Abstract
Carbon−hydrogen (C−H) and carbon−carbon (C−C) bonds are the main constituents of organic matter. The recent advancement of C−H functionalization technology has vastly expanded our toolbox for organic synthesis1. In contrast, C−C activation methods that allow for editing the molecular skeleton remain limited2–7. To date, a number of methods have appeared for catalytic C−C activation, particularly with ketone substrates, which are typically promoted either by ring-strain release as a thermodynamic driving force4,6 or using directing groups5,7 (DGs) to control the reaction outcome. While effective, these strategies require highly strained ketone substrates or those containing a preinstalled DG, or are limited to more specialist substrate classes5. Here, we report a general C−C activation mode driven by aromatization of an in situ-formed pre-aromatic intermediate. This reaction suitable for various ketone substrates, is catalyzed by an iridium/phosphine combination, and is promoted by a hydrazine reagent and 1,3-dienes. Specifically, the acyl group is removed from the ketone, transformed to a pyrazole, and the resulting alkyl fragment undergoes various transformations. These include the deacetylation of methyl ketones, carbenoid-free formal homologation of aliphatic linear ketones, and deconstructive pyrazole synthesis from cyclic ketones. Given that ketones are prevalent in feedstock chemicals, natural products and pharmaceuticals, these transformations could offer new strategic bond disconnections in the synthesis of complex bioactive molecules.
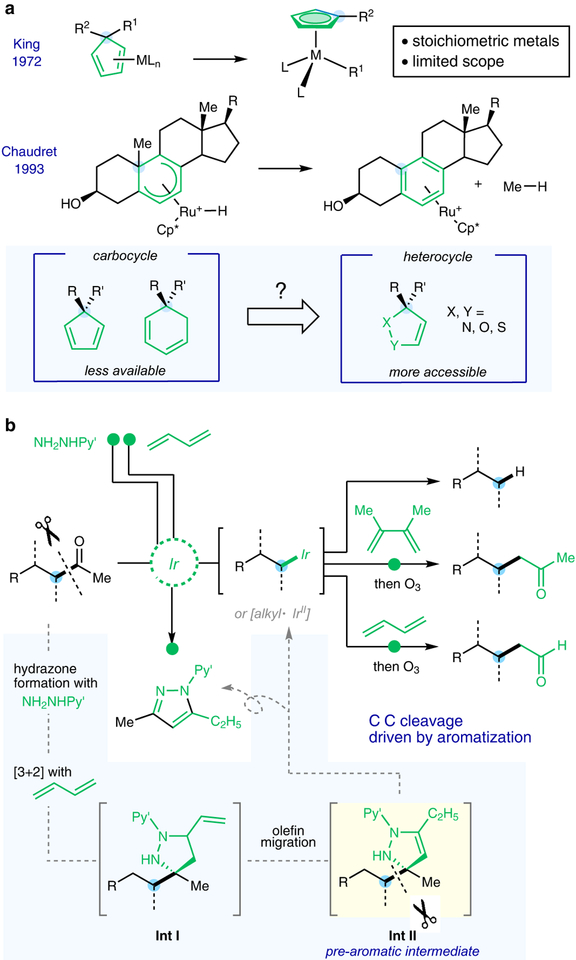
Aromaticity has been known as an important thermodynamic driving force8 for various synthetic and enzymatic transformations. For example, in the biosynthesis of estrogens, aromatase converts testosterone to estradiol through a multi-step oxidative C−C cleavage process9. Forming aromatic compounds has also been known to promote the transition metal-mediated C−C activation since 197210, which unfortunately has been largely underappreciated with only scattered examples10–14 (Fig. 1a). These reactions use a pre-aromatic substrate to complex with a low-valent transition metal, and subsequent C−C cleavage leads to stable arene-metal species, such as Cp-metal complexes (Cp: cyclopentadienyl)10,11,14. However, to utilize such an approach for catalytic synthetic applications, a number of challenges still exist. First, special high-energy pre-aromatic substrates, e.g. cyclopentadienes, are generally needed; thus, it is concerned that whether readily available compounds can be used as substrates. In addition, the aromatic products generated in this reaction typically coordinate strongly with metals; thus, enabling catalyst turnover could be a significant issue10–12,14. Moreover, given the narrow reaction scope, developing attractive and synthetically valuable transformations with aromatization-driven C−C activation represent another difficulty.
Figure 1 |. C−C activation driven by aromatization.
a, Known examples of transition metal-mediated C−C cleavage driven by aromatization. b, This work: catalytic deacylative transformations of common unstrained ketones. M, transition metal; Cp*, pentamethylcyclopentadienyl; Py’, 4-methyl-2-pyridyl.
In contrast to the less accessible carbocyclic pre-aromatics, a number of heterocycles, prepared from readily available chemicals through 1,3-dipolar addition15, could potentially serve as precursors to form heteroarenes. We found such a pre-aromatized heterocycle could serve as the key intermediate for the aromatization-promoted C−C activation, thereby enabling deacylative transformations of common ketones (Fig. 1b). This reaction likely involves a three-component coupling of a ketone, a 1,3-diene and a substituted hydrazine to generate a dihydropyrazole intermediate (Int II) that subsequently undergoes C−C cleavage to form a pyrazole16,17 and an activated alkyl species (e.g. an alkyl-Ir intermediate).
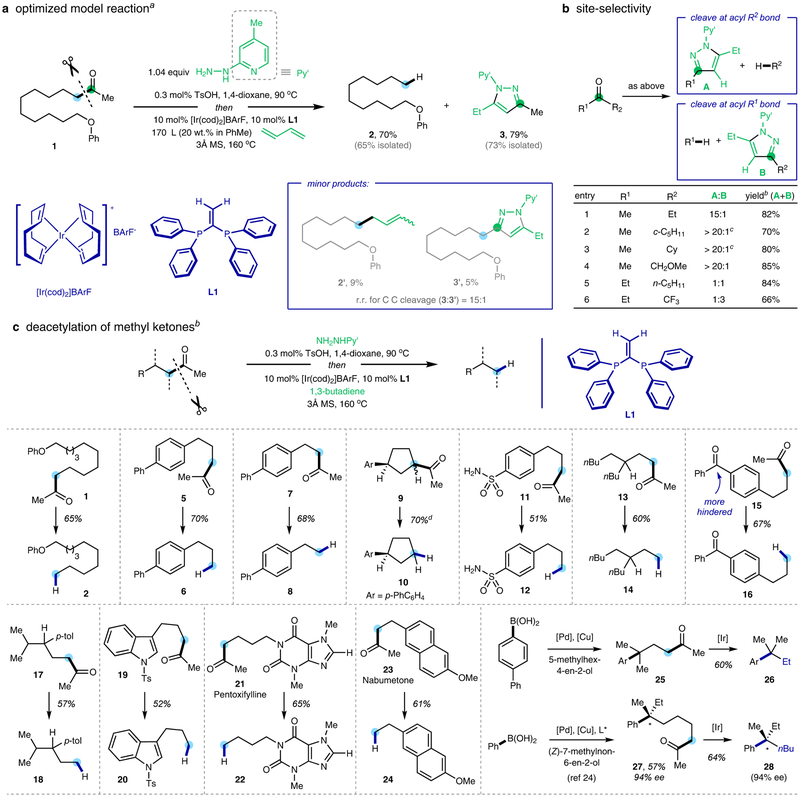
The initial reaction mode was discovered during our exploration of the Rh-catalyzed β-C−H functionalization of ketones with 1,3-butadiene18, in which a small amount of pyrazoles (ca. 5%) were surprisingly yielded as a side product. This reaction was further optimized using 12-phenoxydodecan-2-one (1) as the model substrate, and iridium was found more reactive than rhodium (see Supplementary Information, Section 3.1). After a systematic survey of reaction parameters, the ketone substrate, upon treatment with 4-methyl-2-pyridyl hydrazine and 1,3-butadiene in toluene, underwent efficient α-C−C bond cleavage using cationic [Ir(cod)2]BArF19 and 1,1-bis(diphenylphosphino)ethylene as the optimal metal/ligand combination (Fig. 2a). Pyrazole 3 was formed in 79% yield, along with deacetylation product 2 in 70% yield, as the two major products. A minor deacylative crotylation product (2’) was also observed in 9% yield, which should come from coupling of the alkyl fragment with 1,3-butadiene. Resulted from cleavage at the alternative methyl side, compound 3’ was isolated in 5% yield, which indicated >15:1 site-selectivity in the C−C activation step. The general trend of site-selectivity in the C−C cleavage process was then examined (Fig. 2b), and the results indicated that the bond scission prefers to occur at more substituted20 or α-to-heteroatom carbons.
Figure 2 |. Iridium-catalyzed cleavage of unstrained ketones.
a, Optimized model reaction using linear ketone 1. b, Site-selectivity of this reaction. c, Deacetylation of methyl ketones. Ts, p-toluenesulfonyl; BArF, tetrakis(3,5-bis(trifluoromethyl)phenyl)borate; cod, 1,5-cyclooctadiene; Cy, cyclohexyl; MS, molecular sieves; p-tol, p-tolyl. a The reactions were conducted with 0.05 mmol 1, 0.052 mmol of 4-methyl-2-pyridyl hydrazine, and 0.5 mmol 1,3-butadiene. b Isolated yield. c The reaction was conducted using (2-(4-methoxyphenyl)ethene-1,1-diyl)bis(diphenylphosphane) (L2) instead of L1 as the ligand. d Using the pre-formed hydrazone as the substrate. For detailed experimental procedures, see Supplementary Information.
Encouraged by the excellent site-selectivity with methyl ketones, we foresaw an opportunity of realizing a redox-neutral approach to remove an acyl (particularly an acetyl) moiety from a linear ketone (Fig. 2c). It is well known that the Tsuji−Wilkinson decarbonylation of aldehydes has been frequently employed in natural product synthesis,21 and recently a de-hydroformylation approach was reported to access unsaturated products.22 Hence, the related deacetylation with readily accessible methyl ketones is also expected to be synthetically valuable from the strategic viewpoint. The scope of this transformation was first explored with various structurally diverse methyl ketones. Indeed, the deacetylation took place smoothly with protonation at primary or cyclic secondary positions. Notably, when two ketone carbonyl groups are present in the substrate, the C−C cleavage occurred selectively at the methyl ketone moiety (16). In addition, functional groups (FGs), such as primary sulfonamides (12), and heteroarenes, such as protected indole (20) and purine (22), were found compatible. This approach also holds promise for post-modification of bioactive compounds. For example, anti-inflammatory drugs, such as Pentoxifylline (21) and Nabumetone (23), underwent facile C−C cleavage to generate deacetylated analogues. Furthermore, tert-amylation of arenes is nontrivial via direct cross coupling approaches23, but it can be realized through first coupling of 4-phenylphenylboronic acid with 5-methylhex-4-en-2-ol using Sigman’s redox-relay oxidative-Heck reaction24 followed by this deacetylation protocol. Finally, enantioselective construction of hydrocarbon quaternary stereocenters that lack nearby polar FGs25 (28) was also achieved using a similar strategy24.
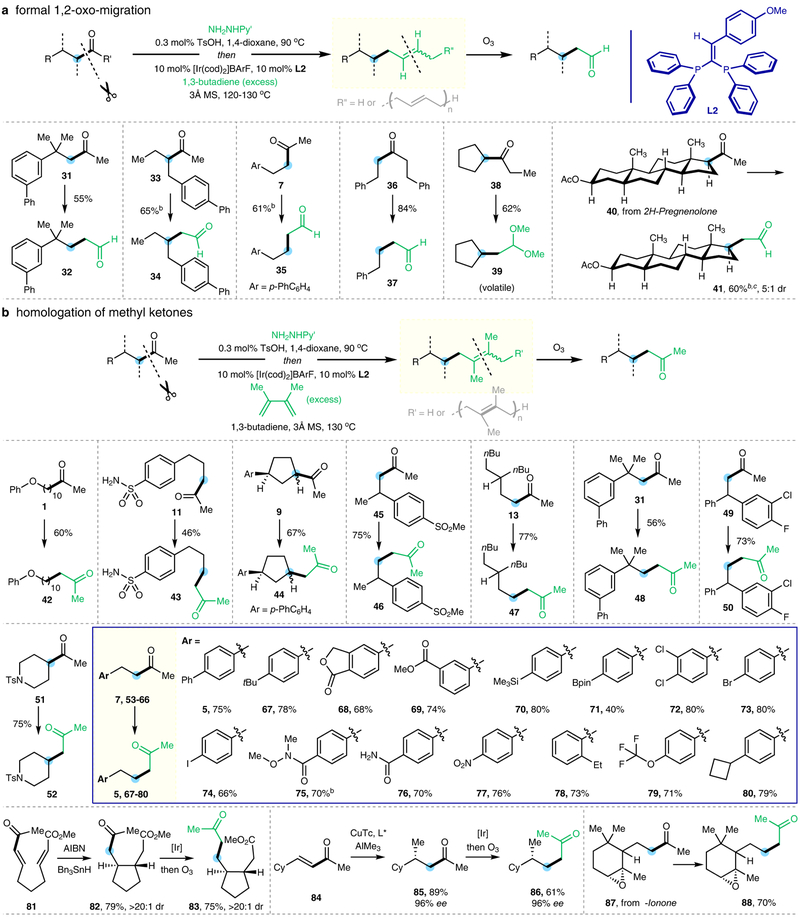
Besides the simple C−H formation, the cleaved alkyl fragment could also be trapped by 1,3-butadiene to give C-allylation products (vide supra, Fig. 2a, compound 2’)26,27. The efficiency of the C-allylation products could be significantly improved using excess 1,3-butadiene at a lower reaction temperature, and high conversion was obtained with L2 as the ligand. Upon facile ozonolysis, a formal “1,2-oxo-migration”, relocating the carbonyl moiety from the internal to the terminal position, was realized, providing the corresponding aldehydes in good yields (Fig. 3a). Ketones containing α (33, 38, 40) and/or β stereocenters (31, 40) could be tolerated. The transformation is not limited to methyl ketones. In particular, selective cleavage and coupling at the cyclopentyl site (versus the ethyl site) in ketone 38 was achieved. Gratifyingly, a steroid natural product, i.e. 2H-pregnenolone (40), was also a competent substrate; the corresponding aldehyde product (41) would be non-trivial to prepare via conventional approaches.
Figure 3 |. Deacylative C−C forming reactions of linear ketones.
a, Formal “1,2-oxo-migration”. After C−C cleavage, through coupling the alkyl fragment with 1,3-butadiene followed by ozonolysis, an aldehyde with the same length of a carbon chain is afforded b, Carbenoid-free homologation of aliphatic linear ketones. With additional 2,3-dimethyl-1,3-butadiene followed by ozonolysis, the sequence offers formal one-carbon homologation selectively at the non-methyl site of the ketone. Py’, 4-methyl-2-pyridyl; Ts, p-toluenesulfonyl; BArF, tetrakis(3,5-bis(trifluoromethyl)phenyl)borate; cod, 1,5-cyclooctadiene; MS, molecular sieves; Bpin, (pinacolato)boryl; AIBN, azobisisobutyronitrile; Tc, thiophene-2-carboxylate. a All yields are isolated yields. b Using the pre-formed hydrazone as the substrate. c The reaction was conducted at 170 °C. For detailed experimental procedures, see Supplementary Information.
Interestingly, when the reaction was run in the presence of a second but bulkier 1,3-diene, such as 2,3-dimethyl-1,3-butadiene, the resulting C-allylation intermediate, upon ozonolysis, led to a formal one-carbon homologation product (Fig. 3b). A variety of ketones with different skeletons, including those with multiple substitutions at α and/or β positions, readily reacted and gave decent yields of the homologation products over two steps. A variety of FGs, such as aryl iodides (74) and bromides (73), aryl boronic esters (71) and silanes (70), epoxides (88), esters (69, 83), lactones (68), nitros (77), amides (75, 76), sulfonamides (43, 52), and sulfones (46) were tolerated. The strained cyclobutane motif remained untouched (80). This formal-homologation approach also allows for preparation of 1,7-ketoesters (83) and enantioselective synthesis of ketones with γ-stereocenters (86) from readily available precursors. Natural product-derived ketones, such as the one from α-ionone (87), could also be used as substrates. Compared with the classical carbenoid-mediated homologation28, this method features high reactivity, excellent site- and chemo-selectivity.
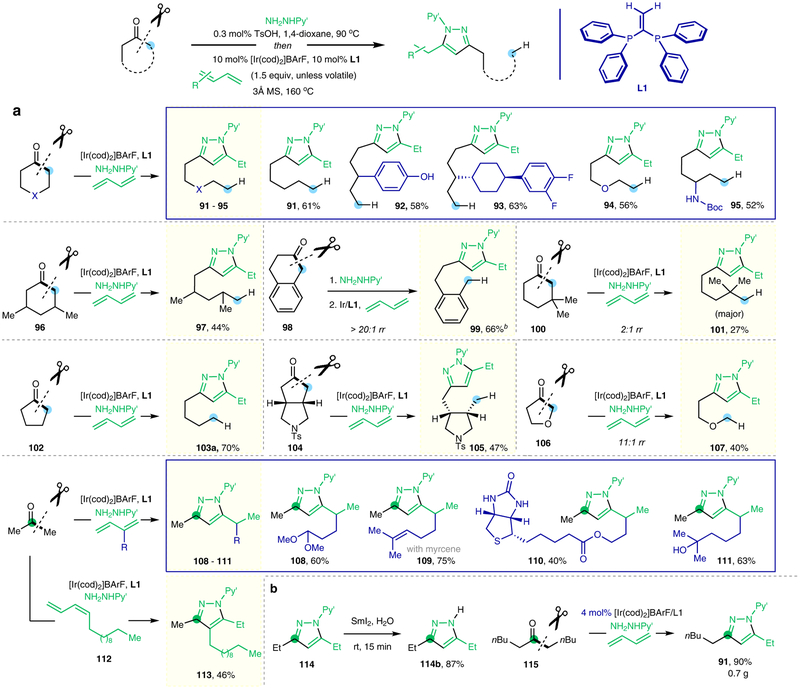
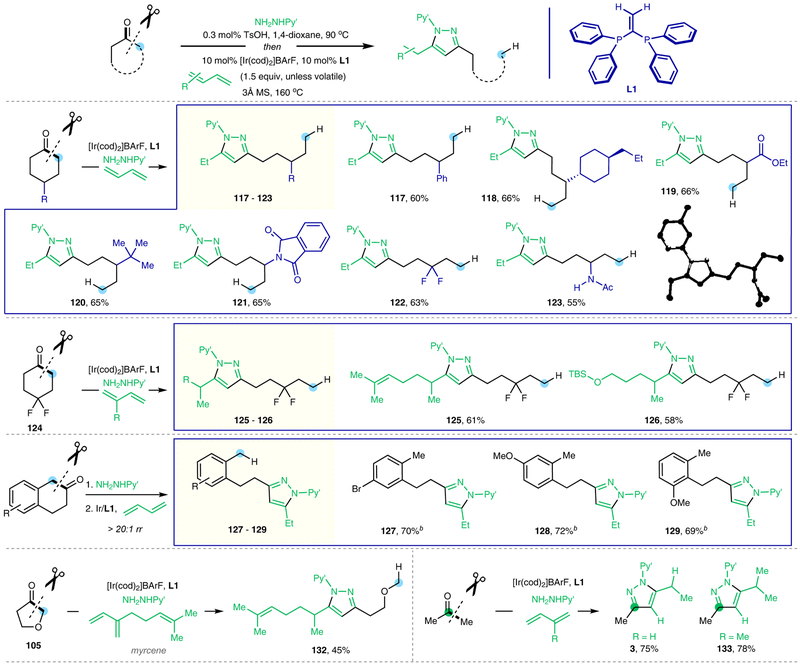
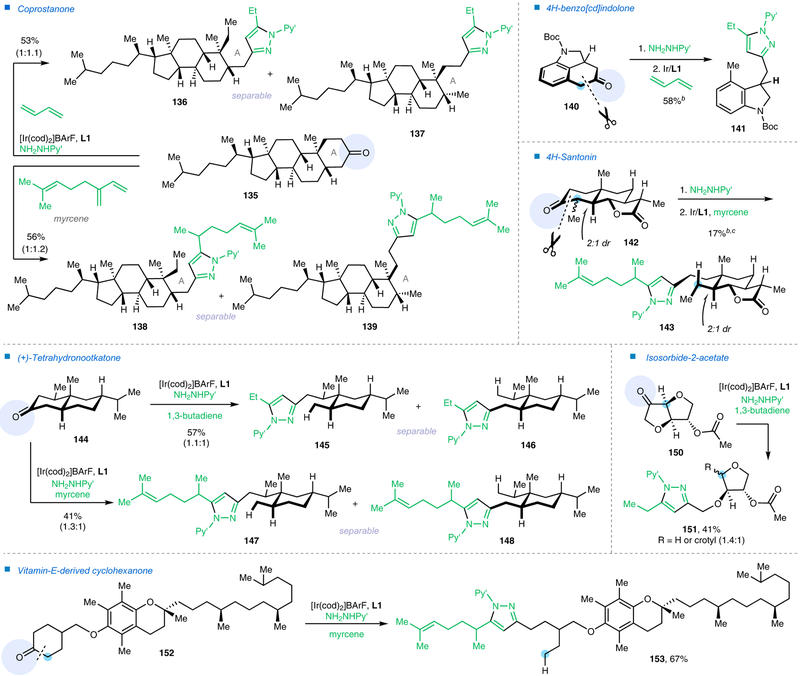
On the other hand, when cyclic ketones were employed as substrates, a redox-neutral deconstructive pyrazole-synthesis method was realized (Fig. 4 and Extended Data Fig. 1)16,17. As a highly important class of pharmacophores, pyrazoles are commonly found in bioactive compounds and approved drugs29, e.g. Celecoxib, Rimonabant, Fomepizole and Sildenafi. While many methods have been developed for pyrazole synthesis29, it could still be attractive to devise a straightforward and oxidant-free approach to prepare complex functionalized pyrazoles from readily available ketones and 1,3-dienes. Under the same conditions shown in Fig. 2c, a variety of cyclic ketones with different substitutions and ring-sizes were converted to the desired pyrazole products that contain rich structural diversity. Similarly, when using unsymmetrical ketones, the C−C bonds at more substituted (101) or benzylic positions (99, 127–129), or α to a heteroatom (107), were activated dominantly. Note that simple acetone is a suitable substrate, and a range of different dienes, such as 1,3-butadiene, isoprene, myrcene, 1,3-pentadecadiene and other functionalized 1,3-dienes, could be readily coupled. The transformation tolerates a broad range of FGs. Moreover, the 2-pyridyl moiety can be readily removed using SmI2 and water, furnishing a free pyrazole product in a high yield. Importantly, the catalyst loading could be reduced to 4 mol% on larger scales, where 0.7 gram of product 91 was isolated in 90% yield (Fig. 4b). Compared with the classical pyrazole syntheses29, this approach only requires a mono-ketone FG to be used as a handle. Thus, it provides a simple and distinct strategy to introduce pyrazole moieties into complex natural products or biologically interesting scaffolds (Extended Data Fig. 2). In addition, simply by switching the 1,3-diene coupling partners, several structurally related and separable pyrazole-derived analogues could be rapidly made available in one step from a single natural product (135 and 144). These pyrazole products would be difficult to access via conventional approaches. Given the wide availability of mono-ketone moieties, this method could offer a useful tool of preparing novel pyrazole-containing analogues for pharmaceutical or agrochemical research.
Figure 4 |. Deconstructive pyrazole synthesis from ketones.
a, Representative substrate scope. b, Further studies on pyridyl group removal and larger scale synthesis. Py’, 4-methyl-2-pyridyl; Ts, p-toluenesulfonyl; BArF, tetrakis(3,5-bis(trifluoromethyl)phenyl)borate; cod, 1,5-cyclooctadiene; MS, molecular sieves; Boc, tert-butoxycarbonyl. a All yields are isolated yields. b Yields refer to the key C−C activation reaction using pre-formed hydrazones as the substrates. See Extended Data Figs. 1 and 2 for additional substrate scope, and see Supplementary Information for detailed experimental procedures.
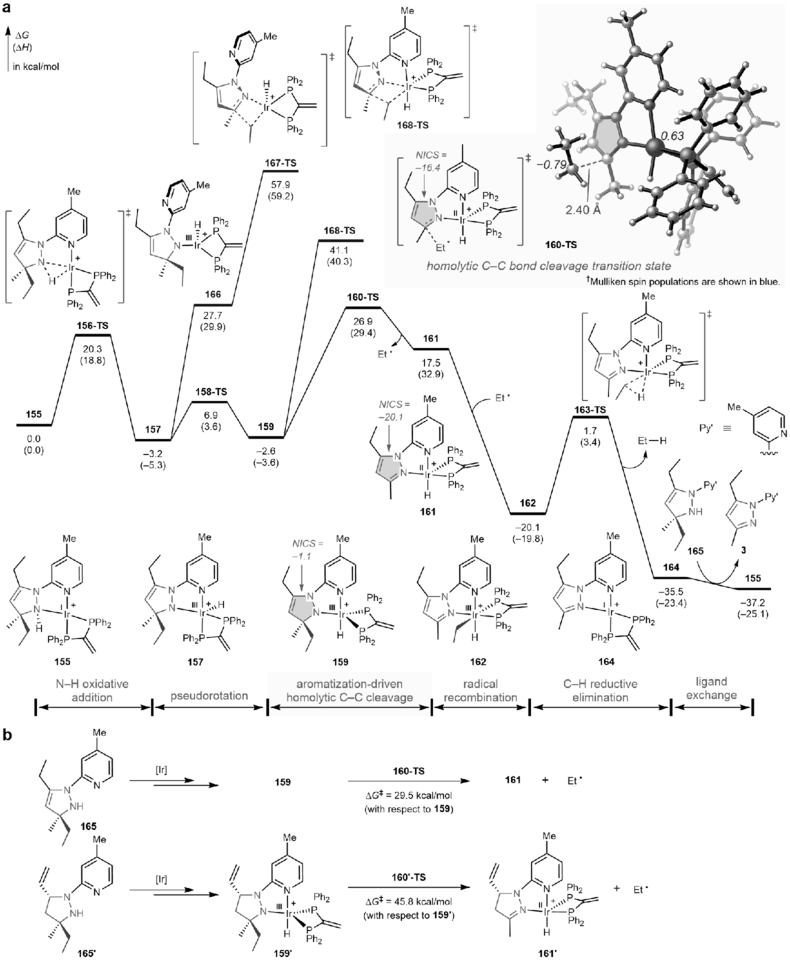
Preliminary mechanistic studies have been performed (see Supplementary Information, Section 3.2) to support the proposed reaction pathway depicted in Fig. 1b. First, the [3+2] cycloaddition adduct (Int I) between 1,3-butadiene and the hydrazone intermediate30 could be isolated during the course of the reaction, and was found to undergo the C‒C cleavage with similar efficiency and selectivity as the standard reaction (Fig. S2). Upon olefin migration, the dihydropyrazole species (Int II), which is one unsaturation degree shy of an aromatic pyrazole structure, is proposed to be the key intermediate for the following C‒C activation. In contrast, no C‒C cleavage was observed without the endocyclic double bond or the ring structure (Fig. S2c). From Int II, an aromatization-promoted homolytic C‒C cleavage/radical recombination mechanism was suggested by the density functional theory (DFT) calculations over other C‒C activation pathways (Extended Data Fig. 3a and Fig. S5). The initial N‒H oxidative addition gives rise to a mixture of Ir(III) hydride isomers (157 and 159), in which the exocyclic C‒C bond of the dihydropyrazole is significantly weakened (BDE = 39.0 and 36.5 kcal/mol for 157 and 159, respectively). From 159, the homolytic C‒C cleavage (160-TS, ΔG‡ = 29.5 kcal/mol with respect to 159) yields a transient Ir(II) species (161) and an alkyl radical, which then rapidly recombine to form 162. The computed NICS(1)zz aromaticity index values revealed a substantial increase of aromaticity of the five-membered ring that stabilizes 160-TS. As a comparison, without the driving force of aromatization, the corresponding C‒C cleavage of pyrazolidine 165’ requires a much higher barrier (Extended Data Fig. 3b). From 162, an alkane and the pyrazole product 3 are formed via C‒H reductive elimination and subsequent ligand exchange. Based on the current mechanistic understanding, future work will focus on enhancing the reaction efficiency and discovering new transformations or applications based on this C−C activation mode.
Methods
General procedure for the deacetylation of methyl ketones.
For a 0.05 mmol scale reaction, a 1,4-dioxane (1 mL) solution of the ketone substrate (0.05 mmol, 1.0 equiv), 2-hydrazineyl-4-methylpyridine (6.4 mg, 0.052 mmol, 1.04 equiv), and p-TsOH.H2O (stock solution in 1,4-dioxane, 0.05 M, 3.0 μL, 0.003 equiv) was heated at 90 °C for 5 hours under N2 atmosphere in a 8 mL vial. After cooled to room temperature, the vial was charged first with [Ir(cod)2]BArF (6.4 mg, 0.005 mmol, 0.1 equiv) and L1 (2.0 mg, 0.005 mmol, 0.1 equiv) under air atmosphere, and then with 3Å molecular sieve (pre-dried, 100 mg) and 1,3-butadiene (20 wt.% in PhMe, 170 μL, ca. 10 equiv) in a glovebox. The vial was sealed and heated at 160 °C under stirring for 72 hours. After cooled to room temperature, the reaction mixture was filtered through Celite, concentrated under reduced pressure, and further purified by flash column chromatography over silica to give the products. General procedures for formal homologation of linear ketones and deconstructive pyrazole synthesis from cyclic ketones, together with full experimental details and characterization of new compounds, can be found in the Supplementary Information.
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information. Additional data are available from the corresponding authors upon request. Metrical parameters for the structure of 123 are available free of charge from the Cambridge Crystallographic Data Centre (https://www.ccdc.cam.ac.uk/) under reference number CCDC 1876535.
Extended Data
Extended Data Fig. 1 |. Additional substrate scope for deconstructive pyrazole synthesis from ketones.
Py’, 4-methyl-2-pyridyl; Ts, p-toluenesulfonyl; BArF, tetrakis(3,5-bis(trifluoromethyl)phenyl)borate; cod, 1,5-cyclooctadiene; MS, molecular sieves; Ac, acetyl. a All yields are isolated yields. b The yield refers to the key C−C activation reaction using pre-formed hydrazone as the substrate. For detailed experimental procedures, see Supplementary Information.
Extended Data Fig. 2 |. Introducing pyrazoles into complex ketones via C−C cleavage.
Py’, 4-methyl-2-pyridyl; BArF, tetrakis(3,5-bis(trifluoromethyl)phenyl)borate; cod, 1,5-cyclooctadiene; Boc, tert-butoxycarbonyl. a All yields are isolated yields. b The yield refers to the key C−C activation reaction using pre-formed hydrazone as the substrate. c 15 mol% Ir catalyst and 15 mol% L1 were used. For detailed experimental procedures, see Supplementary Information.
Extended Data Fig. 3 |. Computational studies of the aromatization-driven C‒C bond activation.
a, Free energy profiles of the aromatization-driven C‒C bond activation of dihydropyrazole 165. Calculations were performed at the M06-L/6–311+G(d,p)‒SDD/SMD(1,4-dioxane)//B3LYP/6–31G(d)‒SDD level of theory. The less favorable β-C elimination pathways with and without pyridine coordination (168-TS and 167-TS, respectively) are shown in blue. The NICS(1)zz aromaticity index was calculated at B3LYP/6–311+G(d,p)‒SDD level of theory to describe the aromaticity of pyrazole ring (highlighted in green) in 159, 160-TS, and 161. The variation of NICS(1)zz values indicates a significant increase of aromaticity during the homolytic C‒C bond cleavage. b, Comparison between homolytic C‒C bond cleavage of dihydropyrazole 165 and pyrazolidine 165ʹ (165ʹ without the driving force of aromatization). See Section 3.2.2 in Supplementary Information for details.
Supplementary Material
Acknowledgements
This project was supported by NIGMS (R01GM109054). Y.X. thanks the Charles H. Viol and William Rainey Harper Dissertation Fellowship from the University of Chicago, and the Bristol-Myers Squibb Graduate Fellowship for financial support. P.Z. thanks the Joint Ph.D. Student Scholarship 2016 from China Scholarship Council (File No. 201603170182). P.L. thanks the NSF (CHE-1654122) for funding. Calculations were performed at the Center for Research Computing at the University of Pittsburgh. Ms. Lin Deng is acknowledged for kind donation of substrate 140. Dr. Jun Zhu is acknowledged for conducting several control experiments.
Footnotes
The authors declare no competing interests.
References:
- 1.Karimov RR & Hartwig JF Transition-Metal-Catalyzed Selective Functionalization of C(sp3)−H Bonds in Natural Products. Angew. Chem. Int. Ed 57, 4234–4241 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murakami M & Ito Y Cleavage of carbon—carbon single bonds by transition metals. Top. Organomet. Chem 3, 97–129 (1999). [Google Scholar]
- 3.Chen F, Wang T & Jiao N Recent advances in transition-metal-catalyzed functionalization of unstrained carbon–carbon bonds. Chem. Rev 114, 8613–8661 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Souillart L & Cramer N Catalytic C–C bond activations via oxidative addition to transition metals. Chem. Rev 115, 9410–9464 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Kim D-S, Park W-J & Jun C-H Metal–organic cooperative catalysis in C–H and C–C bond activation. Chem. Rev 117, 8977–9015 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Fumagalli G, Stanton S & Bower JF Recent methodologies that exploit C–C single-bond cleavage of strained ring systems by transition metal complexes. Chem. Rev 117, 9404–9432 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Dreis A; Douglas C In C–C Bond Activation; Dong G, Ed.; Springer: Berlin; 346, 85–110 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Schleyer PVR & Pühlhofer F Recommendations for the evaluation of aromatic stabilization energies. Org. Lett 4, 2873–2876 (2002). [DOI] [PubMed] [Google Scholar]
- 9.Santen RJ, Brodie H, Simpson ER, Siiteri PK & Brodie A History of aromatase: saga of an important biological mediator and therapeutic target. Endocr. Rev 30, 343–375 (2009). [DOI] [PubMed] [Google Scholar]
- 10.King RB & Efraty A Pentamethylcyclopentadienyl derivatives of transition metals. II. Synthesis of pentamethylcyclopentadienyl metal carbonyls from 5-acetyl-1,2,3,4,5-pentamethylcyclopentadiene. J. Am. Chem. Soc 94, 3773–3779 (1972). [Google Scholar]
- 11.Crabtree RH, Dion RP, Gibboni DJ, Mcgrath DV & Holt EM Carbon–carbon bond cleavage in hydrocarbons by iridium complexes. J. Am. Chem. Soc 108, 7222–7227 (1986). [Google Scholar]
- 12.Halcrow MA, Urbanos F & Chaudret B Aromatization of the B-ring of 5,7-dienyl steroids by the electrophilic ruthenium fragment “[Cp*Ru]+”. Organometallics 12, 955–957 (1993). [Google Scholar]
- 13.Youn SW, Kim BS & Jagdale AR Pd-catalyzed sequential C–C bond formation and cleavage: evidence for an unexpected generation of arylpalladium(II) species. J. Am. Chem. Soc 134, 11308–11311 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Smits G, Audic B, Wodrich MD, Corminboeuf C & Cramer N A β-Carbon elimination strategy for convenient in situ access to cyclopentadienyl metal complexes. Chem. Sci 8, 7174–7179 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Padwa A & Pearson WH Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry toward Heterocycles and Natural Products (John Wiley & Sons, 2003). [Google Scholar]
- 16.Fevre GL & Hamelin J Existence d’une forme N–H stable de pyrazoline-4 lors de l’aromatisation de pyrazolidines 3,3-disubstituees en pyrazole. Mecanisme de la reaction. Tetrahedron Lett. 19, 4503–4506 (1978). [Google Scholar]
- 17.Tian M, Shi X, Zhang X & Fan X Synthesis of 4-acylpyrazoles from saturated ketones and hydrazones featured with multiple C(sp3)–H bond functionalization and C–C bond cleavage and reorganization. J. Org. Chem 82, 7363–7372 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Xu Y, Young MC & Dong G Catalytic coupling between unactivated aliphatic C–H bonds and alkynes via a metal–hydride pathway. J. Am. Chem. Soc 139, 5716–5719 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Pan S & Shibata T Recent advances in iridium-catalyzed alkylation of C–H and N–H bonds. ACS Catal. 3, 704–712 (2013). [Google Scholar]
- 20.Xia Y, Lu G, Liu P & Dong G Catalytic activation of carbon–carbon bonds in cyclopentanones. Nature 539, 546–550 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuji J & Ohno K Organic syntheses by means of noble metal compounds XXI. Decarbonylation of aldehydes using rhodium complex. Tetrahedron Lett. 6, 3969–3971 (1965). [Google Scholar]
- 22.Murphy SK, Park J-W, Cruz FA & Dong VM Rh-catalyzed C–C bond cleavage by transfer hydroformylation. Science 347, 56–60 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zultanski SL & Fu GC Nickel-catalyzed carbon–carbon bond-forming reactions of unactivated tertiary alkyl halides: Suzuki arylations. J. Am. Chem. Soc 135, 624–627 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mei T-S, Patel HH & Sigman MS Enantioselective construction of remote quaternary stereocentres. Nature 508, 340–344 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fessard TEC, Andrews SP, Motoyoshi H & Carreira EM Enantioselective preparation of 1,1-diarylethanes: aldehydes as removable steering groups for asymmetric synthesis. Angew. Chem. Int. Ed 46, 9331–9334 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Chu L, Ohta C, Zuo Z & Macmillan DWC Carboxylic acids as a traceless activation group for conjugate additions: a three-step synthesis of (±)-Pregabalin. J. Am. Chem. Soc 136, 10886–10889 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin T et al. Nickel-catalyzed Barton decarboxylation and Giese reactions: A practical take on classic transforms. Angew. Chem. Int. Ed 56, 260–265 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Candeias NR, Paterna R & Gois PMP Homologation reaction of ketones with diazo compounds. Chem. Rev 116, 2937–2981 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Karrouchi K et al. Synthesis and pharmacological activities of pyrazole derivatives: A review. Molecules 23, 134 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong X et al. Mechanism and selectivity of N-triflylphosphoramide catalyzed (3 + 2) cycloaddition between hydrazones and alkenes. J. Am. Chem. Soc 136, 13769–13780 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available within the article and its Supplementary Information. Additional data are available from the corresponding authors upon request. Metrical parameters for the structure of 123 are available free of charge from the Cambridge Crystallographic Data Centre (https://www.ccdc.cam.ac.uk/) under reference number CCDC 1876535.