Abstract
How do brain systems evaluate the affective valence of a stimulus — that is, its quality of being good or bad? One possibility is that a neural subsystem, or ‘module’ (such as a subregion of the brain, a projection pathway, a neuronal population or an individual neuron), is permanently dedicated to mediate only one affective function, or at least only one specific valence — an idea that is termed here the ‘affective modules’ hypothesis. An alternative possibility is that a given neural module can exist in multiple neurobiological states that give it different affective functions — an idea termed here the ‘affective modes’ hypothesis. This suggests that the affective function or valence mediated by a neural module need not remain permanently stable but rather can change dynamically across different situations. An evaluation of evidence for the ‘affective modules’ versus ‘affective modes’ hypotheses may be useful for advancing understanding of the affective organization of limbic circuitry.
Nature has placed mankind under the governance of two sovereign masters, pain and pleasure. It is for them alone to point out what we ought to do, as well as to determine what we shall do.
Jeremy Bentham, 1789 (REF.1).
The affective valence of a stimulus or event is its quality of being perceived as ‘good’ versus ‘bad’. Affective valence is never simply an inherent quality of any external stimulus but rather must be actively generated by the brain’s limbic systems and translated into hedonic, emotional and/or motivational reactions2–4. Affective valence has both hedonic and motivational aspects. The hedonic aspects of affective valence include positive hedonic impact (pleasure) or negative hedonic impact (displeasure or pain). These are the affective kernels of rewards and punishments as well as components of many emotions5–8. The motivational aspects of affective valence include functions that promote pursuit of rewards (such as incentive salience and declarative goals) and functions that mediate threat avoidance (such as fearful salience and passive avoidance)9–11.
Affective valence is commonly experienced consciously as a subjective feeling, but valence also influences many objectively measurable reactions, both behavioural and physiological12–14 (Box 1). Consequently, even in adult humans, objective affective reactions can occur either with conscious feelings or without them, depending on neural and psychological circumstances3,4,8,12,15,16. That is to say, subjective hedonic experience is a usual feature of affective valence but is not always a necessary feature. Therefore, in this article, I use quotation marks to distinguish between objective affective reactions (such as orofacial ‘liking’ reactions) and the subjective feelings denoted by the same words (such as the subjective feeling of liking).
Box 1 |. Affective reactions that can be objectively measured.
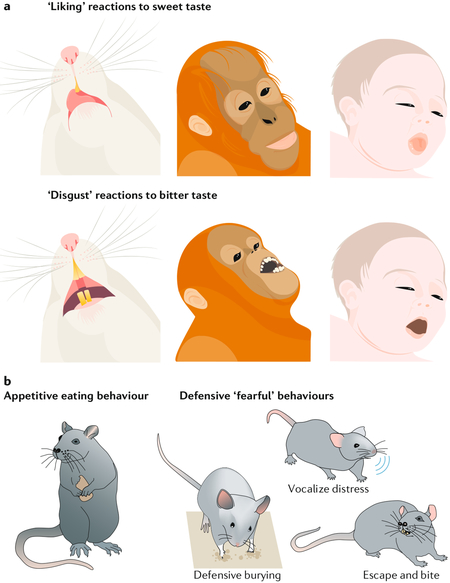
Examples of objective behavioural affective reactions include facial expressions of ‘liking’ or ‘disgust’, which reflect the hedonic impact of sweet or bitter tastes, respectively. Positive-valenced ‘liking’ reactions (such as lip licking and tongue protrusions) are elicited by sweet tastes in human infants, apes, monkeys and rodents, whereas negative-valenced ‘disgust’ reactions (including gaping, headshaking and forearm flailing) are elicited by bitter tastes (see the figure, part a)108,109. These affective reactions can be modified by factors that alter the hedonic impact of a particular taste, including physiological factors (such as hunger and/or satiety states), psychological factors (such as learned preferences and/or aversions) and neurobiological manipulations (such as neural inhibition or stimulation) (reviewed in REF.2). Similarly, appetitive food seeking and eating are positively valenced behaviours that can reflect ‘wanting’ food rewards (see the figure, part b). The negatively valenced objective reactions of animals also include a variety of passive and active ‘fearful’ reactions to threatening stimuli (see the figure, part b). For example, an auditory Pavlovian conditioned stimulus predicting footshock elicits freezing in place as a passive defensive reaction in rats or mice110–112, whereas a moving ‘robogator’ predator elicits active defensive or avoidance responses from rats86. Similarly, a rattlesnake, a shock-delivering object or another localized aversive threat elicits active defensive burying reactions that can deflect the perceived threat in squirrels, rats and mice113–116.
Measurable valenced reactions can also be emotionally specific; for example, ‘disgust’ and ‘fear’ are both negatively valenced defensive reactions but are qualitatively different from each other psychologically and behaviourally, elicited by distinct stimuli and have distinguishable (although partly overlapping) neural mechanisms12,70. Even ‘fear’ itself may describe any of a collection of different subtypes of valenced reactions86,117.
How do brain systems generate the difference between positive valence and negative valence? A traditional answer to this question is given by what I call here the ‘affective modules’ hypothesis (FIG. 1). This hypothesis (perhaps better described as a set of similar hypotheses) posits specific neural systems (which I here term ‘modules’) to be each reliably and permanently dedicated to the generation of a single affective valenced reaction in a one-to-one fashion. The proponents of this idea have applied it to neurobiological units of various types and scales. As such, I here use the term neural module to describe any of the following: an anatomical subregion within a brain structure, a specific neurotransmitter signal in a structure, a specific neuronal subpopulation or even a single neuron. A neural module will be further defined as becoming also a putative affective module when experimenters assign it a specific role related to an affective function2,17–36. Examples of proposed affective modules include an ‘appetitive projection’, a ‘reward subregion’, an ‘aversive subpopulation of neurons’ or a ‘defensive neuron’21,25–35.
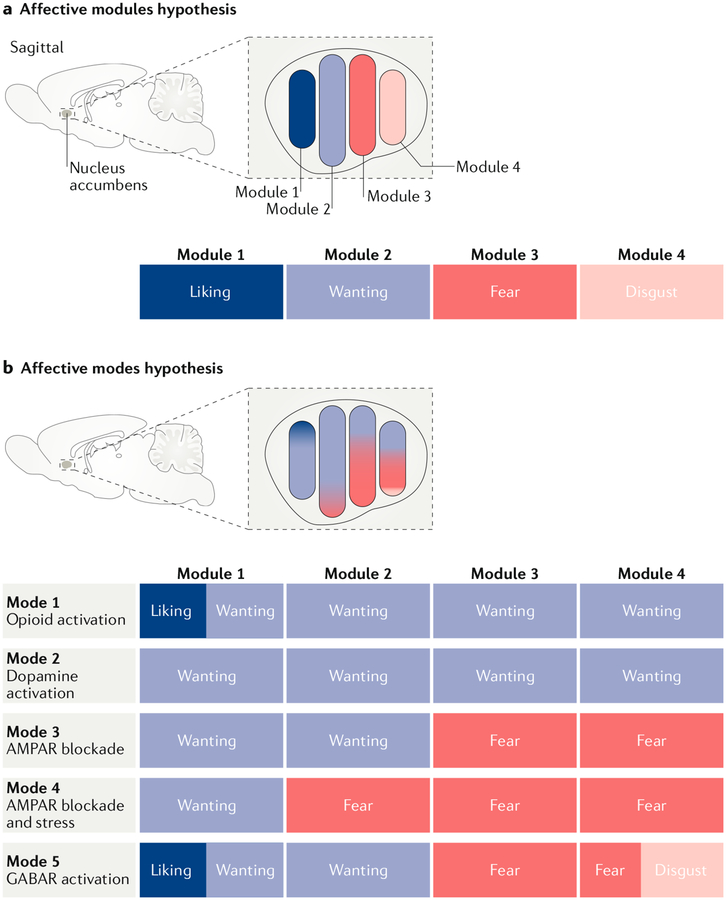
Fig. 1 |. The affective modules and affective modes hypotheses.
a | An affective modules hypothesis posits that a given neuron, neural system, projection or subregion reliably mediates only a single affective function. In the example shown here, which is based on some of the findings described in this article, a hypothesis of affective modules suggests that there are at least four affective modules within the nucleus accumbens shell. Each module is dedicated to mediating just one of four affective functions (the positive-valenced ‘liking’ and ‘wanting’ reactions and the negative-valenced ‘fear’ and ‘disgust’ reactions2,61,62,70) and is activated by particular manipulations of the nucleus accumbens (for example, ‘liking’ enhancement is triggered by opioid stimulation in the rostrodorsal quadrant of the medial shell62). b | An affective modes hypothesis that accounts for the same data would allow a given affective module (a given neuron, projection, neural system or subregion) to have more than one mode. For example, in the schematic example, module 1 (corresponding to a rostrodorsal site in the nucleus accumbens shell) can generate either pure ‘wanting’ (after dopamine receptor stimulation or AMPA receptor (AMPAR) blockade)45,47,70 or ‘liking’ plus ‘wanting’ (in response to opioid receptor stimulation47,62). Module 4 (corresponding to the caudal shell) can generate ‘wanting’ alone (after dopamine receptor or μ-opioid receptor stimulation)50,62, ‘fear’ alone (after AMPA receptor blockade)41,70 or ‘disgust’ plus ‘fear’ (after GABA agonist microinjection)61,70. However, a particular module may still have unique features that distinguish it from other modules (for example, only one of the modules illustrated has the capacity to enhance ‘liking’47,62 and only one has the capacity to generate ‘disgust’61,70). A particular module also may retain a valence bias across modes (for example, module 1 has strong positive-valenced bias41,62,70, whereas module 4 has a negative-valenced bias41,70) but still be capable of generating affective reactions of the opposite valence in particular modes (such as the switching between generation of ‘wanting’ and ‘fear’ by intermediate modules23,41, or the ‘wanting’ enhancement obtained from negatively biased module 4 in the dopamine receptor or μ-opioid receptor stimulation modes45,50,62). GABAR, GABA receptor.
Affective module hypotheses predominate in neuroscience today because they correspond to a traditional view of brain function in which the brain is decomposable into functional anatomical units, each with its own task. This view has been in place at least since the 1835 publication of Müller’s ‘law of specific nerve energies’37, which legitimately posited that different cranial nerves mediate distinct sensory modalities. Within sensory modalities, a modular organization in the brain also gives rise to topographic maps such as somatotopic homunculi, retinotopic maps and tonotopic maps38. Indeed, a module-based organization underlies essentially all localizations of brain function. When applied to affective valence, this view treats limbic neural modules as ‘affective labelled lines’, each carrying a permanently assigned affective valence-related function.
An alternative answer to the question of how brain systems mediate valence is what I will call here the ‘affective modes’ hypothesis39–41. This hypothesis suggests that a given neural module may not be permanently dedicated to just one affective function but rather have multiple neurobiological affective modes as states or conditions change, which give it different affective valence-related functions (FIG. 1). An affective mode is thus defined as one of several potential states of the same neural module, with different affective functions, and could correspond to a particular pattern of neurochemical stimulation or genomic activation of the module, a particular frequency or temporal pattern of electrical excitation within the module, a particular neurobiological state of the larger circuitry in which the neural module is embedded or other factors that alter the final output. Affective modes might shift with changes of internal physiological body states that modify neurobiological signalling and with changes of external stimuli, environmental ambience or other psychological conditions that modify relevant neurobiological states. It is proposed that these dynamic switches between affective modes can alter the affective function of a neural module as conditions change. Different neural modes might even shift some putative affective modules back and forth between positive-valence and negative-valence reactions. As described below, the affective modes hypothesis is consistent with evidence that much of the same limbic circuitry becomes activated by stimuli associated with both positive and negative valence or during many different emotions39,42.
In the remainder of this article, evidence for the affective modules and affective modes hypotheses is described and compared, focusing on the functions of proposed affective modules in the nucleus accumbens and the amygdala as useful examples (although these hypotheses would apply just as well to the hypothalamus or other limbic structures). In evaluating the evidence, I assume that the best criterion for concluding that an affective module is permanently dedicated to a single affective function would be its capacity to resist changes in affective function under a wide range of conditions. By contrast, evidence that an affective module switches its affective functions under particular conditions would support the conclusion that it has multiple affective modes.
Evidence from the nucleus accumbens
Role of mesolimbic dopamine in reward: ‘liking’, learning or ‘wanting’?.
The search for affective modules in the nucleus accumbens has been ongoing for several decades (FIG. 1). It began in the 1980s with manipulations of the mesolimbic dopamine system that projects from the midbrain to the nucleus accumbens, which many expected to be a positive-valence hedonic module for pleasure43. Most researchers in the field at this time were guided by a dominant ‘dopamine anhedonia’ hypothesis, which stated that suppression of dopamine’s actions reduced the psychological hedonic impact or pleasure of sensory rewards44. The experiments supporting this hypothesis had used dopamine receptor antagonists23,41 to suppress dopamine signalling, for example, causing rats to gradually cease working for or consuming sensory rewards (such as food, sex or brain stimulation) as though the hedonic impact of these rewards had drained away44.
It was therefore surprising when, around the year 1990, the first results from experiments measuring affective facial ‘liking’ reactions to sweetness in rats (a more direct measure of hedonic impact of a sensory pleasure that did not depend on voluntary pursuit or consumption) revealed that dopamine disruption, either by antagonist administration or by near elimination of all mesolimbic dopamine (via large 6-hydroxydopamine lesions that destroyed dopaminergic projections), did not suppress the ‘liking’ reactions to rewards after all. Instead, these manipulations suppressed only ‘wanting’ for the same rewards expressed as pursuit or consumption43. Subsequent stimulation of dopamine signalling by mesolimbic electrical stimulation or by drug microinjections in the nucleus accumbens confirmed that mesolimbic dopamine signalling caused increases in ‘wanting’ but not ‘liking’ the same reward2,45–47.
Dopamine neurons are often activated by predictive cues for reward48,49, and studies showed that incentive salience or ‘wanting’ was the psychological form of cue-triggered motivation mediated by mesolimbic dopamine, creating temporary urges to seek and consume the signalled reward12,50–53 and making reward cues more attractive and attention riveting54–57. In short, mesolimbic dopamine neurons appeared to be part of a module for cued incentive salience within the reward system rather than part of a module for hedonic impact48,49,58.
Nucleus accumbens hedonic hot spot.
A different neural module in the nucleus accumbens was eventually found that did amplify the hedonic impact of sensory pleasure. This was one of what later were found to be several interactive opioid-related ‘hedonic hot spots’ that form a network for enhancing ‘liking’ reactions to sweetness and were identified originally in rats2,47,59–63. These hedonic hot spots are small (1–6 mm3 volume in rats), bilateral subregions that are distributed and functionally interactive across the orbitofrontal cortex, insula, nucleus accumbens, ventral pallidum and brainstem2. Each hedonic hot spot is neurochemically restricted to only a few neurotransmitter signals that are responsible for amplifying ‘liking’ reactions2. For example, in hedonic hot spots, neurochemical stimulations of opioid, endocannabinoid or orexin signalling (but never dopamine signalling) have been found capable of enhancing ‘liking’ reactions to sweetness2,47,59–63. Thus, the network of these hedonic hot spots could be considered a ‘liking’ module in that they collectively amplify the hedonic impact of pleasant rewards. In the nucleus accumbens hedonic hot spot, which is located in its rostrodorsal medial shell (FIG. 2a), even stimulation of κ-opioid receptors amplified positive hedonic reactions, although κ-opioid receptor stimulation generates aversive reactions at many other brain sites62,64. However, in most of the nucleus accumbens shell outside the hedonic hot spot, opioid stimulation mimics the effects of dopamine stimulation described above; that is, it enhances only ‘wanting’ without ‘liking’, while even simultaneously suppressing ‘liking’ at some sites50,62,65–67. Perhaps underlying the ability of the hot spot of the nucleus accumbens to act as a ‘liking’ module, this subregion has unique anatomical features when compared with the remainder of the nucleus accumbens68,69. However, as I describe below, the nucleus accumbens hedonic hot spot also has other functional neurochemical modes that turn it into a pure ‘wanting’ module2,47,62,70.
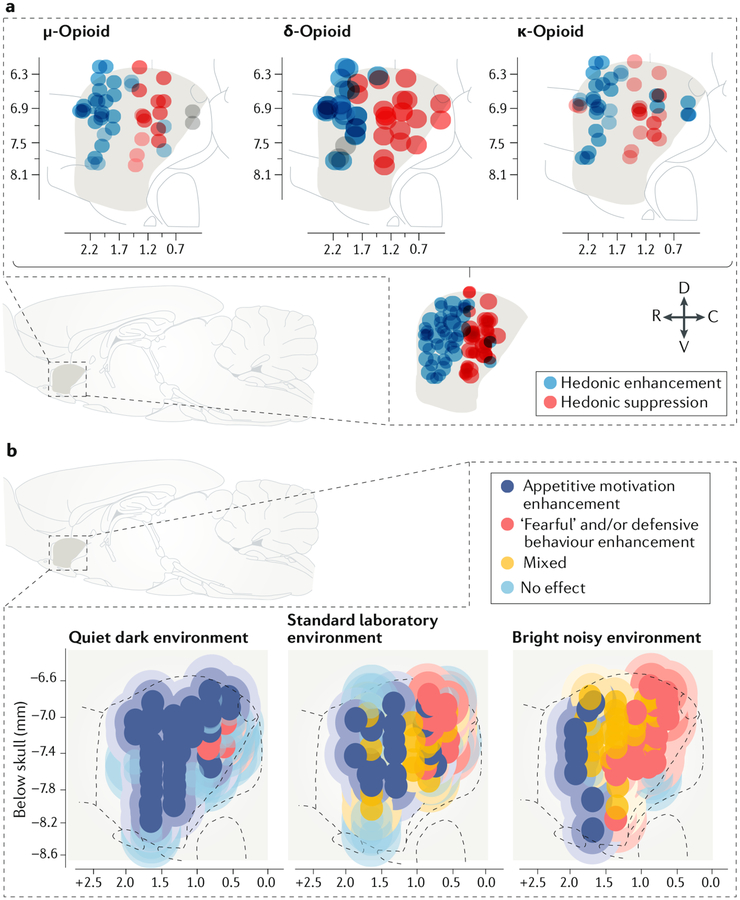
Fig. 2 |. Evidence for affective modules and affective modes in the nucleus accumbens.
a | A sagittal view of the medial shell of the rat nucleus accumbens shows the rostrodorsal ‘hedonic hot spot’, here revealed as the sites at which microinjections of μ, δ or κ-opioid receptor agonists all enhanced the hedonic impact of sucrose (blue circles; enhancement defined as 200–300% increases in taste-elicited orofacial ‘liking’ reactions)2,62. The axis numerals mark stereotaxic coordinates relative to Bregma. Conversely, in a caudal ‘hedonic cold spot’, the same opioid microinjections suppressed ‘liking’ reactions by ~50% (red circles). The bottom section shows the location of a shared opioid hot spot for hedonic enhancement and shared cold spot for hedonic suppression. b | A sagittal view of the rat nucleus accumbens shows a bivalent rostrocaudal gradient pattern of affective modules in the medial shell, revealed by microinjections of a glutamate AMPA receptor antagonist. Dark blue circles indicate microinjection sites that produced selective increases in appetitive motivation to eat food41 (similar results were shown in REF.23). Yellow circles show microinjection sites that enhanced both mixed appetitive and defensive behaviours, with these behaviours typically alternating in the same rat in the hour after microinjection. Red circles show microinjection sites that enhanced only actively defensive or ‘fearful’ behaviours. This study revealed shifts of the valence function of many microinjection sites driven by changes in environmental ambience. When rats were tested in a quiet, dark home environment, microinjection at most sites enhanced only appetitive behaviour. In a standard laboratory environment with moderate illumination and background sound levels, the nucleus shell was evenly divided between rostral appetitive, central mixed and caudal defensive zones. In a stressful highly illuminated and noisy laboratory environment, defensive and mixed zones expanded whereas the appetitive zone shrank to only the far-anterior edge. C, caudal; D, dorsal; R, rostral; V, ventral. Part a republished with permission of the Society for Neuroscience, from Opioid hedonic hotspot in nucleus accumbens shell: Mu, delta, and kappa maps for enhancement of sweetness “liking” and “wanting”. Castro, D. C. & Berridge, K. C. 34(12), 2014; permission conveyed through Copyright Clearance Center, Inc. (REF.62). Part b is adapted from REF.41, Springer Nature Limited.
Nucleus accumbens motivation modules.
In addition to the hedonic hot spots described above, a different type of nucleus accumbens modular organization has been reported, consisting of a rostrocaudal gradient of regions generating respectively intense appetitive-defensive reactions (indicative of bipolar motivational aspects of affective valence) in the nucleus accumbens medial shell23,41. This form of motivation generation supports the hypothesis that local neuronal inhibition of GABAergic output projections from the nucleus accumbens can disinhibit downstream targets in the ventral tegmentum, ventral pallidum and lateral hypothalamus to produce motivation for reward and extends that principle to defensive motivations as well71,72. These different nucleus accumbens motivational modules were first revealed by the results of experiments using inhibitory drug microinjections to alter local amino acid signalling in rats. These included antagonists of glutamate (AMPA) receptors (such as 6,7-dintroquinoxaline-2,3-dione (DNQX)) and GABAA receptor agonists (such as muscimol)70,73–75. At rostral nucleus accumbens shell sites, such microinjections cause increases in appetitive eating and can establish positive learned preferences for an associated place (FIG. 2b). By contrast, at caudal sites, the same drug microinjections instead generate intense defensive behaviours, such as anti-predator burying, establish a negative place avoidance and cause an approaching human hand to elicit distress calls, escape jumps and even bites from normally tame rats70,73–75. Rostral and caudal sites in the medial shell of the nucleus accumbens thus appear to be different affective modules generating motivations of opposite valences in the sense that appetitive versus defensive reactions are produced by the same microinjection drug, depending on its placement along the rostrocaudal gradient.
Multiple modes of anatomical nucleus accumbens modules.
Despite the initial evidence for the presence of affective modules in the nucleus accumbens shell, further experiments (described below) revealed that a given anatomical site in the rat nucleus accumbens shell can change modes to switch its affective function (FIGS 1b,2b). Not only can many sites in the medial shell switch affective modes in response to different neurochemical states, but some can also switch between positive and negative affective valence simply owing to a shift in the emotional ambience of the outside environment, even when they are in the same neurochemical state23,41. For example, in a test environment that is comfortably quiet, dark and similar to a rat’s home cage, AMPA receptor-blocking microinjections at most sites in the nucleus accumbens generate reactions of purely appetitive motivation23,41. However, when the environment is stressfully brighter and noisier than a standard laboratory, identical AMPA receptor-blocking microinjections at most of the same sites instead generate mostly ‘fearful’ reactions23,41 (FIG. 2b). Clearly, most sites within the nucleus accumbens medial shell are not permanently tuned to one affective valence function but rather have multiple modes that can dynamically flip to generate motivations of opposite affective valence as conditions change.
This reversal of affective valence mediated by the same nucleus accumbens module may depend on activation patterns of the larger mesocorticolimbic circuitry in which the nucleus accumbens is embedded, on the pattern of presynaptic input to the nucleus accumbens site or on other neurobiological factors influenced by the current environment23,41. Perhaps pointing to an underlying neurobiological mechanism for this switch within the nucleus accumbens, it has been shown that this environmental ‘retuning’ of affective valence also alters the neurochemical mode of neurons at the shell site of microinjection. For example, nucleus accumbens sites were shown to require only endogenous D1 dopamine receptor (DRD1) stimulation in order for the AMPA-receptor-blocking microinjection to generate appetitive behaviour in the comfortable environment; however, in the stressful environment, simultaneous endogenous stimulation of both D2 dopamine receptors (DRD2) and DRD1 was required at the same sites for the drug microinjection to generate defensive behaviour23. Mesolimbic dopamine is similarly reported to facilitate ‘fearful’ avoidance of aversive events76 as well as motivation for rewards.
Of course, some might suggest that a shift in DRD2 dependence indicates that there has been a shift between two different nucleus accumbens modules (composed of DRD1-expressing versus DRD2-expressing neurons) rather than between modes of the same module. After all, DRD1 and DRD2 are expressed on mostly different medium spiny neurons in the nucleus accumbens77. Medium spiny projection neurons that express DRD1 in the nucleus accumbens are often considered to mediate reward motivation, whereas DRD2-expressing neurons have been proposed by many to either suppress reward motivation and/or to mediate aversive motivation17–22. On the other hand, DRD1 neuronal stimulation also remained necessary for ‘fear’ generation by nucleus accumbens glutamate blockade. Furthermore, the idea that nucleus accumbens DRD2-expressing neurons are anti-reward modules faces some challenges that also call into question their modular identity. For example, it has been reported that nucleus accumbens DRD2-expressing neurons have a role in some forms of positive affective valence, such as positive appetitive motivation for reward, as well as in negative affective valence78–82. For example, DRD2-Cre mice are willing to work at least at moderate levels in order to optogenetically self-stimulate DRD2 neurons in the nucleus accumbens under some conditions78.
Further evidence for affective modes comes from experiments showing that shifting the neurochemical manipulation of the nucleus accumbens can add ‘disgust’ to the ‘fear’ reactions elicited by particular sites. When in a GABAergic mode (that is, after GABAA agonist microinjections), caudal sites in the rat nucleus accumbens shell produce intense affective ‘disgust’ reactions even in response to sucrose taste, in addition to the amplified ‘fearful’ defensive reactions described above61,70,73. By contrast, when these sites are in a glutamate-blocked mode (that is, after microinjections of an AMPA receptor antagonist), only defensive motivation-related reactions are enhanced and ‘liking’ reactions to sucrose remain unsuppressed. Conversely, at rostral nucleus accumbens shell sites, the GABAergic mode can generate enhancement of hedonic ‘liking’ reactions to sucrose, whereas the glutamate-blockade mode generates only the appetitive motivation to eat70,75,83,84. The addition of hedonic affective reactions in the form of ‘liking’ and ‘disgust’ mode might be explained by a stronger inhibitory hyperpolarization of nucleus accumbens neurons by the GABA agonist microinjection (which opens Cl− ion gates), which might disinhibit downstream structures more effectively than AMPA receptor blockade (which merely prevents glutamatergic depolarizations)70. Another difference in the neuronal state underlying the affective shift is that endogenous dopamine is needed at the nucleus accumbens site for the local glutamate blockade to generate appetitive or defensive motivations but is not needed for the GABAergic mode to generate ‘liking’ or ‘disgust’ at the same site23,73,85.
In short, both anatomical sites and specific neuronal subpopulations (such as DRD1-expressing or DRD2-expressing neurons) in the nucleus accumbens that have been proposed to be affective modules can flip between having a role in positive affective valence versus having a role in negative affective valence under some conditions. This flipping suggests that, although nucleus accumbens modules (defined as neural subsystems with distinguishable affective capabilities and affective valence biases) do exist, most may not be exclusively dedicated to mediating a single affective valence. That is, nucleus accumbens modules may be better thought of as having multiple affective modes.
Evidence from the amygdala
Some might consider the amygdala to be an especially likely region to contain permanent, labelled-line affective modules. Depending on the author, amygdala affective modules have been suggested to be particular amygdala nuclei or subregions of nuclei, particular point-to point projections, particular neuronal subtypes or individual neurons21,25–35. However, as outlined below, there is some evidence to suggest that at least some of these putative affective modules can switch functional modes86,87.
Roles of the amygdala in valence.
The amygdala as a structure has historically been portrayed as a mostly negative-valenced module that is primarily dedicated to ‘fear’ and defensive reactions, a reputation that has arisen in large part owing to the many studies of Pavlovian fear learning mediated by the basolateral nucleus of the amygdala (BLA) and the central nucleus of the amygdala (CeA)24,25,35,88. For example, a recent discussion of the roles of the amygdala suggested that various views “all imply that if through psychotherapy or pharmaceutical treatment you successfully reduce amygdala activity … pathological fear should be ameliorated”24. However, there is evidence that the role of the amygdala in ‘fear’ motivation may vary with situation, depending on whether the type of defensive behaviour being controlled is passive conditioned freezing, passive avoidance, active anti-predator reactions or active instrumental avoidance (reviewed in REF.86). Beyond fear, both the BLA and the CeA are now widely recognized to participate also in appetitive motivation for rewards and in positive emotions21,25–35, even by neuroscientists who mostly study ‘fear’ learning and defence responses25,35,88. Given that the amygdala contributes to both negative valence and positive valence, many neuroscientists have proposed a division of amygdala circuitry into two opposing groups of smaller modules: some permanently dedicated to positive valence and others to negative valence21,25–35.
Amygdala projection modules.
Connectivity-based amygdala hypotheses suggest that specific point-to-point projection modules, which send axons from one brain structure or subregion to another particular target, distinguish positive valence from negative valence25–27,89. For example, projections to the BLA from a ‘sweet-coding’ zone of the gustatory insula cortex have been suggested to mediate positive valence89, whereas projections to the CeA from a slightly posterior ‘bitter-coding’ cortical zone in the insula were suggested to mediate negative valence. Similarly, projections to the lateral capsule of the CeA ascending from the parabrachial nucleus of the pons, which express calcitonin gene-related peptide, have been suggested to carry negative-valenced signals of pain or other threats89,90. Within the amygdala itself, defensive motivation is traditionally viewed as being mediated by a serial relay of projections from the BLA to the CeA25–27,91.
Appetitive motivation for reward has instead been suggested to be mediated by parallel projections through the BLA and CeA and by projections from the BLA to the nucleus accumbens26,28,29. However, it is worth noting that stimulation of the BLA–nucleus accumbens pathway also has been reported to suppress appetitive seeking and alcohol consumption92 and that, conversely, BLA projections to the CeA also have been implicated in appetitive motivation31,93. Thus, at least some of the projection modules that have been proposed to be exclusively dedicated to one valence may switch to different valence functions under some conditions.
Neuronal population modules in the amygdala.
Many neuronal subpopulation modules have been proposed for the amygdala: according to these, specific types of neuron mediate positive valence whereas other types of neuron are proposed to mediate negative valence. Within the BLA, separate populations of neurons have been suggested to respectively code rewarding nicotine and aversive footshock stimuli30. Furthermore, only stimulation of the footshock-coding neurons causally established Pavlovian freezing as a learned defensive response, consistent with a role for these neurons in mediating negative valence. Another study proposed that defensive behaviours are mediated by a BLA population of magnocellular R-spondin 2-expressing (RSPO2+) neurons31. These RSPO2+ neurons may also have a projection-based and subregion-based modular identity in that they project specifically to the lateral half of the CeA (CeL). By contrast, appetitive behaviours have been suggested to be promoted by a different BLA population that projects to the CeA — parvocellular neurons that express protein phosphatase 1 and project to both the CeL and the medial CeA (CeM)32,94. However, it has recently been suggested that many BLA neurons that respond to reward-related stimuli may respond to threat-related stimuli in different conditions, raising the possibility that these neuronal populations have different affective modes87.
Within the CeA, a specific population of neurons that express DRD2 and protein kinase C δ-type (PRKCD) have been proposed to mediate defensive ‘fear’ reactions32. Anatomically, these neurons are located primarily in the anterior CeA and in the capsular nucleus of the CeA. Similarly, a population of CeA neurons that release corticotropin releasing factor (CRF) have been proposed for decades to be part of a negative-valence stress circuit activating to mediate the aversiveness of stressful stimuli as well as the unpleasantness of drug withdrawal in addiction95–97. Conversely, positive valence of appetitive motivation in the CeA has been proposed to be mediated by several neurochemically diverse populations of neurons that are generally distributed anatomically throughout the entire CeA32,98,99.
A different, more anatomical division of valence in CeA has also been proposed on the basis of tyramide-amplified immunohistochemistry–fluorescence in situ hybridization (TAI-FISH) measures of two successive waves of immediate gene expression triggered in neurons respectively by serial rewarding and aversive stimuli21. This proposal suggests that aversive valence is mediated by medial neurons in the CeM, whereas reward is mediated by neurons located in the lateral half of the CeL. However, a somewhat contrasting proposal is that RSPO2+ neurons specifically in the CeL mediate negative-valence functions31, and this is consistent with another suggestion that PRKCD-expressing neurons located in the CeL exert an anti-appetitive role and suppress food intake36. Finally, another hypothesis suggested that CeA 5-hydroxytryptamine receptor 2A (HTR2A)-expressing neurons (which lack PRKCD) mediate appetitive increases in eating33 and inhibit conditioned fearful reactions34.
There may be merit to all these modular amygdala proposals, but there is also substantial divergence across proposals and across the findings from laboratory to laboratory. This makes it difficult to extract any strong unifying conclusion that will apply as a general rule for parsing valence among modules in the amygdala. Instead, some may view discrepancies as adding support to the possibility that putative amygdala modules may not be permanently dedicated to a single affective function. Indeed, it may be worth considering alternative explanations, including the possibility that such modules are capable of more flexible modes of valence operation.
Evidence for affective modes in the amygdala.
Some recent evidence from studies that have used optogenetic stimulation to investigate the roles of the amygdala in incentive motivation supports the hypothesis that some CeA modules described above actually may have multiple affective modes. For example, it has been shown that optogenetic stimulation of neurons generally at widespread sites in the CeA can magnify positive incentive motivation for sucrose or cocaine51,100, although the stimulation would have recruited many of the CeA modules that have been posited to mediate negative affective valence.
In these studies, a particular sensory reward (such as one of two sucrose options, or one of two cocaine options) was paired with brief optogenetic stimulation of neurons in the CeA in rats51,100. For example, rats were offered opportunity to press either of two levers to earn identical sucrose rewards while CeA stimulation was paired with only one of those sucrose rewards, or the rats were offered opportunity to earn identical intravenous cocaine rewards while CeA stimulation was paired with only one of those drug rewards51,100. In both cases, the CeA stimulation magnified and focused incentive motivation specifically upon the laser-paired reward; that is, rats escalated effort and pursued and consumed only their laser-paired reward, mostly ignoring the other option. These incentive-focusing effects were achieved using a synapsin promoter to express channelrhodopsin (the target of the laser stimulation), which would infect most neuronal subpopulations. Equivalent incentive enhancements were produced by laser stimulation at many anatomical sites widely scattered throughout CeA, including sites in both the CeL and CeM51,100 (FIG. 3). Thus, neuronal subpopulations and anatomical sites that have been proposed to mediate negative valence would be recruited by such CeA stimulations, but the behavioural outcome in these situations was uniformly to magnify positive appetitive motivation. Although it is impossible to rule out the possibility that there are some negative-valence modules within the CeA, such near-universal CeA-mediated generation of positive-valence reactions would seem unlikely if powerful CeA modules permanently dedicated to negative valence were being activated. Instead, the widespread amplification of appetitive motivation suggests that most CeA affective modules either participated in enhancing the positive-valence incentive valuation of the paired sensory reward or became functionally masked in the appetitive situation despite co-activation. At the least, any negative-valence CeA modules that were co-stimulated gave no functional sign of their existence under these conditions.
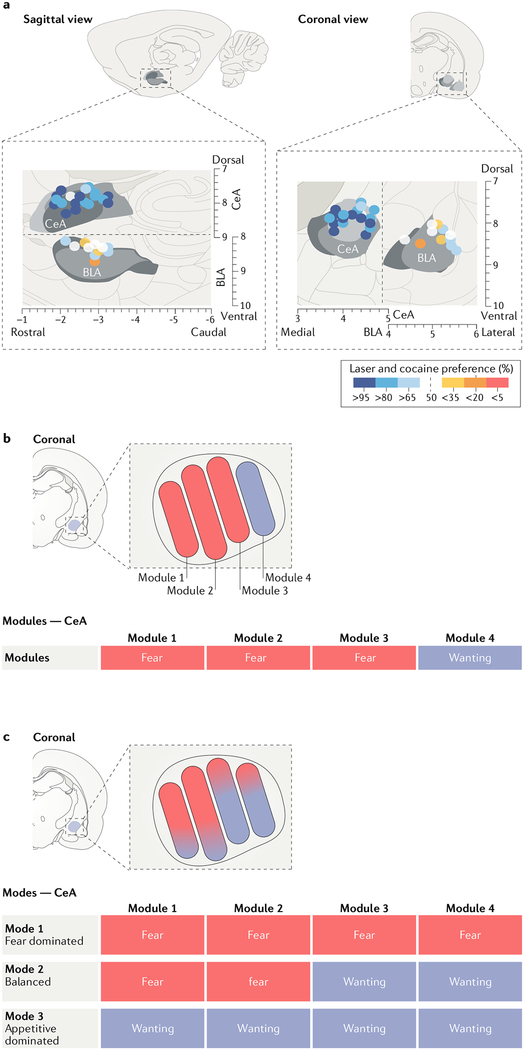
Fig. 3 |. Evidence for affective modules and affective modes in the central amygdala.
a | An anatomical depiction of sites within the central amygdala (CeA) at which optogenetic neuronal stimulation magnified and narrowly focused appetitive motivation to seek and consume a cocaine reward that was paired with the stimulation, compared with seeking alternative cocaine without amygdala stimulation (blue circles indicate increased appetitive motivation)51. Control sites in the basolateral amygdala (BLA) are also shown, at which optogenetic stimulation failed to enhance appetitive motivation (white circles indicate no change and yellow and orange circles indicate relative avoidance of optogenetic-paired cocaine). Sites throughout most of the CeA supported amplification of appetitive motivation in both the lateral (CeL) and medial (CeM) subdivisions of this nucleus51. b | A synthesis of affective modular hypotheses that view most CeA modules as negative-valenced modules that permanently mediate ‘fear’ or defensive reactions while allowing some other CeA modules to mediate positive-valenced motivations such as appetitive ‘wanting’21,25–35. This figure shows negative-valence dominance in the CeA, in accordance with popular views, but some modular hypotheses might posit an equal balance of positive and negative modules. c | An affective modes hypothesis allows each CeA neuronal module to have more than one mode and to mediate either positive valence or negative valence. According to this hypothesis, a particular CeA module may have a valence bias (for example, here modules at the left have negative-valence biases whereas modules at the right have positive-valence biases), but any given module is capable of switching to the opposite valence in at least one mode. Three potential modes are shown in rows at the bottom, consistent with data suggesting that some, or conceivably even all, CeA modules may be capable of mediating either appetitive or defensive motivations39,51,100. The optogenetic appetitive CeA mode described in part a51 corresponds to the third mode in the bottom row. A mixed compromise hypothesis could add one or two pure-valence modules if single-valence neural modules are ever proved to exist in the amygdala (not shown). Part a is adapted with permission from REF.51, Society for Neuroscience.
In addition, preliminary findings from a study that targeted optogenetic stimulation to CeA CRF-releasing neurons101, one of the proposed negative valence modules in the amygdala95–97,102, using Crh-Cre transgenic rats103 reproduced some of the same incentive motivation amplification effects of CeA optogenetic stimulation described above101. Similarly, other reports suggest that CeA CRF-releasing neurons can specifically participate in appetitive motivation32. Thus, CRF-releasing neurons of the CeA may not be dedicated affective modules for negative valence.
It is important to note that this is not proof against the existence of negative-valence modules in the amygdala and related structures: CRF-releasing neurons in the bed nucleus of the stria terminalis (BNST; including CeA subpopulations that specifically project to the BNST) may more robustly mediate fear and avoidance than CeA CRF neurons generally and therefore might well still turn out to be purely dedicated to negative-valence functions101,104. If this is the case, the module’s putative valence role should remain stable across a range of situations and experimental approaches and this should be a focus for future studies. Nevertheless, these observations do indicate that at least some putative CeA negative-valence modules do not live up to the assumption that their stimulation produces only defensive motivation, aversion or ‘fear’. Instead, these observations suggest that some CeA modules previously proposed to mediate negative valence may well be more flexible in function and able to switch to a positive-valence mode at least in some situations32,51,100,101 (FIG. 3). The hypothesis that CeA neural modules can alter functional modes, to participate in both positive and negative valence under different conditions, seems consistent with a recent proposal that “the same CeA output signal could give rise to very different behavioural responses depending on the functional states of other brain areas reflecting external and internal factors, such as context, anxiety, hunger or thirst.”39
Conclusions: modules or modes?
The findings described above indicate that affective modules do exist, at least in a limited sense as neural subunits that differ from each other in their affective capacities and in valence biases under standard laboratory conditions. However, they also may indicate a need for caution: our confidence in modular valence stability should be meted out only by degrees as evidence gradually builds, explicitly noting the range of conditions in which valence stability is thus far known to be maintained. It should be kept in mind that such modules may still be capable of switching modes and valenced roles in other, as yet untested, situations41,87. Some readers will point out that there is as yet insufficient evidence to prove the multiple modes hypothesis, just as there is insufficient evidence to prove permanent modules. However, this lack of evidence puts both modules and modes into the same degree of doubt. It does not provide a protective umbrella to simply assume the stable affective module view is true until proved otherwise.
Some may also believe that defining affective modules in terms of smaller neural units is a tactic able to identify a module with true valence stability, even if larger units appear to switch affective modes. For example, as noted by one of the reviewers of this article, what looks like modes of function in a moderately large neural system (such as a projection pathway or brain subregion) conceivably may be accounted for by the heterogeneity present within that system. The logic of this suggestion presumes that a smaller subpopulation within the larger module, or a single neuron within it, will prove to keep truly stable valence coding even when put to strong tests. Perhaps in some cases this will prove to be the case; however, few if any neuronal modules have been put to such robust tests thus far. Typically, we merely have evidence that an individual neuron repeatedly discriminates a reward stimulus from an aversive stimulus or that activation of a subpopulation in a particular setting has reliable valenced consequences. However, this is often tested in just one or two situations, and mere repetition of valence reliability in one condition is not sufficient evidence to conclude that it will also show valence fidelity as conditions become much different.
In the future, experiments may be better designed to test valence stability across more dramatically different conditions than has been done in the past. These could include different external stressful, neutral and rewarding situations or different internal physiological and/or motivational states. A step in that direction has been provided by a recent report comparing valence coding by serotonin neurons in mice when they were freely moving with that when their head was fixed in one position; results showed that increasing stress levels (due to head fixation) did not alter the serotonin neuronal tuning to reward-related stimuli but only changed the amplitude of positive-valence responses105. Future experiments might use a wider range of situations to probe the stability of valence coding by a neuronal module and use converging monitoring and manipulation techniques to confirm valence conclusions. In this way, confidence in any claim for stable coding by an affective module would grow.
Conversely, the strongest evidence for a multiple modes view would arise if the module proposed to have a negative-valence function in one condition, or by one laboratory, turns out to have a positive-valence function in experiments conducted in another condition or by another laboratory. Examples in which this situation has already arisen might be the disputed roles of DRD2 neurons in the nucleus accumbens or of CRF-releasing neurons in the CeA23,32,78–80,95–97,103,106. However, contradiction between claims does not mean that one valence claim is necessarily wrong. It might alert us instead to the fact that modular valence assignments are conditional to the particular experimental approaches and situations used to obtain them.
The possibility of multiple modes does not preclude the use of modular function labels such as ‘defensive system’, ‘fear circuit’, ‘hedonic hot spot’, ‘wanting circuit’ or ‘disgust system’. These are still useful terms because they describe real functional identities that can be reliably demonstrated, at least within a certain range of conditions. However, the same system or circuit might take on a different affective identity with shifts in neurobiological modes beyond those conditions. Recognition that limbic neural modules may have multiple neurobiological modes, with different affective functions, would change the way we think about those modules and carries implications for related clinical disorders. Awareness of the possibility of multiple modes may also help to avoid prematurely positing affective module hypotheses that later must be discarded if it turns out the module is also capable of other affective functions.
There is, admittedly, an explanatory gap between recognizing that multiple affective modes may exist on the one hand and specifying the mechanisms by which these mode switches occur on the other hand. What mechanism actually shifts a neural module between modes of affective function? The mechanism might relate to patterns of activity in larger circuitry in which it is embedded, to local neuromodulation of the state of the module itself, to synaptic plasticity in the circuitry and so forth. It is unsatisfying to have such explanatory gaps, but this may need to be tolerated until future research can fill the gaps by identifying the actual mechanisms involved in a mode shift. In the meantime, becoming alert to the possibility of multiple modes may help to facilitate the posing of research questions needed to identify mechanisms of mode shift. As Pasteur noted long ago, questions left unasked are rarely answered, whereas discovery favours the prepared mind107. Conversely, as another saying goes, ‘if you have only a hammer, everything looks like a nail’. Neuroscience has an excellent century-old hammer in its concept of functional modules, but it may now be time to add the idea of switchable modes to our conceptual toolbox.
Acknowledgements
The author thanks J. Olney, E. Naffziger, I. Morales, H. Baumgartner and the manuscript reviewers for helpful comments on an earlier version of the manuscript, and S. Warlow for assistance in the initial figure preparation. Research in the laboratory of K.C.B. has been supported by US National Institutes of Health grants DA015188 and MH063649.
Reviewer information
Nature Reviews Neuroscience thanks P. Janak and K. Tye, and other anonymous reviewer(s), for their contribution to the peer review of this work.
Footnotes
Competing interests
The author declares no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bentham J An Introduction to the Principles of Morals and Legislation (T. Payne and Son, 1789). [Google Scholar]
- 2.Berridge KC & Kringelbach ML Pleasure systems in the brain. Neuron 86, 646–664 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson DJ & Adolphs R A framework for studying emotions across species. Cell 157, 187–200 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Damasio A & Carvalho GB The nature of feelings: Evolutionary and neurobiological origins. Nat. Rev. Neurosci 14, 143–152 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Wundt W Outlines of Psychology (Engelmann, 1907). [Google Scholar]
- 6.Zajonc RB Feeling and thinking: preferences need no inferences. Am. Psychol 35, 151–175 (1980). [Google Scholar]
- 7.Russell JA & Barrett LF Core affect, prototypical emotional episodes, and other things called emotion: dissecting the elephant. J. Pers. Soc. Psychol 76, 805–819 (1999). [DOI] [PubMed] [Google Scholar]
- 8.Frijda NH & Parrott WG Basic emotions or ur-emotions? Emot. Rev 3, 406–415 (2011). [Google Scholar]
- 9.Schneirla TC An evolutionary and developmental theory of biphasic processes underlying approach and withdrawal. Nebr. Symp. Motiv 7, 1–42 (1959). [Google Scholar]
- 10.Bindra D How adaptive behavior is produced: a perceptual-motivation alternative to response reinforcement. Behav. Brain Sci 1, 41–91 (1978). [Google Scholar]
- 11.Toates F Motivational Systems (Cambridge Univ. Press, 1986). [Google Scholar]
- 12.Berridge KC Evolving concepts of emotion and motivation. Front. Psychol 9, 1647 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frijda NH The evolutionary emergence of what we call “emotions”. Cogn. Emot 30, 609–620 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Damasio A The Strange Order of Things: Life, Feeling, and the Making of Cultures (Pantheon, 2018). [Google Scholar]
- 15.Winkielman P, Berridge KC & Wilbarger JL Unconscious affective reactions to masked happy versus angry faces influence consumption behavior and judgments of value. Pers. Soc. Psychol. Bull 31, 121–135 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Winkielman P & Gogolushko Y Influence of suboptimally and optimally presented affective pictures and words on consumption-related behavior. Front. Psychol 8, 2261 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kravitz AV & Kreitzer AC Striatal mechanisms underlying movement, reinforcement, and punishment. Physiology 27, 167–177 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Francis TC & Lobo MK Emerging role for nucleus accumbens medium spiny neuron subtypes in depression. Biol. Psychiatry 81, 645–653 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koo JW et al. Loss of BDNF signaling in D1r-expressing NAc neurons enhances morphine reward by reducing GABA inhibition. Neuropsychopharmacology 39, 2646–2653 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volman SF et al. New insights into the specificity and plasticity of reward and aversion encoding in the mesolimbic system. J. Neurosci 33, 17569–17576 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiu J et al. Visualizing an emotional valence map in the limbic forebrain by TAI-FISH. Nat. Neurosci 17, 1552–1559 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Ward RD et al. Dissociation of hedonic reaction to reward and incentive motivation in an animal model of the negative symptoms of schizophrenia. Neuropsychopharmacology 37, 1699–1707 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richard JM & Berridge KC Nucleus accumbens dopamine/glutamate interaction switches modes to generate desire versus dread: D1 alone for appetitive eating but D1 and D2 together for fear. J. Neurosci 31, 12866–12879 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LeDoux JE & Hofmann SG The subjective experience of emotion: a fearful view. Curr. Opin. Behav. Sci 19, 67–72 (2018). [Google Scholar]
- 25.Moscarello JM & LeDoux JE The contribution of the amygdala to aversive and appetitive Pavlovian processes. Emot. Rev 5, 248–253 (2013). [Google Scholar]
- 26.Beyeler A et al. Divergent routing of positive and negative information from the amygdala during memory retrieval. Neuron 90, 348–361 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Britt JP et al. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron 76, 790–803 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Namburi P et al. A circuit mechanism for differentiating positive and negative associations. Nature 520, 675–678 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stuber GD et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475, 377–380 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gore F et al. Neural representations of unconditioned stimuli in basolateral amygdala mediate innate and learned responses. Cell 162, 134–145 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J, Pignatelli M, Xu S, Itohara S & Tonegawa S Antagonistic negative and positive neurons of the basolateral amygdala. Nat. Neurosci 19, 1636–1646 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim J, Zhang X, Muralidhar S, LeBlanc SA & Tonegawa S Basolateral to central amygdala neural circuits for appetitive behaviors. Neuron 93, 1464–1479 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Douglass AM et al. Central amygdala circuits modulate food consumption through a positive-valence mechanism. Nat. Neurosci 20, 1384–1394 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Isosaka T et al. Htr2a-expressing cells in the central amygdala control the hierarchy between innate and learned fear. Cell 163, 1153–1164 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Fanselow MS & Wassum KM The origins and organization of vertebrate Pavlovian conditioning. Cold Spring Harb. Perspect. Biol 8, a021717 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cai H, Haubensak W, Anthony TE & Anderson DJ Central amygdala pkc-δ+ neurons mediate the influence of multiple anorexigenic signals. Nat. Neurosci 17, 1240–1248 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Müller J Elements of Physiology (Taylor & Walton, 1842). [Google Scholar]
- 38.Kaas JH Topographic maps are fundamental to sensory processing. Brain Res. Bull 44, 107–112 (1997). [DOI] [PubMed] [Google Scholar]
- 39.Fadok JP, Markovic M, Tovote P & Luthi A New perspectives on central amygdala function. Curr. Opin. Neurobiol 49, 141–147 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Lindquist KA, Wager TD, Kober H, Bliss-Moreau E & Barrett LF The brain basis of emotion: a meta-analytic review. Behav. Brain Sci 35, 121–143 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reynolds SM & Berridge KC Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens. Nat. Neurosci 11, 423–425 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barrett LF & Wager TD The structure of emotion: evidence from neuroimaging studies. Curr. Dir. Psychol 15, 79–83 (2006). [Google Scholar]
- 43.Berridge KC & Robinson TE What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Res. Rev 28, 309–369 (1998). [DOI] [PubMed] [Google Scholar]
- 44.Wise RA The anhedonia hypothesis: mark III. Behav. Brain Sci 8, 178–186 (1985). [Google Scholar]
- 45.Wyvell CL & Berridge KC Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. J. Neurosci 20, 8122–8130 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berridge KC & Valenstein ES What psychological process mediates feeding evoked by electrical stimulation of the lateral hypothalamus? Behav. Neurosci 105, 3–14 (1991). [DOI] [PubMed] [Google Scholar]
- 47.Smith KS, Berridge KC & Aldridge JW Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proc. Natl Acad. Sci. USA 108, E255–E264 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schultz W Reward functions of the basal ganglia. J. Neural Transm. (Vienna) 123, 679–693 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Berke JD What does dopamine mean? Nat. Neurosci 21, 787–793 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pecina S & Berridge KC Dopamine or opioid stimulation of nucleus accumbens similarly amplify cue-triggered ‘wanting’ for reward: entire core and medial shell mapped as substrates for pit enhancement. Eur. J. Neurosci 37, 1529–1540 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Warlow SM, Robinson MJF & Berridge KC Optogenetic central amygdala stimulation intensifies and narrows motivation for cocaine. J. Neurosci 37, 8330–8348 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DiFeliceantonio AG & Berridge KC Dorsolateral neostriatum contribution to incentive salience: opioid or dopamine stimulation makes one reward cue more motivationally attractive than another. Eur. J. Neurosci 43, 1203–1218 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berridge KC From prediction error to incentive salience: mesolimbic computation of reward motivation. Eur. J. Neurosci 35, 1124–1143 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Flagel SB & Robinson TE Neurobiological basis of individual variation in stimulus-reward learning. Curr. Opin. Behav. Sci 13, 178–185 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hikosaka O, Ghazizadeh A, Griggs W & Amita H Parallel basal ganglia circuits for decision making. J. Neural Transm. (Vienna) 125, 515–529 (2018). [DOI] [PubMed] [Google Scholar]
- 56.Hickey C & Peelen MV Neural mechanisms of incentive salience in naturalistic human vision. Neuron 85, 512–518 (2015). [DOI] [PubMed] [Google Scholar]
- 57.Robinson MJ & Berridge KC Instant transformation of learned repulsion into motivational “wanting”. Curr. Biol 23, 282–289 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salamone JD, Correa M, Yang JH, Rotolo R & Presby R Dopamine, effort-based choice, and behavioral economics: basic and translational research. Front. Behav. Neurosci 12, 52 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Castro DC & Berridge KC Opioid and orexin hedonic hotspots in rat orbitofrontal cortex and insula. Proc. Natl Acad. Sci. USA 114, E9125–E9134 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Castro DC, Terry RA & Berridge KC Orexin in rostral hotspot of nucleus accumbens enhances sucrose ‘liking’ and intake but scopolamine in caudal shell shifts ‘liking’ toward ‘disgust’ and ‘fear’. Neuropsychopharmacology 41, 2101–2111 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ho CY & Berridge KC Excessive disgust caused by brain lesions or temporary inactivations: mapping hotspots of the nucleus accumbens and ventral pallidum. Eur. J. Neurosci 40, 3556–3572 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Castro DC & Berridge KC Opioid hedonic hotspot in nucleus accumbens shell: mu, delta, and kappa maps for enhancement of sweetness “liking” and “wanting”. J. Neurosci 34, 4239–4250 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peciña S & Berridge KC Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J. Neurosci 25, 11777–11786 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Al-Hasani R et al. Distinct subpopulations of nucleus accumbens dynorphin neurons drive aversion and reward. Neuron 87, 1063–1077 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kelley AE, Gauthier AM & Lang CG Amphetamine microinjections into distinct striatal subregions cause dissociable effects on motor and ingestive behavior. Behav. Brain Res 35, 27–39 (1989). [DOI] [PubMed] [Google Scholar]
- 66.Zhang M, Balmadrid C & Kelley AE Nucleus accumbens opioid, gabaergic, and dopaminergic modulation of palatable food motivation: contrasting effects revealed by a progressive ratio study in the rat. Behav. Neurosci 117, 202–211 (2003). [DOI] [PubMed] [Google Scholar]
- 67.Bakshi VP & Kelley AE Striatal regulation of morphine-induced hyperphagia - an anatomical mapping study. Psychopharmacology 111, 207–214 (1993). [DOI] [PubMed] [Google Scholar]
- 68.Thompson RH & Swanson LW Hypothesis-driven structural connectivity analysis supports network over hierarchical model of brain architecture. Proc. Natl Acad. Sci. USA 107, 15235–15239 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zahm DS, Parsley KP, Schwartz ZM & Cheng AY On lateral septum-like characteristics of outputs from the accumbal hedonic ‘hotspot’ of Peciña and Berridge with commentary on the transitional nature of basal forebrain ‘boundaries’. J. Comp. Neurol 521, 50–68 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Faure A, Richard JM & Berridge KC Desire and dread from the nucleus accumbens: cortical glutamate and subcortical GABA differentially generate motivation and hedonic impact in the rat. PLOS ONE 5, e11223 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meredith GE, Baldo BA, Andrezjewski ME & Kelley AE The structural basis for mapping behavior onto the ventral striatum and its subdivisions. Brain Struct. Funct 213, 17–27 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carlezon WA Jr & Thomas, M. J. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology 56(Suppl. 1), 122–132 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Richard JM, Plawecki AM & Berridge KC Nucleus accumbens GABArgic inhibition generates intense eating and fear that resists environmental retuning and needs no local dopamine. Eur. J. Neurosci 37, 1789–1802 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reynolds SM & Berridge KC Glutamate motivational ensembles in nucleus accumbens: rostrocaudal shell gradients of fear and feeding. Eur. J. Neurosci 17, 2187–2200 (2003). [DOI] [PubMed] [Google Scholar]
- 75.Reynolds SM & Berridge KC Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking”/”disliking” reactions, place preference/avoidance, and fear. J. Neurosci 22, 7308–7320 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wenzel JM et al. Phasic dopamine signals in the nucleus accumbens that cause active avoidance require endocannabinoid mobilization in the midbrain. Curr. Biol 28, 1392–1404 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Humphries MD & Prescott TJ The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Prog. Neurobiol 90, 385–417 (2010). [DOI] [PubMed] [Google Scholar]
- 78.Cole SL, Robinson MJF & Berridge KC Optogenetic self-stimulation in the nucleus accumbens: D1 reward versus D2 ambivalence. PLOS ONE 13, e0207694 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Soares-Cunha C et al. Activation of D2 dopamine receptor-expressing neurons in the nucleus accumbens increases motivation. Nat. Commun 7, 11829 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Steinberg EE et al. Positive reinforcement mediated by midbrain dopamine neurons requires D1 and D2 receptor activation in the nucleus accumbens. PLOS ONE 9, e94771 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Trifilieff P et al. Increasing dopamine D2 receptor expression in the adult nucleus accumbens enhances motivation. Mol. Psychiatry 18, 1025–1033 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Song R et al. Blockade of d3 receptors by yqa14 inhibits cocaine’s rewarding effects and relapse to drug-seeking behavior in rats. Neuropharmacology 77, 398–405 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stratford TR, Swanson CJ & Kelley A Specific changes in food intake elicited by blockade or activation of glutamate receptors in the nucleus accumbens shell. Behav. Brain Res 93, 43–50 (1998). [DOI] [PubMed] [Google Scholar]
- 84.Wirtshafter D & Stratford TR Evidence for motivational effects elicited by activation of GABA-A or dopamine receptors in the nucleus accumbens shell. Pharmacol. Biochem. Behav 96, 342–346 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Faure A, Reynolds SM, Richard JM & Berridge KC Mesolimbic dopamine in desire and dread: enabling motivation to be generated by localized glutamate disruptions in nucleus accumbens. J. Neurosci 28, 7184–7192 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Paré D & Quirk GJ When scientific paradigms lead to tunnel vision: lessons from the study of fear. NPJ Sci. Learn 2, 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kyriazi P, Headley DB & Pare D Multi-dimensional coding by basolateral amygdala neurons. Neuron 99, 1315–1328 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Maren S Parsing reward and aversion in the amygdala. Neuron 90, 209–211 (2016). [DOI] [PubMed] [Google Scholar]
- 89.Wang L et al. The coding of valence and identity in the mammalian taste system. Nature 558, 127–131 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Palmiter RD The parabrachial nucleus: CGRP neurons function as a general alarm. Trends Neurosci. 41, 280–293 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pare D, Quirk GJ & Ledoux JE New vistas on amygdala networks in conditioned fear. J. Neurophysiol 92, 1–9 (2004). [DOI] [PubMed] [Google Scholar]
- 92.Millan EZ, Kim HA & Janak PH Optogenetic activation of amygdala projections to nucleus accumbens can arrest conditioned and unconditioned alcohol consummatory behavior. Neuroscience 360, 106–117 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Janak PH & Tye KM From circuits to behaviour in the amygdala. Nature 517, 284–292 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tye KM et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471, 358–362 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Partridge JG et al. Stress increases gabaergic neurotransmission in crf neurons of the central amygdala and bed nucleus stria terminalis. Neuropharmacology 107, 239–250 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wise RA & Koob GF The development and maintenance of drug addiction. Neuropsychopharmacology 39, 254–262 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Koob GF Brain stress systems in the amygdala and addiction. Brain Res. 1293, 61–75 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gallagher M, Graham PW & Holland PC The amygdala central nucleus and appetitive Pavlovian conditioning: Lesions impair one class of conditioned behavior. J. Neurosci 10, 1906–1911 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Holland PC, Petrovich GD & Gallagher M The effects of amygdala lesions on conditioned stimulus-potentiated eating in rats. Physiol. Behav 76, 117–129 (2002). [DOI] [PubMed] [Google Scholar]
- 100.Robinson MJ, Warlow SM & Berridge KC Optogenetic excitation of central amygdala amplifies and narrows incentive motivation to pursue one reward above another. J. Neurosci 34, 16567–16580 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baumgartner HM, Schulkin J & Berridge KC in Neuroscience 2018 600.8 (Society for Neuroscience, San Diego, CA, 2018). [Google Scholar]
- 102.Flandreau EI, Ressler KJ, Owens MJ & Nemeroff CB Chronic overexpression of corticotropin-releasing factor from the central amygdala produces hpa axis hyperactivity and behavioral anxiety associated with gene-expression changes in the hippocampus and paraventricular nucleus of the hypothalamus. Psychoneuroendocrinology 37, 27–38 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pomrenze MB et al. A transgenic rat for investigating the anatomy and function of corticotrophin releasing factor circuits. Front. Neurosci 9, 487 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Asok A et al. Optogenetic silencing of a corticotropin-releasing factor pathway from the central amygdala to the bed nucleus of the stria terminalis disrupts sustained fear. Mol. Psychiatry 23, 914–922 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhong W, Li Y, Feng Q & Luo M Learning and stress shape the reward response patterns of serotonin neurons. J. Neurosci 37, 8863–8875 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lobo MK et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science 330, 385–390 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vallery-Radot R The Life of Pasteur (McLure, Phillips & Co., 1902). [Google Scholar]
- 108.Steiner JE, Glaser D, Hawilo ME & Berridge KC Comparative expression of hedonic impact: affective reactions to taste by human infants and other primates. Neurosci. Biobehav Rev 25, 53–74 (2001). [DOI] [PubMed] [Google Scholar]
- 109.Steiner JE The gustofacial response: observation on normal and anencephalic newborn infants. Symp. Oral Sens. Percept 4, 254–278 (1973). [PubMed] [Google Scholar]
- 110.LeDoux J Rethinking the emotional brain. Neuron 73, 653–676 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maren S, Phan KL & Liberzon I The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat. Rev. Neurosci 14, 417–428 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fanselow MS & Pennington ZT A return to the psychiatric dark ages with a two-system framework for fear. Behav. Res. Ther 100, 24–29 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Coss RG & Owings DH Snake-directed behavior by snake naive and experienced california ground squirrels in a simulated burrow. Z. Tierpsychol 48, 421–435 (1978). [Google Scholar]
- 114.Treit D, Engin E & McEown K Animal models of anxiety and anxiolytic drug action. Curr. Top. Behav. Neurosci 2, 121–160 (2010). [DOI] [PubMed] [Google Scholar]
- 115.Treit D, Pinel JP & Fibiger HC Conditioned defensive burying: a new paradigm for the study of anxiolytic agents. Pharmacol. Biochem. Behav 15, 619–626 (1981). [DOI] [PubMed] [Google Scholar]
- 116.Reynolds SM & Berridge KC Fear and feeding in the nucleus accumbens shell: rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. J. Neurosci 21, 3261–3270 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bolles RC & Fanselow MS A perceptual-defensive-recuperative model of fear and pain. Behav. Brain Sci 3, 291–323 (1980). [Google Scholar]