight-shifting tasks. Before starting the iTMT, the research coordinator described the protocol to the subject. After starting the iTMT test, the research coordinator did not provide any further guidance; only the interactive interface provided the necessary guidance and instructions, as described by the following.
For trail-making, the subject needed to stand upright (always in double stance) and move the hip in the anterior-posterior (AP) direction to generate dorsiflexion/plantarflexion at the ankle without lifting the heels or toes (Figure 2A). The subject navigated the cursor to correct targets in a certain order, by rotating the ankle joint, defined as an ankle-rotation task [24].
Figure 2.
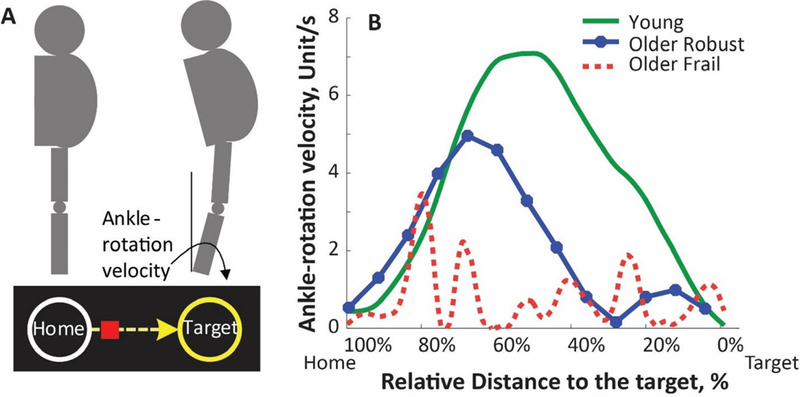
(A) For trail-making, the subject needed to stand upright (always in double stance) and move the hip in the anterior-posterior (AP) direction in order to generate dorsiflexion/plantarflexion at the ankle without lifting heels or toes. The rotation of ankle was mapped into movement of a computer-cursor. A reaching task is defined by navigating the cursor (by rotating ankle joint) from a home circle and stopping the cursor at the middle of a target circle. If the subject achieved to complete the reaching to correct target circle, the target would explode with a rewarding sound. Different visual and audio feedbacks were provided to encourage and assist successful completion of the iTMT. (B) Ankle-rotation velocity for a typical young subject (green solid curve), a typical older robust subject (blue solid curve with filled circles), and a typical frail subject (dash-curve in red).
In the iTMT test, 6 circles appeared on the screen, 1 home circle in white and 5 target circles in yellow (Figure 1B). The target circles were located in a fanwise position in front of the home circle. Each target circle had a number (“1”, “2”, or “3”) or letter (“A” or “B”) located in the center (the order of numbers and letters was randomized). At the beginning of the iTMT, the position of the cursor was automatically calibrated to the center of the home circle, as described earlier. By rotating the ankle joint, the subject navigated the cursor from the home circle to the center of target circles with numbers and letters alternately, defined as the iTMT trial. To be specific, the subject navigated the cursor from the home circle to the center of the first target circle (with number “1” inside, iTMT trial #1). Then the subject navigated the cursor back to the home circle and went to the second target circle (with letter “A” inside, iTMT trial #2), and came back to the home circle and went to the third target circle (with number “2” inside, iTMT trial #3), and went on. When the cursor stopped at the center of the last target circle (with number “3” inside, iTMT trial #5), 1 round of the test was completed, defined as the iTMT round. The whole iTMT test contains 3 continuous rounds. For the iTMT test, besides performing the motor task, the subject also needed to observe and figure out the correct location of the next target and remember to switch between number and letter sequences (cognitive tasks associated) [16, 17]. If the subject navigated the cursor to the correct target circle, the border of the target circle would turn red; and the target circle would explode with a rewarding sound. If the subject navigated the cursor to a wrong target circle, a visual and audio feedback indicating a mistake would be played. When that occurred, the subject had to go back to the home circle and continue the trail-making task from where he/she made the previous mistake. If the subject made 3 consecutive mistakes, a visual cue (flashing of correct target circle) would appear to guide him/her to correct the sequence.
iTMT-derived Parameters
Several measurable parameters were derived from the sensor data, including iTMT time, iTMT Velocity, iTMT Power, iTMT Exhaustion, and iTMT Variability (Table 1). The iTMT time was defined as the time needed to successfully complete the iTMT test and was shown to be correlated with cognitive function [16, 17]. In this study, we explored other iTMT-derived parameters.
During the post-processing phase, we used data recorded from sample-to-sample cursor velocity during each iTMT trial to estimate the ankle-rotation velocity curve (Figure 2B). In summary, the norm of recorded cursor velocities in directions of X and Y was estimated. Next, a low pass filter using wavelet transfer (mother wavelet: db5, cut of frequency: 6.25Hz) was applied. The maximum value of ankle-rotation velocity for each iTMT trial was estimated. The iTMT Velocity was defined as the average of maximum ankle-rotation velocities across the first 15 iTMT trials. This was done for a fair comparison between subjects, considering that some subjects (in particular, those with cognitive impairment) may have more than 15 iTMT trials (due to mistakes they made) to complete the iTMT test. To estimate the power, ankle-rotation velocity and acceleration were multiplied for each iTMT trial. The ankle-rotation acceleration was estimated with a single derivation of ankle-rotation velocity [25]. The iTMT Power was calculated as the maximum power during the iTMT test, averaged for the first 15 iTMT trials. The iTMT Exhaustion was calculated as the percentage of decline in maximum ankle-rotation velocity from the 1-to-5 iTMT trials toward the 11-to-15 iTMT trials. If the maximum ankle-rotation velocity was higher in the 11-to-15 iTMT trials, the iTMT Exhaustion was considered as 0%. The iTMT Variability was calculated as the coefficient of variation (CoV) of ankle-rotation velocity during the first 15 iTMT trials.
Figure 2B illustrates patterns of ankle-rotation velocity during a single trail-making, for a typical young subject (green solid curve), older robust subject (blue solid curve with filled circles), and older frail subject (dash-curve in red). At the beginning of the target reaching, the typical young subject first accelerated the ankle-rotation velocity to navigate the cursor on the computer-screen to the target. When the subject reached the maximum ankle-rotation velocity, he/she started to decelerate to achieve zero ankle-rotation velocity for stopping the cursor at the center of the target. For this typical young subject, the ankle-rotation velocity curve had only one peak, with a relatively large magnitude (Figure 2B). For the typical older robust subject, he/she also had a relatively smooth acceleration and deceleration of ankle-rotation velocity. However, the peak velocity was lower than that of the typical young subject. For the typical older frail subject, multiple ankle-rotation velocity peaks could be observed from Figure 2B, indicating large variability. The peak velocity was lower even when compared to the typical older robust subject.
Statistical Analysis
All continuous data were presented as mean±SD. All categorical data were expressed as count(percentage). The Shapiro-Wilk test was applied for testing normality of data.Analysis of variance (ANOVA) was used for comparing the iTMT-derived parameters between different groups and between the presence and absence of each frailty phenotype. Fisher’s least significant difference-based post-hoc test was performed for pairwise comparison to explore significant main effects or interactions. The effect size to discriminate between groups was estimated using Cohen’s d effect size and represented as d in the Results section. Values ranging from 0.20 to 0.49 indicate small effects, and values between 0.50 and 0.79 indicate medium effects. Values ranging from 0.80 to 1.29 indicate large effects, and values above 1.30 indicate very large effects. Values less than 0.20 are considered as having no noticeable effect [26]. The Pearson correlation coefficient was used to evaluate the degree of agreement between the iTMT Velocity and conventional gait speed. Logistic regression analysis was employed to examine the relationship between each study variable and frailty. First, univariate logistic regression was employed to investigate the relationship of the test variables using “non-robust/robust” as the dependent variable. This strategy reflects the exploratory character of the study. The odds ratio (OR) was calculated for each explanatory variable. Second, stepwise multivariate logistic regression, using variables found with p<0.20 in the univariate analysis, was performed to investigate the independent effects of variables in predicting frailty. To examine whether the iTMT-derived parameters may yield similar results as gait speed, we used 2 different models. Variables in one of the models (Model 2) included gait speed and other variables found with p<0.20 in the univariate analysis, except the iTMT-derived parameters. In Model 3, we replaced gait speed in Model 2 by the iTMT-derived parameters. In Model 1 (reference model), we used all variables found with p<0.20 in the univariate analysis, except gait speed and the iTMT-derived parameters. The receiver-operating-curve (ROC) and area-under-curve (AUC) were calculated for different frailty-prediction models. A 2-sided p<0.050 was considered to be statistically significant. All statistical analyses were performed using IBM SPSS Statistics 24 (IBM, IL, USA).
Results
All subjects were able to complete the iTMT test, with an average completion time of 125±85 seconds. None of the subjects stopped or were overtaxed during the test, indicating high feasibility of the test. No adverse events, including loss of balance, were observed during the iTMT test.
Table 2 summarizes demographic and clinical data. The older subjects’ ages ranged from 60 to 93 years. No between-group difference was observed for older subjects’ gender, height, weight, BMI, daily number of prescription medicines, daily number of over-the-counter medicines, and prevalence of depression (p>0.050). However, as expected, the average age of the robust group was significantly younger than that of the pre-frail and frail groups (p=0.035). The pre-frail and frail groups had higher prevalence of using of walking assistance, fall history, and high concern about falling than the robust group. However the differences did not reach statistical significance. For individual frailty phenotypes, as expected, the frail group had significantly higher prevalence of presence than the pre-frail group for every individual phenotype. For the gait assessment, with progression in frailty, a significant decline of gait speed was observed.
Table 2.
General characteristics of the study population.
| Variable (mean±SD) | Older Adults |
p-value (between Older Adults) |
Young (n = 17) |
||
|---|---|---|---|---|---|
| Robust (n = 24) |
Pre-frail (n = 29) |
Frail (n = 8) |
|||
| Age, years (range) |
68.8±8.1 (60–89) |
75.0±9.8 (62–91) |
76.8±11.9 (65–93) |
0.035 | 29.0±7.2 (20–35) |
| Female, % | 38% | 35% | 50% | 0.725 | 35% |
| Height, cm | 171.7±9.8 | 169.5±11.3 | 162.9±8.6 | 0.125 | 170.4±9.7 |
| Weight, kg | 79.7±18.2 | 78.9±15.5 | 73.9±19.8 | 0.711 | 76.2±14.8 |
| BMI, kg/m2 | 27.3±5.6 | 27.3±3.7 | 27.9±7.2 | 0.963 | 26.2±4.6 |
| Daily # of Prescription Medicines, n | 5±4 | 5±3 | 6±3 | 0.699 | - |
| Daily # of Over the Counter Medicines, n | 2±2 | 3±2 | 1±1 | 0.243 | - |
| Using Walking Assistance, % | 5% | 27% | 14% | 0.131 | - |
| Fall history, % | 17% | 28% | 29% | 0.607 | - |
| High Concern about Falling, % | 17% | 35% | 29% | 0.342 | - |
| Depression, % | 25% | 14% | 38% | 0.298 | - |
| Slowness, % | 0 | 14% | 88% | <0.001 | - |
| Weakness, % | 0 | 52% | 88% | <0.001 | - |
| Exhaustion,% | 0 | 35% | 50% | 0.002 | - |
| Inactivity, % | 0 | 41% | 88% | <0.001 | - |
| Gait Speed, m/s | 1.06±0.19 | 0.99±0.23 | 0.75±0.18 | 0.002 | 1.19±0.18 |
BMI: body-mass-index
Significant difference between groups were indicated in bold
Results suggested that the iTMT Velocity, iTMT Power, iTMT Exhaustion, and iTMT Variability enable significant discrimination between the presence and absence, as determined by the FFC, of slowness, weakness, exhaustion, and inactivity phenotypes, respectively (Figure 3). In summary, the iTMT Velocity distinguished between the presence and absence of slowness with very large effect size (d=1.40, p<0.001, Figure 3A). Similarly, the iTMT Power determined the presence of weakness (d=1.38, p<0.001, Figure 3B), the iTMT Exhaustion determined the presence of exhaustion (d=0.98, p=0.003, Figure 3C), and the iTMT Variability determined the presence of inactivity (d=0.90, p<0.001, Figure 3D), all with large-to-very-large effect sizes.
Figure 3.
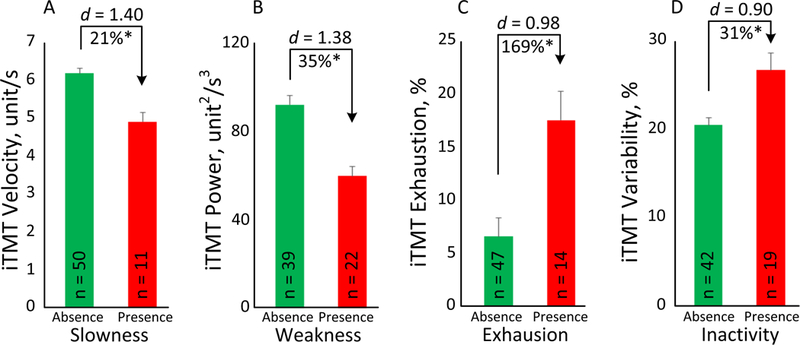
The iTMT derived parameters enabled significant discrimination between the presence and absence of each frailty phenotype as determined by the Fried Frailty Criteria, including slowness (Fig. 3A), weakness (Fig. 3B), exhaustion (Fig. 3C), and inactivity (Fig. 3D).Error bars represent the standard errors. ‘n’ denotes number of subjects per group. ‘*’ denotes when the between group comparison achieved a statistically significant level (p<0.050).
Table 3 summarizes gait speed and the iTMT-derived parameters for older robust subjects, older non-robust subjects, and young subjects. The older non-robust group had poorer performance for gait speed and all of the parameters, as measured by the iTMT, than the robust and young subjects. Among these results, the iTMT Velocity had the largest effect sizes to distinguish between the 3 groups (Figure 4). The iTMT Velocity was 6.31±0.98 unit/s in the older robust group and was significantly decreased on average by 10% in the non-robust group (d=0.62, p=0.025). When compared with the older groups, a significantly higher iTMT Velocity of 7.30±1.13 unit/s was observed in the young group (Figure 4). Using the iTMT Velocity, we further compared between the pre-frail and frail subjects. Results suggested that the iTMT Velocity was able to discriminate pre-frail and frail with very large effect size (d=1.50, p=0.002).
Table 3.
Between group comparisons for gait speed and the iTMT derived parameters.
| Older Adults | Young (n = 17) |
Non-robust vs. Robust | Robust vs. Young | Non-robust vs. Young | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Robust (n = 24) |
Non-robust (n = 37) |
p-value | d | 95% CI | p-value | d | 95% CI | p-value | d | 95% CI | ||
| Gait Speed, m/s | 1.06±0.19 | 0.94±0.24 | 1.19±0.18 | 0.032 | 0.56 | 0.01–0.24 | 0.050 | 0.71 | 0.01–0.27 | <0.001 | 1.21 | 0.13–0.38 |
| iTMT Velocity, unit/s | 6.31±0.98 | 5.67±1.09 | 7.30±1.13 | 0.025 | 0.62 | 0.08–1.20 | 0.005 | 0.94 | 0.32–1.66 | <0.001 | 1.47 | 1.01–2.25 |
| iTMT Power, unit2/s3 | 90.56±26.73 | 73.70±28.47 | 113.81±39.94 | 0.040 | 0.61 | 0.78–32.94 | 0.020 | 0.68 | 3.81–42.70 | <0.001 | 1.16 | 22.14–58.09 |
| iTMT Exhaustion, % | 8.23±15.19 | 9.41±10.58 | 4.60±6.68 | 0.698 | 0.09 | −7.23–4.86 | 0.325 | 0.31 | −10.94–3.68 | 0.160 | 0.54 | −11.57–1.94 |
| iTMT Variability, % | 20.92±4.94 | 23.05±7.84 | 17.15±6.99 | 0.241 | 0.33 | −5.73–1.46 | 0.088 | 0.62 | −8.12–0.58 | 0.005 | 0.79 | 1.88–9.92 |
The Older Adults Non-robust group included pre-frail and frail subjects
iTMT: instrumented trail-making task
Significant difference between groups were indicated in bold
Effect sizes were calculated as Cohen’s d
Figure 4.
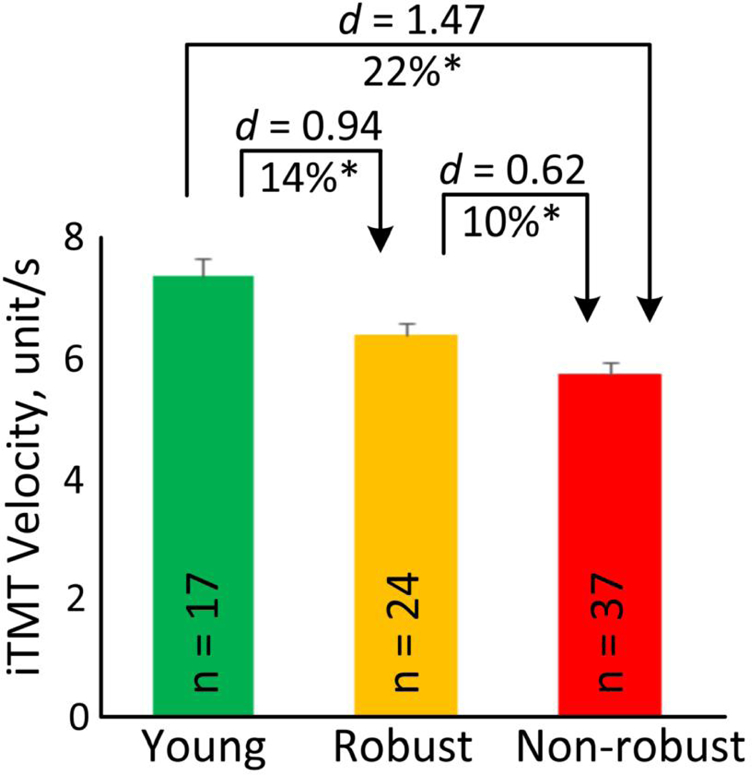
Average of the iTMT Velocity for young, older robust, and older non-robust groups. Error bars represent the standard errors. ‘n’ denotes number of subjects per group. ‘*’ denotes when the pairwise group comparison achieved a statistically significant level (p<0.050).
Figure 5 demonstrated the correlation between the iTMT and over ground gait test at habitual speed. A significant correlation was observed between the iTMT Velocity and gait speed (r=0.684, p<0.001).
Figure 5.
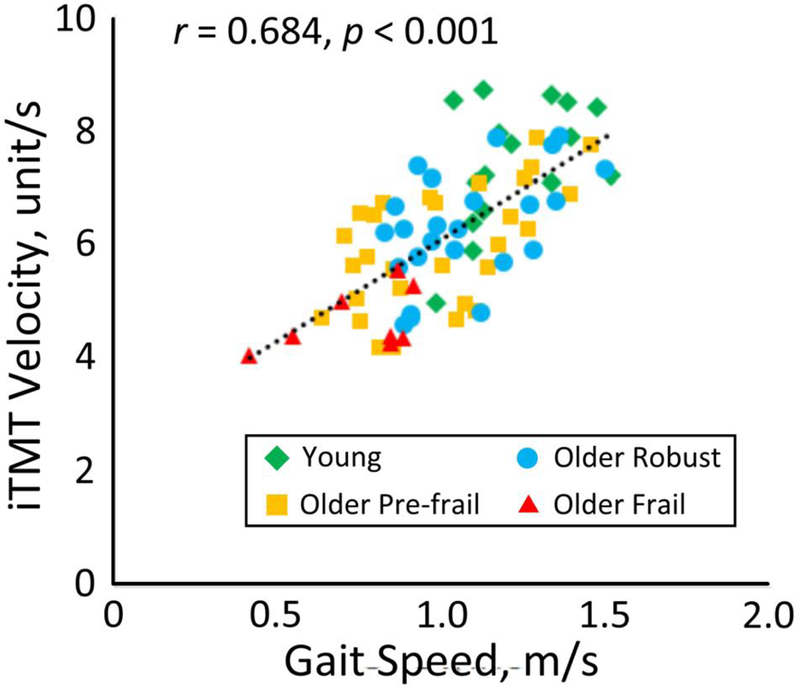
A significant correlation was observed between the iTMT Velocity and over ground gait speed.
In the univariate regression analysis, 4 variables were significantly associated with frailty: age, gait speed, iTMT Velocity, and iTMT Power (Table 4). In Model 1, only age remained in the model. In Model 2, only age and gait speed remained in the model. Similarly, in Model 3, age and iTMT Velocity remained in the model. The ROCs for the 3 models were displayed in Figure 6. The AUC for Model 1 was 0.708, with a sensitivity of 78.4% and specificity of 45.8% for predicting frailty. The AUC for Model 2 was 0.795, with a sensitivity of 79.3% and specificity of 61.9% for predicting frailty. The highest AUC (0.834) was obtained by Model 3, with a sensitivity of 82.8% and specificity of 66.7% for distinguishing non-robust cases.
Table 4.
Results of univariate logistic regression.
| Variable | Robust (n = 24) |
Non-robust (n = 37) |
OR | 95% CI | p-value |
|---|---|---|---|---|---|
| Age, years | 68.8±8.1 | 75.4±10.1 | 1.078 | 1.015 – 1.145 | 0.015 |
| Female, % | 38% | 38% | 1.014 | 0.351 – 2.929 | 0.979 |
| Height, cm | 171.7±9.8 | 168.1±11.0 | 0.966 | 0.918 – 1.017 | 0.186 |
| Weight, kg | 79.7±18.2 | 77.8±16.4 | 0.994 | 0.964 – 1.024 | 0.678 |
| BMI, kg/m2 | 27.3±5.6 | 27.4±4.6 | 1.005 | 0.904 – 1.116 | 0.930 |
| Daily # of Prescription Medicines, n | 5±4 | 5±3 | 1.002 | 0.855 – 1.174 | 0.979 |
| Daily # of Over the Counter Medicines, n | 2±2 | 2±2 | 1.019 | 0.786 – 1.320 | 0.887 |
| Using Walking Assistance, % | 5% | 24% | 6.364 | 0.719 – 56.353 | 0.096 |
| Fall history, % | 17% | 28% | 1.923 | 0.525 – 7.041 | 0.323 |
| High Concern about Falling, % | 17% | 33% | 2.500 | 0.697 – 8.971 | 0.160 |
| Depression, % | 25% | 19% | 0.700 | 0.203 – 2.412 | 0.572 |
| Gait Speed, m/s | 1.06±0.19 | 0.94±0.24 | 0.082 | 0.007 – 0.947 | 0.045 |
| iTMT Velocity, unit/s | 6.31±0.98 | 5.67±1.09 | 0.557 | 0.330 – 0.938 | 0.028 |
| iTMT Power, unit2/s3 | 90.56±26.73 | 73.70±28.47 | 0.979 | 0.960 – 0.998 | 0.030 |
| iTMT Exhaustion, % | 8.23±15.19 | 9.41±10.58 | 1.008 | 0.966 – 1.052 | 0.717 |
| iTMT Variability, % | 20.91±4.94 | 23.05±7.84 | 1.051 | 0.967 – 1.144 | 0.243 |
Figure 6.
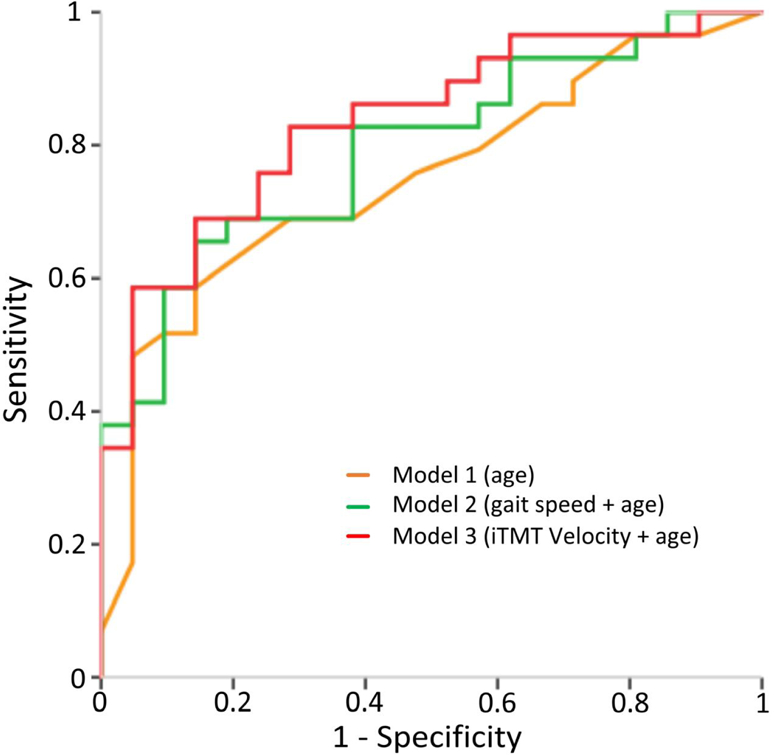
ROCs of different models for predicting frailty: Model 1 used ‘age’ (AUC=0.708), Model 2 used a combination of ‘gait speed’ and ‘age’ (AUC=0.795), and Model 3 used a combination of the ‘iTMT Velocity’ and ‘age’ (AUC=0.834).
Discussion
This study demonstrates feasibility and ability of an innovative iTMT to quantify physical frailty phenotypes. In addition, it examined the ability of the iTMT to distinguish between robust individuals and those with frailty (pre-frail or frail), as determined by the FFC. Results demonstrated that the iTMT-derived parameters were able to identify the presence and absence of key physical frailty phenotypes (slowness, weakness, exhaustion, and inactivity) with large-to-very-large effect sizes (d=0.90–1.40). In addition, the iTMT Velocity was able to discriminate between robust and pre-frail or frail individuals with medium effect size (d=0.62). Comparing with other iTMT-derived parameters, the iTMT Velocity had the largest power to distinguish different frailty stages, as determined by the FFC. This finding was in agreement with previous works, which demonstrated that gait speed is the most important indicator of frailty syndrome [27].
Key advantages of the iTMT platform include its practicality for a busy setting (unlike the FFC, it does not require a walking test to determine physical frailty), relatively low cost (as it only needs a single inertial sensor), time efficiency (as the whole test takes less than 5 minutes) and objectivity (as a fully computerized test to determine frailty).
From the ease of administration standpoint, this study demonstrates that the interactive interface of the iTMT and computerized instructions provided to the subjects were clear enough to administer the test with minimal supervision from the examiner. This allows reducing the potential bias related to the examiner’s experience. It may also provide the opportunity to administer the test by non-expert staff and those with minimum training, making the administration of the test feasible, irrespective of setting, including in-clinic and in-home. Furthermore, it may facilitate integration of the test for the purpose of telemedicine application, in which the individual could perform the test while standing in front of a telemedicine tablet or screen. More importantly, all subjects, including frail ones and those who needed walking assistance, were able to complete the test. In our study, 16% of subjects were unable to walk without assistance or walking aid; and 23% had history of falls. For these individuals, gait assessment might be challenging or difficult to safely administer. No adverse event or difficulty was observed in the current study for the iTMT test.
From the time-efficacy standpoint, our results suggested that the completion time of the iTMT test was, on average, 125±85 seconds; and subject preparation and sensor setup were less than 2 minutes, making the whole operation less than 5 minutes. This adds advantages when comparing to the FFC assessment, which usually takes 15 to 20 minutes [28], thus making the iTMT suitable and potentially cost effective for busy clinics.
Previous studies [27, 29] have often evaluated slowness by recording an individual’s walking speed from one point to another. For the iTMT, we use the subject’s ankle-rotation velocity to determine slowness, while standing in front of a computer with no need of a walking test. In addition, subjects can hold a chair or table placed in front of them to avoid falling or to maintain balance if needed. This has superior benefits compared to the FFC walking test, since administrating the FFC walking test in a busy clinical setting is often impractical; could be hazardous, depending on the subject’s risk of falling; and may be biased by the type of footwear, walking accessories (e.g., cane and walker), and walking test condition [20, 30]. On the other hand, the iTMT test requires devices, including a single inertial sensor and a standard computer, which may add to the cost when comparing to the conventional FFC walking test. We believe this tradeoff may still be acceptable, considering the time saved by approximately a factor of 3 when comparing to the FFC. In addition, the iTMT test does not need walking space, which makes it more convenient than conventional physical frailty assessments based on the FFC walking analysis. Although there is a significant correlation between the iTMT Velocity and gait speed, it should be recognized that the iTMT may not provide all useful features from gait tests for all clinical applications. However, it may be an acceptable surrogate to determine slowness and frailty stages.
Our results suggest that the iTMT Velocity has the largest effect size to identify the presence of slowness phenotype, as determined by the FFC. This was in agreement with another finding of the current study, in which we demonstrated that the iTMT Velocity has significant correlation with gait speed. The finding was also consistent with other studies, which have demonstrated that speed of joint rotation can be used as a surrogate of gait speed. For example, Toosizadeh et al. have demonstrated that speed of elbow rotation during a repetitive 20-second elbow flexion-extension task is correlated with gait speed, which can determine slowness and frailty in older adults [31]. We found the percentage of decline in maximum ankle-rotation velocity (iTMT Exhaustion) has the largest effect size to identify the presence of exhaustion phenotype in the FFC. This result is supported by the finding in a study by Toosizadeh et al. [31] that the speed reduction of elbow flexion-extension in 20 seconds is able to quantify exhaustion frailty phenotype. We found the power of ankle-rotation (iTMT Weakness) is able to identify the presence of weakness. This finding was consistent with previous works, which have demonstrated that power generated during joint rotation is in agreement with grip strength and weakness phenotype in older adults [25, 31]. Among the iTMT parameters, the iTMT Variability had the largest effect size to identify inactivity phenotype. According to the cycle of frailty suggested by Fried et al. [32], inactivity is a marker of reduction in total energy expenditure in frail people. We speculated that low total energy expenditure could also be explained by poor motor efficiency and high motor variability. These can be measurable by wearable sensors and can be quantified by the iTMT Variability. This is supported by prior studies, which suggest that large gait variability is associated with increasing in metabolic cost and decreasing physical activity level [33, 34]. In this study, we did not find any iTMT-derived parameter, which can identify the presence of shrinking phenotype. This may be due to insufficient power, as our sample included only 4 cases with the presence of shrinking phenotype.
The results of regression analysis suggested that age alone (Model 1) has poor specificity to distinguish frailty. When adding gait speed into the model (Model 2), the specificity was increased from 46% to 62% with modest increase in sensitivity from 78% to 79%. When replacing gait speed with the iTMT Velocity (Model 3), both sensitivity and specificity improved and reached 83% and 67%, respectively. Together, we concluded that the iTMT could be a good surrogate for the FFC walking test to determine frailty status independent of age. But future studies are needed to demonstrate whether the proposed model is clinically meaningful to predict health outcomes in geriatric populations.
In our previous studies [16, 17], we have demonstrated that the iTMT is able to assess cognitive impairment in older adults. The iTMT test has significant agreement (r>0.65) with conventional cognitive screening tools, including Trail Making Task (TMT A&B) and Montreal Cognitive Assessment (MoCA). Combined with the results of this study, we may speculate that the iTMT enables assessing “cognitive frailty” (frailty together with cognitive impairment) [35], which has been shown to be a strong and independent predictor of cognitive decline over time [36, 37]. If this hypothesis is confirmed by future studies, the iTMT may have the potential to detect the subtle progression of cognitivedecline over time, which in turn may facilitate early intervention and prevention of further cognitive and functional decline [38].
Limitations and Future Directions
One limitation of this study is that our subjects were recruited from specialized outpatient clinics instead of older adults dwelling in the community. Thus, our sample may not represent the general older population. The observation needs to be confirmed in a larger study representing the general older population.
In this study, the number of frail subjects was very small (n=8, 13% of older participants in our sample). This number was in agreement with the prevalence of frailty in the general population, which was estimated to be 12% [1, 39]. However, for the purpose of between-group comparison, 8 subjects were considered as underpowered for a fair comparison. Thus, we merged pre-frail and frail subjects into a single group. However, when we compared frail and pre-frail groups, the iTMT Velocity was able to distinguish between groups, with a very large effect size (d=1.50). According to this effect size, to achieve a minimum power of 80%, a minimum sample size of 9 subjects per group is required to observe a statistical significance of 5% or lower, using 2-tailed independent-sample comparisons.
In this study, we did not adjust for age because frailty is a geriatric symptom; and the likelihood of frailty increases with age. In addition, our reference to determine different frailty stages was the FFC, which has considered age to determine cutoff points for the presence and absence of frailty phenotypes. The purpose of this study was to evaluate the feasibility and ability of using the iTMT to quantify frailty phenotypes and distinguish different frailty stages, as determined by the FFC. Thus, considering that our gold standard has been already adjusted by age, further adjustment was determined to be unnecessary for the purpose of this study. Furthermore, when age and the iTMT-derived parameters were used in a multivariate model, the iTMT Velocity remained a significant predictor of non-robustness, independent of age. Another study is needed to demonstrate that the iTMT, irrespective of age, is also able to predict adverse health outcomes in the geriatric population.
We have demonstrated that, when gait speed was replaced by the iTMT Velocity, the power of the model to distinguish frailty status was improved. However, we did not explore other gait parameters and other gait test conditions (e.g., dual task test), which may help to improve the accuracy of a gait-based model to determine frailty status [27]. Thus, this study cannot conclude that the iTMT is superior than the gait test to determine physical frailty but rather that it is more practical in some circumstances in which the administration of gait testing is cumbersome or impractical.
In this study, we demonstrated that the iTMT has a significant correlation with the continuous frailty phenotype variable, such as gait speed. However, a continuous scale to describe frailty stages similar to the Rockwood Frailty index has not been developed because of the selection of the FFC as our gold standard. Another study is required to develop a continuous frailty model similar to a validated frailty meter with a continuous scale [25, 40]. We anticipate that a continuous frailty index may be more sensitive to change than a categorical frailty model, as suggested in previous studies [25, 40]. In addition, we anticipate that measurement of both physical frailty and cognitive function, as offered by the iTMT, would be more sensitive to track the changes in life independency and pathway to dementia, as suggested by literature on cognitive decline and frailty [36, 37]. These hypotheses should be validated in future studies.
Conclusions
To our knowledge, the iTMT is the first platform enabling the simultaneous and objective measurements of both physical frailty and cognitive performance in the geriatric population. Other key advantages of the iTMT platform are its objectivity, time efficiency, and ability to objectively quantify different frailty phenotypes such as slowness, exhaustion, weakness and inactivity, with a practical test protocol that does not need self-report or conventional FFC walking examination. Future studies are required to confirm the observation in a larger sample, as well as examine the ability of the iTMT to track changes in physical frailty over time, its sensitivity to intervention, and its ability to predict post-intervention adverse outcomes.
Acknowledgments
Partial support was provided by the National Institutes of Health/National Institute on Aging (award number 2R42AG032748) and National Institutes of Health/National Cancer Institute (award number 1R21CA190933-01A1), and Baylor College of Medicine, Michael E. DeBakey Department of Surgery. The content is solely the responsibility of the authors and does not necessarily represent the official views of sponsors. We thank Ana Enriquez, Ivan Marin, and Noreen Siddiqi for assisting with data collection and coordination of this research study between involved key investigators.
Footnotes
Conflict of Interest: The iTMT is protected by a patent (pending). HZ and BN are listed as co-inventors. However they do not claim any financial conflict of interest relevant to this study.
Reference
- 1.Fried LP, et al. , Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci, 2001. 56(3): p. M146–56. [DOI] [PubMed] [Google Scholar]
- 2.Morley JE, Diabetes, sarcopenia, and frailty. Clin Geriatr Med, 2008. 24(3): p. 455–69, vi. [DOI] [PubMed] [Google Scholar]
- 3.Janssen I, et al. , Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol, 2004. 159(4): p. 413–21. [DOI] [PubMed] [Google Scholar]
- 4.Makary MA, et al. , Frailty as a predictor of surgical outcomes in older patients. Journal of the American College of Surgeons, 2010. 210(6): p. 901–908. [DOI] [PubMed] [Google Scholar]
- 5.Mohler MJ, et al. , The Frailty syndrome: clinical measurements and basic underpinnings in humans and animals. Exp Gerontol, 2014. 54: p. 6–13. [DOI] [PubMed] [Google Scholar]
- 6.Podsiadlo D and Richardson S, The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. Journal of the American geriatrics Society, 1991. 39(2): p. 142–148. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein JP, Andrew MK, and Travers A, Frailty in older adults using pre-hospital care and the emergency department: a narrative review. Canadian geriatrics journal: CGJ, 2012. 15(1): p. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchman A, et al. , Change in frailty and risk of death in older persons. Experimental aging research, 2009. 35(1): p. 61–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.da Câmara SMA, et al. , Using the Short Physical Performance Battery to screen for frailty in young‐old adults with distinct socioeconomic conditions. Geriatrics & gerontology international, 2013. 13(2): p. 421–428. [DOI] [PubMed] [Google Scholar]
- 10.Verghese J and Xue X, Identifying frailty in high functioning older adults with normal mobility. Age and ageing, 2010. 39(3): p. 382–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goel R, et al. , Assessing Somatosensory Utilization during Unipedal Postural Control. Frontiers in systems neuroscience, 2017. 11: p. 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang C, et al. , Low-Power Fall Detector Using Triaxial Accelerometry and Barometric Pressure Sensing. IEEE Transactions on Industrial Informatics, 2016. 12(6): p. 2302–2311. [Google Scholar]
- 13.Razjouyan J, et al. , Wearable Sensors and the Assessment of Frailty among Vulnerable Older Adults: An Observational Cohort Study. Sensors (Basel, Switzerland), 2018. 18(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zahiri M, et al. , Design and evaluation of a portable laparoscopic training system using virtual reality. Journal of Medical Devices, 2017. 11(1): p. 011002. [Google Scholar]
- 15.Nguyen H, et al. , Using inertial sensors to automatically detect and segment activities of daily living in people with Parkinson’s disease. IEEE Transactions on Neural Systems and Rehabilitation Engineering, 2018. 26(1): p. 197–204. [DOI] [PubMed] [Google Scholar]
- 16.Zhou H, et al. , Instrumented trail-making task to differentiate persons with no cognitive impairment, amnestic mild cognitive impairment, and Alzheimer disease: a proof of concept study. Gerontology, 2017. 63(2): p. 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou H, et al. , Motor planning error: toward measuring cognitive frailty in older adults using wearables. Sensors, 2018. 18(3): p. 926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delbaere K, et al. , The falls efficacy scale international (FES-I). A comprehensive longitudinal validation study. Age and ageing, 2010. 39(2): p. 210–216. [DOI] [PubMed] [Google Scholar]
- 19.Weissman MM, et al. , Assessing depressive symptoms in five psychiatric populations: a validation study. American journal of epidemiology, 1977. 106(3): p. 203–214. [DOI] [PubMed] [Google Scholar]
- 20.Najafi B, et al. , Does walking strategy in older people change as a function of walking distance? Gait Posture, 2009. 29(2): p. 261–6. [DOI] [PubMed] [Google Scholar]
- 21.Grewal G, et al. , Virtualizing the assessment: a novel pragmatic paradigm to evaluate lower extremity joint perception in diabetes. Gerontology, 2012. 58(5): p. 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Najafi B, et al. , Assessing postural control and postural control strategy in diabetes patients using innovative and wearable technology. J Diabetes Sci Technol, 2010. 4(4): p. 780–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Najafi B, et al. , Estimation of Center of Mass Trajectory using Wearable Sensors during Golf Swing. J Sports Sci Med, 2015. 14(2): p. 354–63. [PMC free article] [PubMed] [Google Scholar]
- 24.Grewal GS, et al. , Sensor-Based Interactive Balance Training with Visual Joint Movement Feedback for Improving Postural Stability in Diabetics with Peripheral Neuropathy: A Randomized Controlled Trial. Gerontology, 2015. 61(6): p. 567–74. [DOI] [PubMed] [Google Scholar]
- 25.Lee H, et al. , Toward Using a Smartwatch to Monitor Frailty in a Hospital Setting: Using a Single Wrist-Wearable Sensor to Assess Frailty in Bedbound Inpatients. Gerontology, 2017. [DOI] [PMC free article] [PubMed]
- 26.Cohen J, Statistical power analysis for the behavioral sciences 2nd 1988, Hillsdale, NJ:erlbaum. [Google Scholar]
- 27.Schwenk M, et al. , Frailty and technology: a systematic review of gait analysis in those with frailty. Gerontology, 2014. 60(1): p. 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gary R, Evaluation of frailty in older adults with cardiovascular disease: incorporating physical performance measures. The Journal of cardiovascular nursing, 2012. 27(2): p. 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwenk M, et al. , Wearable sensor-based in-home assessment of gait, balance, and physical activity for discrimination of frailty status: baseline results of the Arizona frailty cohort study. 606 Gerontology, 2015. 61(3): p. 258–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Najafi B, et al. , The impact of footwear and walking distance on gait stability in diabetic patients with peripheral neuropathy. J Am Podiatr Med Assoc, 2013. 103(3): p. 165–73. [DOI] [PubMed] [Google Scholar]
- 31.Toosizadeh N, Mohler J, and Najafi B, Assessing upper extremity motion: an innovative method to identify frailty. Journal of the American Geriatrics Society, 2015. 63(6): p. 1181–1186. 611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fried LP, Walston J Hazzard WR, Blass JP, Ettinger WH Jr., Halter JB and Ouslander J, Principles of Geriatric Medicine and Gerontology 4th ed. McGraw Hill; 1998, New York: pp. 1387–1402. [Google Scholar]
- 33.O’Connor SM, Xu HZ, and Kuo AD, Energetic cost of walking with increased step variability. Gait & posture, 2012. 36(1): p. 102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montero-Odasso M, et al. , Gait variability is associated with frailty in community-dwelling older 6adults. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences, 2011. 66(5): p. 568–576. [DOI] [PubMed] [Google Scholar]
- 35.Shimada H, et al. , Cognitive Frailty and Incidence of Dementia in Older Persons. The journal of prevention of Alzheimer’s disease, 2018. 5(1): p. 42–48. [DOI] [PubMed] [Google Scholar]
- 36.Kelaiditi E, et al. , Cognitive frailty: rational and definition from an (IANA/IAGG) international consensus group. The journal of nutrition, health & aging, 2013. 17(9): p. 726–734. [DOI] [PubMed] [Google Scholar]
- 37.Ruan Q, et al. , Cognitive frailty, a novel target for the prevention of elderly dependency. Ageing research reviews, 2015. 20: p. 1–10. [DOI] [PubMed] [Google Scholar]
- 38.Dierckx E, et al. , Mild cognitive impairment: what’s in a name? Gerontology, 2007. 53(1): p. 28– 62635. [DOI] [PubMed] [Google Scholar]
- 39.Buckinx F, et al. , Burden of frailty in the elderly population: perspectives for a public health challenge. Archives of Public Health, 2015. 73(1): p. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joseph B, et al. , Upper-extremity function predicts adverse health outcomes among older adults hospitalized for ground-level falls. Gerontology, 2017. 63(4): p. 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]


