Abstract
Exclusive breastfeeding (EBF) has numerous maternal health benefits. However, EBF rates are lower in mothers with obesity. We sought to better understand whether maternal body composition measurements in early pregnancy are also predictive of lower rates of EBF. Healthy pregnant women with prepregnancy body mass index (BMI) of 17.5–51 kg/m2 underwent determination of percent body fat (% body fat) in early (12–16 weeks) and late (37 weeks) gestation. Intent and duration of EBF were determined by surveys completed at 6 weeks and 6 months postpartum (PP). Unadjusted and adjusted analyses were performed to compare EBF rates and weaning by maternal BMI and % body fat. Increasing BMI and % body fat in early pregnancy were significantly associated with lower rates of EBF among women intending EBF. Women with BMI ≥ 25 were less likely to be EBF at 6 weeks and 6 months PP compared with women of normal BMI (67 and 37% vs. 91 and 79%, P value 0.005 and 0.001, respectively). Among primiparous women intending EBF, 100% of women in the lowest two body fat quartiles in early pregnancy were EBF at 6 weeks PP compared with 66.7 and 63.6% of women in the higher quartiles (P = 0.03). Lactation cessation by 6 months PP was higher with increasing maternal BMI (P = 0.001). Maternal obesity in early gestation is associated with lower EBF rates among women intending EBF and earlier weaning. Excess adiposity in early pregnancy may impede EBF.
Keywords: exclusive breastfeeding, obesity, weaning
Key messages.
Elevated prepregnancy BMI and body fat percent in early gestation are associated with significantly lower rates of exclusive breastfeeding at 6 weeks postpartum among women intending exclusive breastfeeding.
Introduction of formula within the first 2 weeks postpartum accounts for 74% of formula introduction within the first 6 weeks among women intending exclusive breastfeeding.
Maternal obesity is associated with lower rates of EBF and higher rates of lactation cessation at 6 months postpartum.
1. INTRODUCTION
Breastfeeding is associated with many maternal health benefits in addition to improved infant health outcomes including lower risk of infection, obesity, diabetes, and sudden infant death syndrome (“Breastfeeding and the use of human milk,” 2012). Women who breastfeed have decreased risk for metabolic syndrome (Choi, Kim, Cho, Kim, & Shim, 2017; Gunderson et al., 2010; Ram et al., 2008), Type 2 diabetes mellitus (T2DM; Ip et al., 2007; Schwarz et al., 2010; Stuebe, Rich‐Edwards, Willett, Manson, & Michels, 2005), cardiovascular disease (CVD; Peters et al., 2017; Schwarz et al., 2009; Stuebe et al., 2009), and cancer (Chowdhury et al., 2015; Ip et al., 2007) and decreased all‐cause mortality (Bartick et al., 2017) in later life compared with women who do not breastfeed. Several studies have reported that the degree of maternal health benefit is proportional to lactation intensity and duration, suggestive of a dose–response relationship (Gunderson et al., 2015; Peters et al., 2017; Ziegler et al., 2012). The greatest long‐term maternal health benefits have been shown in women who follow the World Health Organization recommendation for exclusive breastfeeding (EBF) for the first 6 months of life without formula supplementation or introduction of other foods or beverages (Organization, 2002).
However, women who are obese are significantly less likely to exclusively breastfeed (Amir & Donath, 2007; Donath & Amir, 2008; Hauff, Leonard, & Rasmussen, 2014). This is reflected in lower intention to breastfeed (Thompson et al., 2013; Wojcicki, 2011), earlier formula supplementation (Donath & Amir, 2008; Hauff et al., 2014; Martinez, Chapman, & Perez‐Escamilla, 2016), and increased risk of disrupted lactation (Stuebe et al., 2014; Vanky et al., 2012), defined as early, undesired weaning attributed to lactation dysfunction. Despite correction for psychosocial factors (Hauff et al., 2014), obesity remains a significant risk factor for non‐EBF, suggesting a biological basis for lactation dysfunction.
EBF requires proper development and normal function of breast glands in order to meet the average newborn's need to feed 8–12 times and consume an average of 710 mL of breastmilk daily (Dewey, 1997). Obesity is implicated as a cause of delayed lactogenesis and low‐milk supply (Nommsen‐Rivers, Chantry, Peerson, Cohen, & Dewey, 2010; Nommsen‐Rivers, Dolan, & Huang, 2012; Preusting, Brumley, Odibo, Spatz, & Louis, 2017), which both contribute to lactation dysfunction due to insufficient milk production requiring early formula supplementation (O'Sullivan, Perrine, & Rasmussen, 2015). Animal studies support this association of increased fat mass and low‐milk supply (Morrow, 1976; Revell, Williams, Mullan, Ranford, & Smits, 1998a, 1998b). The “preset” hypothesis suggests that maternal metabolic factors present before delivery determine lactation initiation and duration as well as later life metabolic risk (Stuebe & Rich‐Edwards, 2009). If true, a candidate link between maternal obesity and decreased EBF rates could be in variations of percent body fat in early gestation.
In order to explore associations among maternal obesity, EBF, and long‐term maternal health, it is important to distinguish between intended EBF versus planned formula use and undesired introduction of formula due to lactation dysfunction. A significant limitation of longitudinal studies supporting the correlation between breastfeeding and improved maternal health is the lack of information regarding maternal EBF intent, which is critical because breastfeeding intention and planned duration are among the strongest factors associated with length of lactation (Scott & Binns, 1999). By studying women who intended EBF and initiated breastfeeding, we can focus on factors other than intention, including physiological factors, which may influence the relationship between increased maternal obesity and decreased rates of EBF.
We therefore sought to address the research gap related to the impact of maternal obesity and body composition in early pregnancy on EBF by focusing on women intending EBF to exclude planned formula use. We also explored if maternal body composition influenced when infant formula supplementation was introduced. Our primary hypothesis was that maternal obesity is a risk factor for decreased rates of EBF and earlier introduction of formula supplementation among women intending EBF. In addition, we hypothesized that maternal obesity is a risk factor for earlier cessation of lactation.
2. MATERIALS AND METHODS
2.1. Study design
This was a two‐cohort longitudinal study of 185 healthy mother–baby pairs of various maternal prepregnancy BMI enrolled at Oregon Health & Science University from October 2015 to April 2018. Ninety‐eight women were early enrollers (12–16 weeks gestation), and 87 were late enrollers (>37 weeks gestation); both were followed through the first year postpartum (Figure 1). Five early enrollers withdrew prior to delivery. Women for whom definitive assessment of lactation status could not be determined through survey response or review of the mother or infant electronic medical record were excluded (n = 28), leaving 152 subjects available for analysis (78 early enrollers and 74 late enrollers). Maternal prepregnancy BMI was determined by self‐reported prepregnancy weight and confirmed with first prenatal visit weight (P < 0.0001, r2 0.98) and measured maternal height. Maternal BMI was categorized using the WHO BMI categories: Normal weight women (BMI 18.5–24.9 kg/m2; n = 65), women who are overweight (25.0–29.9 kg/m2; n = 52), and women with obesity (BMI ≥ 30.0 kg/m2; n = 33). Underweight women (BMI < 18.5 kg/m2; n = 2) were included in the normal weight category. Exclusion criteria included active maternal infection, documented fetal congenital anomalies, substance abuse, chronic illness requiring regular medication use, maternal diabetes, chorioamnionitis, significant medical conditions (active cancers, cardiac, renal, hepatic, and pulmonary), or, for late enrollers, an abnormal 2‐h 75‐g glucose tolerance test. The OHSU institutional review board approved the study protocol, and each subject provided signed informed consent prior to enrollment.
Figure 1.
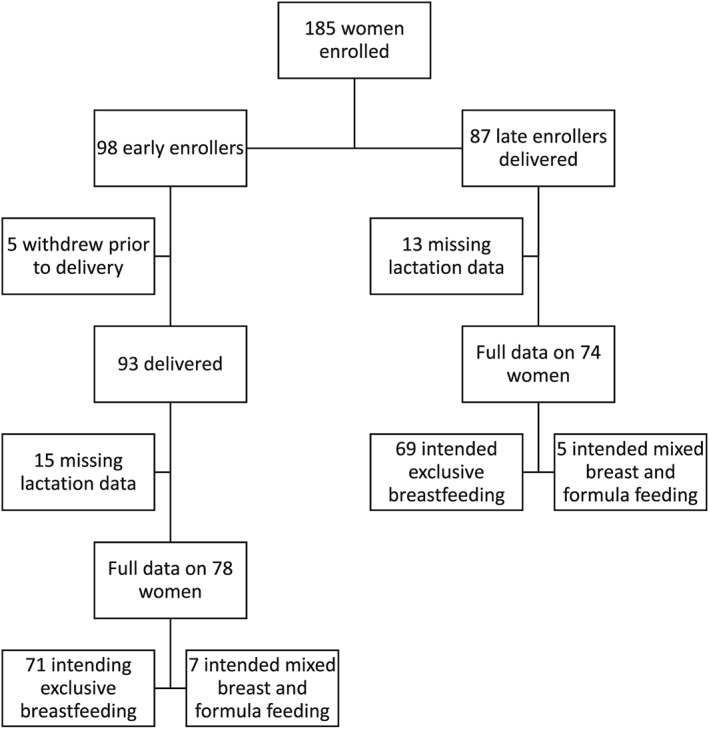
Subject flow chart
Subjects presented for their study visits in the morning following an overnight fast. Maternal fat mass was determined by air displacement plethysmography via the BodPod (Life Measurement Inc.) in early pregnancy (12–16 weeks) and in late pregnancy (>37 weeks gestation). The calculations from von Raaij (van Raaij, Peek, Vermaat‐Miedema, Schonk, & Hautvast, 1988) were used to correct for changes in fat free mass density based on gestational age, as previously described (Marshall et al., 2016). Percent body fat was determined by dividing maternal fat mass (kg) as calculated above by total maternal weight (kg) × 100. Subjects were then separated into % body fat quartiles in early and late gestation.
Our primary outcome was the rate of EBF at 6 weeks and 6 months by maternal BMI category and % body fat quartile in early and late gestation. Subjects completed modified Infant Feeding Practice Study II surveys (Fein et al., 2008) online at 6 weeks and 6 months postpartum. These time points were chosen as 6 weeks postpartum is the traditional timing for the routine postpartum exam, and the majority of employed women have not yet returned to work, and 6 months is the recommended duration for EBF prior to initiating solid food supplementation. Breastfeeding intention (breast, formula, and both) and intended months of breastfeeding duration were assessed. The end of EBF was determined by self‐report of infant age in weeks at first use of formula supplementation. Exclusive breastfeeding was confirmed on the 6‐week and 6‐month postpartum surveys. As the primary intent of this study was to evaluate introduction of formula supplementation, women whose infants started complementary solid foods between 4 and 6 months but did not receive formula supplementation were considered to be EBF (n = 45). Duration of lactation was determined by maternal survey response at 6 weeks and 6 months postpartum. Women were considered to have stopped lactating when they were no longer providing breastmilk to their infant through either breastfeeding or pumping.
Given the physiologic and psychosocial differences between primiparous and multiparous mothers, we also examined the relationship between body composition and EBF stratified by parity.
2.2. Statistical analysis
We used descriptive statistics to characterize the demographic profile of our cross‐sectional sample. We analysed unadjusted associations between BMI and the end of EBF via Kaplan Meier survival analysis by graphing the prevalence of formula introduction over time with women of each BMI category using a unique line. We employed multivariable logistic regression modelling to assess the association between maternal BMI and % body fat in late gestation and EBF among women intending EBF. We controlled for potential confounding variables between the BMI categories, including maternal age, gestational weight gain, smoking status, and intended duration of breastfeeding less than 6 months. Due to universal rates of EBF in the lowest quartiles for % body fat in early gestation, we were unable to perform adjusted analysis. To evaluate the impact of parity on EBF, we analysed unadjusted rates of EBF at 6 weeks and 6 months postpartum stratified by maternal BMI and % body fat quartile and separated by maternal parity.
3. RESULTS
Women with prepregnancy BMI ≥30 kg/m2 were less likely to intend EBF compared with women of normal weight and overweight (78.8% vs. 95.5 and 96.2%, respectively, P = 0.006, Table SS1). Women with BMI ≥25 kg/m2 were less likely to intend to breastfeed for at least 12 months compared with women of lower BMI (P = 0.002). All subjects intended to breastfeed and initiated breastfeeding. By 6 weeks postpartum, three women had stopped lactating, and there was no association of cessation of lactation likelihood with maternal body composition. No subjects intending EBF had stopped lactating by 6 weeks. However, by 6 months postpartum, increasing maternal prepregnancy BMI was significantly associated with earlier lactation cessation (Figure SS1 and Table S2). No women with normal BMI or % body fat in the first quartile in early or late gestation had stopped lactating at 6 months postpartum versus over 20% for women with BMI ≥25 kg/m2 (P = 0.001) or % body fat in the third and fourth quartiles in early or late gestation (P = 0.02 and 0.01, respectively).
The remainder of the results refer to the final analytic sample of 140 subjects who intended EBF, which included 71 early enrollers and 69 late enrollers. Women who were obese were more likely to be younger, current smokers, and have lower gestational weight gain (Table 1). There were no differences in parity, mode of delivery, birthweight, gestational age at delivery, or returning to work. Women with normal BMI were significantly more likely to be EBF during the first 6 months postpartum compared with women who were overweight or obese (Figure 2). EBF duration was not different between women who were overweight compared with those who were obese. Seventy‐four percent of women who started formula supplementation within the first 6 weeks after delivery despite EBF intent did so within the first 2 weeks. Of the 27 women who initiated formula in the first 6 weeks, seven started prior to hospital discharge, five started within the first week, and eight started within 2 weeks after delivery.
Table 1.
Demographics of subjects intending exclusive breastfeeding
| Total n = 140 | Normal weight n = 64 | Overweight n = 50 | Obese n = 26 | P value | |
|---|---|---|---|---|---|
| Maternal agea (years) | 33.0 (5.5) | 32.8 (4.6) | 34.5 (4.8) | 30.4 (7.8) | 0.008 |
| Race/ethnicityb (%) | |||||
| White | 84.29 | 87.5 | 80.0 | 84.6 | 0.55 |
| Black | 2.86 | 0 | 6.0 | 3.85 | 0.15 |
| Hispanic | 9.4 | 9.52 | 10.20 | 7.69 | 0.88 |
| Asian | 6.43 | 6.25 | 10.0 | 0 | 0.24 |
| American Indian | 5.71 | 3.13 | 6.00 | 11.54 | 0.30 |
| Pacific islander | 1.43 | 3.13 | 0 | 0 | 0.30 |
| Declined/unknown | 2.86 | 1.56 | 6.0 | 0 | 0.23 |
| Nulliparous (%) | 57.1 | 59.4 | 48.0 | 69.2 | 0.18 |
| Prenatal smoking (%) | 0.71 | 0 | 0 | 3.85 | 0.04 |
| GWGa (lbs.) | 13.1 (6.1) | 14.5 (4.2) | 13.1 (6.4) | 9.5 (8.1) | 0.002 |
| Lactation class (%) | 26.5 | 26.2 | 21.7 | 36.0 | 0.43 |
| BF experience (%) (multiparous only) | 96.4 | 92.0 | 100 | 100 | 0.28 |
| Intended BF duration | 0.058 | ||||
| <6 months | 2.24 | 0 | 6.38 | 0 | |
| ≥6 months | 97.76 | 100 | 93.6 | 100 | |
| Intended BF durationa (months) | 14.1 (6.7) | 15.2 (4.9) | 13.8 (9.2) | 11.8 (4.4) | 0.11 |
| GA at deliverya (weeks) | 39.4 (1.3) | 39.5 (1.4) | 39.2 (1.3) | 39.8 (1.1) | 0.15 |
| Mode of delivery (%) | 0.28 | ||||
| Vaginal | 75.7 | 75.0 | 78.0 | 73.1 | |
| Assisted vaginal | 0.71 | 1.56 | 0 | 0 | |
| Scheduled caesarean | 6.43 | 3.13 | 12.0 | 3.85 | |
| Unscheduled caesarean | 17.1 | 20.31 | 10.0 | 23.1 | |
| Birthweighta (kg) | 3.43 (0.47) | 3.42 (.51) | 3.40 (0.46) | 3.52 (0.38) | 0.56 |
| Fetal sex (male) | 49.3 | 50.0 | 48.0 | 50.0 | 0.98 |
| Initiated BF | 100 | 100 | 100 | 100 | |
| Return to work (%) | 75.4 | 78.7 | 70.3 | 75.0 | 0.64 |
| Age at return to worka (months) | 2.99 (1.2) | 3.04 (1.2) | 2.77 (1.2) | 3.25(1.4) | 0.47 |
Note. BF: breastfeeding; EBF: exclusive breastfeeding; EFF: exclusive formula feeding; GA: gestational age; MBFF: mixed breast & formula feeding.
mean (SD).
reported all that apply.
Figure 2.
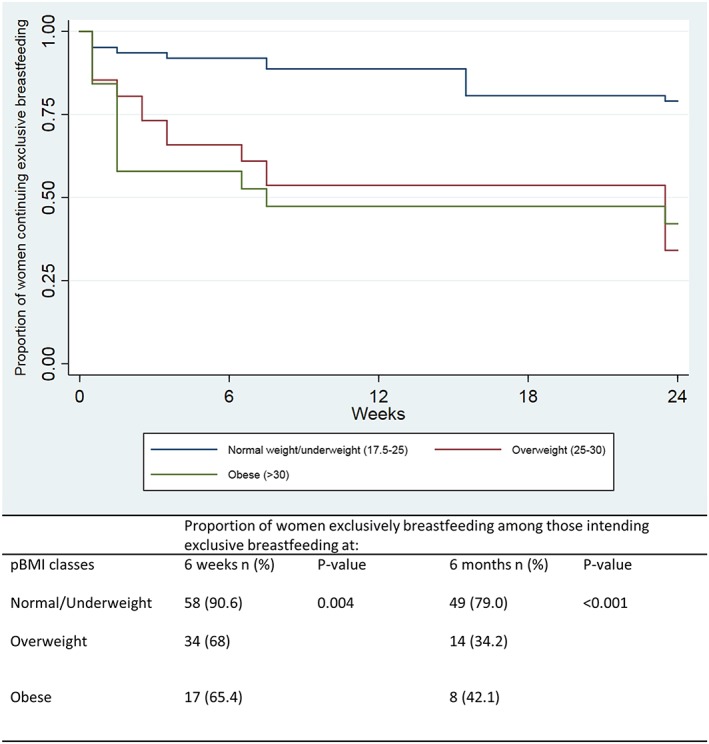
Kaplan–Meier survival analysis for end of exclusive breastfeeding by infant age in weeks over the first 6 months of life due to introduction of formula, among women intending exclusive breastfeeding stratified by prepregnancy body mass index
After adjustment for confounders, the odds ratio for EBF at 6 weeks postpartum for women who were overweight/obese was 0.24 (95% confidence interval, CI [0.08–0.75], P = 0.01; TTable 2). Women in the fourth quartile for % body fat in late pregnancy were significantly less likely to be EBF compared with women in the first quartile. By 6 months postpartum, women who were overweight or obese and who had a % body fat greater than the first or second quartile in late gestation were all significantly less likely to be EBF.
Table 2.
Association of exclusive breastfeeding at 6 weeks and 6 months postpartum and maternal body composition in unadjusted and adjusted analyses among women intending exclusive breastfeeding (n = 140)
| EBF at 6 weeks N (%) | P value | Odds ratio[Link] | 95% CI | P value | EBF at 6 months N (%) | P value | Odds ratio[Link] | 95% CI | P value | |
|---|---|---|---|---|---|---|---|---|---|---|
| BMI category | 0.001 | <0.001 | ||||||||
| Normal | 58 (90.6) | 1.0 | — | — | 49 (79.0) | 1.0 | — | — | ||
| Overweight/obese | 51 (67.1) | 0.24 | 0.08–0.75 | 0.01 | 22 (36.7) | 0.17 | 0.07–0.41 | <0.001 | ||
| % body fat in late pregnancy | 0.08 | 0.015 | ||||||||
| First quartile (ref) | 33 (91.7) | 1.0 | — | — | 27 (79.4) | 1.0 | — | — | ||
| Second quartile | 27 (77.1) | 0.32 | 0.06–1.77 | 0.19 | 18 (54.6) | 0.34 | 0.11–1.08 | 0.07 | ||
| Third quartile | 23 (69.7) | 0.22 | 0.04–1.21 | 0.08 | 12 (42.9) | 0.20 | 0.06–0.67 | 0.009 | ||
| Fourth quartile | 19 (67.9) | 0.14 | 0.03–0.77 | 0.02 | 9 (45.0) | 0.20 | 0.05–0.73 | 0.015 |
adjusted for age, gestational weight gain, smoking status, intended duration of breastfeeding.
After separation by parity, primiparous women with a BMI ≥25 kg/m2 had significantly decreased rates of EBF at 6 weeks postpartum compared with normal weight women (P = 0.01; Table 3). All of the primiparous women with % body fat in the first or second quartile in early pregnancy were EBF at 6 weeks postpartum compared with 66.7% of women in the third quartile and 63.6% of women in the fourth quartile (P = 0.03). These differences were not apparent when separated by % body fat quartile in late gestation. Multiparous women who were overweight or obese prior to pregnancy were significantly less likely to be EBF at 6 weeks postpartum (P = 0.02) compared with women of normal weight, but there were no differences by % body fat quartile in early or late gestation. At 6 months postpartum, maternal BMI ≥25 kg/m2 remained significantly associated with decreased rates of EBF, with 76.3% of primiparous women with normal BMI reporting EBF, compared with 19.4% of primiparous women who were overweight or obese (P = 0.001) and none of the women in the fourth quartile for % body fat in early or late gestation. In contrast to 6 weeks postpartum, there were significant variations in EBF rates among multiparous women by % body fat in late gestation as well as by prepregnancy BMI, with no difference by % body fat in early gestation.
Table 3.
Association of maternal body composition and parity on exclusive breastfeeding rates at 6 weeks and 6 months postpartum among women intending exclusive breastfeeding (primiparous n = 80, multiparous n = 60), n (%)
| 6 weeks | 6 months | |||||||
|---|---|---|---|---|---|---|---|---|
| Primiparous | P value | Multiparous | P value | Primiparous | P value | Multiparous | P value | |
| BMI category | 0.01 | 0.02 | 0.001 | 0.03 | ||||
| Normal weight | 33 (86.8) | 25 (96.2) | 29 (76.3) | 20 (83.3) | ||||
| Overweight/obese | 26 (61.9) | 25 (73.5) | 6 (19.4) | 16 (55.2) | ||||
| % body fat in early pregnancy | 0.03 | 0.48 | 0.001 | 0.16 | ||||
| First quartile | 14 (100) | 4 (100) | 12 (85.7) | 4 (100) | ||||
| Second quartile | 7 (100) | 10 (83.3) | 6 (85.7) | 8 (76.7) | ||||
| Third quartile | 8 (66.7) | 5 (83.3) | 3 (30.0) | 2 (40.0) | ||||
| 7 (63.6) | 3 (60.0) | 0 | 2 (40.0) | |||||
| % body fat in late pregnancy | 0.10 | 0.16 | 0.004 | 0.003 | ||||
| First quartile | 18 (85.7) | 15 (100) | 14 (66.7) | 13 (100) | ||||
| Second quartile | 15 (83.3) | 12 (70.6) | 11 (64.7) | 7 (43.8) | ||||
| Third quartile | 13 (65.0) | 10 (76.9) | 7 (41.2) | 5 (45.5) | ||||
| Fourth quartile | 8 (53.3) | 11 (84.6) | 0 | 9 (81.8) | ||||
4. DISCUSSION
Among women intending to exclusively breastfeed, maternal obesity was significantly associated with lower rates of EBF at 6 weeks and 6 months postpartum, despite correction for maternal age, gestational weight gain, smoking status, and intended duration of breastfeeding. Importantly, we found that primiparous women with elevated prepregnancy BMI and % body fat in early pregnancy were at the greatest risk for early introduction of formula. Although these women all intended EBF, maternal obesity was significantly associated with lower EBF rates, as evidenced by greater formula introduction rates primarily within the first 2 weeks postpartum. This suggests that physiologic differences associated with increased fat mass, which manifest as lactation dysfunction early in the postpartum period, may be driving the differences in EBF rates by maternal obesity more than psychosocial factors.
Although BMI is commonly used as a surrogate for % body fat, we have shown it to poorly correlate with actual % body fat on an individual level (Marshall et al., 2016). Thus, % body fat is important to distinguish from BMI when attempting to determine the impact of maternal body composition on capacity for EBF. Among primiparous women intending EBF, 86.8% in the normal BMI category were EBF at 6 weeks postpartum, whereas 100% of women in the first and second quartile for % body fat in early gestation were EBF. By late pregnancy, maternal % body fat was no longer significantly associated with lower rates of EBF. The physiological links that explain an association between maternal % body fat in early gestation and EBF are yet to be determined. Our data suggest that % body fat may have a threshold effect rather than a dose–response effect or may be one component of multiple physiologic factors responsible for breast milk production. One possibility would be an impairment of normal breast glandular development in early pregnancy. Although breast glandular maturation begins in puberty, terminal differentiation is not complete until after full term pregnancy and lactation (Martinson, Lyons, Giles, Borges, & Schedin, 2013). In animal studies, abnormalities in fat mass are associated with abnormal mammary gland development and low‐milk supply. In pigs, increased fat mass associates with up to 15% lower milk production compared with normal weight sows, which the authors speculated may be due to poor mammary development during pregnancy (Revell et al., 1998a, 1998b). Obese mice, as a result of a high‐fat diet, have been shown to have abnormal ductal branching and reduced alveolar development that associates with impaired lactogenesis (Flint, Travers, Barber, Binart, & Kelly, 2005). In addition, rat pups born to obese dams on a high‐fat diet were more likely to die in the first 3 days of life compared with pups born to dams on a control diet, which was postulated to be related to delayed lactogenesis and insufficient milk supply (Shaw, Rasmussen, & Myers, 1997). These animal models provide a plausible biological link between increased maternal % body fat, reduced mammary gland development, and decreased milk production, which could result in lower EBF rates as shown in our study population. It is also possible that these differences are driven by different postnatal practices or social confounders, which need to be assessed in greater detail.
Elevated maternal prepregnancy BMI was associated with significantly higher rates of stopping breastfeeding by 6 months postpartum, with over 20% of women who were overweight/obese having ceased to provide breastmilk compared with no women of normal BMI. Because several studies have suggested that length of lactation plays an important role in long‐term maternal health benefits (Gunderson et al., 2010; Ram et al., 2008; Schwarz et al., 2009; Stuebe et al., 2005; Stuebe et al., 2009), it is important to more closely examine why women with higher prepregnancy BMI are stopping breastfeeding earlier compared with their normal weight peers.
Although the primary intent of this study was to explore the relationship between maternal obesity and EBF among women intending EBF, it was notable that women with elevated BMI were much more likely to report intended mixed breast and formula feeding. Future studies are needed to explore the factors that influence the intention to combine breast and formula feeding rather than EBF as recommended.
Limitations to this study include a limited number of subjects, which was compounded by the loss of subjects due to incomplete survey completion. Although this may have resulted in an overestimation of differences in EBF rates, it is potentially more likely that findings should have been biased towards the null. Additionally, EBF intention was assessed at 6 weeks postpartum, which may not have accurately reflected predelivery intention. Strengths of this study include the detailed information on maternal EBF intent, % body fat, and breastfeeding behaviours for the first 6 months among a population of women with high‐EBF intention, which allows for characterization of the relationship between maternal % body fat and EBF without confounding related to intention differences. The majority of large‐database studies examining the impact of breastfeeding on maternal health outcomes to date have been hampered by limited data regarding the intensity of breastfeeding, as most relied on maternal recollection several years later as to whether they breastfed (yes or no) and how long they breastfed, which has been shown to both over and underestimate breastfeeding duration (Burnham et al., 2014). As such, the ability to establish EBF is substantially limited. A critical limitation of these studies, in contrast to our work, is the lack of distinction between desired formula use and undesired formula introduction due to lactation dysfunction, which is essential to determine the role of breastfeeding in maternal long‐term health.
In summary, this study found that among women intending EBF, elevated prepregnancy BMI and higher % body fat in early pregnancy were significantly associated with lower rates of EBF and higher rates of lactation cessation as of 6 months postpartum. It is possible that the difference in rates of EBF by maternal BMI are driven by confounding factors, and it is important for future studies to fully explore this possibility. Although some studies that have attempted to increase EBF rates among women who are obese have shown improvement in breastfeeding initiation, rates of EBF remain unchanged (Chapman et al., 2013; Martin, MacDonald‐Wicks, Hure, Smith, & Collins, 2015). Our findings suggest that a primary focus on psychosocial factors in these studies would miss important physiologic barriers related to increased fat mass in early gestation that contributed to low‐EBF rates. It would also miss the opportunity to speculate that intervention prior to pregnancy or in early pregnancy may be an important tool to increase rates of EBF. A similar implication of our data is that unrecognized physiologic barriers present prepregnancy and early in gestation, rather than failure to EBF, may also be responsible for increased long‐term maternal risk for diabetes, obesity, T2DM, and CVD and that future studies on breastfeeding and long‐term maternal health need to address lactation intention and dysfunction, not just duration. By better understanding the early physiological contributors to breastfeeding success, we can develop more effective interventions.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
All authors read and approved the final manuscript. NM, JP and KT designed the research study. NM performed the research. NM, BL, JP and KT analyzed the data. NM, JP and KT wrote the paper.
Supporting information
Figure S1: Kaplan Meier survival analysis for continuation of lactation by infant age in weeks stratified by prepregnancy BMI.
Table S1: Demographics of complete study cohort
Table S2: Association of weaning and maternal body composition among all subjects (no subjects intending exclusive lactation had weaned by 6 weeks, 10 had weaned by 6 months)
ACKNOWLEDGMENTS
We thank Mekhala Dissanayake for her assistance with figure generation. We gratefully acknowledge the mothers and babies who participated in the Maternal Body Composition Study.
Marshall NE, Lau B, Purnell JQ, Thornburg KL. Impact of maternal obesity and breastfeeding intention on lactation intensity and duration. Matern Child Nutr. 2019;15:e12732 10.1111/mcn.12732
REFERENCES
- Amir, L. H. , & Donath, S. (2007). A systematic review of maternal obesity and breastfeeding intention, initiation and duration. BMC Pregnancy and Childbirth, 7, 9 10.1186/1471-2393-7-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartick, M. C. , Schwarz, E. B. , Green, B. D. , Jegier, B. J. , Reinhold, A. G. , Colaizy, T. T. , … Stuebe, A. M. (2017). Suboptimal breastfeeding in the United States: Maternal and pediatric health outcomes and costs. Maternal & Child Nutrition, 13(1). 10.1111/mcn.12366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breastfeeding and the use of human milk . (2012). Breastfeeding and the Use of Human Milk. Pediatrics, 129(3), e827–e841. 10.1542/peds.2011-3552 [DOI] [PubMed] [Google Scholar]
- Burnham, L. , Buczek, M. , Braun, N. , Feldman‐Winter, L. , Chen, N. , & Merewood, A. (2014). Determining length of breastfeeding exclusivity: Validity of maternal report 2 years after birth. Journal of Human Lactation, 30(2), 190–194. 10.1177/0890334414525682 [DOI] [PubMed] [Google Scholar]
- Chapman, D. J. , Morel, K. , Bermudez‐Millan, A. , Young, S. , Damio, G. , & Perez‐Escamilla, R. (2013). Breastfeeding education and support trial for overweight and obese women: a randomized trial. Pediatrics, 131(1), e162–e170. 10.1542/peds.2012-0688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, S. R. , Kim, Y. M. , Cho, M. S. , Kim, S. H. , & Shim, Y. S. (2017). Association Between Duration of Breast Feeding and Metabolic Syndrome: The Korean National Health and Nutrition Examination Surveys. Journal of Women's Health (2002), 26(4), 361–367. 10.1089/jwh.2016.6036 [DOI] [PubMed] [Google Scholar]
- Chowdhury, R. , Sinha, B. , Sankar, M. J. , Taneja, S. , Bhandari, N. , Rollins, N. , … Martines, J. (2015). Breastfeeding and maternal health outcomes: A systematic review and meta‐analysis. Acta Paediatrica, 104(467), 96–113. 10.1111/apa.13102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey, K. G. (1997). ENERGY AND PROTEIN REQUIREMENTS DURING LACTATION. Annual Review of Nutrition, 17(1), 19–36. 10.1146/annurev.nutr.17.1.19 [DOI] [PubMed] [Google Scholar]
- Donath, S. M. , & Amir, L. H. (2008). Maternal obesity and initiation and duration of breastfeeding: Data from the longitudinal study of Australian children. Maternal & Child Nutrition, 4(3), 163–170. 10.1111/j.1740-8709.2008.00134.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein, S. B. , Labiner‐Wolfe, J. , Shealy, K. R. , Li, R. , Chen, J. , & Grummer‐Strawn, L. M. (2008). Infant Feeding Practices Study II: Study Methods. Pediatrics, 122(Supplement 2), S28–S35. 10.1542/peds.2008-1315c [DOI] [PubMed] [Google Scholar]
- Flint, D. J. , Travers, M. T. , Barber, M. C. , Binart, N. , & Kelly, P. A. (2005). Diet‐induced obesity impairs mammary development and lactogenesis in murine mammary gland. American Journal of Physiology: Endocrinology and Metabolism, 288(6), E1179–E1187. 10.1152/ajpendo.00433.2004 [DOI] [PubMed] [Google Scholar]
- Gunderson, E. P. , Hurston, S. R. , Ning, X. , Lo, J. C. , Crites, Y. , Walton, D. , … Type 2 Diabetes After, G. D. M. P. I (2015). Lactation and progression to Type 2 diabetes mellitus after gestational diabetes mellitus: A prospective cohort study. Annals of Internal Medicine, 163(12), 889–898. 10.7326/M15-0807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson, E. P. , Jacobs, D. R. Jr. , Chiang, V. , Lewis, C. E. , Feng, J. , Quesenberry, C. P. Jr. , & Sidney, S. (2010). Duration of lactation and incidence of the metabolic syndrome in women of reproductive age according to gestational diabetes mellitus status: A 20‐Year prospective study in CARDIA (Coronary Artery Risk Development in Young Adults). Diabetes, 59(2), 495–504. 10.2337/db09-1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauff, L. E. , Leonard, S. A. , & Rasmussen, K. M. (2014). Associations of maternal obesity and psychosocial factors with breastfeeding intention, initiation, and duration. American Journal of Clinical Nutrition, 99(3), 524–534. 10.3945/ajcn.113.071191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip, S. , Chung, M. , Raman, G. , Chew, P. , Magula, N. , DeVine, D. , … Lau, J. (2007). Breastfeeding and maternal and infant health outcomes in developed countries. Evid Rep Technol Assess (Full Rep), 153, 1–186. [PMC free article] [PubMed] [Google Scholar]
- Marshall, N. E. , Murphy, E. J. , King, J. C. , Haas, E. K. , Lim, J. Y. , Wiedrick, J. , … Purnell, J. Q. (2016). Comparison of multiple methods to measure maternal fat mass in late gestation. American Journal of Clinical Nutrition, 103(4), 1055–1063. 10.3945/ajcn.115.113464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, J. , MacDonald‐Wicks, L. , Hure, A. , Smith, R. , & Collins, C. E. (2015). Reducing postpartum weight retention and improving breastfeeding outcomes in overweight women: A pilot randomised controlled trial. Nutrients, 7(3), 1464–1479. 10.3390/nu7031464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez, J. L. , Chapman, D. J. , & Perez‐Escamilla, R. (2016). Prepregnancy obesity class is a risk factor for failure to exclusively breastfeed at hospital discharge among Latinas. Journal of Human Lactation, 32(2), 258–268. 10.1177/0890334415622638 [DOI] [PubMed] [Google Scholar]
- Martinson, H. A. , Lyons, T. R. , Giles, E. D. , Borges, V. F. , & Schedin, P. (2013). Developmental windows of breast cancer risk provide opportunities for targeted chemoprevention. Experimental Cell Research, 319(11), 1671–1678. 10.1016/j.yexcr.2013.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow, D. A. (1976). Fat cow syndrome. Journal of Dairy Science, 59(9), 1625–1629. 10.3168/jds.S0022-0302(76)84415-3 [DOI] [PubMed] [Google Scholar]
- Nommsen‐Rivers, L. A. , Chantry, C. J. , Peerson, J. M. , Cohen, R. J. , & Dewey, K. G. (2010). Delayed onset of lactogenesis among first‐time mothers is related to maternal obesity and factors associated with ineffective breastfeeding. American Journal of Clinical Nutrition, 92(3), 574–584. 10.3945/ajcn.2010.29192 [DOI] [PubMed] [Google Scholar]
- Nommsen‐Rivers, L. A. , Dolan, L. M. , & Huang, B. (2012). Timing of stage II lactogenesis is predicted by antenatal metabolic health in a cohort of primiparas. Breastfeeding Medicine, 7(1), 43–49. 10.1089/bfm.2011.0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization, W. H . (2002). The Optimal Duration of Exclusive Breastfeeding: Report of an Expert Consultation. Retrieved from Geneva: http://apps.who.int/iris/bitstream/10665/67208/1/WHO_NHD_01.08.pdf?ua=1
- O'Sullivan, E. J. , Perrine, C. G. , & Rasmussen, K. M. (2015). Early breastfeeding problems mediate the negative association between maternal obesity and exclusive breastfeeding at 1 and 2 months postpartum. Journal of Nutrition, 145(10), 2369–2378. 10.3945/jn.115.214619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, S. A. E. , Yang, L. , Guo, Y. , Chen, Y. , Bian, Z. , Du, J. , … Chen, Z. (2017). Breastfeeding and the risk of maternal cardiovascular disease: A prospective study of 300 000 Chinese women. Journal of the American Heart Association, 6(6). 10.1161/jaha.117.006081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preusting, I. , Brumley, J. , Odibo, L. , Spatz, D. L. , & Louis, J. M. (2017). Obesity as a Predictor of Delayed Lactogenesis II. Journal of Human Lactation, 33(4), 684–691. 10.1177/0890334417727716 [DOI] [PubMed] [Google Scholar]
- Ram, K. T. , Bobby, P. , Hailpern, S. M. , Lo, J. C. , Schocken, M. , Skurnick, J. , & Santoro, N. (2008). Duration of lactation is associated with lower prevalence of the metabolic syndrome in midlife‐‐SWAN, the study of women's health across the nation. American Journal of Obstetrics and Gynecology, 198(3), 268), e261–e266. 10.1016/j.ajog.2007.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revell, D. K. , Williams, I. H. , Mullan, B. P. , Ranford, J. L. , & Smits, R. J. (1998a). Body composition at farrowing and nutrition during lactation affect the performance of primiparous sows: I. Voluntary feed intake, weight loss, and plasma metabolites. Journal of Animal Science, 76(7), 1729–1737. 10.2527/1998.7671729x [DOI] [PubMed] [Google Scholar]
- Revell, D. K. , Williams, I. H. , Mullan, B. P. , Ranford, J. L. , & Smits, R. J. (1998b). Body composition at farrowing and nutrition during lactation affect the performance of primiparous sows: II. Milk composition, milk yield, and pig growth. Journal of Animal Science, 76(7), 1738–1743. 10.2527/1998.7671738x [DOI] [PubMed] [Google Scholar]
- Schwarz, E. B. , Brown, J. S. , Creasman, J. M. , Stuebe, A. , McClure, C. K. , Van Den Eeden, S. K. , & Thom, D. (2010). Lactation and maternal risk of type 2 diabetes: A population‐based study. American Journal of Medicine, 123(9), 863), e861–e866. 10.1016/j.amjmed.2010.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz, E. B. , Ray, R. M. , Stuebe, A. M. , Allison, M. A. , Ness, R. B. , Freiberg, M. S. , & Cauley, J. A. (2009). Duration of lactation and risk factors for maternal cardiovascular disease. Obstetrics and Gynecology, 113(5), 974–982. 10.1097/01.AOG.0000346884.67796.ca [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, J. A. , & Binns, C. W. (1999). Factors associated with the initiation and duration of breastfeeding: a review of the literature. Breastfeeding Review, 7(1), 5–16. [PubMed] [Google Scholar]
- Shaw, M. A. , Rasmussen, K. M. , & Myers, T. R. (1997). Consumption of a high fat diet impairs reproductive performance in Sprague‐Dawley rats. Journal of Nutrition, 127(1), 64–69. 10.1093/jn/127.1.64 [DOI] [PubMed] [Google Scholar]
- Stuebe, A. M. , Horton, B. J. , Chetwynd, E. , Watkins, S. , Grewen, K. , & Meltzer‐Brody, S. (2014). Prevalence and risk factors for early, undesired weaning attributed to lactation dysfunction. Journal of Women's Health (2002), 23(5), 404–412. 10.1089/jwh.2013.4506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuebe, A. M. , Michels, K. B. , Willett, W. C. , Manson, J. E. , Rexrode, K. , & Rich‐Edwards, J. W. (2009). Duration of lactation and incidence of myocardial infarction in middle to late adulthood. American Journal of Obstetrics and Gynecology, 200(2), 138), e131–e138. 10.1016/j.ajog.2008.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuebe, A. M. , & Rich‐Edwards, J. W. (2009). The reset hypothesis: Lactation and maternal metabolism. American Journal of Perinatology, 26(1), 81–88. 10.1055/s-0028-1103034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuebe, A. M. , Rich‐Edwards, J. W. , Willett, W. C. , Manson, J. E. , & Michels, K. B. (2005). Duration of lactation and incidence of type 2 diabetes. Jama, 294(20), 2601–2610. 10.1001/jama.294.20.2601 [DOI] [PubMed] [Google Scholar]
- Thompson, L. A. , Zhang, S. , Black, E. , Das, R. , Ryngaert, M. , Sullivan, S. , & Roth, J. (2013). The association of maternal pre‐pregnancy body mass index with breastfeeding initiation. Maternal and Child Health Journal, 17(10), 1842–1851. 10.1007/s10995-012-1204-7 [DOI] [PubMed] [Google Scholar]
- van Raaij, J. M. , Peek, M. E. , Vermaat‐Miedema, S. H. , Schonk, C. M. , & Hautvast, J. G. (1988). New equations for estimating body fat mass in pregnancy from body density or total body water. The American Journal of Clinical Nutrition, 48(1), 24–29. 10.1093/ajcn/48.1.24 [DOI] [PubMed] [Google Scholar]
- Vanky, E. , Nordskar, J. J. , Leithe, H. , Hjorth‐Hansen, A. K. , Martinussen, M. , & Carlsen, S. M. (2012). Breast size increment during pregnancy and breastfeeding in mothers with polycystic ovary syndrome: A follow‐up study of a randomised controlled trial on metformin versus placebo. BJOG: An International Journal of Obstetrics and Gynaecology, 119(11), 1403–1409. 10.1111/j.1471-0528.2012.03449.x [DOI] [PubMed] [Google Scholar]
- Wojcicki, J. M. (2011). Maternal prepregnancy body mass index and initiation and duration of breastfeeding: A review of the literature. Journal of Women's Health (2002), 20(3), 341–347. 10.1089/jwh.2010.2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler, A. G. , Wallner, M. , Kaiser, I. , Rossbauer, M. , Harsunen, M. H. , Lachmann, L. , … Hummel, S. (2012). Long‐term protective effect of lactation on the development of type 2 diabetes in women with recent gestational diabetes mellitus. Diabetes, 61(12), 3167–3171. 10.2337/db12-0393 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Kaplan Meier survival analysis for continuation of lactation by infant age in weeks stratified by prepregnancy BMI.
Table S1: Demographics of complete study cohort
Table S2: Association of weaning and maternal body composition among all subjects (no subjects intending exclusive lactation had weaned by 6 weeks, 10 had weaned by 6 months)


